Abstract
In March 2014, the American Academy of Sleep Medicine (AASM) Board of Directors requested the Scoring Manual Editorial Board develop rules, terminology, and technical specifications for scoring sleep/wake states in full-term infants from birth to 2 mo of age, cognizant of the 1971 Anders, Emde, and Parmelee Manual for Scoring Sleep in Newborns. On July 1, 2015, the AASM published rules for scoring sleep in infants, ages 0–2 mo. This evidence-based review summarizes the background information provided to the Scoring Manual Editorial Board to write these rules. The Anders Manual only provided criteria for coding physiological and behavioral state characteristics in polysomnograms (PSG) of infants, leaving specific sleep scoring criteria to the individual investigator. Other infant scoring criteria have been published, none widely accepted or used.
The AASM Scoring Manual infant scoring criteria incorporate modern concepts, digital PSG recording techniques, practicalities, and compromises. Important tenets are: (1) sleep/wake should be scored in 30-sec epochs as either wakefulness (W), rapid eye movement, REM (R), nonrapid eye movement, NREM (N) and transitional (T) sleep; (2) an electroencephalographic (EEG) montage that permits adequate display of young infant EEG is: F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1; additionally, recording C3-Cz, Cz-C4 help detect early and asynchronous sleep spindles; (3) sleep onsets are more often R sleep until 2–3 mo postterm; (4) drowsiness is best characterized by visual observation (supplemented by later video review); (5) wide open eyes is the most crucial determinant of W; (6) regularity (or irregularity) of respiration is the single most useful PSG characteristic for scoring sleep stages at this age; (7) trace alternant (TA) is the only relatively distinctive EEG pattern, characteristic of N sleep, and usually disappears by 1 mo postterm replaced by high voltage slow (HVS); (8) sleep spindles first appear 44–48 w conceptional age (CA) and when present prompt scoring N; (9) score EEG activity in an epoch as “continuous” or “discontinuous” for inter-scorer reliability; (10) score R if four or more of the following conditions are present, including irregular respiration and rapid eye movement(s): (a) low chin EMG (for the majority of the epoch); (b) eyes closed with at least one rapid eye movement (concurrent with low chin tone); (c) irregular respiration; (d) mouthing, sucking, twitches, or brief head movements; and (e) EEG exhibits a continuous pattern without sleep spindles; (11) because rapid eye movements may not be seen on every page, epochs following an epoch of definite R in the absence of rapid eye movements may be scored if the EEG is continuous without TA or sleep spindles, chin muscle tone low for the majority of the epoch; and there is no intervening arousal; (12) Score N if four or more of the following conditions are present, including regular respiration, for the majority of the epoch: (a) eyes are closed with no eye movements; (b) chin EMG tone present; (c) regular respiration; and (d) EEG patterns of either TA, HVS, or sleep spindles are present; and (13) score T sleep if an epoch contains two or more discordant PSG state characteristics (either three NREM and two REM characteristics or two NREM and three REM characteristics).
These criteria for ages 0–2 mo represent far more than baby steps. Like all the other AASM Manual rules and specifications none are fixed in stone, all open for debate, discussion and revision with the fundamental goal to provide standards for comparison of methods and results.
Commentary:
A commentary on this article appears in this issue on page 291.
Citation:
Grigg-Damberger MM. The visual scoring of sleep in infants 0 to 2 months of age. J Clin Sleep Med 2016;12(3):429–445.
Keywords: infant sleep scoring, neonatal sleep scoring, polysomnography, sleep scoring criteria, neonatal polysomnography
INTRODUCTION
At increasingly younger ages, children are being referred to sleep specialists, technologists, laboratories, and centers in the United States. Criteria for scoring sleep in children developed by the American Academy of Sleep Medicine (AASM) are for children 2 mo of age or older.1 Members of the AASM requested rules and recommendations for scoring sleep in neonates and infants. In March 2014, the AASM Board of Directors requested the Scoring Manual Editorial Board develop rules, terminology, and technical specifications for scoring sleep/wake states in full-term infants from birth to 2 mo of age,2 cognizant of the 1971 Anders, Emde, and Parmelee Manual for Scoring Sleep in Newborns (Anders Manual).3 Rules for scoring respiratory events or arousals in infants this age were not devised, reflecting a belief that the current rules for scoring these would suffice for now. On July 1, 2015, the AASM published rules for scoring sleep/wake states in infants 0–2 mo of age.2 This review summarizes the background information provided to the Scoring Manual Editorial Board to write these rules.
DEFINITIONS OF AGE
Knowing an infant's conceptional age (CA) is crucial for interpreting the normalcy, immaturity, or abnormality on an electroencephalogram (EEG) or polysomnogram (PSG) because the brain and the EEG continue to develop and mature at a similar rate independent of whether the infant is in utero or post-delivery. Except when stressed, or in situations involving encephalopathy, seizures, or medication-related factors, the PSG in infants 6 mo or younger reflects the developmental age of the brain.4–6 The PSG of a normal low-risk premature infant born at 32 w gestational age (GA) whose chronological or legal age is 8 w should resemble that of a normal infant of 40 w GA born 2 days earlier.
CA is the GA at birth plus the number of weeks postpartum.7 GA is the time elapsed between the first day of the mother's last menstrual period and the day of delivery expressed in completed weeks. If the pregnancy was achieved using assisted reproductive technology, GA is calculated by adding 2 w to the CA. Chronological age (or postnatal or legal age) is the time elapsed since birth (and can be expressed in days, months, or years). The American Academy of Pediatrics recommends postmenstrual age (PMA) be used instead of CA, but CA is used here (and in the scoring criteria) because the evidence cited uses CA.7 At birth, an infant is classified as being born premature (< 37 w gestation), full-term (37–42 w); or postterm (born after 42 w). A neonate is a child during the first 28 days after birth; an infant is a child between 1–12 mo of age.7
METHODS USED TO WRITE THIS REVIEW
To write this review, the author did a literature search using PubMed and searching for terms: infant or neonatal or fetal AND sleep OR polysomnography. The author additionally searched for development and sleep, sleep spindles, eye movements and sleep, and infant. More than 3,000 abstracts and papers were found. The author reviewed the abstracts of these, and then identified relevant papers. From references contained in some of these, the author found other papers that were then reviewed. The author wrote a preliminary version of this review, which was submitted to the Scoring Manual Editorial Board to assist them in writing the rules. Illustrative figures of PSG epochs were provided from the author's sleep laboratory demonstrating wakefulness (W), rapid eye movement (REM, R), nonrapid eye movement (NREM, N) and transitional sleep (T), and different EEG patterns seen in these states.
The author later suggested preliminary scoring rules and technical recommendations to the Scoring Manual Editorial Board for their consideration. The Scoring Manual Board reviewed these and wrote the rules and recommendations, submitting these to the AASM Board of Directors for revision and approval. The author reviewed the final rules and recommendations and revised this paper, summarizing these. The Scoring Manual Board requested only rules for scoring sleep/wake states in from 0–2 mo of age. Rules for scoring respiratory events or arousals were not requested, nor were rules for scoring sleep/wake states in premature infants.
HISTORY OF DEVELOPMENT OF NEONATAL AND INFANT POLYSOMNOGR APHY AND INFANT SLEEP SCORING CRITERIA
The earliest studies of sleep and waking in infants were based on observations of caregivers watching bedside.8–10 The first observations of cyclic fluctuating levels of arousal and motor behavior in humans were studied in infants, not adults. The discovery of REM sleep was made possible by Aserinsky and Kleitman,11 who decided to simultaneously record eye movements (electro-oculography, EOG) and EEG in infants because they thought eye movements were a better marker of state changes than EEG. To their surprise, clusters of REMs were observed when infants fell asleep. In 1955, they published their findings that infants when sleeping exhibited regularly recurring periods of eye motility and other concomitant motor behaviors.12 This discovery soon led to the recognition of REM sleep and its association with dreaming in adults by Dement and Kleitman in 1957.13
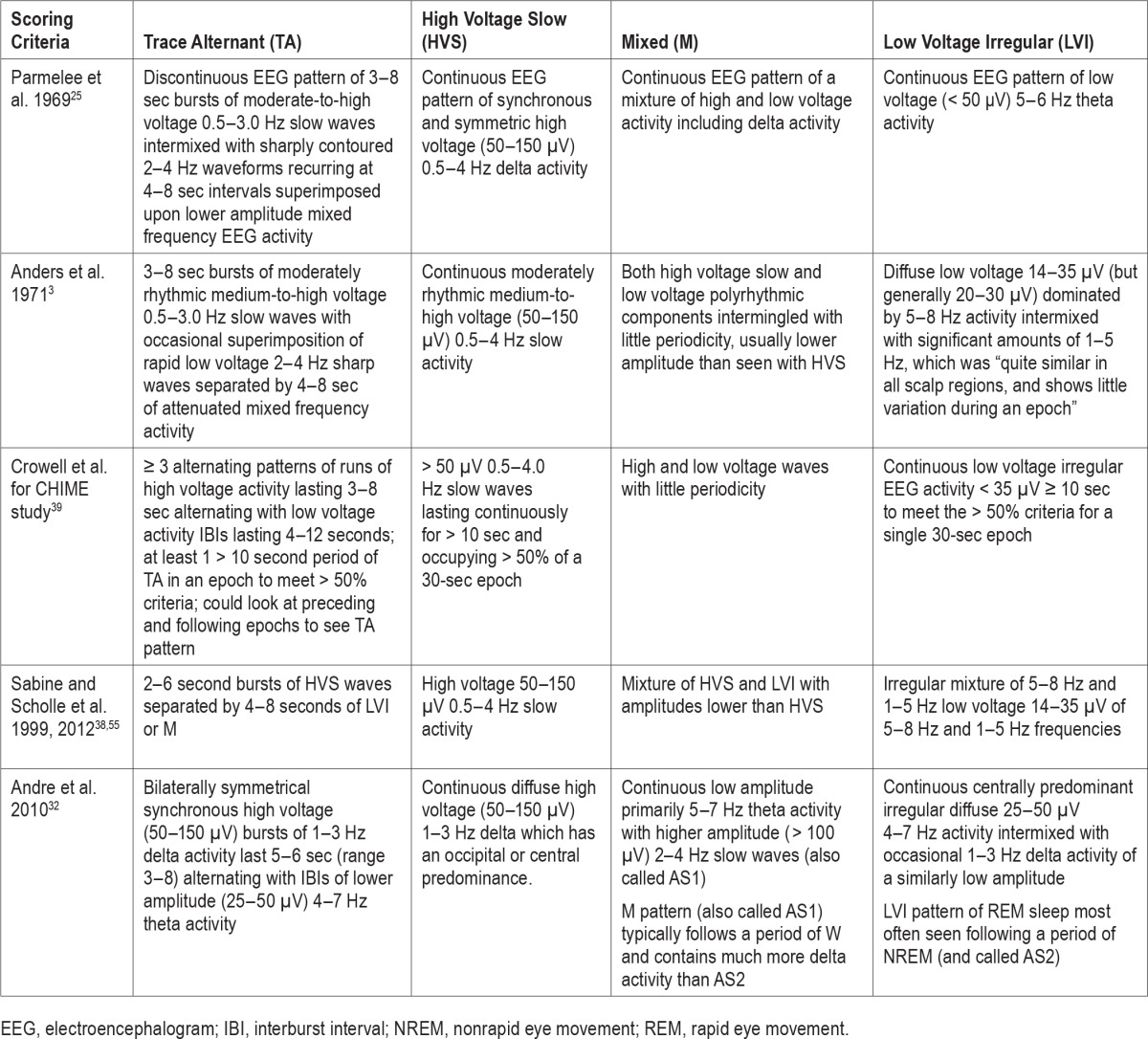
Beginning in the late 1950s, neonatal electroencephalographers14–17 and infant sleep researchers18–24 systematically studied the EEG, PSG and behaviors of sleep and wake in full-term infants. Dreyfus-Brisac et al.,14 Dreyfus-Brisbac and Monod,15 Monod and Pajot,16 and Parmalee et al.17,25 identified REM and NREM sleep states, respectively, terming them active sleep (AS) and quiet sleep (QS). Infants during QS/NREM were “very quiet” with regular respiration and heart rates, preserved chin electromyography (EMG) activity, and no or rare vertical eye movements. Whereas, during AS/REM sleep, REMs, small movements of the limbs or face, irregular respiration and heart rates, and no chin muscle activity were noted. They identified and named four different EEG patterns seen in term infants when sleeping: tracé alternant (TA), high voltage slow (HVS), activité moyenne (mixed, M), and low voltage irregular (LVI)15,26–28 (Figure 1). They recognized that normal infants had many periods (or epochs) of sleep that were not easily categorized as a particular sleep state because of discordant features between the physiological markers of sleep/wake states (EEG, EOG, chin EMG, respiration, and body movements).16,26,29 These were variously called transitional, indeterminate, or intermediate sleep.
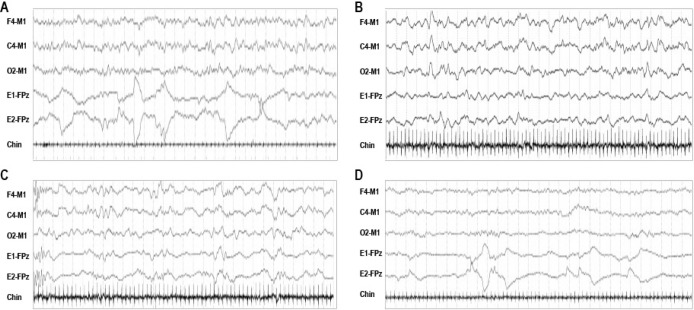
Figure 1. Electroencephalogram (EEG) patterns of sleep in infants 0–2 mo of age.
Thirty-second epochs of each of the four sleep EEG patterns seen in infants between ages 0–2 mo: (A) mixed (M) EEG pattern characterized by both high voltage slow (HVS) and low voltage polyrhythmic components intermingled with little periodicity; (B) high voltage slow (HVS) EEG pattern characterized by continuous synchronous symmetrical predominantly high voltage (100–150 μV) 1–3 Hz delta activity, which often has an occipital or central predominance; (C) trace alternant (TA) pattern characterized by ≥ 3 alternating runs of bilaterally symmetrical synchronous high voltage (50–150 μV) bursts of 1–3 Hz delta activity lasting 5–6 sec (range, 3–8 sec) alternating with periods of lower amplitude (25–50 μV) 4–7 Hz theta activity (range, 4–12 sec); and (D) low voltage irregular (LVI) EEG pattern characterized by continuous low voltage mixed-frequency activity with delta and predominantly theta activity. The only EEG pattern distinctive of a particular sleep/wake state is trace alternant (TA) which when present suggests NREM sleep. The other three EEG patterns can be observed in more than one sleep/wake state. The (M pattern can be seen in W or R, rarely N; LVI in either R or W; and HVS most often in N, rarely R. The EEG pattern of R sleep before a period of R is often M, and LVI after a period of N (respectively termed AS1 and AS2).
By the 1960s, neonatal electroencephalographers had delineated how the characteristic EEG patterns of AS and QS evolve in premature infants with increasing CA based on hundreds of neonatal EEG studies.17,30,31 These studies found that the behavioral correlates (and cycles) of REM sleep are seen as early as 25–30 w CA when the EEG was still undifferentiated.32,33 Recognizable EEG patterns of REM and NREM sleep first appeared at 32–34 w CA.31 In 1968, Parmelee et al.17 published a seminal catalog of EEG patterns observed in infants at each CA. With the catalog, the authors were able to predict an infant's CA within 2 w in 85% of infant EEGs scored blindly.17 In 2010, Andre et al.32 published a comprehensive and well-illustrated glossary of the EEG features and neonatal behavioral states at different CA, which incorporates digitized EEG tracings and cites available evidence for the patterns of both normal and pathological neonatal EEGs, a worthy tool when reading PSGs in children this age.
During the same period, infant sleep researchers Prechtl18,20,34,35 and Anders et al.21–24 developed scales for “staging” sleep in infants solely based on behavioral observations (Table 1). They argued that particular combinations of behaviors more reliably identified sleep/wake states and even normalcy of development than EEG. Although this approach continues to be used by Anders and his colleagues, the two schools merged into one to develop the Anders Manual for scoring sleep in newborn infants published in 1971.3
Table 1.
Anders and Chalemian methodology for scoring sleep in infants solely based on behavioral observations.

The Anders, Emde, and Parmelee Manual for Scoring Sleep in Newborns
In 1969, the Association for the Psychophysiological Study of Sleep (APSS, the earlier name of the Sleep Research Society) chartered an ad hoc committee to develop a guide for scoring sleep in infants.3 This committee (chaired by Anders, Emde, and Parmelee) developed consensus-derived terminology, criteria for coding sleep/wake states, and a manual containing illustrative examples for scoring sleep in healthy normal full-term infants. The authors of A Manual for Standardized Terminology, Techniques, and Criteria for Scoring of States of Sleep and Wakefulness in Newborn Infants emphasized it was meant only to provide criteria for coding physiological and behavioral parameters in PSGs of healthy full-term infants, leaving “specific criteria for scoring states…to the investigator.”3
Anders et al.3 reported they “had considerable difficulty reaching a consensus on the criteria for scoring states” in part because of debates about recognizing or ignoring indeterminate or transitional sleep with discordant features for the particular sleep state. Committee members used to scoring sleep primarily in adults “were inclined to ignore indeterminate sleep” while infant sleep researchers did not. By hard-won consensus, the Committee agreed to score either 20- or 30-sec epochs from lights out to lights on as either wakefulness (W), quiet (Q), active (A), or indeterminate (I) sleep. Anders et al.3 presumed that QS and AS, respectively, were antecedents of NREM and REM sleep.
Contained within the Anders Manual are important observations that continue to serve when scoring sleep/wake in newborns. Transitions from W to sleep are particularly difficult to score in neonates because (1) sleep onset is most often REM sleep; (2) there is no dominant posterior rhythm in wakefulness to drop out; (3) the EEG backgrounds of W and REM sleep are quite similar, both “continuous” mixed frequencies; and (4) short periods of drowsiness often alternate with REM sleep. Epochs of indeterminate or transitional sleep are most often observed at sleep onset, upon arousal, and especially in transitions from REM to NREM sleep. The EEG pattern of TA is only seen in NREM sleep, LVI in REM sleep, but HVS and M can be seen in either NREM or REM sleep. Regularity (or irregularity) of respiration is the single PSG characteristic most useful for scoring sleep states in infants. They defined regular respiration (characteristic of NREM sleep) as a period in which the respiratory rate varies by < 20 breaths per minute; irregular respiration > 20 breaths per minute.3,16 They thought regularity of respiration could usually be determined by visual inspection.3 If more precise measurement was needed, they advised measuring the longest and shortest respiratory cycles in an epoch, and extrapolating the respiratory rates of each of these for 1 min.
Other Earlier Infant Sleep Scoring Criteria
Since the publication of the Anders manual in 1971,3 several groups have published criteria for scoring sleep in PSG of newborns and young infants, but none have been widely accepted or used.36–38 All said these were based on the Anders Manual criteria. In 1987, Hoppenbrouwers et al.36 published criteria for scoring sleep/wake in infants between birth and 6 mo of age using 60-sec epochs and recognizing four states: wakefulness (AW), active sleep (AS), quiet sleep (QS), indeterminate sleep (IS). In 1997, Crowell et al.39 developed detailed PSG recording and sleep/wake scoring criteria for the Collaborative Home Infant Monitoring Evaluation (CHIME) study (a prospective multicenter collaborative home infant monitoring study of 415 healthy infants ages 34–64 w CA). These criteria use C4-A1 as the primary EEG derivation and C3-A2 as a “back-up channel” in case the first malfunctioned. A particular EEG pattern was scored if it occupied > 50% of a 30-sec epoch. Wakefulness they thought was most easily scored when the infant's eyes were open, crying, or being fed. Transitional (T) sleep was scored if a 30-sec epoch contained too many discordant state markers (e.g., three NREM + two REM state characteristics or two NREM + three REM state characteristics). They recommended ignoring respiratory pauses lasting > 3 sec and small movements when assessing regularity or irregularity of respiration in a given epoch. Table 2 summarizes the definitions of sleep EEG patterns in different infant sleep scoring criteria.
Table 2.
Definitions of sleep electroencephalogram patterns in different infant sleep scoring criteria.
INFANT POLYSOMNOGR APHY TECHNICAL SPECIFICATIONS
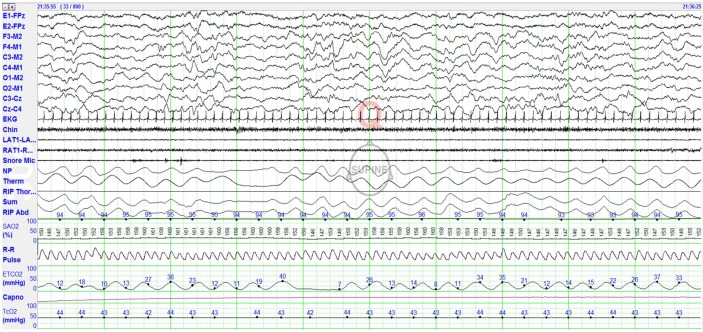
In general, the technical specifications for recording PSG in infants 0–2 mo of age are much the same as those recommended by the AASM Manual for scoring children 2 mo to 18 y of age. However, a few important differences should be mentioned. Infant EEG is often of much higher voltage requiring sensitivity settings of 10–15 μV/mm (but an initial gain of 7 μV/mm is appropriate).32 It is important to record EEG over the mid-line (Cz, vertex) because sleep spindles appear here first, and wise to display left and right central EEG derivations because sleep spindles are more often asynchronous at this age. An EEG montage that permits adequate display of young infant EEG is: F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1, adding C3-Cz, Cz-C4 to optimize detection of asynchronous sleep spindles. Figure 2 shows a PSG montage the author's laboratory uses to record infants 0–2 mo of age. Lead I electrocardiographic electrodes are placed on the right and left arms which permits simultaneous recording of electrocardiographic as well as arm movements. Recording video to correlate behaviors with other PSG features is crucial for recognizing sleep/ wake states, especially W. However, even more important are real time observations and detailed documentation of behaviors by the sleep technologist during collection to confirm if a restless baby is in W, R, or T.
Figure 2. Polysomnographic (PSG) montage for recording infants 0–2 mo of age.
Eye channels (left eye [E1], right eye [E2]) are referenced to the midline frontopolar electrode (FPz). Electroencephalogram (EEG montage is left frontal-right mastoid (F3-M2), right frontal-left mastoid (F4-M1), left central-right mastoid (C3-M2), right central-left mastoid (C4-M1), left occipital-right mastoid (O1-M2), right occipital-left mastoid (O2-M1), left central-midline central (C3-CZ), and midline central-right central (CZ-C4). This EEG montage allows easy recognition of low voltage 12–14 Hz sleep spindles, which may be seen as early as 43 w, usually present by 45–48 w CA. Other PSG signals recorded include: EKG, chin electromyogram (EMG), left and right anterior tibialis (L Leg, R Leg) muscles, snore microphone, nasal pressure (NP), thermal sensor, respiratory inductance plethysmography (RIP) thoracic, RIP abdomen, RIP SUM, pulse oxygen saturation (SpO2), R-R interval, pulse waveform, endtidal carbon dioxide (EtCO2), capnogram, and transcutaneous carbon dioxide (tcCO2).
SLEEP/WAKE PATTERNS IN HEALTHY INFANTS, 0–2 MONTHS OF AGE
Sleep is the penultimate behavioral state of the fetus, neonate, and young infant. The ontogenetic development of sleep follows orderly reproducible patterns.40–44 The behavioral correlates of REM sleep are seen as early as 28–30 w CA when the EEG was still undifferentiated; recognizable EEG patterns of REM and NREM sleep first appear at 32–34 w CA.32,33,45,46 Piaget47 and Piers and Piaget48 thought play was the major “work” of children, but for infants it is sleep. Newborn full-term infants typically spend 16–18 h per day sleeping. 70–80% of sleep time is spent in REM sleep in preterm infants and 50% in term infants, falling to 30% by 6 mo of age, and adult levels of 20–25% by 5 y of age.49 The greater time spent sleeping in infants and early childhood is thought to reflect the crucial role sleep plays in fostering optimal brain development, cognition, and behavior. The time course of REM sleep development (and decline) in humans corresponds well with critical periods of brain maturation.68 Why do infants spend so much time in REM sleep? Roffwarg and Dement50 and Marks et al.51 were first to speculate greater time spent in REM sleep could provide sensory experiences in the absence of external stimuli in utero. Sleep in premature infants in utero is not a passive state of rest, waiting for birth, but a time in which neuronal networks underlying vision, olfaction, somatosensory, and motor pathways are being devised. Infants twitch far more when sleeping than adults, even more so when they are premature. Premature infants from 24–35 w CA are constantly moving and twitching, far more than those born closer to term.
In utero muscle twitches (often tens of thousands per day) are neither random nor purposeless. Evidence from studies in human infants and infant animal models show feedback from a twitching limb guides organization of spinal sensorimotor circuits and trigger 1-sec bursts of electrical activity in appropriate regions of the developing somatosensory cortex. These bursts of electrical activity are triggered by proprioceptive feedback from the twitching limb.40,52 These bursts of EEG activity are none other than the quintessential EEG signature of premature infants, delta brushes. Scalp EEG recorded delta brushes reflect bursts of 8–25 Hz electrical activity within the cortex which is most prominent and prevalent during the third trimester of gestation. Myoclonic twitching during sleep in early development are thought to: (1) sculpt nascent neuronal circuits in the cerebral cortex; (2) guide the formation, rearrangement, and elimination of synapses; and (3) construct sensory maps for auditory, motor, touch, and noxious topographic regions of the brain and the infant connectome.53 Blumberg et al. (2014) argue that myoclonic twitches during REM sleep in infants represent a form of motor exploration which help human infants and other mammals explore limb biomechanics, build motor synergies, and lay a foundation for complex, automatic, and goal-directed movements when awake.44,45
Sleep Architecture and Cycling in Healthy Infants 0–2 Months of Age
Until approximately 44 w CA, sleep cycles repeat in a polyphasic pattern across the 24-h day interrupted approximately every 3–4 h by waking for care and feeding.54 Sleep onset is more often REM sleep in infants until 2–3 mo of age (Figure 3). Sleep cycles in healthy infants at term typically last a mean of 50–60 min (range, 30–70 min).55 At 43–48 w CA, sleep cycles average 60 min, wake periods are primarily to feed, the first half of the night sleep is more restless, and artifact more likely to obscure sleep scoring particularly in REM sleep. Sleep at this age is more restless in the first half of the night.55 Wakefulness usually accounts for only 8–10% of a 24-h day in infants up to 48 w CA.54
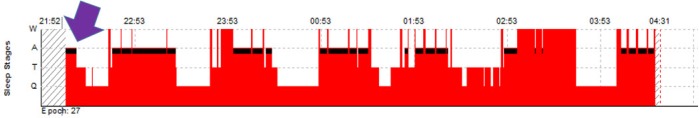
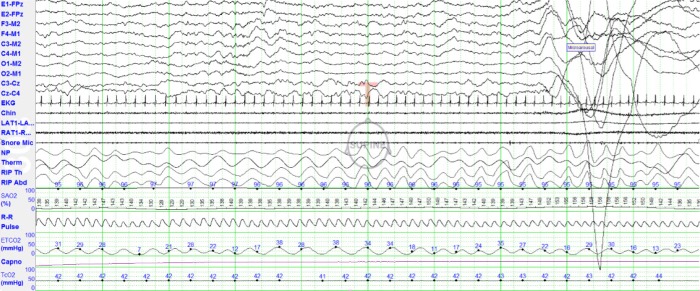
Figure 3. Hypnogram showing rapid eye movement (REM) sleep onset in an infant.
Sleep onsets more often are REM sleep in healthy infants until 2–3 mo postterm. Note sleep onset (purple arrow) is REM sleep (black bars). Note more than half the sleep time is spent in REM sleep, typical for infants this age.
Within a given REM-NREM-REM sleep cycle, REM sleep lasts 10–45 (mean 25) min, NREM sleep 20 min, and T sleep 10 min.16,54 The EEG pattern seen in the first REM sleep of a REM-NREM-REM sleep cycle after W is typically Mixed and comprises 25–30% of the cycle (Figure 4A). Of note, NREM sleep following a first period of REM sleep often begins with a brief period of HVS for 3–5% of the cycle (Figure 4B), then a longer period of TA (Figure 4C) for 25%. The sleep cycle then ends with another period of REM sleep but typically with LVI (Figure 4D) and for 25% of the cycle. 10–15% of epochs of sleep within the REM-NREM-REM ultradian sleep cycle are best scored as T sleep (Figure 5); these most often occurring at sleep onset, stage shifts (especially W to REM sleep (less often REM to NREM sleep) and following arousals or awakenings. A recently published study recording comprehensive home PSG on 84 healthy normal infants 44–48 w CA scored using infant sleep scoring they had adapted using AASM 2012 sleep scoring criteria.66 They reported mean time in bed was 719 ± 82 min, total sleep time (TST) 477 ± 59 min, and percentages of TST in REM sleep 37 ± 7%, NREM sleep 42 ± 5%, and T sleep 22 ± 6%. Using current AASM Manual scoring criteria, awakenings averaged 8.5 ± 2.1/h, arousals 19.3 ± 3.7/h of sleep, mean apneahypopnea indexes (AHI) 13.7 ± 14.1/h, obstructive AHI 0.0 ± 0.0/h, and 3% desaturation indexes of 20.9 ± 16.9/h.
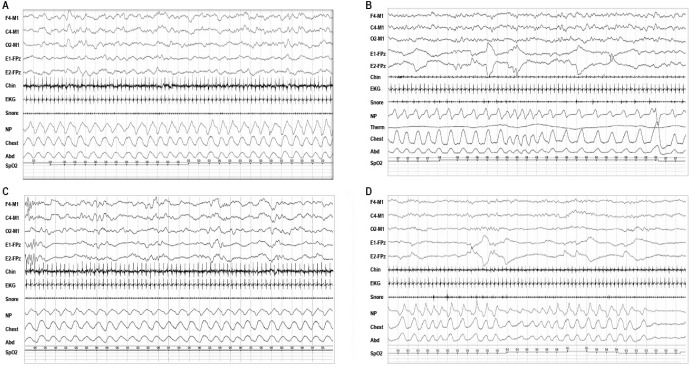
Figure 4. Representative examples of polysomnographic patterns of sleep in infants 0–2 mo of age.
(A) 30-sec epoch of rapid eye movement (REM) sleep with mixed (M) electroencephalogram (EEG) pattern, REMs and low chin electromyogram (EMG) tone typically seen following a period of wakefulness; (B) 30-sec epoch of NREM sleep with high voltage slow (HVS) EEG pattern, no eye movements, preserved chin EMG, and regular respiration, which often precedes longer period of trace alternant (TA) in infants at 38–42 w conceptual age (CA); (C) 30-sec epoch of nonrapid eye movement (NREM) sleep with trace alternant (TA) EEG pattern, no eye movements, regular respiration; and (D) 30-sec epoch of REM sleep with a low voltage irregular (LVI) EEG pattern, rapid eye movements (REMs), irregular respiration, and low chin EMG tone.
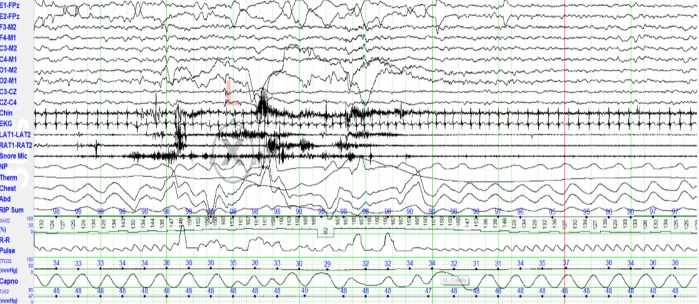
Figure 5. A 30-second epoch of sleep best scored as transitional sleep (T).
The discontinuous EEG pattern of trace alternant, absence of eye movements and the regular heart rate suggest stage N, but irregular respiration and body movements are more consistent with REM sleep (stage R). The AASM Scoring rules for scoring sleep in infants recommend scoring stage T if 3 NREM + 2 REM characteristics or 2 REM and 3 NREM characteristics are discordant. See Figure 2 for explanation of the various channels displayed in the montage.
Limited Reference Values for Scoring Respiratory Events in Infants 0–2 Months of Age
Sleep studies in infants 0–2 mo of age are most often done to evaluate for sleep disordered breathing (SDB).56–58 Several authors recently summarized current information on normal values for SDB in PSG done neonates and young infants.59–64,65 They found that: (1) apneas are common in normal infants this age; (2) the majority of apneas observed are central in type (70–80%) and typically last ≥ 3 but < 10 sec; (3) central apneas are commonly seen in REM sleep, after a sigh breath or a body movement, and during transition from wakefulness to sleep; (4) apneas lasting > 10 seconds are usually mixed apneas; and (5) periodic breathing is common typically averaging < 5% of sleep time for infants 1 and 3 months of age. Based on these studies (albeit limited), Ng et al.66 proposed an upper limit of normal central apnea index (CAI) was 45 central apneas per hour of sleep for 1-mo-olds and 30/h for 2-mo-olds. Ng et al. emphasized that obstructive apneas are uncommon in healthy neonates and young infants and suggested the upper limits of normal for both obstructive and mixed apneas are indexes of < 1.0/h of sleep. More normative data is needed.
Wakefulness (W)
The medical literature suggests the most crucial determinant of W (Figure 6) in a normal healthy term infant is open eyes (although transient eye closures often occur especially when crying). Characteristic features of W in infants 0–2 mo of age are: (1) open eyes (which sometimes pursue visual targets); (2) elevated chin EMG tone; (3) elevated limb EMG tone; (4) rapid and irregular heart rates; (5) rapid and irregular respiratory patterns; (6) spontaneous movements; (7) crying; and (8) a continuous synchronous symmetric irregular mixed frequency EEG with superimposed frequent movement artifacts.54,68
Figure 6. A 30-second epoch of sleep best scored as wakefulness (W).
The technologist noted eyes open, moving head and crying. The EEG is continuous mixed EEG frequencies, rapid eye movements are present, chin muscle tone is high, respiration irregular and rapid, and movement and muscle artifact mar the tracing. See Figure 2 for explanation of the various channels displayed in the montage.
Infants this age have a variety of eye movements when awake, including eye blinks, conjugate 0.5–2 Hz vertical eye movements, and conjugate irregular sharply peaked rapid eye movements with an initial deflection of < 500 msec.43 Lambda waves have been observed as early as 2 w following birth in calm awake newborns looking at a visual target.69 These are characterized by sharply contoured waveforms over the occipital regions lasting 250–350 msec.69 Sucking and puckering mouth movements and/or dysconjugate eye movements are common in newborn infants when awake and are not to be mistaken for seizures.
The EEG background in infants 37–48 w CA is continuous and can include mixtures of either: (1) irregular theta and delta activity with amplitudes up to 100 μV maximal over the occipital regions; (2) diffuse irregular alpha and beta activity with amplitudes up to 30 μV; or (3) rhythmic theta activity with amplitudes up to 50 μV, and often maximal over the central regions.
W at 46 w CA is characterized by mixed frequency EEG activity, artifacts from body movements, eye movements, bursts of muscle activity in the chin and anterior tibialis EMG, irregular respiration, and pronounced heart rate variability. The EEG background awake at this age consists of a symmetrical continuous mixture of mixed EEG frequencies (Figure 6).
Of note, the AASM Manual recommends scoring a 30-sec epoch as W if either a, b, or c is present for the majority of the 30-sec epoch: (a) eyes are wide open (for the majority of the epoch); (b) vocalization (whimpering, crying, etc.) or actively feeding; and (c) all of the following conditions are met: (1) eyes are open intermittently; (2) rapid eye movements or scanning eye movements; (3) sustained chin EMG tone with bursts of muscle activity; (4) irregular respiration, and (5) the EEG shows low voltage irregular (LVI) or mixed (M) patterns.
Wake/Sleep Transitions
Recognizing sleep onset in infants 0–2 mo of age is especially difficult. Sleep onsets are typically REM sleep (often preceded by a few epochs of sleep best scored as T). Sustained eye closure is the best physiological marker of drowsiness in an infant this age. Other behaviors suggestive of drowsiness are absence of focused attention, relative immobility, and intermittent opening and closing of eyes. If an infant's eyes remain closed for greater than 3 min, the infant is considered asleep.
By 44–48 w CA, more sleep onsets may be NREM sleep.70,71 REM sleep latencies at this age may be short (< 8 min) or longer (> 16 min). The EEG background in wake/sleep transitions may show increases in the amplitudes of theta and delta activity especially over the frontal regions. Isolated generalized myoclonic jerks can be seen as an infant wakes from sleep, not to be mistaken for seizures.
NREM Sleep (N)
Characteristic features of NREM (N, Quiet) sleep at 38–42 w CA include: (1) no body movements; (2) no eye movements; (3) regular respiration; (4) regular heart rate; (5) some chin EMG activity; and/or (6) EEG pattern of either TA or HVS activity.26,29,72 The AASM Manual recommends scoring stage N if four or more the following are present, including regular respiration for the majority of the 30-sec epoch: (a) eyes closed with no eye movements; (b) chin EMG tone present; (c) regular respiration (postsigh respiratory pauses may occur); (d) TA, HVS, or sleep spindles present; and (e) reduced movement relative to wake.
Regularity of respiration during NREM/quiet sleep is reliably present in infants 40 w CA.26,73 However, chin EMG activity may be inappropriately absent or low in 15–20% of the N sleep time in infants 40 w CA.29 Similar percentages of absent chin EMG tone during N sleep are also seen 6-mo-old infants26,74,75 and even in adults.76 Two PSG markers best identify N sleep in infants 37–44 w CA: the discontinuous EEG pattern of TA and regularity of respiration.
It is worthwhile to appreciate the distinctive features of the TA pattern: bilaterally symmetrical and synchronous high voltage (50–150 μV) bursts of 1–3 Hz delta activity which typically last 5–6 sec (range, 3–8 sec) alternating with lower amplitude (25–50 μV) 4–7 Hz theta activity inter-burst intervals (IBIs) of similar duration (Figure 4C).32 Intermixed alpha and beta activity and isolated sharp waves in the theta range up to 100 μV are observed in the bursts of TA. Temporal asynchrony of the EEG background for several minutes can be seen during transition from REM to NREM sleep, and is of no pathological significance.
The other EEG pattern of N sleep is HVS (Figure 4A) characterized by continuous symmetrical and synchronous high voltage (50–150 μV) 1–3 Hz delta which often has an occipital or central predominance. Between 37–42 w CA, HVS accounts for only 3–8% of the N sleep time. HVS at this age occurs as a brief period following a REM sleep onset associated with the mixed (AS1) EEG pattern. HVS precedes a longer period of TA within the same NREM period. Between 42–44 w CA, the IBIs of TA shorten to 1–2 sec and the amplitude difference between bursts and IBIs lessens, making it harder to recognize and score. TA usually disappears by 44 w CA (up to 46 w) replaced by HVS.
Sleep spindles first appear as early as 3–9 w postterm in both infants born preterm (30–33 weeks) and term.70,77 Sleep spindles in infants 43–48 w CA typically first appear over the midline central (Cz, vertex), and shift from side to side over left and right central regions (C3 and C4). Multiple different studies have confirmed that sleep spindles are usually present by 12 w term,78,79 43–48 w CA,80 and 2 mo term.81–84 Studies using quantitative frequency analysis found they usually first appear 4–9 w term.85,86 Three longitudinal studies using EEG, PSG, or most recently magnetoencephalography (MEG) have confirmed that sleep spindles are usually present by 2 mo term.82–84
REM Sleep (R)
Electroencephalographers are taught to recognize two types of REM sleep EEG patterns (called AS1 and AS2). Both are continuous. AS1 typically occurs following W and AS2 following a period of NREM sleep. AS2 consists of primarily irregular synchronous low voltage (25–50 μV) 4–7 Hz theta activity intermixed with occasional similarly low voltage 1–3 Hz delta activity and called low voltage irregular (LVI, Figure 4D). AS1 contains generous amounts of intermixed high amplitude (> 100 μV) 2–4 Hz delta activity intermixed with lower voltage theta activity, is termed mixed (M, Figure 4A). Rarely, HVS may be seen during R sleep.32
As mentioned earlier, the most reliable physiological marker to distinguish N from R sleep in infants is the regularity or irregularity of respiration. REM sleep is characteristically associated with irregularities in respiration including differences in rate, depth, and minute ventilation with brief periods of relative bradypnea or tachypnea.43,87 REMs occur singly or in clusters during R. Irregular respiration and heart rate especially during phasic periods of REM when rapid eye movements are more prevalent.29 Chin EMG tone is most often low or absent during R, but may not be atonic (state dissociation reflecting immaturity of skeletal muscle atonia development). Last, an extraordinary panoply of motor behaviors accompany R at this age, including: sucking; facial grimaces; clonic jaw jerks; fine limb muscle twitches; chin, body and limb tremors; intermittent stretching; and large athetoid limb movements.88
Transitional Sleep (T)
Depending on how strictly criteria for NREM or REM sleep are defined, 10–15% or 20–40% of 30-sec epochs of sleep in infants at term could be scored as T sleep.89 Epochs best scored as T sleep are observed in transitions to sleep, especially transitions from W to R (less often R to N) and following arousals. Earlier infant scoring criteria have tried to reduce the percentages of T sleep scored by permitting scoring if only one, or better yet, two PSG correlates within an epoch are discordant for the sleep state.24,90 The percentage of sleep time spent in T sleep decreases with increasing infant maturation. Figure 5 shows an example of T sleep.
A ASM SCORING MANUAL CRITERIA FOR SCORING SLEEP IN INFANTS 0–2 MONTHS OF AGE (37–48 WEEKS CA)
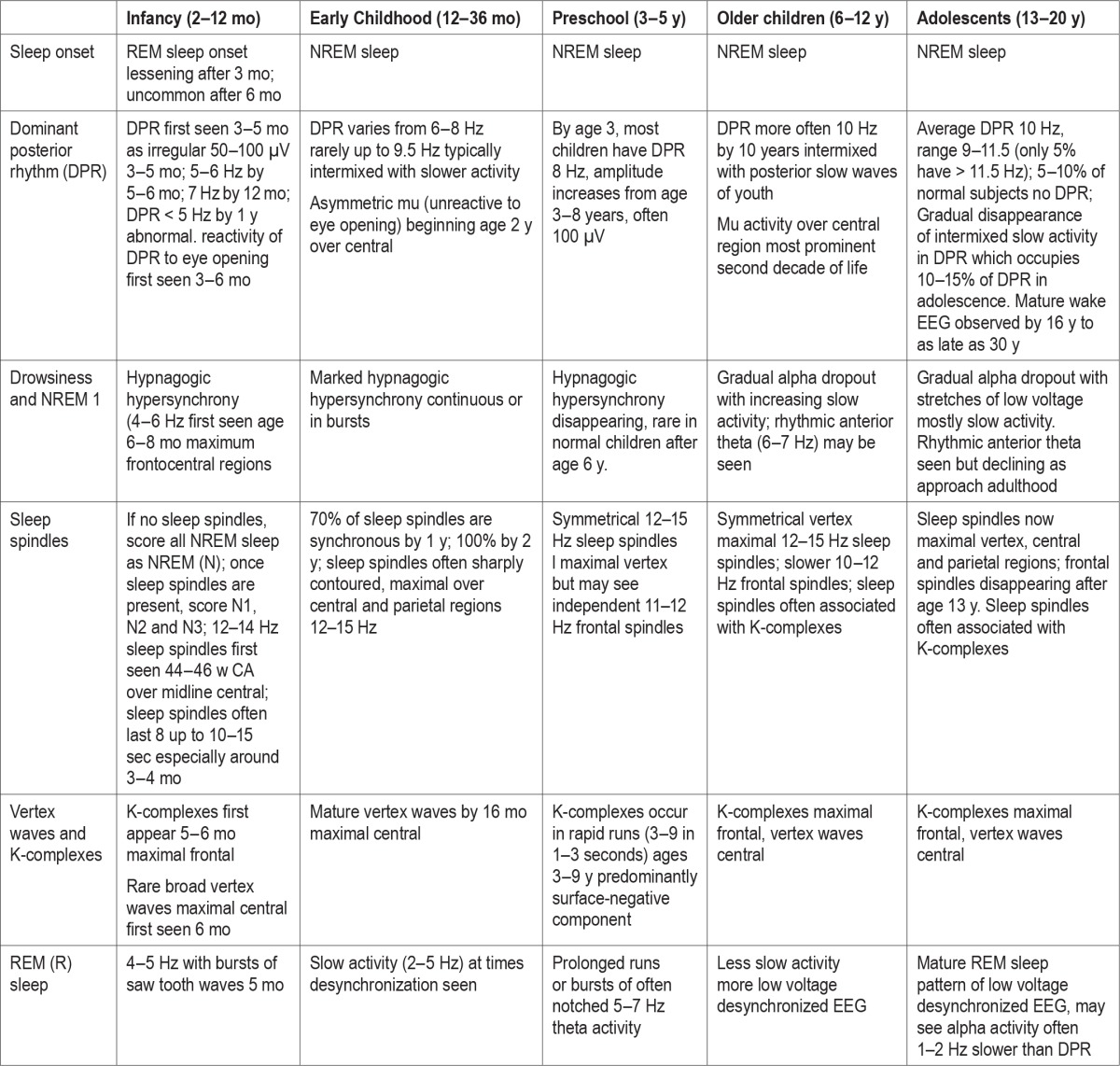
The AASM published the rules for staging sleep in infants, ages 0–2 mo, with the hope these would become widely used, thereby permitting comparisons among sleep laboratories, test results, and research. These were based on the Anders Manual coding criteria but rightly incorporated modern concepts, digital PSG recording techniques, more recent knowledge, realities, practicalities, and compromises. Table 3 compares earlier infant sleep scoring criteria. Table 4 summarizes the AASM Scoring Manual definitions for the four sleep EEG patterns (TA, HVS, M, and LVI) and Table 5 the sleep/wake state scoring characteristics. Table 6 shows the development of the EEG features of sleep and wakefulness from infancy through adolescence.
Table 3.
Earlier infant sleep/wake scoring criteria.
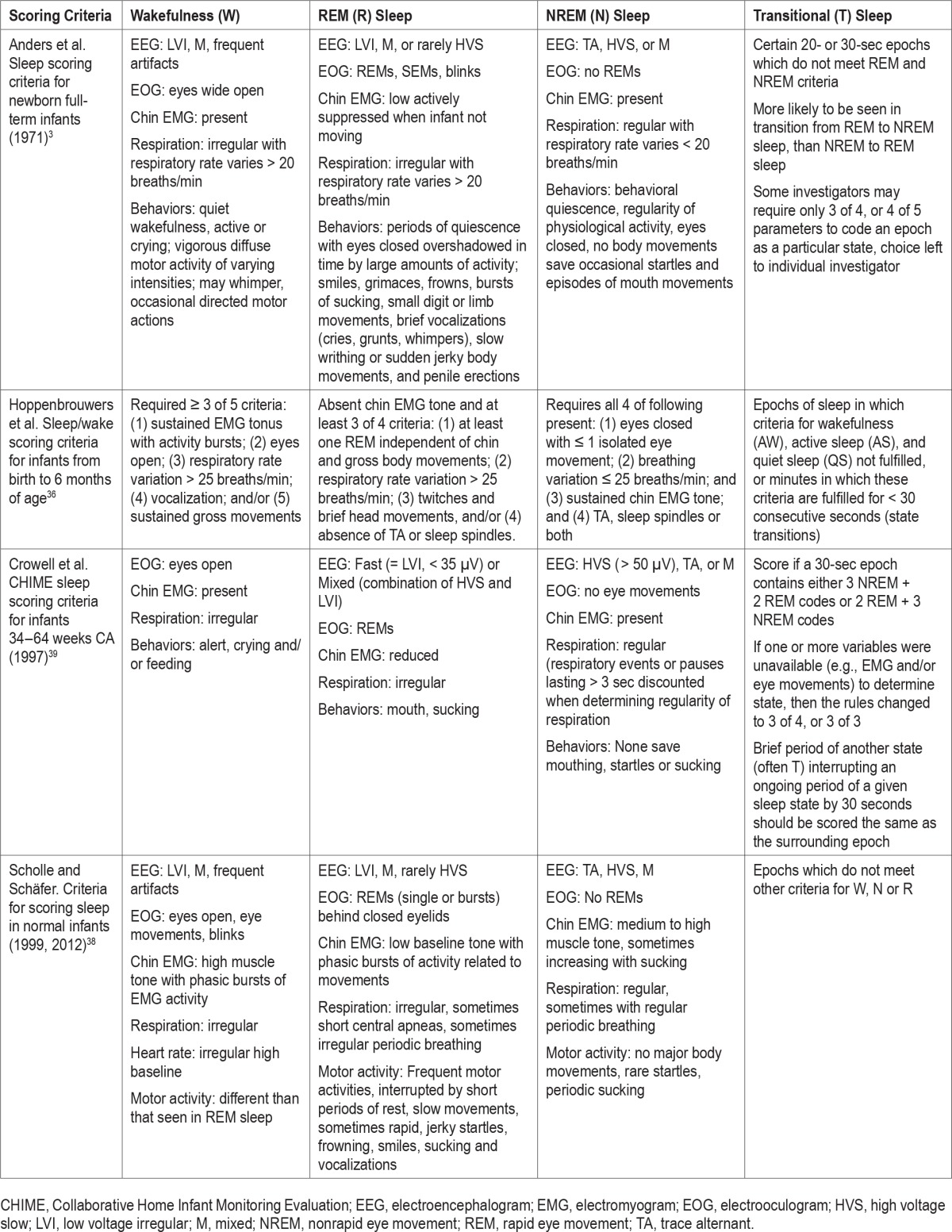
Table 4.
American Academy of Sleep Medicine Scoring Manual definitions of electroencephalogram patterns of sleep and wake in infants 0–2 months.
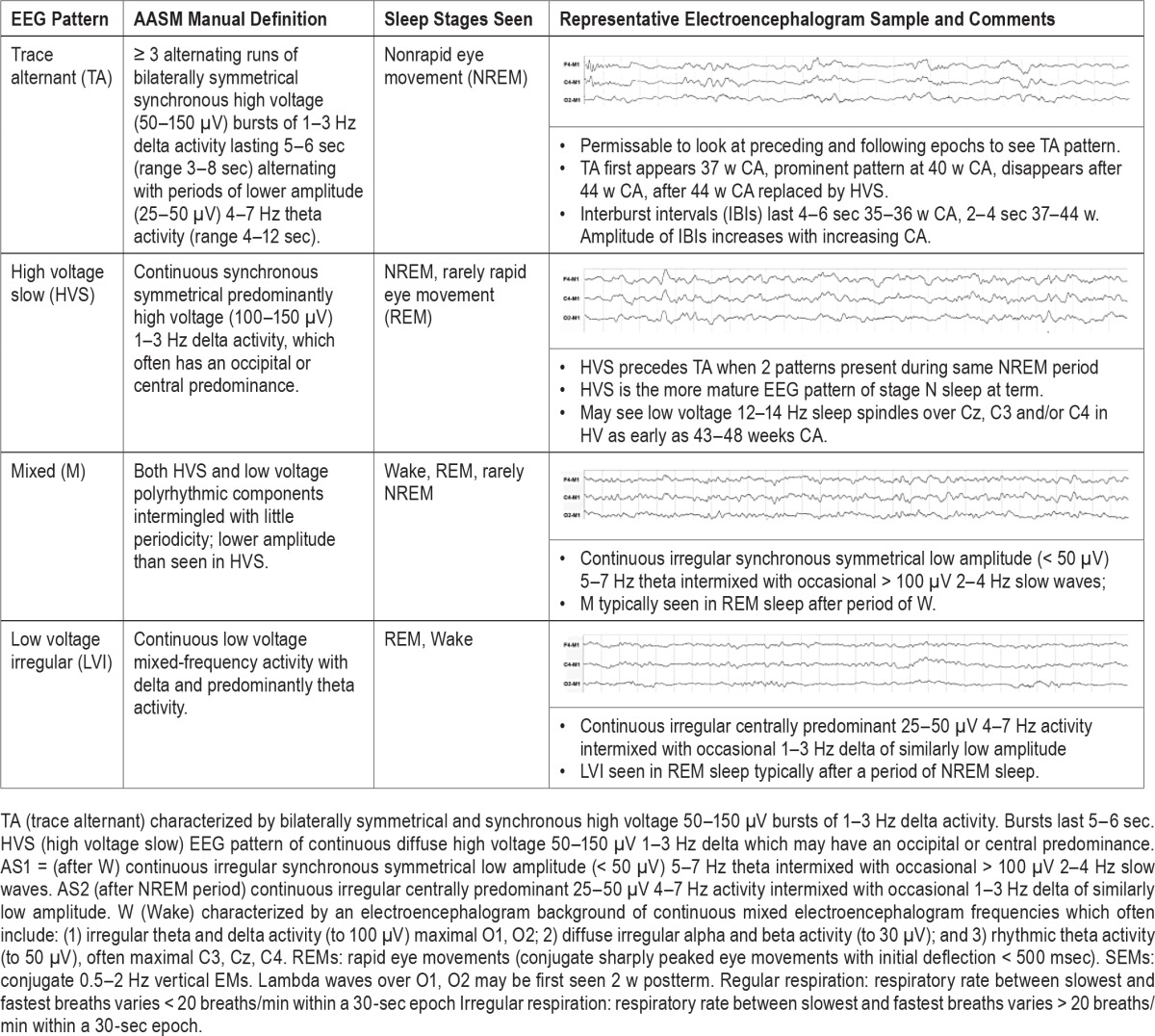
Table 5.
Summary of American Academy of Sleep Medicine Manual sleep/wake state characteristics for scoring sleep in infants 0–2 months of age.
Table 6.
Development of electroencephalogram features of sleep and wakefulness: infants to adolescents.
Overview of Infant Sleep Scoring Criteria
Sleep/wake should be scored in 30-sec epochs as either W, NREM, REM, or T sleep. Scoring sleep at this age begins by assessing whether in each 30-sec epoch: (1) eyes are open or closed; (2) the EEG background is continuous or discontinuous; (3) chin tone present, decreasing, or absent; (4) REMs are present or absent; and (5) respiration is irregular or regular. The most crucial behavioral determinant for scoring stage W is eyes wide open (with or without crying) because many of the distinctive EEG features of W are not seen until 2 mo postterm. Knowing whether the eyes are open or closed depends upon careful observations and notations made by the technologist when recording the PSG, supplemented by video review.
Regularity or irregularity of respiration is the most important PSG characteristic when scoring sleep/wake states in infants this age. Respiration is scored as regular if the variability between the slowest and fastest breaths is less than 20 breaths/min (and typical of N sleep); irregular if > 20 breaths/ min (seen in W, R, or T). However, regularity (or lack of it) is often judged solely by visual inspection. Respiratory events or pauses ≥ 3 sec are not used to determine respiratory irregularity. Preservation of chin EMG tone is often but not always present in N sleep at this age, present in only 80–85% of epochs of N sleep; its presence useful, its absence not.
Only one relatively distinctive EEG pattern in infants 0–2 mo of age is discontinuous TA; all the rest are continuous with varying combinations and frequencies in either W, N, R, or T. Given this, simply identifying EEG activity within a PSG epoch as “continuous” or “discontinuous” might suffice when scoring sleep in infants this age, especially because other PSG characteristics are better markers at this age. Sleep spindles may be seen as early as 43 w CA; if present, they warrant scoring an epoch as N (not N2 at this age). Simultaneous recording and scoring of left, right, and midline central EEG derivations (C3, C4, and CZ) provide the greatest likelihood of these often low amplitude rudimentary and asynchronous waveforms.
Wakefulness (W)
Given these tenets, W is scored in an epoch if either are present: (1) eyes are open intermittently; (2) vocalization (whimpering, crying, etc.) or actively feeding observed; or (3) all of the following are met: (a) eyes are open intermittently; (b) REMs or scanning eye movements; (c) sustained chin EMG tone with bursts of muscle activity; (d) irregular respiration; and (e) an EEG of either continuous LVI or M. The AASM Scoring Manual advises that: W is most reliably scored by behaviors observed because many of the distinctive EEG features of W are not seen until after 2 mo postterm. The EEG background may include: (a) irregular theta and delta activity (to 100 μV) maximal in O1, O2; (b) diffuse irregular alpha and beta activity (to 30 μV); (c) rhythmic theta activity (to 50 μV) often maximal in C3, Cz, C4; or (d) artifacts from body movements, and eye movements.
Wakefulness/Sleep Transitions
Sleep onsets are more often R sleep until 2–3 mo postterm. Drowsiness is best characterized at this age by visual observation (supplemented by video recordings) during which the infant appears relatively immobile, lacks focused attention, and may exhibit brief intermittent opening and closing of eyes. By consensus, the Committee decided if a healthy infant's eyes remain closed for ≥ 3 min, it was reasonable to consider the infant asleep.
NREM Sleep (N)
NREM sleep is scored if four or more of the following conditions are present, including regular respiration, for the majority of the epoch: (1) eyes are closed with no eye movements; (2) chin EMG tone present; (3) regular respiration (postsigh respiratory pauses may occur); and (4) EEG patterns of either TA, HVS, or sleep spindles are present. The AASM Scoring Manual emphasizes that EMG in stage N is variable; it is generally lower than W and higher than in stage R. So, if chin EMG activity is present (higher than stage R) this favors scoring the epoch as NREM. However, stage N can still be scored with low EMG tone provided more than four other criteria for stage N including regular respiration are met.
REM Sleep (R)
REM sleep is scored in epochs with four or more of the following criteria present, including irregular respiration and REM(s): (1) low chin EMG (for the majority of the epoch); (2) eyes closed with at least one REM (concurrent with low chin tone); (3) irregular respiration; (4) mouthing, sucking, twitches, or brief head movements; and (5) EEG exhibits a continuous pattern without sleep spindles. In infants younger than 3 mo, the first epoch of sleep is commonly REM sleep. Given the difficulty in determining sleep onset, an epoch of definite stage R is required to begin scoring REM sleep. Because REMs may not be seen on every page, epochs following an epoch of definite REM sleep in the absence of REMs may be scored if all of the following conditions are present: (a) EEG shows low or medium voltage mixed frequencies without TA or sleep spindles; (b) chin muscle tone is low for the majority of the epoch; and (c) there is no intervening arousal using the same rules for scoring arousal in children and adults. Epochs of REM sleep containing REM sleep without atonia are not unusual in infants.
Transitional Sleep (T)
The AASM Scoring Editorial Board thought recognizing and scoring T sleep was needed because it is common in developing infants (accounting for 10–40% of epochs of sleep depending upon how stage T is defined) and a marker of development and maturation.89 They chose to call indeterminate sleep T because it most often occurs in transitions from W to R, before awakening, and at sleep onset. They further recommended scoring a 30-sec epoch as T if it contains either three NREM and two REM characteristics or two NREM and three REM characteristics. However, an epoch can be scored as stage N, stage R, or stage W if only one PSG characteristic is discordant for the sleep state.
CHALLENGES AND COMPLEXITIES WHEN SCORING INFANT SLEEP STUDIES
Sleep specialists and technologists with little experience in scoring sleep and wakefulness in infants 0–2 mo of age are challenged by: (1) many epochs of sleep are better identified by regularity or irregularity of respiration and other behavioral correlates than the EEG background; (2) EEG transients, which are sharply contoured but not epileptic in nature, especially prominent in NREM sleep in infants 38–42 w CA; and (3) tendency for infants when stressed, sick, or born premature to exhibit EEG patterns that would classify them as younger than their CA (termed dysmature).
EEG of full-term newborns contains a variety of distinctive EEG transients that are often sharply contoured, easily mistaken by the inexperienced as epileptiform in appearance. Frontal sharp transients (FSTs) are isolated or repeated bursts of unilateral or bilateral 50–200 μV biphasic waves each lasting 0.5–0.75 sec over the frontal regions in infants, especially between 35–44 w CA, which are often more frequent and of higher amplitude during N sleep (especially at onset), but can be seen in W or R (especially AS1) sleep. FSTs disappear by 49 w CA. Sharp transients are also observed in infants 0–2 mo of age over the central and temporal regions. These are more likely to be considered abnormal if: excessively frequent for CA; appear in short runs; are consistently unilateral, occur frequently during the IBI of TA; and/or persist during R or W.
Delta brushes, an EEG transient most abundant in premature infants, are still seen at term, but usually in rare numbers mostly during N sleep and then usually localized to the occipital regions. At 38 w CA, the number of delta brushes observed in 30 min of N sleep are 60–125, only 6–8 in 30 min of R sleep. By 40 w, delta brushes average 0–24 in 30 min of N sleep, 0–6 in NREM. Delta brushes are normally only seen in N sleep in healthy infants 42 weeks then averaging 0–12 in 30 minutes of recording. Slow anterior dysrhythmia (short bursts of regular or irregular 50–100 μV 1–3 Hz delta waves are another EEG transient observed between 38–41 w CA, especially in AS1-REM sleep.
IMPACT OF POOR SLEEP IN INFANTS ON LATER COGNITIVE FUNCTIONING
Poor-quality sleep in premature and term infants has lasting effects on later cognitive functioning.91–93 Sleep architecture of infants born premature may lag behind that of infants of a similar CA born at term with longer sleep cycles, more TA, less REM sleep, fewer and shorter duration arousals, fewer body movements, and less REMs.6 How well sleep architecture is organized in a neonatal EEG or PSG may predict long-term cognitive outcomes.91–94 Less REM sleep time in 81 infants born 32–36 w CA was associated with poorer developmental outcomes on the Bayley II at 6 mo.91 Whereas premature infants who had longer periods of sustained sleep, more time spent in REM sleep, and more periods of REM sleep with REMs had better cognitive outcomes. Another prospective study of 65 infants born premature found those who slept poorly as neonates exhibited poorer attention and greater distractibility at 4 and 18 mo than those who slept well.92 Another study of 143 infants born mean age 32 w CA that particular sleep/wake transitions predicted neurobehavior, emotional and cognitive growth at age 5 y.93 Sleep/wake transitions from NREM to wake were associated with greater neonatal neuromaturation, less negative emotionality, and better verbal, symbolic, and executive competences at age 5 y. Whereas, short episodes of REM and NREM sleep or REM sleep to wakefulness with cry were associated with poorer outcomes. Such findings have prompted interventions to improve premature sleep in neonatal intensive care units including environmental noise reduction, ear muffs when sleeping, lights on from 07:00–19:00 (and off the rest of the time) and nonpharmacological treatments for pain.95–99
INTERSCORER RELIABILIT Y WHEN SCORING SLEEP IN INFANTS
Very few studies have been published evaluating reliability of individuals scoring sleep studies in infants. Crowell et al.39 calculated inter-reliabilities and intra-reliabilities for sleep parameters and sleep stages for three senior investigators and four sleep technologists scoring 408 randomly selected 30-sec epochs from overnight home PSG recorded in 15 CHIME home PSG records.39 They found the kappa statistics (Κ) for agreement in scoring among the investigators were 0.56 for EEG, 0.51 EOG, 0.54 body movements, and 0.67 respiration. After intensive training, Κ agreement for the technologists was 0.76 for EEG, 0.62 EOG, 0.68 body movements, and 0.73 for respiration. Crowell et al. found that intensive uniform training and careful attention to specification of scoring criteria improved inter-scorer reliability.
Stefanski et al.100 reported an overall inter-scorer agreement of 87% for scoring sleep EEG patterns in 23 infants using a scoring system they developed and validated.100 The greatest inter-scorer disagreement occurred when scoring premature infants. Hoppenbrouwers35 analyzed scoring agreement in their work and others.70,101 The following conditions were found: (1) infant scoring criteria that required three or four of five criteria to score N sleep significantly altered the percentage of N scored; (2) scoring agreements were “uniformly low” (50%) when scoring indeterminate sleep; (3) correctly scoring N sleep in infants 1–2 mo of age was especially challenging because TA had disappeared and sleep spindles were usually absent; and (4) using behavioral observations including respiration may have helped reduce inter-scorer disagreement when faced with distinguishing the M EEG background of REM sleep from HVS of NREM sleep at this age.
Satomaa et al.67 scored comprehensive home PSGs in 84 healthy 1-mo-old infants using sleep scoring criteria they adapted from the 2012 AASM criteria.67 Infants ranged in age from 44–48 w CA. Inter-scorer agreement between two independent scorers was 80.6% and a kappa score of 0.73 indicating substantial agreement. Scoring agreement was > 80% for REM, NREM, and W, but only 46% for T sleep. The most common mismatch between sleep stages were: T/REM (26.5%), Tl/ NREM (16.1%), and T/W (8.9%). W/REM, REM/NREM, and W/NREM mismatches accounted for only 10%, 5%, and 2% of all mismatches, respectively.
Time and again when AASM scoring rules are written, pragmatism wins. Rules are developed (and later revised) to be as simple as possible. Some pediatric sleep specialists and researchers lament the AASM Manual lumping stages 3 and 4 sleep into NREM 3, saying the “richness and complexity” of slow wave activity is lost. However, inter-scoring reliability is far better when stages 3 and 4 are combined, and so reason overruled wish. The Scoring Manual Editorial Board's bold decision the EEG in infant PSG be scored as continuous or discontinuous (rather than LVI, HVS, M) reflects recognition that TA is the only neonatal EEG pattern that can be reliably recognized even by experts.
FUTURE DIRECTIONS
These criteria for scoring sleep/wake states between ages 0–2 mo represents far more than baby steps. These rules, like all the other AASM Scoring Manual rules, are not fixed in stone, and are open for debate, discussion, and revision. Future studies are needed to: (1) evaluate inter-scorer and intra-scoring reliability of these sleep staging rules for ages 0–2 mo; (2) confirm or refute that scoring simply as continuous or discontinuous will suffice; (3) confirm whether these rules can be used in infants when stressed, sick or born premature; and (4) develop normative data for these by comparing results between patients, laboratories, and research.
DISCLOSURE STATEMENT
This was not an industry supported study. The author has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
I would like to acknowledge my dear husband, Stanley Damberger, who edited the manuscript, and Dr. Mark Scher who was a member of the advisory committee. The literature searches, evidence review, rationale and writing of this entire document was solely done by the author and submitted to the AASM Scoring Manual Editorial Board.
ABBREVIATIONS
- A1
left auricular electrode
- A2
right auricular electrode
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index (mean number of apneas and hypopneas per hour of sleep)
- APSS
Association for the Psychophysiological Study of Sleep
- A
active (REM) sleep
- AS
active (REM) sleep
- ASI
REM sleep EEG pattern following wakefulness
- AS2
REM sleep EEG pattern following a period of NREM sleep
- AW
wakefulness
- CA
conceptional age
- CAI
central apnea index
- C3
left central electrode
- C4
right central electrode
- CHIME
Collaborative Home Infant Monitoring Evaluation study
- Cz
vertex electrode
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electro-oculogram
- F3
left frontal electrode
- F4
right frontal electrode
- FST
frontal sharp transients
- GA
gestational age
- HVS
high voltage slow
- Hz
hertz
- IBIs
interburst intervals
- IS
indeterminate (transitional) sleep
- LVI
low voltage irregular EEG pattern
- κ
kappa statistics
- μV
microvolts
- mm
millimeter(s)
- M1
left mastoid electrode
- M2
right mastoid electrode
- M
mixed EEG pattern
- MEG
magnetoencephalography
- mo
month(s)
- N
nonrapid eye movement sleep
- NREM
nonrapid eye movement sleep
- O1
left occipital electrode
- O2
right occipital electrode
- PMA
post menstrual age
- PSG
polysomnogram
- Q
quiet (NREM) sleep
- QS
quiet (NREM) sleep
- R
rapid eye movement sleep
- REM
rapid eye movement sleep
- REMs
rapid eye movements
- SDB
sleep disordered breathing
- sec
second(s)
- T
transitional sleep
- TA
trace alternant
- TST
total sleep time
- W
wakefulness
- w
week(s)
- y
year(s)
REFERENCES
- 1.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2014. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, Version 2.0.3. www.aasmnet.org. [Google Scholar]
- 2.Berry RB, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2015. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, Version 2.2. www.aasmnet.org. [Google Scholar]
- 3.Anders T, Emde R, Parmelee A. Los Angeles, CA: UCLA Brain Information Service, NINDS Neurological information Network; 1971. A manual of standardized terminology, techniques and criteria for scoring states of sleep and wakefulness in newborn infants. [Google Scholar]
- 4.Holmes GL, Logan WJ, Kirkpatrick BV, Meyer EC. Central nervous system maturation in the stressed premature. Ann Neurol. 1979;6:518–22. doi: 10.1002/ana.410060610. [DOI] [PubMed] [Google Scholar]
- 5.Nunes ML, Da Costa JC, Moura-Ribeiro MV. Polysomnographic quantification of bioelectrical maturation in preterm and fullterm newborns at matched conceptional ages. Electroencephalogr Clin Neurophysiol. 1997;102:186–91. doi: 10.1016/s0013-4694(96)95191-7. [DOI] [PubMed] [Google Scholar]
- 6.Scher MS, Steppe DA, Dahl RE, Asthana S, Guthrie RD. Comparison of EEG sleep measures in healthy full-term and preterm infants at matched conceptional ages. Sleep. 1992;15:442–8. doi: 10.1093/sleep/15.5.442. [DOI] [PubMed] [Google Scholar]
- 7.Engle WA the American Academy of Pediatrics Committee on Fetus and Newborn. Age terminology during the perinatal period. Pediatrics. 2004;114:1362–4. doi: 10.1542/peds.2004-1915. [DOI] [PubMed] [Google Scholar]
- 8.Wagner IF. Curves of sleep depth in newborn infants. J Genet Psychology. 1939;55:121–35. [Google Scholar]
- 9.Illingworth RS. Sleep problems in the first three years. J R Inst Public Health. 1952;15:191–4. [PubMed] [Google Scholar]
- 10.Moore T, Ucko LE. Night waking in early infancy. I. Arch Dis Child. 1957;32:333–42. doi: 10.1136/adc.32.164.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aserinsky E, Kleitman N. Two types of ocular motility occurring during sleep. J Applied Physiology. 1955;8:1–10. doi: 10.1152/jappl.1955.8.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Aserinsky E, Kleitman N. A motility cycle in sleeping infants as manifested by ocular and gross bodily activity. J Appl Physiol. 1955;8:11–8. doi: 10.1152/jappl.1955.8.1.11. [DOI] [PubMed] [Google Scholar]
- 13.Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9:673–90. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- 14.Dreyfus-Brisac C, Fischgold H, Samson-Dollfus D, et al. Veille, sommeil et al réactivité sensorielle chez le prématuré, le nouveau-né et le nourisson. Clin Neurophysiol. 1957;(Suppl 6):417–40. [Google Scholar]
- 15.Dreyfus-Brisac C, Monod N. Sleep of premature and full-term neonates--a polygraphic study. Proc R Soc Med. 1965;58:6–7. doi: 10.1177/003591576505800104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monod N, Pajot N. [The sleep of the full-term newborn and premature infant. I. Analysis of the polygraphic study (rapid eye movements, respiration and E.E.G.) in the full-term newborn]. Biologia neonatorum. Neo-natal studies. 1965;8:281–307. [PubMed] [Google Scholar]
- 17.Parmelee AH, Jr, Schulte FJ, Akiyama Y, Wenner WH, Schultz MA, Stern E. Maturation of EEG activity during sleep in premature infants. Electroencephalogr Clin Neurophysiol. 1968;24:319–29. doi: 10.1016/0013-4694(68)90193-4. [DOI] [PubMed] [Google Scholar]
- 18.Prechtl HF. Polygraphic studies in newborn infants. Electroencephalogr Clin Neurophysiol. 1967;23:494. [PubMed] [Google Scholar]
- 19.Prechtl HF. Clinical neurophysiology of early life. Electroencephalogr Clin Neurophysiol Suppl. 1978:57–66. [PubMed] [Google Scholar]
- 20.Prechtl HF. The organization of behavioral states and their dysfunction. Semin Perinatol. 1992;16:258–63. [PubMed] [Google Scholar]
- 21.Anders TF, Keener MA, Kraemer H. Sleep-wake state organization, neonatal assessment and development in premature infants during the first year of life. II. Sleep. 1985;8:193–206. doi: 10.1093/sleep/8.3.193. [DOI] [PubMed] [Google Scholar]
- 22.Anders TF, Keener M. Developmental course of nighttime sleep-wake patterns in full-term and premature infants during the first year of life. I. Sleep. 1985;8:173–92. doi: 10.1093/sleep/8.3.173. [DOI] [PubMed] [Google Scholar]
- 23.Anders TF. Home-recorded sleep in 2- and 9-month-old infants. J Am Acad Child Psychiatry. 1978;17:421–32. doi: 10.1016/s0002-7138(09)62298-6. [DOI] [PubMed] [Google Scholar]
- 24.Anders TF, Sostek AM. The use of time lapse video recording of sleep-wake behavior in human infants. Psychophysiology. 1976;13:155–8. doi: 10.1111/j.1469-8986.1976.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 25.Parmelee AH, Jr, Akiyama Y, Schulz MA, Wenner WH, Schulte FJ. Analysis of the electroencephalogram of sleeping infants. Act Nerv Super (Praha) 1969;11:111–5. [PubMed] [Google Scholar]
- 26.Dreyfus-Brisac C. Ontogenesis of sleep in human prematures after 32 weeks of conceptional age. Dev Psychobiol. 1970;3:91–121. doi: 10.1002/dev.420030203. [DOI] [PubMed] [Google Scholar]
- 27.Curzi-Dascalova L, Peirano P, Morel-Kahn F. Development of sleep states in normal premature and full-term newborns. Dev Psychobiol. 1988;21:431–44. doi: 10.1002/dev.420210503. [DOI] [PubMed] [Google Scholar]
- 28.Torres F, Anderson C. The normal EEG of the human newborn. J Clin Neurophysiol. 1985;2:89–103. doi: 10.1097/00004691-198504000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Parmelee AH, Stern E, Harris MA. Maturation of respiration in prematures and young infants. Neuropadiatrie. 1972;3:294–304. doi: 10.1055/s-0028-1091768. [DOI] [PubMed] [Google Scholar]
- 30.Dreyfus-Brisac C, Minkowski A. [Electroencephalographic maturation and too low birth weight] Rev Neurol (Paris) 1968;119:299–301. [PubMed] [Google Scholar]
- 31.Dreyfus-Brisac C, Larroche JC. Discontinuous electroencephalograms in the premature newborn and at term. Electro-anatomo-clinical correlations. Rev Electroencephalogr Neurophysiol Clin. 1971;1:95–9. doi: 10.1016/s0370-4475(71)80022-9. [DOI] [PubMed] [Google Scholar]
- 32.Andre M, Lamblin MD, d'Allest AM, et al. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiol Clin. 2010;40:59–124. doi: 10.1016/j.neucli.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Scher MS, Johnson MW, Holditch-Davis D. Cyclicity of neonatal sleep behaviors at 25 to 30 weeks' postconceptional age. Pediatr Res. 2005;57:879–82. doi: 10.1203/01.PDR.0000157678.84132.A8. [DOI] [PubMed] [Google Scholar]
- 34.Prechtl HF. Qualitative changes of spontaneous movements in fetus and preterm infant are a marker of neurological dysfunction. Early Hum Dev. 1990;23:151–8. doi: 10.1016/0378-3782(90)90011-7. [DOI] [PubMed] [Google Scholar]
- 35.Prechtl HF. State of the art of a new functional assessment of the young nervous system. An early predictor of cerebral palsy. Early Hum Dev. 1997;50:1–11. doi: 10.1016/s0378-3782(97)00088-1. [DOI] [PubMed] [Google Scholar]
- 36.Hoppenbrouwers T. New York City, NY: Raven Press; 1987. Sleep in infants. [Google Scholar]
- 37.Crowell DH, Kulp TD, Kapuniai LE, et al. Infant polysomnography: reliability and validity of infant arousal assessment. J Clin Neurophysiol. 2002;19:46–83. doi: 10.1097/00004691-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Scholle S, Schafer T. Atlas of states of sleep and wakefulness in infants and children. Somnologie. 1999;3:163–241. [Google Scholar]
- 39.Crowell DH, Brooks LJ, Colton T, et al. Infant polysomnography: reliability. Collaborative Home Infant Monitoring Evaluation (CHIME) Steering Committee. Sleep. 1997;20:553–60. [PubMed] [Google Scholar]
- 40.Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Current Biology. 2013;23:R532–7. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blumberg MS. Homology, correspondence, and continuity across development: the case of sleep. Dev Psychobiol. 2013;55:92–100. doi: 10.1002/dev.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Heuvel MP, Kersbergen KJ, de Reus MA, et al. The neonatal connectome during preterm brain development. Cereb Cortex. 2015;25:3000–13. doi: 10.1093/cercor/bhu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheldon SH. Development of sleep in infants and children. In: Sheldon SH, Ferber R, Kryger MH, Gozal D, editors. Development of sleep in infants and children. Chicago, IL: Elsevier Saunders; 2014. pp. 17–24. [Google Scholar]
- 44.Blumberg MS, Gall AJ, Todd WD. The development of sleep-wake rhythms and the search for elemental circuits in the infant brain. Behav Neurosci. 2014;128:250–63. doi: 10.1037/a0035891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selton D, Andre M, Debruille C, Deforge H, Fresson J, Hascoet JM. EEG at 6 weeks of life in very premature neonates. Clin Neurophysiol. 2010;121:818–22. doi: 10.1016/j.clinph.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 46.Scher MS. Ontogeny of EEG sleep from neonatal through infancy periods. Handb Clin Neurol. 2011;98:111–29. doi: 10.1016/B978-0-444-52006-7.00008-3. [DOI] [PubMed] [Google Scholar]
- 47.Piaget J. New York, NY: Norton; 1951. Play, dreams, and imitation in childhood. [Google Scholar]
- 48.Piers MW, Piaget J Erikson Institute. New York City, NY: Norton; 1972. Loyola University Chicago school of psychology play and development: a symposium. [Google Scholar]
- 49.Horne J. Why REM sleep? Clues beyond the laboratory in a more challenging world. Biol Psychology. 2013;92:152–68. doi: 10.1016/j.biopsycho.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152:604–19. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- 51.Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- 52.Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol. 2013;23:R532–7. doi: 10.1016/j.cub.2013.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chipaux M, Colonnese MT, Mauguen A, et al. Auditory stimuli mimicking ambient sounds drive temporal “delta-brushes” in premature infants. PloS One. 2013;8:e79028. doi: 10.1371/journal.pone.0079028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lamblin MD, Walls Esquivel E, Andre M. The electroencephalogram of the full-term newborn: review of normal features and hypoxic-ischemic encephalopathy patterns. Neurophysiol Clin. 2013;43:267–87. doi: 10.1016/j.neucli.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Scholle S, Feldmann-Ulrich E. Landsberg, Germany: Ecomed Medizin; 2012. Polysomnographic atlas of sleep-wake states during development from infancy to adolescence. [Google Scholar]
- 56.Qubty WF, Mrelashvili A, Kotagal S, Lloyd RM. Comorbidities in infants with obstructive sleep apnea. J Clin Sleep Med. 2014;10:1213–6. doi: 10.5664/jcsm.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramgopal S, Kothare SV, Rana M, Singh K, Khatwa U. Obstructive sleep apnea in infancy: a 7-year experience at a pediatric sleep center. Pediatr Pulmonol. 2014;49:554–60. doi: 10.1002/ppul.22867. [DOI] [PubMed] [Google Scholar]
- 58.Reddy KR, Lim MT, Lee TJ, Goh DY, Ramamurthy MB. Pediatric polysomnographic studies at a tertiary-care hospital in Singapore. Indian Pediatr. 2014;51:484–6. doi: 10.1007/s13312-014-0433-9. [DOI] [PubMed] [Google Scholar]
- 59.Schlüter B, Buschatz D, Trowitzsch E. Polysomnographic reference curves for the first and second year of life. Somnologie. 2001;5:3–16. [Google Scholar]
- 60.Kato I, Franco P, Groswasser J, Kelmanson I, Togari H, Kahn A. Frequency of obstructive and mixed sleep apneas in 1,023 infants. Sleep. 2000;23:487–92. [PubMed] [Google Scholar]
- 61.Franco P, Szliwowski H, Dramaix M, Kahn A. Influence of ambient temperature on sleep characteristics and autonomic nervous control in healthy infants. Sleep. 2000;23:401–7. [PubMed] [Google Scholar]
- 62.Rebuffat E, Groswasser J, Kelmanson I, Sottiaux M, Kahn A. Polygraphic evaluation of night-to-night variability in sleep characteristics and apneas in infants. Sleep. 1994;17:329–32. [PubMed] [Google Scholar]
- 63.Guilleminault C, Ariagno R, Korobkin R, Coons S, Owen-Boeddiker M, Baldwin R. Sleep parameters and respiratory variables in “near miss” sudden infant death syndrome infants. Pediatrics. 1981;68:354–60. [PubMed] [Google Scholar]
- 64.Kahn A, Groswasser J, Franco P, Kelmanson IA, Kato I, Dan B, Scaillet S. Breathing during sleep in infancy. In: Loughlin GM, Carroll JL, Marcus CL, editors. Sleep and breathing in children: a developmental approach. New York: Marcel Dekker, Inc.; 2000. pp. 405–22. [Google Scholar]
- 65.Ng DK, Chan CH. A review of normal values of infant sleep polysomnography. Pediatr Neonatol. 2013;54:82–7. doi: 10.1016/j.pedneo.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Ng DK, Leung LC, Tong TF, Chan CH, Wong SF. Airway obstruction in premature newborns: a missing link. Pediatrics. 2005;115:1789. doi: 10.1542/peds.2005-0495. author reply 1789–90. [DOI] [PubMed] [Google Scholar]
- 67.Satomaa AL, Saarenpaa-Heikkila O, Paavonen EJ, Himanen SL. The adapted American Academy of Sleep Medicine sleep scoring criteria in one month old infants: a means to improve comparability? Clin Neurophysiol. 2016;127:1410–8. doi: 10.1016/j.clinph.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 68.Mirmiran M, Maas YG, Ariagno RL. Development of fetal and neonatal sleep and circadian rhythms. Sleep Med Rev. 2003;7:321–34. doi: 10.1053/smrv.2002.0243. [DOI] [PubMed] [Google Scholar]
- 69.Moussalli F, Arfel G. [Lambda waves in the neonate (author's transl)] Rev Electroencephalogr Neurophysiol Clin. 1977;7:361–4. doi: 10.1016/s0370-4475(77)80015-4. [DOI] [PubMed] [Google Scholar]
- 70.Ellingson RJ, Peters JF. Development of EEG and daytime sleep patterns in normal full-term infant during the first 3 months of life: longitudinal observations. Electroencephalogr Clin Neurophysiol. 1980;49:112–24. doi: 10.1016/0013-4694(80)90357-0. [DOI] [PubMed] [Google Scholar]
- 71.De Weerd AW, Despland PA, Plouin P. Neonatal EEG. Electroencephalogr Clin Neurophysiol. 1999;(Suppl 52):149–57. [PubMed] [Google Scholar]
- 72.Kahn A, Dan B, Groswasser J, Franco P, Sottiaux M. Normal sleep architecture in infants and children. J Clin Neurophysiol. 1996;13:184–97. doi: 10.1097/00004691-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Curzi-Dascalova L, Gaudebout C, Dreyfus-Brisac C. Respiratory frequencies of sleeping infants during the first months of life: correlations between values in different sleep states. Early Hum Dev. 1981;5:39–54. doi: 10.1016/0378-3782(81)90069-4. [DOI] [PubMed] [Google Scholar]
- 74.Eliet-Flescher J, Dreyfus-Brisac C. [The sleep of the full-term newborn and premature infant. II. Electroencephalogram and chin muscle activity during maturation] Biol Neonat. 1966;10:316–39. [PubMed] [Google Scholar]
- 75.Schloon H, O'Brien MJ, Scholten CA, Prechtl HF. Muscle activity and postural behaviour in newborn infants. A polymyographic study. Neuropadiatrie. 1976;7:384–415. doi: 10.1055/s-0028-1091640. [DOI] [PubMed] [Google Scholar]
- 76.Salzaulo P. L'atonie msculaire pendant le sommeil chez l'homme. Riv Psicol. 1968;62:201–20. [Google Scholar]
- 77.Ellingson RJ, Peters JF. Development of EEG and daytime sleep patterns in low risk premature infants during the first year of life: longitudinal observations. Electroencephalogr Clin Neurophysiol. 1980;50:165–71. doi: 10.1016/0013-4694(80)90333-8. [DOI] [PubMed] [Google Scholar]
- 78.Metcalf DR. The effect of extrauterine experience on the ontogenesis of EEG sleep spindles. Psychosom Med. 1969;31:393–9. doi: 10.1097/00006842-196909000-00005. [DOI] [PubMed] [Google Scholar]
- 79.Metcalf DR. EEG sleep spindle ontogenesis. Neuropadiatrie. 1970;1:428–33. doi: 10.1055/s-0028-1091828. [DOI] [PubMed] [Google Scholar]
- 80.Curzi-Dascalova L. [Waking and sleeping E.E.G. in normal babies before 6 months of age (author's transl)] Rev Electroencephalogr Neurophysiol Clin. 1977;7:316–26. doi: 10.1016/s0370-4475(77)80010-5. [DOI] [PubMed] [Google Scholar]
- 81.Lenard HG. The development of sleep spindles in the EEG during the first two years of life. Neuropadiatrie. 1970;1:264–76. doi: 10.1055/s-0028-1091818. [DOI] [PubMed] [Google Scholar]
- 82.Louis J, Zhang J, Revol M, et al. Ontogenesis of nocturnal organization of sleep spindles: a longitudinal study during the first six months of life. Electroencephalogr Clin Neurophysiol. 1992;83:289–96. doi: 10.1016/0013-4694(92)90088-y. [DOI] [PubMed] [Google Scholar]
- 83.Jenni OG, Borbely AA, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R528–38. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- 84.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 85.Sterman MB, McGinty DJ, Harper RM, Hoppenbrouwers T, Hodgman JE. Developmental comparison of sleep EEG power spectral patterns in infants at low and high risk for sudden death. Electroencephalogr Clin Neurophysiol. 1982;53:166–81. doi: 10.1016/0013-4694(82)90021-9. [DOI] [PubMed] [Google Scholar]
- 86.Schulte FJ, Bell EF. Bioelectric brain development. An atlas of EEG power spectra in infants and young children. Neuropadiatrie. 1973;4:30–45. doi: 10.1055/s-0028-1091726. [DOI] [PubMed] [Google Scholar]
- 87.Kirjavainen T, Cooper D, Polo O, Sullivan CE. Respiratory and body movements as indicators of sleep stage and wakefulness in infants and young children. J Sleep Res. 1996;5:186–94. doi: 10.1046/j.1365-2869.1996.t01-1-00003.x. [DOI] [PubMed] [Google Scholar]
- 88.Roffwarg H, Dement W, Fisher C. Preliminary observations of the sleep-wake pattern in neonates, infants, children, and adults. In: Harms E, editor. Preliminary observations of the sleep-wake pattern in neonates, infants, children, and adults. New York City, NY: Macmillan; 1964. pp. 60–72. [Google Scholar]
- 89.de Weerd AW, van den Bossche RA. The development of sleep during the first months of life. Sleep Med Rev. 2003;7:179–91. doi: 10.1053/smrv.2002.0198. [DOI] [PubMed] [Google Scholar]
- 90.Parmelee AH, Jr, Wenner WH, Akiyama Y, Schultz M, Stern E. Sleep states in premature infants. Dev Med Child Neurol. 1967;9:70–7. doi: 10.1111/j.1469-8749.1967.tb02212.x. [DOI] [PubMed] [Google Scholar]
- 91.Arditi-Babchuk H, Feldman R, Eidelman AI. Rapid eye movement (REM) in premature neonates and developmental outcome at 6 months. Infant Behav Dev. 2009;32:27–32. doi: 10.1016/j.infbeh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Geva R, Yaron H, Kuint J. Neonatal sleep predicts attention orienting and distractibility. J Atten Disord. 2016;20:138–50. doi: 10.1177/1087054713491493. [DOI] [PubMed] [Google Scholar]
- 93.Weisman O, Magori-Cohen R, Louzoun Y, Eidelman AI, Feldman R. Sleep-wake transitions in premature neonates predict early development. Pediatrics. 2011;128:706–14. doi: 10.1542/peds.2011-0047. [DOI] [PubMed] [Google Scholar]
- 94.Nunes ML, Khan RL, Gomes Filho I, Booij L, da Costa JC. Maturational changes of neonatal electroencephalogram: a comparison between intra uterine and extra uterine development. Clin Neurophysiol. 2014;125:1121–8. doi: 10.1016/j.clinph.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 95.Hwang SS, O'Sullivan A, Fitzgerald E, Melvin P, Gorman T, Fiascone JM. Implementation of safe sleep practices in the neonatal intensive care unit. J Perinatol. 2015;35:862–6. doi: 10.1038/jp.2015.79. [DOI] [PubMed] [Google Scholar]
- 96.Almadhoob A, Ohlsson A. Sound reduction management in the neonatal intensive care unit for preterm or very low birth weight infants. Cochrane Database Syst Rev. 2015;1:CD010333. doi: 10.1002/14651858.CD010333.pub2. [DOI] [PubMed] [Google Scholar]
- 97.Voos KC, Terreros A, Larimore P, Leick-Rude MK, Park N. Implementing safe sleep practices in a neonatal intensive care unit. J Matern Fetal Neonatal Med. 2015;28:1637–40. doi: 10.3109/14767058.2014.964679. [DOI] [PubMed] [Google Scholar]
- 98.Szymczak SE, Shellhaas RA. Impact of NICU design on environmental noise. J Neonatal Nurs. 2014;20:77–81. doi: 10.1016/j.jnn.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mason B, Ahlers-Schmidt CR, Schunn C. Improving safe sleep environments for well newborns in the hospital setting. Clin Pediatr. 2013;52:969–75. doi: 10.1177/0009922813495954. [DOI] [PubMed] [Google Scholar]
- 100.Stefanski M, Schulze K, Bateman D, et al. A scoring system for states of sleep and wakefulness in term and preterm infants. Pediatr Res. 1984;18:58–62. [PubMed] [Google Scholar]
- 101.Emde RN, Walker S. Longitudinal study of infant sleep: results of 14 subjects studied at monthly intervals. Psychophysiology. 1976;13:456–61. doi: 10.1111/j.1469-8986.1976.tb00861.x. [DOI] [PubMed] [Google Scholar]
- 102.Anders TF, Chalemian RJ. The effects of circumcision on sleep-wake states in human neonates. Psychosom Med. 1974;36:174–9. doi: 10.1097/00006842-197403000-00009. [DOI] [PubMed] [Google Scholar]
- 103.Wolff PH. The causes, controls, and organization of behavior in the neonate. Psychol Issues. 1966;5:1–105. [PubMed] [Google Scholar]