Abstract
Citrobacter amalonaticus is a bacterium that has rarely been reported as a human pathogen. Here we report four cases of C. amalonaticus infections occurring in patients hospitalized in Marseille, France, and review all cases described in the published literature.
Keywords: Bacteria, Citrobacter, epidemiology, infection, MALDI-TOF, urine
Introduction
Citrobacter amalonaticus, formerly called Levinea amalonatica, was first studied and described in 1971 after being isolated from various human samples from hospitalized patients, especially faeces [1]. Since then this bacterial species has been isolated from the environment [2], [3] as well as sporadically isolated from humans, mainly in faecal, urine, wound and respiratory samples [1], [4], [5], [6], [7], [8], [9]. Recently our syndromic clinical laboratory-based surveillance system, called BALYSES (the BActerial real-time LaboratorY-based SurveillancE System) [10], detected two consecutive C. amalonaticus kidney infections in two renal transplant recipients hospitalized in the same nephrology unit in Conception Hospital, Marseille, France. An additional two cases of C. amalonaticus infection were then detected over the following few weeks at other university hospitals in Marseille. We report here all cases from the literature to date.
Case Reports
Case 1
A 75-year-old woman with chronic renal failure due to membranoproliferative glomerulonephritis who had received a transplant in December 2010 was admitted to the nephrology unit of Conception Hospital for a regular check of her renal transplant. Since her transplantation the patient had developed membranoproliferative glomerulonephritis in the kidney transplant, urinary tract infections (UTIs) and diabetes as a result of immunosuppressive therapy. At admission in December 2014 analysis of a urine sample revealed leukocyturia (29.8 elements/mm3) and bacteriuria (104/mL C. amalonaticus, identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF; Bruker Daltonics, Leipzig, Germany)). Antimicrobial susceptibility testing revealed that the isolate was resistant to amoxicillin and susceptible to third-generation cephalosporins, carbapenem, cotrimoxazole, ciprofloxacin and amoxicillin/clavulanate. The patient was treated with amoxicillin/clavulanate during 7 days and was considered cured.
Case 2
A 61-year-old man who had received a renal transplant in November 2014 visited the nephrology unit at Conception Hospital for his weekly checkup in December 2014. In the urine samples collected, leukocyturia was 25.2 elements/mm3, and bacteriuria was 104/mL with C. amalonaticus, which was identified by MALDI-TOF. In this case C. amalonaticus was resistant to amoxicillin, ticarcillin and cotrimoxazole and susceptible to amoxicillin/clavulanate, ticarcillin/clavulanate, third-generation cephalosporins, carbapenems, nitrofurantoin, gentamicin and ciprofloxacin. The patient was treated a first time in December with ciprofloxacin during 7 days; antibiotic therapy was recommended only during urologic surgery. He underwent treatment a second time from 11 March 2015 to 3 April 2015.
Case 3
A 4-year-old child with Leigh syndrome, which is a mitochondrial cytopathy due to a heterozygote mutation on the SURF1 gene, was admitted to the intensive care unit at Timone Hospital for cardiogenic shock due to Epstein-Barr virus infection on 16 February 2015. On 6 April 2015 a urine sample was collected; analysis revealed that leukocyturia was 35 elements/mm3, hematuria 8 elements/mm3 and bacteriuria 104/mL. The bacterium C. amalonaticus, identified by MALDI-TOF, was resistant to amoxicillin, amoxicillin/clavulanate, ticarcillin and ticarcillin/clavulanate and susceptible to carbapenems, third-generation cephalosporins, carbapenems, nitrofurantoin, ciprofloxacin and cotrimoxazole. The patient was treated with cotrimoxazole and was cured after 7 days of treatment.
Case 4
A 27-day-old newborn girl was brought into the paediatric unit in North Hospital on 4 March 2015 for rhinitis and fever (temperature 38.3°C). A urine sample was collected; analysis revealed leukocyturia at 13.2 elements/mm3 and bacteriuria 107/mL C. amalonaticus. The strain was susceptible to amoxicillin/clavulanate, ticarcillin/clavulanate, ciprofloxacin, cotrimoxazole, nitrofurantoin, carbapenems and third-generation cephalosporins but resistant to amoxicillin and ticarcillin. The patient was symptomatically treated with paracetamol for fever; no antibiotic treatment was provided for asymptomatic urinary colonization.
Epidemiologic features
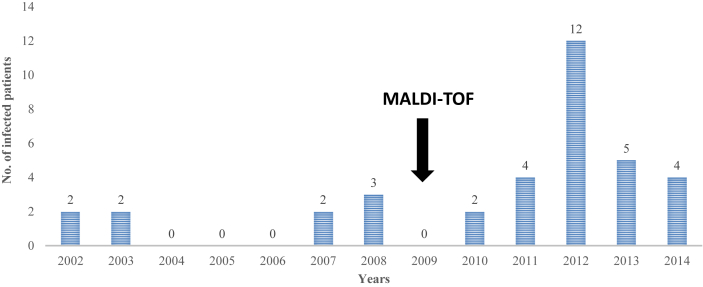
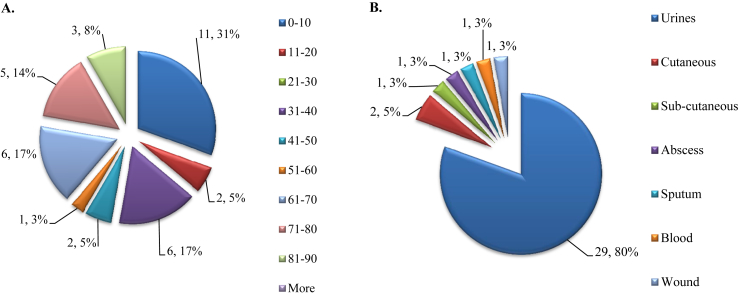
Because the two initial cases were reported in the nephrology unit in renal transplant recipients, we looked at our updated 13-year historical database [11] and retrospectively found that 36 patients had experienced C. amalonaticus infections before 2015 (Fig. 1). Most of the infections occurred in male subjects (21 male and 15 female patients), were hospital-acquired infections (22 infections, 62%) and were UTIs (29/36, 80%) (Fig. 2). We also identified a peak in the number of infected patients in 2012: a total of 12 patients were infected that year (Fig. 1). When we compared the global number of patients experiencing C. amalonaticus UTIs before 2012 and since 2012 to the number of patients experiencing Escherichia coli UTIs over the same two time periods, based on our updated 13-year historical database [11], we observed that there were statistically more UTIs due to C. amalonaticus after the 2012 peak than before (11 in 25 789 patient–bacteria pairs from 2002 to 2011 vs. 18 of 13 502 patient–bacteria pairs from 2012 to 2014; p 0.003). Moreover, we observed that the majority of the strains were collected from young children (11 patients, 31% of all infected patients). When we compared the proportion of patients experiencing C. amalonaticus infection who were younger than 11 years old to that of E. coli infection, based on our historical database [11], we noted that the proportion of patients under 11 years infected by C. amalonaticus was statistically higher than that of E. coli (11 of 6361 patient–bacteria pairs vs. 25 of 43 169 patient–bacteria pairs; p 0.003). Furthermore, we statistically identified more patients infected with C. amalonaticus after the introduction of MALDI-TOF technology for routine bacteria identification in our laboratory [11] in 2009 than before (nine of 82 436 patient–bacteria pairs from 2002 to 2008 vs. 27 of 115 922 patient–bacteria pairs from 2009 to 2014; p 0.04) (Fig. 1).
Fig. 1.
Annual evolution of number of patients with Citrobacter amalonaticus infections at our hospital from January 2002 to December 2014.
Fig. 2.
Main features of 36 patients with Citrobacter amalonaticus infections. (A) Age distribution of patients infected with C. amalonaticus. (B) Different kinds of samples from which bacterium was isolated.
Discussion
C. amalonaticus has only rarely been isolated in humans (Table 1). In 2009, a Chinese woman who had received a kidney transplant contracted peritonitis due to C. amalonaticus. In another study, a 63-year-old woman who had undergone bone marrow transplantation developed wound infection caused by C. amalonaticus. Another case concerned a 75-year-old man with pancreatic cancer who contracted a biliary tract infection and bacteraemia due to C. amalonaticus. In Italy, it was recovered from a urine sample in one patient who had received a renal graft 10 months earlier. In the United States, between 1972 and 1978, at the Seattle Veterans Administration Medical Center, C. amalonaticus was identified in urine and fluid samples from five patients. Among these patients, two had UTIs. In Thailand, a man contracted enteric fever, and C. amalonaticus was incriminated.
Table 1.
Characteristics of patients with Citrobacter amalonaticus infections reported in our studies and elsewherea
| Case, no. of patient (country) | Age (years)/sex | Infection type | Underlying condition | Sampling date | Identification method | Antibiotic therapy | Issue | Reference |
|---|---|---|---|---|---|---|---|---|
| 1, 1 (China) | 47/F | Peritonitis | IgA nephropathy, renal graft, intermittent peritoneal dialysis | NA | Biochemical tests and16S rRNA | CAZ AK | Cure | [9] |
| 2, 1 (Italy) | 63/F | Wound infection | ABMT, AML, intracranial haemorrhages | NA | Vitek 2 system | TG | Cure | [4] |
| 3, 1 (Taiwan) | 75/M | Bacteraemia | Ampulla vater cancer | NA | Phoenix automated system | CMZ | Cure | [6] |
| 4, 1 (Italy) | NA | NA | Renal allograft 10 months earlier | NA | API 20E | NA | NA | [12] |
| 5, 1 (Thailand) | 53/M | Enteric fever | Fever, water diarrhoea, headache, travel to Asia | NA | Biochemical tests | CRO SXT | Cure | [8] |
| 6, 5 (USA) | 2 patients | NA | Urinary tract abnormality, diabetes, malignancy | NA | Biochemical tests | NA | NA | [7] |
| 3 patients | NA | Diabetes mellitus | NA | NA | NA | |||
| 1, 1 (France) | 77/F | Urinary tract infection | Renal graft, chronic nephropathy, diabetes | 02/03/15 | MALDI-TOF | AMC | Cure | Our study |
| 2, 1 (France) | 61/M | Urinary tract infection | Renal graft, lymphatic cyst of graft, ureter stenosis, diabetes | 10/12/14 04/03/15 | MALDI-TOF | CIP | Cure | Our study |
| 3, 1 (France) | 4/M | Urinary tract infection | Leigh syndrome | 04/02/15 | MALDI-TOF | SXT | Cure | Our study |
| 4, 1 (France) | 0/F | Asymptomatic urinary colonization | Fever, rhinitis | 04/03/15 | MALDI-TOF | No | Cure | Our study |
ABMT, allogenic bone marrow transplantation; AMC, amoxicillin/clavulanate; AMT, acute myelogenous leukaemia; CAZ, ceftazidime; CIP, ciprofloxacin; CMZ, cefmetazole; CRO, ceftriaxone; Ig, immunoglobulin; MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; NA data not available; SXT, cotrimoxazole; TG, tigecycline.
Case = number of cases previously described in the literature; no of patient = number of patients infected in the case report.
Only studies reporting fully described infections with well-identified C. amalonaticus are included.
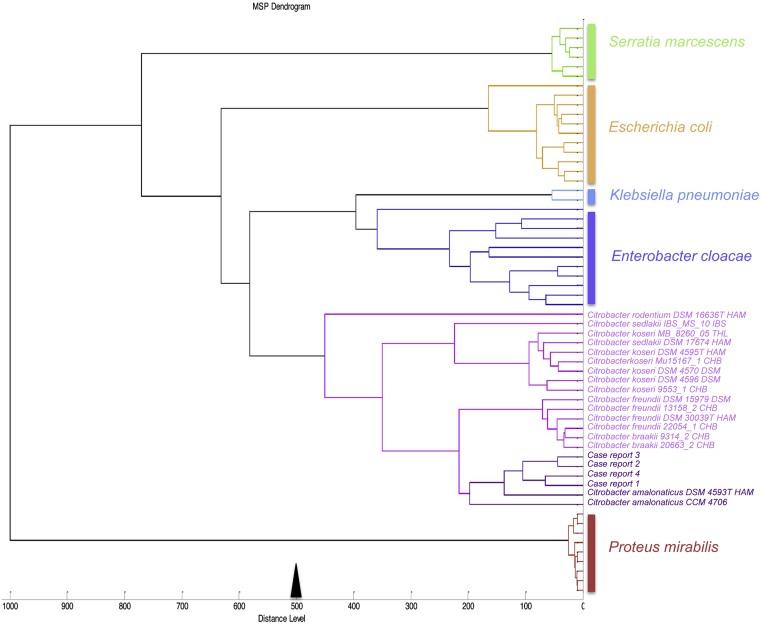
In our study, we identified four cases of C. amalonaticus infection by MALDI-TOF. The four spectra were then included in a dendrogram built with Bruker MALDI Biotyper 3.0 software (Fig. 3). Among all cases reported, including ours, we observed five patients who had undergone kidney transplantation or who had a urinary tract abnormality (case 6) [7]. Also, we observed that patients were mainly immunocompromised, including a patient with a renal graft [9], [12], a newborn patient (case 4) and patients with leukaemia and pancreatic cancer (cases 2 and 3) [4], [6]. Taken together, our observations and those in previously published reports (Table 1) lead us to think that this bacterium is an opportunistic pathogen, especially in patients with urinary tract failure. The fact that C. amalonaticus infection was rarely reported in the past may be explained by the fact that this bacterium is difficult to identify (Table 1). Thus, conventional methods such as the API system and RapID onE may underestimate their prevalence by misidentifying the strains [7], [13], [14], [15], [16]. The fact we statistically identified more C. amalonaticus infections after we instituted the routine use of the MALDI-TOF may have resulted in an improvement in the identification of this bacterial species. However, we found a statistically higher prevalence of the number of UTIs due to this bacterium even after routine use of MALDI-TOF (after 2012), suggesting that it is likely that this bacteria could be an emerging pathogen responsible for UTIs in immunocompromised patients.
Fig. 3.
Main-spectrum dendrogram of four Citrobacter amalonaticus isolates built from protein spectra.
Acknowledgements
Supported in part by the Centre National de la Recherche Scientifique and the IHU Méditerranée Infection. We thank Trad Online for English-language editing.
Conflict of Interest
None declared.
References
- 1.Young V.M., Kenton D.M., Hobbs B.J., Moody M.R. Levinea, a new genus of the family Enterobacteriaceae. Int J Syst Bacteriol. 1971;21:58–63. [Google Scholar]
- 2.Nawaz M., Khan A.A., Khan S., Sung K., Steele R. Isolation and characterization of tetracycline-resistant Citrobacter spp. from catfish. Food Microbiol. 2008;25:85–91. doi: 10.1016/j.fm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Urbanova E., Pacova Z. Identification of Citrobacter species and their occurrence in raw products and foods. Vet Med (Praha) 1997;42:87–91. [PubMed] [Google Scholar]
- 4.Aschbacher R., Pagani L., Doumith M., Pike R., Woodford N., Spoladore G. Metallo-beta-lactamases among Enterobacteriaceae from routine samples in an Italian tertiary-care hospital and long-term care facilities during 2008. Clin Microbiol Infect. 2011;17:181–189. doi: 10.1111/j.1198-743X.2010.03225.x. [DOI] [PubMed] [Google Scholar]
- 5.Farmer J.J., III, Davis B.R., Hickman-Brenner F.W., McWhorter A., Huntley-Carter G.P., Asbury M.A. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol. 1985;21:46–76. doi: 10.1128/jcm.21.1.46-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai C.C., Tan C.K., Lin S.H., Liu W.L., Liao C.H., Huang Y.T. Bacteraemia caused by non-freundii, non-koseri Citrobacter species in Taiwan. J Hosp Infect. 2010;76:332–335. doi: 10.1016/j.jhin.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Lipsky B.A., Hook E.W., III, Smith A.A., Plorde J.J. Citrobacter infections in humans: experience at the Seattle Veterans Administration Medical Center and a review of the literature. Rev Infect Dis. 1980;2:746–760. doi: 10.1093/clinids/2.5.746. [DOI] [PubMed] [Google Scholar]
- 8.Suwansrinon K., Wilde H., Sitprija V., Hanvesakul R. Enteric fever–like illness caused by infection with Citrobacter amalonaticus. J Med Assoc Thai. 2005;88:837–840. [PubMed] [Google Scholar]
- 9.Wong M.Y., Lau S.K., Tang S.C., Curreem S.O., Woo P.C., Yuen K.Y. First report of peritoneal dialysis–related peritonitis caused by Citrobacter amalonaticus. Perit Dial Int. 2012;32:224–225. doi: 10.3747/pdi.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abat C., Chaudet H., Colson P., Rolain J.M., Raoult D. 2015 Jul 13. Real-time microbiology laboratory surveillance system to detect abnormal events and emerging infections, Marseille, France; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seng P., Abat C., Rolain J.M., Colson P., Lagier J.C., Gouriet F. Identification of rare pathogenic bacteria in a clinical microbiology laboratory: impact of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol. 2013;51:2182–2194. doi: 10.1128/JCM.00492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mugnaioli C., Luzzaro F., De L.F., Brigante G., Amicosante G., Rossolini G.M. Dissemination of CTX-M-type extended-spectrum beta-lactamase genes to unusual hosts. J Clin Microbiol. 2005;43:4183–4185. doi: 10.1128/JCM.43.8.4183-4185.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffery J., Sulaiman S., Oothuman P., Vellayan S., Zainol-Ariffin P., Paramaswaran S. Domiciliary cockroaches found in restaurants in five zones of Kuala Lumpur Federal Territory, peninsular Malaysia. Trop Biomed. 2012;29:180–186. [PubMed] [Google Scholar]
- 14.Kitch T.T., Jacobs M.R., Appelbaum P.C. Evaluation of RapID onE system for identification of 379 strains in the family Enterobacteriaceae and oxidase-negative, Gram-negative nonfermenters. J Clin Microbiol. 1994;32:931–934. doi: 10.1128/jcm.32.4.931-934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan M.G., Stuart C., Leanord A.T., Enright M., Cole G.F. Citrobacter diversus brain abscess: case reports and molecular epidemiology. J Med Microbiol. 1992;36:273–278. doi: 10.1099/00222615-36-4-273. [DOI] [PubMed] [Google Scholar]
- 16.York M.K., Brooks G.F., Fiss E.H. Evaluation of the autoSCAN-W/A rapid system for identification and susceptibility testing of Gram-negative fermentative bacilli. J Clin Microbiol. 1992;30:2903–2910. doi: 10.1128/jcm.30.11.2903-2910.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]