Abstract
Background
Long QT syndrome type 3 (LQT3) is a lethal disease caused by gain-of-function mutations in the SCN5A gene, coding for the alpha-subunit of the sodium channel NaV1.5. Mexiletine is used to block late sodium current and to shorten QT interval in LQT3 patients.
Objectives
The aim of this study was to determine whether mexiletine prevents arrhythmic events (arrhythmic syncope, aborted cardiac arrest, or sudden cardiac death) in LQT3 patients.
Methods
The endpoint of this retrospective cohort study, which studied consecutive LQT3 patients who were referred to our center and treated with mexiletine, was to evaluate the antiarrhythmic efficacy of mexiletine by comparing the number of arrhythmic events per patient and the annual rate of arrhythmic events during observation periods of equal duration before and after the beginning of therapy with mexiletine.
Results
The study population comprised 34 LQT3 patients, 19 (56%) of whom were male. The median age at beginning of treatment with mexiletine was 22 years, and median QTc interval before therapy 509 ms. The median duration of oral mexiletine therapy was 36 months, at an average daily dose of 8 ± 0.5 mg/kg. Mexiletine significantly shortened QTc (by 63 ± 6 ms; p < 0.0001) and reduced the percentage of patients with arrhythmic events (from 22% to 3%; p = 0.031), the mean number of arrhythmic events per patient (from 0.43 ± 0.17 to 0.03 ± 0.03; p = 0.027), and the annual rate of arrhythmic events (from 10.3% to 0.7%; p = 0.0097).
Conclusions
Besides shortening QTc interval, mexiletine caused a major reduction of life-threatening arrhythmic events in LQT3 patients, thus representing an efficacious therapeutic strategy.
Key Words: beta-blocker, mutation, SCN5A, sodium channel, sudden cardiac death
Abbreviations and Acronyms: CI, confidence interval; ECG, electrocardiogram; LQT3, long QT syndrome type 3; LQTS, long QT syndrome
Long QT syndrome type 3 (LQT3) is a rare variant of long QT syndrome (LQTS) caused by gain-of-function mutations in the gene SCN5A, coding for the sodium channel NaV1.5 (1). Based on reports that the incidence of LQTS in neonates is 1:2,000 (2) and that LQT3 accounts for approximately 10% of LQTS patients (3), the incidence of LQT3 is estimated at 1:20,000.
LQT3 is characterized by a severe prognosis 3, 4 and an incomplete response to beta-blockers (5), as compared to LQTS type 1, the most common LQTS variant. Since arrhythmic events in LQT3 occur predominantly at rest (6), the value of beta-blocker therapy has been questioned 5, 7, leaving the management of LQT3 patients uncertain.
This study is an extension of 2 pilot works we conducted in the late 1990s, when we demonstrated the ability of mexiletine to abbreviate the duration of ventricular repolarization in an animal model of LQT3 (8) and in LQT3 patients (9). Those preliminary observations led to an off-label use of mexiletine in LQT3 patients, targeted to shorten the QT interval; this off-label use is now recommended in clinical practice guidelines (10). Unfortunately, there is no evidence to support the hypothesis that, by shortening the QTc, this drug may also reduce the occurrence of life-threatening arrhythmias.
The objective of the study was to evaluate the ability of mexiletine to reduce the occurrence of arrhythmic events in LQT3 patients.
Methods
We enrolled consecutive LQT3 patients referred to our center who accepted treatment with oral mexiletine. For each individual, we collected demographic data, personal and family history, symptoms, arrhythmic events, and therapy at follow-up, which was then stored in a customized database. Additionally, we stored electrocardiogram (ECG) parameters for rhythm, heart rate, and duration of PR, QRS, and QT intervals. In addition, we obtained 12-lead ECGs (paper speed 25 mm/s and 10 mm/mV sensitivity) at stable heart rates close to 60 beats/min during daylight hours to limit the confounding effect of diurnal variability of QT interval (11). The QT interval duration before and after therapy was measured in lead II, selecting beats preceded by similar RR intervals, as required when applying Bazett’s formula.
Terms and Definitions
Arrhythmic events included sudden cardiac death, aborted cardiac arrest (including appropriate implantable cardioverter-defibrillator [ICD] shocks), or syncope occurring in the absence of alternative explanations (e.g., orthostatic hypotension or vagally mediated episodes).
Symptomatic patients were defined as individuals who had experienced ≥1 arrhythmic event(s) before starting mexiletine.
We used several electrocardiographic terms. Baseline ECG was the ECG recorded at first visit, before starting mexiletine. ECG on therapy was used to describe the first ECG recorded after initiation of mexiletine, when the maximum-tolerated dose of mexiletine was administered. To verify whether the effect of mexiletine was maintained across the 24-h period, we added measurements of ECG parameters from the first Holter monitor recording obtained at our center on therapy, comparing QTc duration during “daytime” hours (at a heart rate of 60 beats/min) versus QTc duration at the slowest heart rate recorded during “night time.”
Statistical Analysis
Statistical analysis was performed using SPSS software version 21 (IBM Corp., Armonk, New York). Continuous variables were expressed as median with interquartile range (IQR) or mean ± SE; comparisons were performed using paired and unpaired nonparametric tests as appropriate. Categorical variables were reported as absolute and relative frequencies and compared by Fisher exact or McNemar tests.
To evaluate the antiarrhythmic efficacy of mexiletine, a matched-period analysis was performed to compare arrhythmic events off and on mexiletine (12). For each patient, periods of equal duration before and after starting mexiletine were identified, with patients serving as their own controls. Two infants who initiated therapy in the first week of life were considered ineligible for the matched-period analysis because they had a pre-drug observation time of only 7 days; therefore, the analysis was conducted on 32 patients.
The number of arrhythmic events was determined for the matched periods and averaged over the number of patients. To assess the effect of mexiletine on event rates, a Poisson regression model was fitted using generalized estimating equations. Robust standard errors were computed to account for intrapatient correlation. The incidence rate ratio with 95% confidence interval (CI) was calculated to measure the impact of mexiletine on event counts over time. A value of p < 0.05 (2 sides) was considered statistically significant.
Results
Population Characteristics
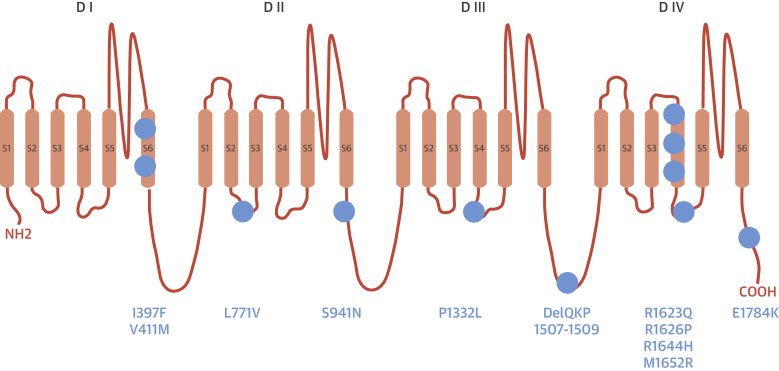
A total of 34 LQT3 patients (19 or 56% males) were enrolled. The mutations identified in the cohort are shown in Figure 1. Patients entered the study at the time of the first clinical visit to our center and the total observation time was 59 months (IQR: 29 to 144 months). Mexiletine was initiated at a median age of 22 years (IQR: 8 to 44 years); the median QTc at baseline ECG was 509 ms (IQR: 490 to 548 ms). Five infants younger than 1 year of age were referred to our center after experiencing a cardiac arrest (4 of 5) or a prolonged loss of consciousness (1 of 5); all of them had a QTc interval >550 ms.
Figure 1.
Localization of LQT3 Mutations
This illustration represents the predicted topology of the alpha subunit of the cardiac sodium channel (Nav1.5) and the position of the mutations identified in the 34 LQT3 patients who were treated with mexiletine. Blue circles = individual mutations.
Before starting mexiletine, 21 of 34 (62%) individuals were asymptomatic and 13 (38%) were symptomatic. Among the symptomatic patients, 7 had experienced ≥1 cardiac arrest: 4 while on beta-blockers and 3 while off therapy. At the time of mexiletine initiation, 21 of 34 (62%) patients were on chronic beta-blocker therapy.
Effect of Mexiletine Treatment
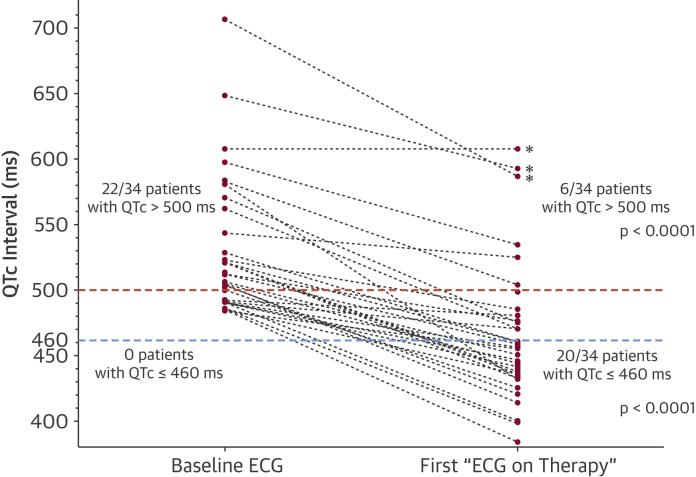
Mexiletine was administered in the 34 patients at a mean dose of 8 ± 0.5 mg/kg/day; the median QTc at the first ECG on therapy was 457 ms (IQR: 434 to 481 ms). Mexiletine induced a mean shortening of the QTc interval of 63 ± 6 ms (p < 0.0001) (Figure 2, Figure 3). Before starting mexiletine, 22 of 34 (65%) patients had QTc values >500 ms, and 16 of 22 (73%) reduced their QTc under this high-risk threshold (3) after drug initiation (p < 0.0001). Moreover, 20 of 34 (59%) patients exhibited “normal” QTc values (i.e., QTc ≤460 ms) after starting mexiletine (p < 0.0001) (Figure 2).
Figure 2.
Effect of Mexiletine on QTc Interval Values
Comparison of QTc interval values for the 34 patients before (Baseline ECG) and after (First “ECG on Therapy”) starting mexiletine. Sixteen of the 22 patients (73%) with QTc values >500 ms (i.e., “high-risk QTc”, red dotted line) reduced their QTc under 500 ms after starting mexiletine (p < 0.0001). Moreover, 20 of 34 (59%) patients “normalized” their QTc values (i.e., QTc ≤460 ms, blue dotted line) after starting the drug (p < 0.0001). Patients who experienced arrhythmic events on mexiletine (asterisks) had “high-risk” QTc values while on treatment. ECG = electrocardiogram.
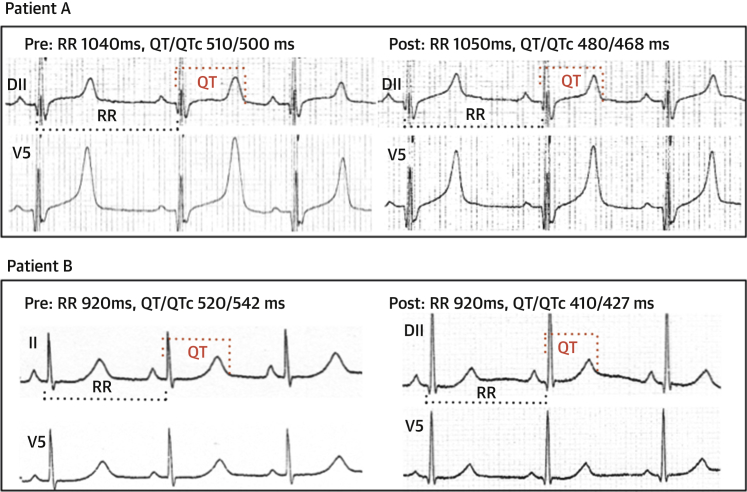
Figure 3.
Effect of Mexiletine in 2 LQT3 Patients
Upper panels (Patient A) show the effect of mexiletine in a child with a baseline QTc of 500 ms who had a syncope at the age of 1 year during fever. After starting mexiletine, his QTc shortened to 468 ms and he has remained asymptomatic for 2 years.
The lower panels (Patient B) show the effect of mexiletine in a girl with a baseline QTc of 542 ms who experienced recurrent syncopal events while receiving nadolol. On mexiletine, the QTc shortened to 427 ms and she remained asymptomatic for 7 years.
The beats used to measure the electrocardiographic parameters before and after starting mexiletine are indicated by the dotted lines (black for the RR interval, red for the QT interval).
There was no statistically significant difference between median QTc values on mexiletine during daytime (464 ms; IQR: 450 to 510 ms) or night time (448 ms; IQR: 432 to 505 ms; p = 0.354). There was also no statistically significant difference between median QTc values on the first “ECG on therapy” and at last follow-up (p = 0.234).
None of the 21 patients who were asymptomatic before mexiletine experienced arrhythmic events after initiating the drug. Among the 13 patients who were symptomatic before mexiletine, 10 remained symptom-free while receiving the drug, whereas 3 patients experienced arrhythmic events on mexiletine (indicated by an asterisk in Figure 2); all 3 had experienced arrhythmic storms in the first 2 months of life while on beta blockade. The first infant received mexiletine in conjunction with an ICD and died from an arrhythmic storm unresponsive to ICD shocks after 11 months of treatment. The second child had a cardiac arrest during fever 2 weeks after starting mexiletine and was resuscitated. The third patient, despite responding initially to mexiletine (13), subsequently demonstrated prolonged QT interval again (14) and died at 4 years. All 3 infants showed extreme QTc prolongation before mexiletine, greatly exceeding 500 ms on treatment (607, 586, and 592 ms) (Figure 2).
Using 12-lead Holter monitor recording, we verified that no patient developed a type 1 Brugada pattern while receiving mexiletine (15).
Arrhythmic Events During Matched Periods
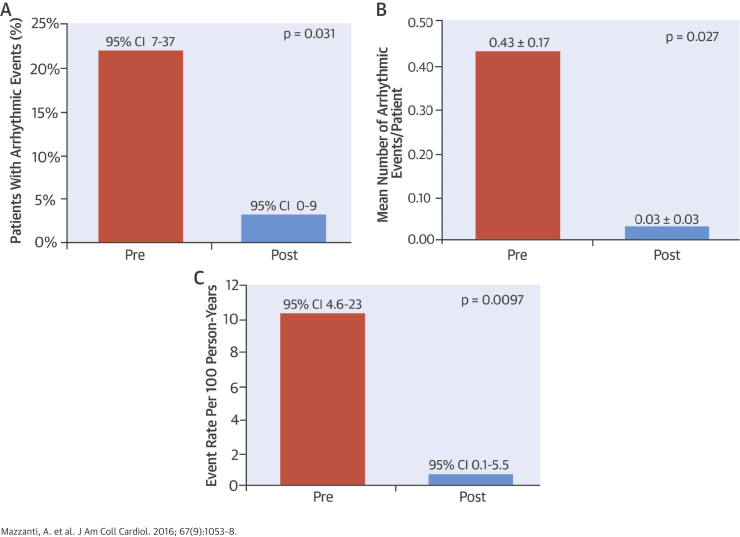
The median duration of risk exposure during matched periods before and after mexiletine was 35 months (IQR: 19 to 64 months), for a total of 136.2 person-years per period. Using mexiletine, there was a significant reduction in the percentage of patients with arrhythmic events (from 22% [95% CI: 7 to 37] to 3% [95% CI: 0 to 9]; p = 0.031), in the mean number of arrhythmic events per patient (from 0.43 ± 0.17 to 0.03 ± 0.03; p = 0.027), and in the rate of arrhythmic events, which dropped by 93% (from 10.3 [95% CI: 4.6 to 23] to 0.7 [95% CI: 0.1 to 5.5] per 100 PY; p = 0.0097) (Central Illustration).
Central Illustration.
Reduction of Arrhythmic Events During Treatment With Mexiletine in Patients With Long QT Syndrome Type 3
We observed a significant reduction of the arrhythmic burden in our cohort of consecutive patients with long QT syndrome type 3 treated with mexiletine during matched periods of a median of 35 months. The percentage of patients with arrhythmic events declined from 22% (95% confidence interval [CI]: 7 to 37) to 3% (95% CI: 0 to 9; p = 0.031, A), the mean number of arrhythmic events per patient decreased from 0.43 ± 0.17 to 0.03 ± 0.03 (p = 0.027, B), and the rate of arrhythmic events dropped by 93%, from 10.3 (95% CI: 4.6 to 23) per 100 PY to 0.7 (95% CI: 0.1 to 5.5) per 100 PY (p = 0.0097, C).
Discussion
Several studies 5, 7 reported that LQT3 patients have an excessive risk of arrhythmic events as compared to LQT1, even with beta-blocker treatment. Using an experimental preparation of LQT3, Shimizu and Antzelevitch (16) speculated that beta-blockers may be proarrhythmic in LQT3 and the efficacy of these drugs in LQT3 remains undefined. For these reasons, alternative therapeutic strategies are needed to reduce the risk of life-threatening events in LQT3 patients.
The use of sodium-channel blockers to shorten QT interval in LQT3 therapy has been supported in recent clinical guidelines (Class IIb, Level of Evidence: C) (10) but, due to the lack of clinical data, no recommendation on the antiarrhythmic efficacy of mexiletine in LQT3 was provided.
We demonstrated here for the first time that, besides shortening the QT interval 4, 8, 9, long-term treatment with mexiletine reduced the occurrence of arrhythmic events in LQT3 patients. During matched-period observations of almost 3 years, both the mean number of arrhythmic events per patient and the annual rate of arrhythmic events were significantly reduced.
Interestingly, more than two-thirds of patients (16 of 22) with “high-risk” QT intervals (3) in baseline conditions reduced their QTc to below the threshold of 500 ms after mexiletine and more than 50% of patients (20 of 34) had a normalization of QTc duration (i.e., QTc ≤460 ms) while on therapy (Figure 2). The only patients who continued to experience arrhythmic events on mexiletine were those who, despite showing a shortening of QTc >100 ms, remained in the “high-risk” category with QTc >500 ms while on therapy. This observation suggests that the therapeutic goal should not be related to the amount of QTc shortening, but rather to obtaining QTc values <500 ms.
Our study provided novel information on the tolerability of long-term mexiletine in young LQT3 patients including infants. Importantly, none of the patients who were asymptomatic before mexiletine experienced an arrhythmic event on the agent.
It is becoming increasingly difficult to find mexiletine in several countries; in Italy the importance of the drug for selected groups of patients has been recognized and the military pharmacy started producing and selling mexiletine. Furthermore, in 2014, the Italian Medicines Agency supported our request to provide free mexiletine to patients with LQTS.
Study Limitations
We acknowledge that our cohort might present a selection bias because patients with a highly malignant form of LQT3 might have died before receiving medical attention and being treated with mexiletine.
Conclusions
We reported here the first observation that a medication, other than beta-blockers, is able to reduce the occurrence of arrhythmic events in LQT3 patients, corroborating the hypothesis that mexiletine may improve survival in these patients. Overall, our data supported the view that precision medicine can be applied to the management of LQTS patients, guiding not only risk stratification, but also the identification of therapies able to modify the molecular mechanism of the disease.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with LQT3 respond incompletely to beta-blockers and face an unfavorable prognosis. Mexiletine exerted effective antiarrhythmic effects in this patient cohort, representing a genotype-specific treatment for an inherited arrhythmogenic substrate.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Mexiletine therapy should be considered for patients with LQT3 who have QTc intervals >500 ms to reduce the risk of life-threatening arrhythmic events.
TRANSLATIONAL OUTLOOK: Clinical trials are needed to establish whether therapy with mexiletine should be adopted as a standard adjunct to the care of patients with LQT3.
Acknowledgment
The authors thank Katherine Underwood, BS, for her editorial contribution.
Footnotes
This work was supported by Telethon grant N. GGP11141, by an unrestricted research grant by Gilead, and by the Ricerca Corrente Funding scheme of the Italian Ministry of Health. Dr. Priori has received research funds from Gilead. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Wang Q., Shen J., Splawski I. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz P.J., Stramba-Badiale M., Crotti L. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priori S.G., Schwartz P.J., Napolitano C. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 4.Blaufox A.D., Tristani-Firouzi M., Seslar S. Congenital long QT 3 in the pediatric population. Am J Cardiol. 2012;109:1459–1465. doi: 10.1016/j.amjcard.2012.01.361. [DOI] [PubMed] [Google Scholar]
- 5.Priori S.G., Napolitano C., Schwartz P.J. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz P.J., Priori S.G., Spazzolini C. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Zareba W., Moss A.J., Schwartz P.J. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 8.Priori S.G., Napolitano C., Cantu F., Brown A.M., Schwartz P.J. Differential response to Na+ channel blockade, beta-adrenergic stimulation, and rapid pacing in a cellular model mimicking the SCN5A and HERG defects present in the long-QT syndrome. Circ Res. 1996;78:1009–1015. doi: 10.1161/01.res.78.6.1009. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz P.J., Priori S.G., Locati E.H. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 10.Priori S.G., Blomstrom-Lundqvist C., Mazzanti A. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv445. [DOI] [PubMed] [Google Scholar]
- 11.Rautaharju P.M., Surawicz B., Gettes L.S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement. J Am Coll Cardiol. 2009;53:982–991. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Moss A.J., Zareba W., Hall W.J. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz P.J., Priori S.G., Dumaine R. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N Engl J Med. 2000;343:262–267. doi: 10.1056/NEJM200007273430405. [DOI] [PubMed] [Google Scholar]
- 14.Ruan Y., Liu N., Bloise R., Napolitano C., Priori S.G. Gating properties of SCN5A mutations and the response to mexiletine in long-QT syndrome type 3 patients. Circulation. 2007;116:1137–1144. doi: 10.1161/CIRCULATIONAHA.107.707877. [DOI] [PubMed] [Google Scholar]
- 15.Priori S.G., Napolitano C., Schwartz P.J., Bloise R., Crotti L., Ronchetti E. The elusive link between LQT3 and Brugada syndrome: the role of flecainide challenge. Circulation. 2000;102:945–947. doi: 10.1161/01.cir.102.9.945. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu W., Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778–786. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]