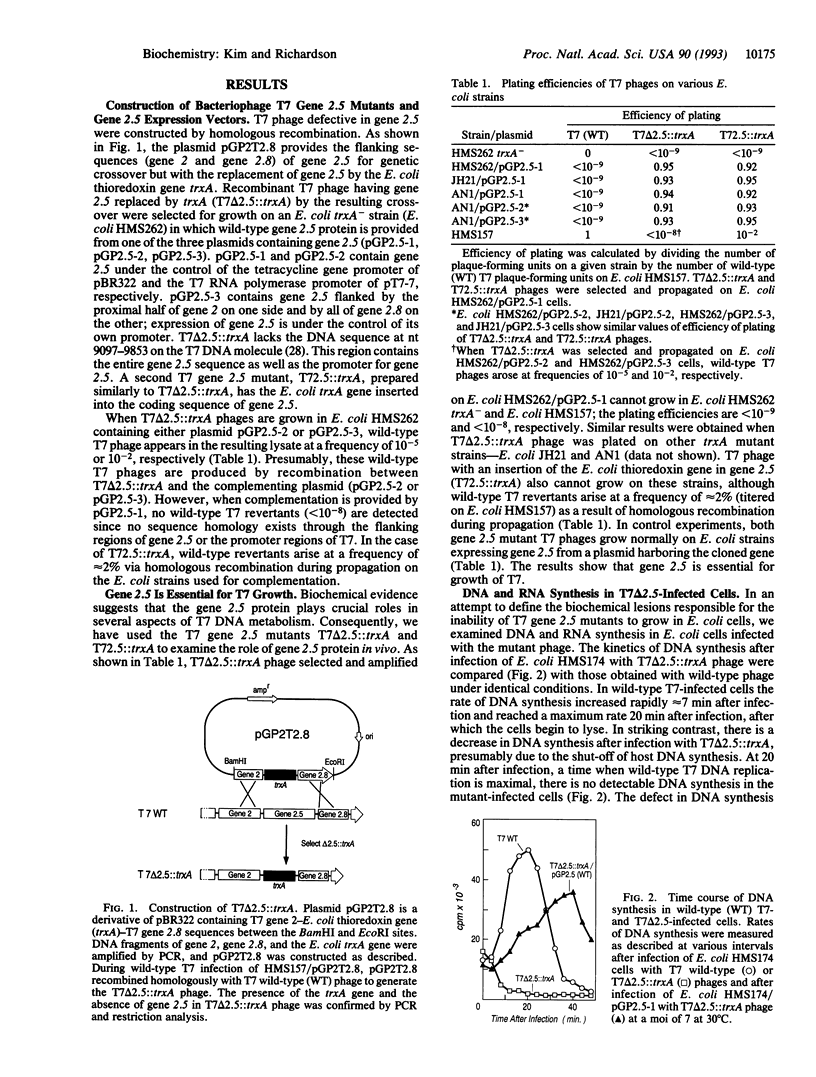
Abstract
The product of gene 2.5 of bacteriophage T7, a single-stranded DNA binding protein, physically interacts with the phage-encoded gene 5 protein (DNA polymerase) and gene 4 proteins (helicase and primase) and stimulates their activities. Genetic analysis of T7 phage defective in gene 2.5 shows that the gene 2.5 protein is essential for T7 DNA replication and growth. T7 phages that contain null mutants of gene 2.5 were constructed by homologous recombination. These gene 2.5 null mutants contain either a deletion of gene 2.5 (T7 delta 2.5) or an insertion into gene 2.5 and cannot grow in Escherichia coli (efficiency of plating, < 10(-8)). After infection of E. coli with T7 delta 2.5, host DNA synthesis is shut off, and phage DNA synthesis is reduced to < 1% of phage DNA synthesis in wild-type T7-infected E. coli cells as measured by incorporation of [3H]thymidine. In contrast, RNA synthesis is essentially normal in T7 delta 2.5-infected cells. The defects in growth and DNA replication are overcome by wild-type gene 2.5 protein expressed from a plasmid harboring the T7 gene 2.5.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Araki H., Ogawa H. A T7 amber mutant defective in DNA-binding protein. Mol Gen Genet. 1981;183(1):66–73. doi: 10.1007/BF00270140. [DOI] [PubMed] [Google Scholar]
- Araki H., Ogawa H. The participation of T7 DNA-binding protein in T7 genetic recombination. Virology. 1981 Jun;111(2):509–515. doi: 10.1016/0042-6822(81)90353-6. [DOI] [PubMed] [Google Scholar]
- Argos P., Tucker A. D., Philipson L. Primary structural relationships may reflect similar DNA replication strategies. Virology. 1986 Mar;149(2):208–216. doi: 10.1016/0042-6822(86)90122-4. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Burke R. L., Alberts B. M., Hosoda J. Proteolytic removal of the COOH terminus of the T4 gene 32 helix-destabilizing protein alters the T4 in vitro replication complex. J Biol Chem. 1980 Dec 10;255(23):11484–11493. [PubMed] [Google Scholar]
- Campbell J. L., Richardson C. C., Studier F. W. Genetic recombination and complementation between bacteriophage T7 and cloned fragments of T7 DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2276–2280. doi: 10.1073/pnas.75.5.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase J. W., Williams K. R. Single-stranded DNA binding proteins required for DNA replication. Annu Rev Biochem. 1986;55:103–136. doi: 10.1146/annurev.bi.55.070186.000535. [DOI] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Formosa T., Burke R. L., Alberts B. M. Affinity purification of bacteriophage T4 proteins essential for DNA replication and genetic recombination. Proc Natl Acad Sci U S A. 1983 May;80(9):2442–2446. doi: 10.1073/pnas.80.9.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassberg J., Meyer R. R., Kornberg A. Mutant single-strand binding protein of Escherichia coli: genetic and physiological characterization. J Bacteriol. 1979 Oct;140(1):14–19. doi: 10.1128/jb.140.1.14-19.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himawan J. S., Richardson C. C. Genetic analysis of the interaction between bacteriophage T7 DNA polymerase and Escherichia coli thioredoxin. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9774–9778. doi: 10.1073/pnas.89.20.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber H. E., Tabor S., Richardson C. C. Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J Biol Chem. 1987 Nov 25;262(33):16224–16232. [PubMed] [Google Scholar]
- Huberman J. A., Kornberg A., Alberts B. M. Stimulation of T4 bacteriophage DNA polymerase by the protein product of T4 gene 32. J Mol Biol. 1971 Nov 28;62(1):39–52. doi: 10.1016/0022-2836(71)90129-x. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Kim Y. T., Tabor S., Bortner C., Griffith J. D., Richardson C. C. Purification and characterization of the bacteriophage T7 gene 2.5 protein. A single-stranded DNA-binding protein. J Biol Chem. 1992 Jul 25;267(21):15022–15031. [PubMed] [Google Scholar]
- Kim Y. T., Tabor S., Churchich J. E., Richardson C. C. Interactions of gene 2.5 protein and DNA polymerase of bacteriophage T7. J Biol Chem. 1992 Jul 25;267(21):15032–15040. [PubMed] [Google Scholar]
- Kowalczykowski S. C. Biochemistry of genetic recombination: energetics and mechanism of DNA strand exchange. Annu Rev Biophys Biophys Chem. 1991;20:539–575. doi: 10.1146/annurev.bb.20.060191.002543. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Felgenhauer Z. Z. Molecular recombination in T4 bacteriophage deoxyribonucleic acid. II. Single-strand breaks and exposure of uncomplemented areas as a prerequisite for recombination. J Virol. 1967 Dec;1(6):1193–1202. doi: 10.1128/jvi.1.6.1193-1202.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman L. V., Richardson C. C. Requirements for primer synthesis by bacteriophage T7 63-kDa gene 4 protein. Roles of template sequence and T7 56-kDa gene 4 protein. J Biol Chem. 1991 Dec 5;266(34):23240–23250. [PubMed] [Google Scholar]
- Meyer R. R., Glassberg J., Kornberg A. An Escherichia coli mutant defective in single-strand binding protein is defective in DNA replication. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1702–1705. doi: 10.1073/pnas.76.4.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Laine P. S. The single-stranded DNA-binding protein of Escherichia coli. Microbiol Rev. 1990 Dec;54(4):342–380. doi: 10.1128/mr.54.4.342-380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. Recombination in bacteriophage T4. Adv Genet. 1970;15:1–53. doi: 10.1016/s0065-2660(08)60070-x. [DOI] [PubMed] [Google Scholar]
- Nakai H., Matsuda H., Takara E., Diksic M., Yamamoto Y. L., Meyer E., Redies C. Changes in lumped and rate constants in experimental cerebral ischemia--intra-animal comparison before and after middle cerebral artery occlusion. Neurol Med Chir (Tokyo) 1988 Jan;28(1):11–17. doi: 10.2176/nmc.28.11. [DOI] [PubMed] [Google Scholar]
- Nakai H., Richardson C. C. The effect of the T7 and Escherichia coli DNA-binding proteins at the replication fork of bacteriophage T7. J Biol Chem. 1988 Jul 15;263(20):9831–9839. [PubMed] [Google Scholar]
- Rabkin S. D., Richardson C. C. Initiation of DNA replication at cloned origins of bacteriophage T7. J Mol Biol. 1988 Dec 20;204(4):903–916. doi: 10.1016/0022-2836(88)90050-2. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Gefter M. L. A DNA-binding protein induced by bacteriophage T7. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1846–1850. doi: 10.1073/pnas.70.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C., Gefter M. L. A deoxyribonucleic acid-binding protein induced by bacteriophage T7. Purification and properties of the protein. J Biol Chem. 1974 Jun 25;249(12):3843–3850. [PubMed] [Google Scholar]
- Richardson C. C. Bacteriophage T7: minimal requirements for the replication of a duplex DNA molecule. Cell. 1983 Jun;33(2):315–317. doi: 10.1016/0092-8674(83)90411-7. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. The 5'-terminal nucleotides of T7 bacteriophage deoxyribonucleic acid. J Mol Biol. 1966 Jan;15(1):49–61. doi: 10.1016/s0022-2836(66)80208-5. [DOI] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Genetic analysis of gene 1.2 of bacteriophage T7: isolation of a mutant of Escherichia coli unable to support the growth of T7 gene 1.2 mutants. J Virol. 1981 Jan;37(1):343–351. doi: 10.1128/jvi.37.1.343-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Litfin F., Jost E. Stimulation of T7 DNA polymerase by a new phage-coded protein. Mol Gen Genet. 1973 Jul 2;123(3):247–262. doi: 10.1007/BF00271243. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Tabor S., Huber H. E., Richardson C. C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J Biol Chem. 1987 Nov 25;262(33):16212–16223. [PubMed] [Google Scholar]
- Tomizawa J. I., Anraku N., Iwama Y. Molecular mechanisms of genetic recombination in bacteriophage. VI. A mutant defective in the joining of DNA molecules. J Mol Biol. 1966 Nov 14;21(2):247–253. doi: 10.1016/0022-2836(66)90095-7. [DOI] [PubMed] [Google Scholar]
- Wackernagel W., Radding C. M. Formation in vitro of infective joint molecules of lambda DNA by T4 gene-32 protein. Proc Natl Acad Sci U S A. 1974 Feb;71(2):431–435. doi: 10.1073/pnas.71.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. R., Yeh Y. C. Requirement of a functional gene 32 product of bacteriophage T4 in UV, repair. J Virol. 1973 Oct;12(4):758–765. doi: 10.1128/jvi.12.4.758-765.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]