Abstract
Study Objectives:
Studies have shown pharmacokinetic differences for hypnotics in women compared to men, but few studies have assessed either short-or long-term differences in efficacy and safety.
Methods:
To evaluate gender differences in the efficacy and safety of chronic nightly zolpidem (10 mg), we did a post hoc assessment of a large clinical trial. In the trial, participants with primary insomnia (n = 89), ages 23–70, meeting DSM-IV-TR criteria for primary insomnia were randomized, double blind, to nightly zolpidem, 10 mg (n = 47) or placebo (n = 42) 30 minutes before bedtime nightly for 12 months. Polysomnographic sleep on 2 nights in months 1 and 8 and likelihood of next-day sleepiness, rebound insomnia, and dose escalation were evaluated in months 1, 4, and 12.
Results:
Relative to placebo, zolpidem significantly increased sleep efficiency and reduced sleep latency and wake after sleep onset assessed at months 1 and 8, with no differences in efficacy between women and men and no diminution of efficacy over months. On a next-day multiple sleep latency test (MSLT), no residual sedation was observed for either women or men. No rebound insomnia or dose escalation was seen with no gender differences in either.
Conclusions:
In adults with primary insomnia, nightly zolpidem administration showed no gender differences in acute or chronic efficacy or in next-day sleepiness. Zolpidem remained efficacious and safe across 12 months.
Clincial Trials Registration:
ClinicalTrials.gov Identifier: NCT01006525; Trial Name: Safety and Efficacy of Chronic Hypnotic Use; http://clinicaltrials.gov/ct2/show/NCT01006525.
Citation:
Roehrs TA, Roth T. Gender differences in the efficacy and safety of chronic nightly zolpidem. J Clin Sleep Med 2016;12(3):319–325.
Keywords: gender differences, primary insomnia, zolpidem
INTRODUCTION
Gender-related pharmacokinetic differences in zolpidem plasma concentration have been reported, with plasma concentrations in women being higher and clearance slower than in men. Men metabolized the 10 mg standard formulation of zolpidem at approximately double the rate of women.1,2 The peak concentration of the sublingual formulation of zolpidem was 45% higher in women than men.3 Some studies have shown that these pharmacokinetic differences are associated with safety differences. At 5 h post daytime zolpidem administration and testing, poorer automobile driving was found3 and in the morning 4 h after middle of the night zolpidem administration, poorer automobile driving was shown in women than men.4 It should be noted that these pharmacokinetic studies were done in healthy volunteers and not patients with insomnia for whom this medication is indicated.
Many insomnia patients use prescription hypnotics chronically, with over half taking hypnotics longer than 4 weeks, likely due to their chronic and frequent symptomatology.5–8 Given this evidence of long-term hypnotic use in insomnia, it is also of interest to assess long-term gender differences in the efficacy and safety of zolpidem. Controlled studies using polysomnography (PSG) to evaluate hypnotic efficacy for 6 months and beyond have shown continued efficacy. But gender-related differences in long-term use have not been explored.9–16
As to safety, short-term placebo controlled trials show no signs of withdrawal, tolerance, rebound insomnia, and next morning residual effects at the maximum recommended therapeutic doses.14–17 However, these short-term studies, with the exception of next morning residual effects, have not compared men and women, and furthermore, no studies have assessed gender-related changes in these safety measures with long-term use. For example, it has been shown that the likelihood of rebound insomnia is related to dose; that is, higher doses are more likely to produce rebound insomnia.18 Given zolpidem plasma concentration is increased in women, are women more likely to experience rebound insomnia in short-term or long-term use?
BRIEF SUMMARY
Current Knowledge/Study Rationale: Studies have shown pharmacokinetic differences for zolpidem, the most frequently prescribed hypnotic in the US, in women compared to men. Few studies have assessed either short- or long-term gender differences in zolpidem 10 mg efficacy and safety. In women given higher plasma concentrations than men one might predict greater loss of efficacy and greater likelihood of dose escalation or rebound insomnia.
Study Impact: These post-hoc gender analyses of a large clinical trial in persons with insomnia that compared 12 months of nightly zolpidem 10 mg versus placebo did not show any loss of efficacy in either men or women. Women did not differ from men in likelihood of dose escalation or rebound insomnia. The data suggest the zolpidem gender-related pharmacokinetic differences do not translate to significant clinical differences.
This paper presents a post hoc analysis of gender-related differences in the efficacy and safety of nightly use of zolpidem in persons with primary insomnia, collected in a large 12-month clinical trial.18–20 Efficacy in this double blind, placebo-controlled investigation was assessed during months 1 and 8 using PSG and safety was evaluated with measures of next-day sleepiness, the likelihood of dose escalation, and rebound insomnia assessed in months 1, 4, and 12.
METHODS
Participants
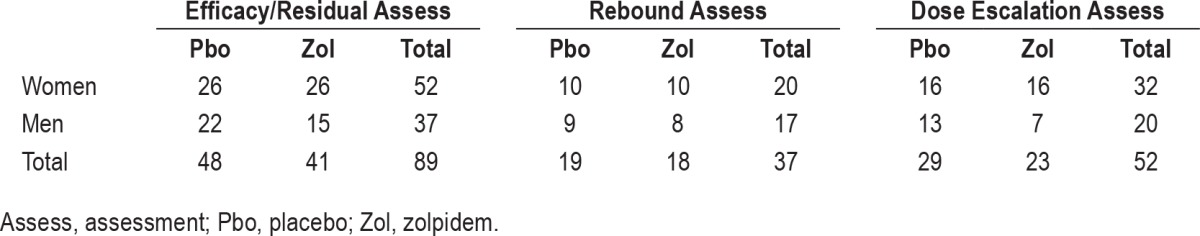
Participants, 52 women and 37 men, average age 50.4 years (range: 23–70 years), met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSMIV-TR) criteria for primary insomnia. They were randomized to receive placebo (n = 48) or zolpidem 10 mg (n = 41) nightly for 12 months. Table 1 outlines the “n” for the various study subgroups (gender, drug, and assessment type). All participants were in good psychiatric and physical health and without comorbid sleep disorders (sleep apnea, restless legs/periodic leg movements), as determined by the screening procedures described in detail previously.18
Table 1.
Number of participants in each assessment.
The Institutional Review Board of the Henry Ford Health System (HFHS) reviewed and approved the study protocol and a Data Safety Monitoring Board oversaw study conduct. All participants provided written informed consent after the procedures and potential side effects associated with zolpidem were verbally explained. They were monetarily compensated each month upon the completion of scheduled protocol activities (monthly clinic visit, laboratory sleep study and/or completion of 4 consecutive weekly telephone interactive voice response calls for medication compliance).
Screening
The complete study eligibility, screening and participant disposition were described previously.18 Briefly, participants were in good physical and mental health, determined by a medical history, physical examination, urinalysis and blood chemistry profiles, and a Structured Clinical Interview for the DSM (SCID). Those consuming > 14 standard alcoholic drinks per week, > 300 mg/day caffeine, unable to refrain from smoking during overnight laboratory visits, using illegal drugs within the past 2 years, or failing the urine drug screen were excluded. Pregnant or lactating females were excluded from study participation. Non-pregnant females were required to use standard birth control methods.
Screening PSG and Study PSG Procedures
The 8-h screening and study PSGs included standard electroencephalograms (EEGs), bilateral horizontal electroculograms (EOG), submental electromyogram (EMG), and electrocardiogram (ECG) recorded with a V5 lead.21 In addition, on the screening night, airflow and tibialis EMG recordings were monitored to assess apnea/hypopnea and leg movements. Subjects with respiratory disturbances (apnea hypopnea index [AHI] > 10) or with periodic limb movement arousal indices (PLMAI) > 10/h were excluded from the study.22 Subjects were required to demonstrate a screening sleep efficiency ≤ 85% (total sleep time / time in bed) and have no other sleep disorders. Subsequent PSGs excluded airflow and leg monitoring.
Rechtschaffen and Kales21 methods for sleep scoring were used. PSGs were scored in 30-sec epochs with scorers blind to treatment conditions and maintained 90% interrater reliability.
Medication Preparation and Dosing
The HFHS Research Pharmacy prepared study medication in size #1 clear capsules. Zolpidem (10 mg) clear capsules contained lactose and zolpidem. Placebo capsules were identical in appearance to zolpidem capsules, containing lactose only. Participants took their assigned medication 30 min before bedtime at home and in the sleep laboratory. A weekly telephone interactive voice response system (IVRS) captured patient reports regarding medication compliance and side effects.
Study Design
All participants had PSGs conducted on 2 consecutive treatment nights during the first week of months 1 and 8. A standard multiple sleep latency test (10:00, 12:00, 14:00, and 16:00) followed the night 2 PSG.23 Participants were assigned to either a dose escalation (n = 37) or rebound insomnia (n = 52) assessment conducted in months 1, 4, and 12 (all participants underwent the efficacy assessment in months 1 and 8).18–20 The subgroup assessments in month 1 were conducted after the efficacy assessment, and in months 4 and 12 the dose escalation or rebound insomnia assessments were preceded by a single PSG night on the assigned medication and followed the next day by MSLT.
The dose escalation assessment involved a 7-night protocol in which the first 2 nights were sampling nights and the next 5 nights were choice nights. For the zolpidem group the color assigned to placebo and zolpidem capsules differed and on sampling nights one of each color-coded capsule was administered. For the placebo group both capsules were placebo, but the colors administered differed each night. Participants were instructed on sampling nights to attend to the capsule color because on choice nights they would select which medication they wanted for a given night based on the capsule color of the sampling nights. On the following 5 choice nights, based on their sampling experience, the participant was instructed to choose a capsule color and then given the option on that night of taking up to 3 capsules of their chosen color. The zolpidem capsules were 5 mg each for a total possible nightly dose of 15 mg. The rebound insomnia assessment involved placebo substitution for 7 nights in both the zolpidem and placebo groups. On the first 2 nights and the last night (night 7), PSGs were collected; we assessed gender-related rebound on the first 2 placebo substitution nights only, as rebound was not found on night 7.
Analyses
The primary efficacy parameters were PSG defined measures of sleep efficiency (SE: sleep time/time in bed × 100%), latency to persistent sleep (LPS: latency to 10 min of continuous sleep), and wake after sleep onset (WASO: minutes of wake after LPS). All efficacy dependent measures were the mean of the 2 consecutive nights in months 1 and 8. The residual sedation measure used was the average sleep latency on the MSLT for months 1, 4, and 12. Likelihood of rebound insomnia was evaluated by determining, on the first 2 placebo substitution nights of months 1, 4, and 12, the number of nights on which sleep efficiency was less than that of the screening night. Rebound insomnia would be reflected in lower placebo substitution sleep efficiencies shown by the zolpidem group relative to the placebo group. The likelihood of dose escalation was assessed by tabulating the total number of placebo and zolpidem capsules chosen on the choice nights of months 1, 4, and 12. Again increased zolpidem choices compared to placebo choices would reflect dose escalation.
Data were analyzed using mixed design multivariate analyses of variance (MANOVA) with gender and drug (placebo and zolpidem) as between-subject factors and time (months 1 and 8 or months 1, 4, and 12 depending on the variable) as within-subject factors. Secondary analyses compared the 4 subgroups (gender and drug) on screening PSG with a between-group ANOVA. To assess night effects in the PSG efficacy analyses the 2 nights of month 1 were analyzed separately from the 2 nights of month 8. The percentage of nights on month 1, 4, and 12 of placebo substitution that showed lower sleep efficiencies was compared among the 4 subgroups with Kruskal-Wallis nonparametric analyses. While this trial was not originally designed to test gender differences, given the observed differences between placebo and zolpidem in the gender groups and the associated standard deviations, the power for a gender by group interaction given a within cell n of ≥ 15 was 0.80 for sleep efficiency, 0.75 for WASO, and 0.95 for LPS (p < 0.05). For the safety assessments the sample sizes were much smaller and the powers were 0.10 for the dose escalation, 0.28 for the next-day sleepiness, and 0.35 for the rebound insomnia assessments.
RESULTS
Efficacy by Gender
The SE, WASO, and LPS for women and men on screening, months 1 and 8 for the placebo and zolpidem groups are presented in Table 2. The 4 subgroups (gender and drug) were compared on the screening night. There were no main effects of groups or interactions for SE and WASO. On screening, the men assigned to the zolpidem group had a significantly shorter LPS than the men and women of the other 3 groups as reflected by post hoc analyses of a significant group by gender interaction (F = 4.11, p < 0.05).
Table 2.
PSG efficacy by gender and drug.
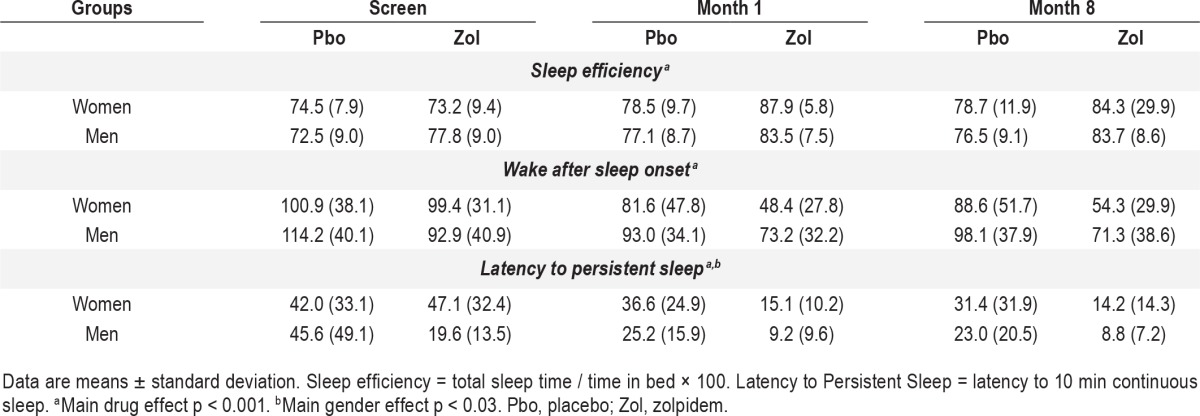
In the mixed design MANOVAs SE was increased with zolpidem compared to placebo (F = 23.8, p < 0.001), and there were no main effects of gender or gender by drug interactions, and no month effects or month interactions with gender or drug. Zolpidem was similarly effective for men and women, and its efficacy was not diminished from month 1 to 8. WASO was reduced by zolpidem (F = 16.6, p < 0.001) with no gender or month main effects or interactions. Finally, LPS was reduced by zolpidem (F = 26.3, p < 0.001), and again there were no gender or month main effects or interactions. As was the case at baseline, there was a main effect of gender (F = 4.7, p < 0.03), with LPS being shorter in the men. The separate month 1 and 8 analyses by night yielded no night effects or night interactions.
Next-day Sleepiness Effects (MSLT) by Gender
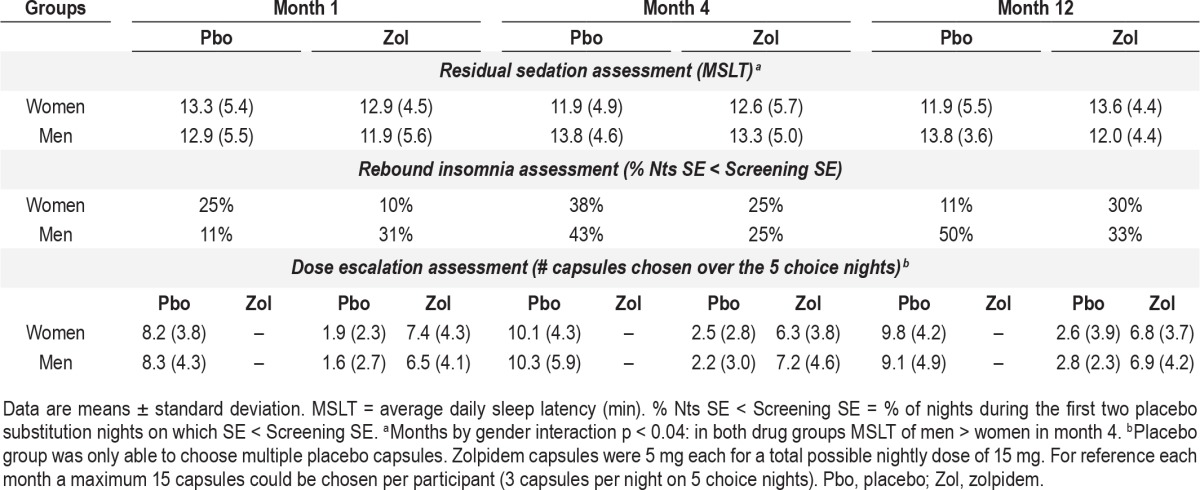
The average daily sleep latency on the MSLT for each gender and drug group in month 1, 4, and 12 is presented in Table 3. There were no main effects of gender or drug and no interaction effects of gender and drug. There was an interaction of gender by month, with the men in both placebo and zolpidem groups having a higher MSLT average daily sleep latency than women in month 4 compared to month 1 and 12 (F = 3.61, p < 0.04), which is likely a spurious finding. The relevant comparison showed there were no zolpidem residual effects in women or men as measured by the MSLT assessments that began 10 h after drug administration.
Table 3.
Safety measures by gender and drug.
Likelihood of Rebound Insomnia by Gender
The percent of nights with greater sleep disturbance on placebo substitution nights 1 and 2 is also presented in Table 3. The presence of rebound insomnia would be reflected in greater sleep disturbance on placebo substitution nights in the zolpidem compared to placebo groups, and there were no main effects of drug or gender and no drug or gender interactions. The rate of rebound did not increase across months. Twenty-seven percent of participants had rebound on more than one month. Repeatability of rebound did not vary as a function of gender or drug group. Three of the women in the placebo group experienced rebound in multiple months, as did 3 men. In the zolpidem group, 2 women and 2 men had rebound insomnia in more than one month. Those with repeated expression of placebo substitution rebound had less WASO on their screening night (i.e., better screening sleep) than those without or with a single night of sleep disturbance (t = 2.66, p < 0.01).
Likelihood of Dose Escalation by Gender
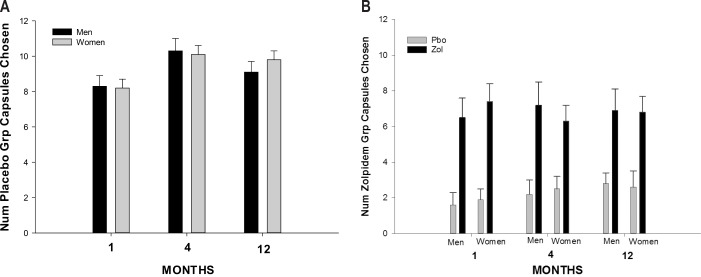
The total number of placebo and zolpidem capsules chosen on the 5 choice nights as a function of gender and drug group in months 1, 4, and 12 is presented in Table 3 and Figure 1. The placebo group was only able to choose placebo (1, 2, or 3 capsules each night), while the zolpidem group could choose zolpidem or placebo and then 1, 2, or 3 of their chosen capsule for that night. As previously reported the zolpidem group chose zolpidem more frequently than placebo (F = 85.6, p < 0.001). There were no gender differences in frequency of active drug choices. There also were no gender differences in the number of placebo choices in the placebo group. There was an increase in the total number of capsules chosen from month 1 to month 4, which remained elevated in month 12 (F = 4.9, p < 0.02), as shown in Figure 1 and Table 3. But, there was no gender difference in this increased capsule choice across months. The increase across months was due to an increase in the number of placebo capsules chosen by the placebo group (Month × Drug group interaction; F = 3.1, p < 0.05; see Figure 1A).
Figure 1.
(A) Total number of placebo capsules chosen by women and men in the placebo group in months 1, 4, and 12. Total possible choices each month 15 (3 on each of 5 nights). Number chosen increased in months 4 and 12 relative to month 1 (Group × Month interaction p < 0.05) in the placebo group with no gender difference. (B) Total number of placebo and zolpidem capsules chosen by men and women in the zolpidem group in months 1, 4, and 12. After choosing placebo or zolpidem on a given night a total of 3 capsules could be taken on each of 5 nights. No significant month or gender differences were found in the zolpidem group. Pbo, placebo; Zol, zolpidem.
DISCUSSION
This paper presents a post hoc analysis of gender differences in the efficacy and safety associated with 12 months of nightly zolpidem use by persons with primary insomnia. The data show the previously reported gender-related pharmacokinetic differences for zolpidem did not translate into gender-related differences in the efficacy and safety of zolpidem during either short- or long-term use.
As seen in Table 2 all the efficacy measures on the screening night were remarkably similar between women and men, except for men in the zolpidem group whose LPS was shorter than the women. This shorter male basal latency actually biased the study against showing a significant zolpidem reduction in LPS and hence a potential gender difference. Despite the shorter latency, zolpidem reduced LPS similarly in both men and women and reduced WASO in both men and women resulting in comparable improved sleep efficiency for both genders. The improved sleep was sustained through month 8 with no gender-related decline in efficacy. Given potentially higher zolpidem plasma concentration and slowed elimination in women compared to men, one might hypothesize greater potential receptor exposure to zolpidem and consequent greater receptor downregulation with long-term use, which would result in reduction or loss of efficacy. That was not observed over the 8 months of this trial. No tolerance development was observed in either men or women.
Consistent with an absence of tolerance development, no dose escalation was found in the zolpidem group and no differential gender-related dose escalation was seen. But “dose escalation” was seen in the placebo group, although the “dose escalation” seen in the placebo group was not gender specific. In a previous paper, the point was made that providing an ineffective (i.e., placebo) hypnotic is what leads to dose escalation.21 While women report higher rates of insomnia, these data would suggest that women are not at a differential risk for dose escalation of either an effective (zolpidem) or ineffective hypnotic (placebo). It is important to note that these data are related to clinical use of zolpidem in persons without an abuse history. It remains to be determined if among sedative abusers the degree of abuse of zolpidem would be different in men and women.
While greater likelihood of rebound insomnia in women than men might be predicted due to the previously reported elevated zolpidem plasma concentrations in women and the enhanced risk of rebound insomnia with high doses, no differential gender-related rebound insomnia was observed. Furthermore, no overall rebound insomnia was seen with zolpidem. For the majority of the participants, lower sleep efficiency on placebo substitution nights relative to their screening night was not a repeatable phenomenon. Among those showing repeated low sleep efficiency, the same number of women as men in both the zolpidem and placebo groups had the disturbance on multiple months.
The reason for lowered sleep efficiencies in those of the placebo group is not clear. It was found that regardless of gender those with the reduced sleep efficiency on placebo substitution nights had higher screening sleep efficiencies. Thus, their reduction may merely reflect a regression to the mean. It could also be that these individuals are more sensitive to changes in their sleep environment. The rebound insomnia assessments occurred after two months of sleeping at home.
Several limitations must be discussed. This paper presents a post hoc analysis of gender effects; the study was not specifically designed to test gender differences. The parent trial was designed to detect effect sizes of 0.60 with a 0.80 power given “n”s of 15–20 in the study's main efficacy, rebound insomnia, and dose escalation outcomes. As seen on Table 1 the “n”s for gender main effects meet these parameters, while the “n”s for gender interaction effects are not as robust. As noted in the analyses section the powers observed in the efficacy analyses for gender by drug interaction were 0.75 and greater. But, the power associated with the safety assessments were much lower given the small “n”s in some of the cells. The gender distribution in this trial, approximately 60% female, reflects the frequently reported female prevalence of insomnia.
Secondly, the objective residual effects assessment using the MSLT did not begin until 10 hours after drug administration. That means the 1.5-h wake period from 8.5 h to 10 h after drug administration was not directly and objectively assessed for residual effects. The study was designed to query for medication side effects on weekly telephone interactive voice response calls and monthly face-to-face clinic visits. In these side effect assessments no reports of residual sedation were received. Participants had been made aware of possible residual effects through the consent process and the written consent document.
Finally actual pharmacokinetic data were not collected in this study and consequently we are unable to relate the efficacy and safety data of this study to possible pharmacokinetic differences of zolpidem in the men and women. It will be important to do gender comparisons on the presence of residual sedation using measures of sleepiness and performance with concurrent measurement of plasma concentrations in the morning 8 h after nighttime use of zolpidem.
In summary, in men and women with primary insomnia both using the same zolpidem 10 mg dose nightly, there were no gender differences in acute or chronic efficacy or in residual effects. Zolpidem remained efficacious and safe across 12 months. There was no differential gender-related rebound insomnia or dose escalation.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was provided by a NIDA grant # R01DA17355 awarded to Dr. Roehrs. Dr. Roehrs has consulted for Lundbeck. Dr. Roth has consulted for Actelion, Addrenex, Cephalon, Eisai, Intec, Merck, Pfizer, Sanofi, Sepracor, Shire, Somaxon, and TransOral; has participated in speaking engagements for Cephalon and Sanofi; and has received research support from Merck.
ACKNOWLEDGMENTS
The authors thank the Henry Ford Sleep Center technical staff for nocturnal recordings as well as G. Koshorek, S. Cameron, A. Rojas and D. Ditri for their meticulous scoring of PSG records.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ECG
electrocardiogram
- EMG
submental electromyogram
- EOG
electroculograms
- HFHS
Henry Ford Health System
- IVRS
interactive voice response system
- LPS
latency to persistent sleep
- MANOVA
multivariate analyses of variance MSLT. Multiple Sleep Latency Test
- PLMAI
periodic limb movement arousal indices
- PSG
polysomnography
- SE
sleep efficiency
- SCID
Structured Clinical Interview for the DSM
- WASO
wake after sleep onset
REFERENCES
- 1.Greenblatt DJ, Harmatz JS, von Moltke LL, et al. Comparative kinetics and response to benzodiazepine agonists triazolam and zolpidem: evaluation of sex-dependent differences. J Pharmacol Exp Ther. 2000;293:435–43. [PubMed] [Google Scholar]
- 2.Olubodun JO, Ochs HR, von Moltke LL, et al. Pharmacokinetic properties of zolpidem in elderly and young adults: possible modulation by testosterone in men. Br J Clin Pharmacol. 2003;56:297–304. doi: 10.1046/j.0306-5251.2003.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenblatt DJ, Harmatz JS, Singh NN, et al. Gender differences in pharmacokinetics and pharmacodynamics of zolpidem following sublingual administration. J Clin Pharmacol. 2014;54:282–90. doi: 10.1002/jcph.220. [DOI] [PubMed] [Google Scholar]
- 4.Verster JC, Roth T. Gender differences in highway driving performance after administration of sleep medication: a review of the literature. Traffic Inj Prev. 2012;13:286–92. doi: 10.1080/15389588.2011.652751. [DOI] [PubMed] [Google Scholar]
- 5.Krakow B, Ulibarri VA, Romero EA. Patients with treatment-resistant insomnia taking nightly prescription medication for sleep: a retrospective assessment of diagnostic and treatment variables. Prim Care Companion J Clin Psychiatry. 2010;12:e1–10. doi: 10.4088/PCC.09m00873bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krakow B, Ulibarri VA, Romero EA. Persistent insomnia in chronic hypnotic users presenting to a sleep medical center: retrospective chart review of 137 consecutive patients. J Nerv Ment Dis. 2010;198:734–41. doi: 10.1097/NMD.0b013e3181f4aca1. [DOI] [PubMed] [Google Scholar]
- 7.Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 New Clinical Drug Evaluation Unit Meeting Symposium. Sleep Med Rev. 2004;8:7–17. doi: 10.1016/S1087-0792(03)00042-X. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal LD, Dolan DC, Taylor DJ, Grieser E. Long term follow up of patients with insomnia. Proc Bayl Univ Med Cent. 2008;21:264–5. doi: 10.1080/08998280.2008.11928409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effects on sleep, quality of life, and work limitations. Sleep. 2007;30:959–68. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, doubled blinded, placebo controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 11.Roth T, Walsh JK, Krystal A, et al. An evaluation of the efficacy and safety of eszopiclone over 12 month in patients with chronic primary insomnia. Sleep Med. 2005;6:487–95. doi: 10.1016/j.sleep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Mayer G, Wang-Weigan S, Roth-Schechter B, et al. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep. 2009;32:351–60. doi: 10.1093/sleep/32.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Richardson GS, Mangano RM, et al. Long-term use of sedative hypnotics in older patients with insomnia. Sleep Med. 2005;6:107–13. doi: 10.1016/j.sleep.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Schlich D, L'Heriter C, Coquelin JP, Attali P, Kryreub HJ. Long-term treatment of insomnia with zolpidem: a multicentre general practitioner study of 107 patients. J Int Med Res. 1991;19:271–9. doi: 10.1177/030006059101900313. [DOI] [PubMed] [Google Scholar]
- 15.Scharf MB, Roth T, Vogel GW, Walsh JK. A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55:192–9. [PubMed] [Google Scholar]
- 16.Maarek L, Cramer P, Attali P, Coquelin JP, Morselli PL. The safety and efficacy of zolpidem in insomniac patients: a long-term open study in general practice. J Int Med Res. 1992;20:162–70. doi: 10.1177/030006059202000208. [DOI] [PubMed] [Google Scholar]
- 17.Ware JC, Walsh JK, Scharf MB, Roehrs T, Roth T, Vogel GW. Minimal rebound insomnia after treatment with 10-mg zolpidem. Clin Neuropharmacol. 1997;20:116–25. doi: 10.1097/00002826-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Roehrs T, Merlotti L, Zorick F, Roth T. Rebound Insomnia in normals and patients with Insomnia after abrupt and tapered discontinuation. Psychopharmacology. 1992;108:67–71. doi: 10.1007/BF02245287. [DOI] [PubMed] [Google Scholar]
- 19.Randall S, Roehrs TA, Roth T. Efficacy of eight months of nightly zolpidem: a placebo controlled study. Sleep. 2012;35:1551–7. doi: 10.5665/sleep.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehrs TA, Randall S, Harris E, Maan R, Roth T. Twelve months of nightly zolpidem does not lead to rebound insomnia or withdrawal symptoms: a prospective placebo-controlled study. J Psychopharmacol. 2012;28:1088–95. doi: 10.1177/0269881111424455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roehrs TA, Randall S, Harris E, Maan R, Roth T. Twelve months of nightly zolpidem does not lead to dose escalation: a prospective placebo-controlled study. Sleep. 2011;34:207–12. doi: 10.1093/sleep/34.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A. Washington D.C.: Public Health Service, U.S. Government Printing Office; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 23.Carskadon MA, Dement WC, Mitler MM. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]