Abstract
Prostaglandin E2 (PGE2) produced by the cyclooxygenase (COX) pathway regulates skeletal muscle protein turnover and exercise training adaptations. The purpose of this study was twofold: 1) define the PGE2/COX pathway enzymes and receptors in human skeletal muscle, with a focus on type I and II muscle fibers; and 2) examine the influence of aging on this pathway. Muscle biopsies were obtained from the soleus (primarily type I fibers) and vastus lateralis (proportionally more type II fibers than soleus) of young men and women (n = 8; 26 ± 2 yr), and from the vastus lateralis of young (n = 8; 25 ± 1 yr) and old (n = 12; 79 ± 2 yr) men and women. PGE2/COX pathway proteins [COX enzymes (COX-1 and COX-2), PGE2 synthases (cPGES, mPGES-1, and mPGES-2), and PGE2 receptors (EP1, EP2, EP3, and EP4)] were quantified via Western blot. COX-1, cPGES, mPGES-2, and all four PGE2 receptors were detected in all skeletal muscle samples examined. COX-1 (P < 0.1) and mPGES-2 were ∼20% higher, while EP3 was 99% higher and EP4 57% lower in soleus compared with vastus lateralis (P < 0.05). Aging did not change the level of skeletal muscle COX-1, while cPGES increased 45% and EP1 (P < 0.1), EP3, and EP4 decreased ∼33% (P < 0.05). In summary, PGE2 production capacity and receptor levels are different in human skeletal muscles with markedly different type I and II muscle fiber composition. In aging skeletal muscle, PGE2 production capacity is elevated and receptor levels are downregulated. These findings have implications for understanding the regulation of skeletal muscle adaptations to exercise and aging by the PGE2/COX pathway and related inhibitors.
Keywords: prostaglandin E2, cyclooxygenase, PGE2 synthase, EP receptor, sarcopenia
over the last decade it has become clear that the cyclooxygenase (COX) pathway, which produces prostaglandins (PGs) and is regulated by common COX inhibiting drugs (38), is a major regulator of skeletal muscle adaptations to aging and exercise (49). PGE2 is one of the key PGs produced by this pathway and is intricately involved in regulating these adaptations (40, 49). As a result, this portion of the pathway has been referred to as the PGE2/COX pathway (49).
While some of the downstream molecular and metabolic signals of this pathway have been clarified (20, 40, 49, 51), the actual enzymes that direct the synthesis of PGE2, as well as the receptors for PGE2, have not been elucidated in human skeletal muscle. In addition, evidence from animal muscle studies (34, 43) and recent findings in older exercising individuals suggest that this pathway may be differentially expressed or regulated between the two major fiber types found in human skeletal muscle (i.e., type I and II) (47, 50). This potential fiber type specificity is important because of the large difference in functional properties and adaptability of type I and II muscle fibers (7, 11, 32, 35, 44, 45).
The purpose of this investigation was twofold: 1) define which PGE2/COX pathway enzymes and receptors are present in human skeletal muscle, with an additional focus on type I and II muscle fibers; and 2) examine the influence of aging on this pathway in a cohort with well-documented sarcopenia. The overarching hypothesis of the investigation was that the PGE2/COX pathway enzymes and receptors would generally be higher in type I muscle fibers and aging skeletal muscle.
MATERIALS AND METHODS
Subjects.
A total of 28 subjects were studied for this investigation. For the first part of the investigation that examined the PGE2/COX pathway and muscle fiber type, muscle biopsies (3) were obtained from the soleus (primarily type I fibers) and vastus lateralis (proportionally more type II fibers than soleus) of recreationally active young men and women (n = 8: 6 male, 2 female; 26 ± 2 yr; 175 ± 4 cm; and 75.2 ± 5.7 kg). For the second part of the investigation that examined the PGE2/COX pathway and aging, muscle biopsies were obtained from the vastus lateralis of a separate group of sedentary young (n = 8: 4 male, 4 female; 25 ± 1 yr; 172 ± 3 cm; and 71.5 ± 3.4 kg) and old (n = 12: 6 male, 6 female; 79 ± 2 yr, range: 70–93 yr; 166 ± 2 cm; and 69.8 ± 4.0 kg) men and women. The older group had well-documented sarcopenia, including an impaired ability (increased time) to climb stairs (80%), rise from a chair (56%), and walk (44%), as well as lower quadriceps muscle volume (−29%), muscle strength (−35%), muscle power (−48%), and strength (−17%) and power (−33%) normalized to muscle size (16). Data on both of these cohorts have been reported previously (16, 22, 33, 44, 48). The muscle biopsies were immediately frozen and stored in liquid nitrogen until analyzed as described below. All procedures, risks, and benefits associated with the experimental testing were explained to the subjects before they signed a consent form adhering to the Institutional Review Board guidelines.
PGE2/COX pathway protein quantification.
The following PGE2/COX pathway proteins, based on identification primarily from other tissues (4, 15, 18, 25, 27–29, 39, 41, 49), were quantified in each muscle sample using Western blot analysis: two COX enzymes (COX-1 and COX-2), three PGE2 synthases (cPGES, mPGES-1, and mPGES-2), and four PGE2 receptors (EP1, EP2, EP3, and EP4) (Fig. 1).
Fig. 1.
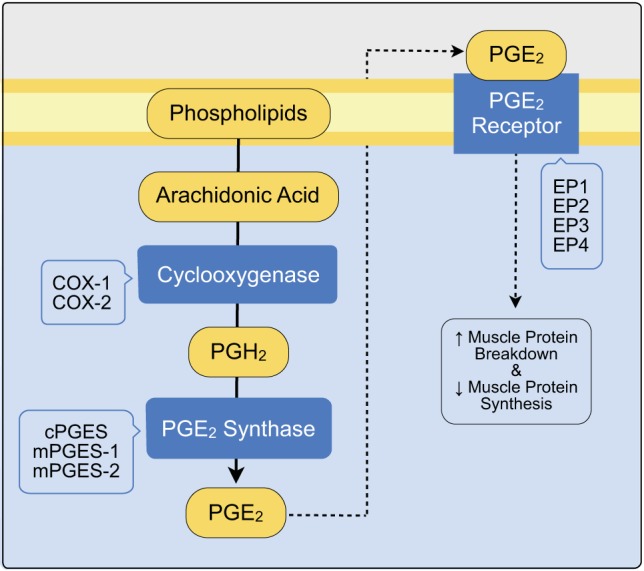
The general prostaglandin E2/cyclooxygenase (PGE2/COX) pathway. The specific enzyme isoforms and receptor subtypes listed in the blue offset boxes have been identified primarily in other tissues and were the focus of the current investigation. The yellow components represent lipid components of the pathway. Downstream effects of this pathway in human skeletal muscle are also shown (49).
Muscle samples (∼10 mg) were weighed at −25°C (Cahn C-35; Orion Research, Beverly, MA) and homogenized using a motorized ground-glass homogenizer (Duall; Kimble Chase, Vineland, NJ) in 40 vol of cold RIPA buffer (25 mM Tris·HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS; Pierce, Rockford, IL) with Halt protease and phosphatase inhibitors (Pierce). Homogenized samples were spun for 5 min at 14,000 g and 4°C. Protein concentration was determined on an aliquot of each supernatant with a BCA assay (Pierce) using bovine serum albumin (BSA) as the standard. Samples were diluted in SDS sample buffer, heated one time for 5 min at 95°C, and further diluted to a common concentration (1 μg/μl) before gel loading.
Proteins were resolved on a 4–20% gradient gel (Pierce) via SDS-PAGE for ∼90 min at 100 V (Mini Protean 3 system; Bio-Rad Laboratories, Hercules, CA) and then transferred to a polyvinylidene fluoride membrane (Immobilon-P; Millipore, Bedford, MA) for 90 min at 40 V and ∼4°C (Table 1). Membranes were blocked with either 5% milk or BSA (EP1 only) diluted in Tris-buffered saline with 1% Tween-20 (TBS-T) for 60 min at room temperature. Incubation with human primary antibodies diluted in 1× TBS-T was completed overnight at 4°C (Table 1). Membranes were incubated with secondary antibody (Table 1) in 5% milk or BSA (EP1 only) for 60 min at room temperature and exposed to an enhanced chemiluminescent substrate [Amersham ECL Prime, GE Healthcare, Piscataway, NJ; or ECL 2 Western blotting Substrate, Pierce (EP1 only)] for protein identification and quantification with a chemiluminescent imaging system (FluorChem SP; Alpha Innotech, San Leandro, CA).
Table 1.
PGE2/COX Pathway Western blot parameters
| Protein Loaded, μg | Primary Antibody Cat. No. | Primary Antibody Dilution | Secondary Antibody Cat. No. | Secondary Antibody Dilution | Positive Control Cat. No. | Negative Control Cat. No. | |
|---|---|---|---|---|---|---|---|
| COX-1 | 20 | 160110 | 1:200 | 10004302 | 1:10,000 | 60100 | — |
| COX-2 | 20 | 160112 | 1:500 | 10004302 | 1:10,000 | 10009624 | 360107 |
| cPGES | 20 | 160150 | 1:200 | 10004301 | 1:10,000 | 10010498 | — |
| mPGES-1 | 20 | 160140 | 1:200 | 10004301 | 1:10,000 | 10009734 | — |
| mPGES-2 | 8 | 160145 | 1:200 | 10004301 | 1:10,000 | SC-2254 | — |
| EP1 | 20 | 101740 | 1:500 | 10004301 | 1:10,000 | HSMSTD | 301740 |
| EP2 | 5 | 101750 | 1:500 | 10004301 | 1:10,000 | 10004722 | 301750 |
| EP3 | 10 | 101760 | 1:500 | 10004301 | 1:10,000 | HSMSTD | 301760 |
| EP4 | 10 | 101775 | 1:500 | 10004301 | 1:10,000 | HSMSTD | 301775 |
All primary antibodies were purchased from Cayman Chemical (Ann Arbor, MI). All commercially purchased positive and negative controls were obtained from Cayman, except mPGES-2, which was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). PGE2, prostaglandin E2; COX, cyclooxygenase; HSMSTD, human skeletal muscle standard (vastus lateralis, obtained from our laboratory), which was used for quantification normalization when a positive control was not available. All positive controls are human except for mPGES-2 (mouse) and EP2 (ram).
Each gel was loaded with soleus and vastus lateralis samples from the same subject or young and old samples in alternating fashion, a molecular mass ladder (MagicMark XP Western Protein Standard; Invitrogen/Life Technologies, Grand Island, NY), and a positive control protein or human skeletal muscle standard (Table 1). Intensities of the protein bands were quantified and normalized to the positive control or standard and expressed as arbitrary units (AU). Verification of equal protein loading was accomplished by Ponceau S staining.
Western blot identification considerations.
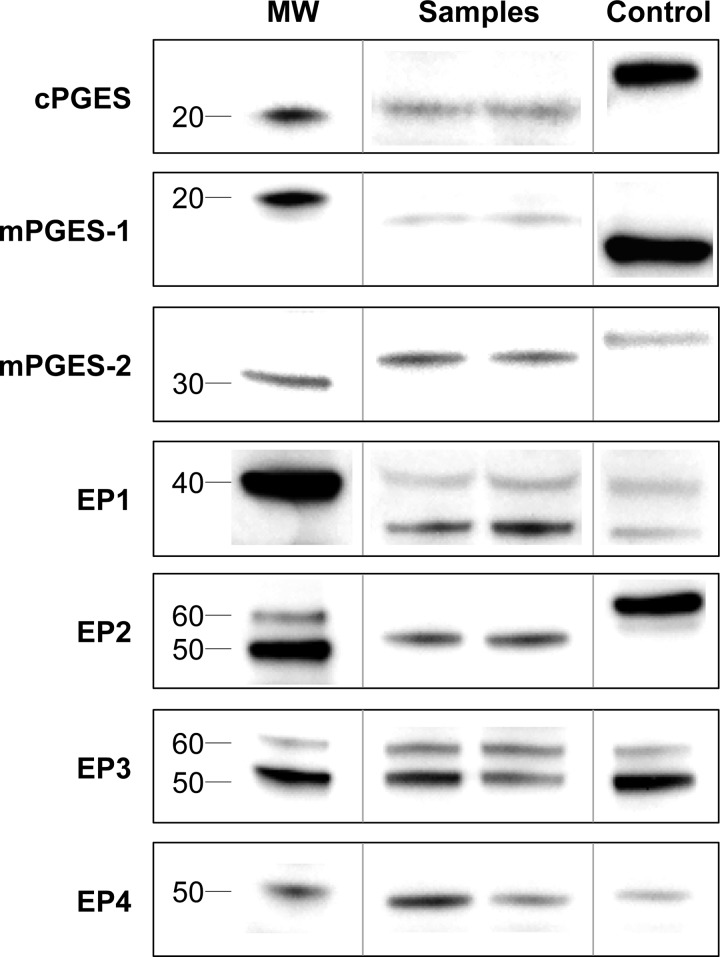
Identification of immunodetected proteins was confirmed by considering the reported molecular mass of the protein and any known posttranslational modifications, along with migration relative to the molecular mass ladder and positive control (Fig. 2). Negative control (blocking peptide) experiments were also completed for COX-2, EP1, EP2, EP3, and EP4. For COX-1, the identified band migrated equivalent to the positive control protein and appropriately relative to the molecular mass ladder as we have previously reported (5, 47). COX-2 was not detected in any of the muscle samples, with a banding pattern similar to the blots we have previously reported and discussed in detail (5, 47). The cPGES-identified band migrated slightly beyond the positive control protein and just above the 20-kDa molecular mass band, which is consistent with previous reports in other human tissues (15, 27, 29). Identification of a mPGES-1 band was not completely conclusive, although a band migrating somewhat beyond the 20-kDa molecular mass band was identified. However, this band did not migrate with or beyond the hexahistidine-tagged positive control (reported as 19 kDa), as might be expected considering the molecular mass of mPGES-1 is ∼16 kDa (13, 15, 27, 29). No other bands at or below 20 kDa were detectable. However, the identified band was quantified and was not different between soleus and vastus lateralis or young and old muscle (data not shown). The mPGES-2 identified band migrated slightly beyond the positive control and just above the 30-kDa molecular mass band, which is consistent with the commonly reported 33-kDa version of the protein (15, 27, 29). No data are presented for the ∼42-kDa version, which did appear as a very faint band near the expected migration distance on the blots.
Fig. 2.
Representative blot images of the PGE2 synthases and receptors. Representative images of the COX enzymes have been presented and described in detail previously (5, 47). Images for a given protein include molecular mass band(s) (MW), a positive control or human skeletal muscle standard (Table 1), and representative samples from the muscle/fiber type specific or aging comparison. See Western blot identification considerations for a complete description of protein identification. Images presented are from noncontiguous lanes of the same blot (as indicated by vertical lines) at the same exposure and reassembled to reproduce exactly the relative migration patterns.
For EP1, two bands migrating near the 40-kDa molecular mass band were identified. One band migrated near (likely representing the expected molecular mass protein, ∼42 kDa) and one band migrated beyond (∼35 kDa), as has been reported previously in myocytes and other tissues (31, 37). These two bands were reduced in intensity with the blocking peptide. The EP2 identified band migrated just above the 50-kDa molecular mass band but beyond the 60-kDa band and (nonhuman) positive control protein. The identified band and positive control band were eliminated with the blocking peptide. This migration is consistent with previous reports in other human tissues (23). EP3 was identified as two distinct bands migrating near the 50 and 60-kDa molecular mass bands, and these two bands were eliminated with the blocking peptide. This banding pattern is consistent with previously reported glycosylations of the EP3 receptor in nonhuman tissues and bands reported in other human tissues (17, 26). The EP4-identified band migrated near the 50-kDa molecular mass band and was eliminated with the blocking peptide. This migration is consistent with what would be expected from human and other tissues (4, 26).
Statistical analysis.
Data were analyzed with paired (soleus vs. vastus lateralis) or unpaired (young vs. old) t-tests. Data are presented as means ± SE.
RESULTS
PGE2/COX pathway components in human skeletal muscle.
COX-1, cPGES, mPGES-2, and all four PGE2 receptors (EP1, EP2, EP3, and EP4) were detected in all young and old muscle samples examined. mPGES-1 was not reliably identified and COX-2 was not detected in any of the muscle samples (see Western blot identification considerations).
Soleus vs. vastus lateralis skeletal muscle.
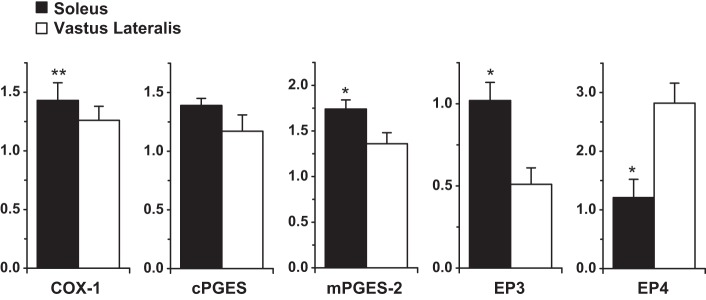
On average, COX-1, cPGES, and mPGES-2 were ∼20% higher in soleus compared with vastus lateralis (Fig. 3). EP1 (35 kDa: 1.34 ± 0.36 vs. 1.51 ± 0.46 AU; 42 kDa: 0.55 ± 0.11 vs. 0.64 ± 0.13 AU) and EP2 (0.56 ± 0.04 vs. 0.50 ± 0.05 AU) were not different between soleus and vastus lateralis, while EP3 was 99% higher and EP4 was 57% lower in soleus (P < 0.05) (Fig. 3).
Fig. 3.
Protein levels for COX-1, cPGES, mPGES-2, EP3, and EP4 in soleus (a surrogate for type I muscle fibers) and vastus lateralis (a surrogate for type II muscle fibers, relative to soleus). *P < 0.05 vs. vastus lateralis. **P < 0.1 vs. vastus lateralis. The y-axis is protein abundance normalized to the positive control or human skeletal muscle standard (Table 1) in arbitrary units. The EP3 data represent the 50-kDa band (no difference was found between muscles for the 60-kDa band, data not shown).
Aging skeletal muscle.
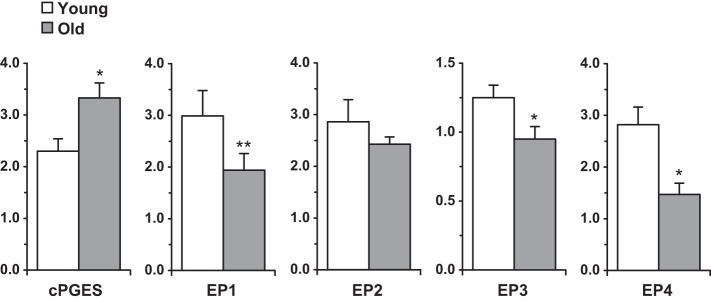
COX-1 (1.26 ± 0.29 vs. 1.00 ± 0.12 AU) and mPGES-2 (0.45 ± 0.03 vs. 0.42 ± 0.03 AU) were not different between young and old, while cPGES was 45% higher in old (P < 0.05) (Fig. 4). On average, the PGE2 receptors were ∼33% lower in old compared with young skeletal muscle (Fig. 4).
Fig. 4.
Protein levels for cPGES and all four PGE2 receptors in young and old skeletal muscle. *P < 0.05 vs. young. **P < 0.1 vs. young. The y-axis is protein abundance normalized to the positive control or human skeletal muscle standard (Table 1) in arbitrary units. The EP1 and EP3 data represent the 35 and 60-kDa bands, respectively (no difference was found between young and old for the other quantified bands for those receptors, data not shown).
DISCUSSION
The primary aim of this investigation was to define the PGE2/COX pathway enzymes and receptors in human skeletal muscle, as well as to understand the potential influence of muscle fiber type and aging on the pathway. Understanding this pathway is important because it is involved in skeletal muscle adaptations to aging and exercise training, and it is regulated by commonly consumed COX inhibiting drugs (49). The findings show that human skeletal muscles with markedly different type I and II muscle fiber composition, as well as skeletal muscle from aging individuals, have altered PGE2 production capacity and PGE2 receptor abundance.
The vastus lateralis and soleus muscle samples studied in this investigation were from 28 healthy men and women ranging from 20 to 93 yr old. Thus the findings provide a broad representation of muscle fiber type, age, and gender. The results define for the first time that COX-1, the PGE2 synthases cPGES and mPGES-2, and the PGE2 receptors EP1, EP2, EP3, and EP4 are constitutively expressed at the protein level in young and old human skeletal muscle and in skeletal muscles with vastly different fiber types. This latter finding provides strong support that all of these proteins are expressed in both type I and type II muscle fibers of men and women. These protein data are supported by the findings that COX-1, cPGES, mPGES-2, and EP4 are constitutively expressed at the mRNA level at relatively high abundance in skeletal muscle (5, 33, 47, 51, 53). However, EP1, EP2, and EP3 are inconsistently expressed at low transcript levels (33). It should be noted that our homogenate measurements could contain some nonmuscle fiber contributions (e.g., intramuscular vasculature, inflammatory cells). This contribution is likely very small in our subjects, since they did not have overt muscle or other inflammatory disease (18, 25, 30). In addition, some of these proteins (and related mRNA) (e.g., COX-1, cPGES, mPGES-2, EP4) have been detected inside muscle fibers using immunolocalization and isolated muscle fiber measurements (18, 25, 30, 33).
Whether or not mPGES-1 was present in the muscle samples studied in this investigation is not completely clear (see Western blot identification considerations). However, it does appear that if present in muscle homogenates, mPGES-1 is expressed at very low levels at both the protein and mRNA levels (18, 25, 33, 51). Furthermore, any contributions to the homogenate measurements likely come from nonmuscle fiber components, even in muscle inflammatory disease states (18, 25). These findings are consistent with the current and previously reported findings of no or very low level expression of COX-2 protein and mRNA in human skeletal muscle (5, 18, 25, 30, 33, 47). This is also consistent with the idea that COX-2 and mPGES-1 appear to be linked in the production of PGE2 in inflammatory diseases of skeletal muscle and pathophysiological conditions in other tissues (1, 18, 25, 27, 29). Collectively, these findings provide further evidence that COX-1 is likely the major source of general PG production in young and old human skeletal muscle (5, 18, 25, 30, 47, 49) and suggest PGE2 specific synthesis is directed by cPGES and mPGES-2. Thus these three constitutively expressed proteins likely regulate the homeostatic processes controlled, at least in part, by PGE2. In skeletal muscle, these processes would include the regulation of hypertrophy and atrophy in young and old individuals.
The influence of muscle fiber type on the PGE2/COX pathway profile was one focus of this investigation because the contractile and metabolic characteristics between fiber types are fundamentally different (7, 35, 44) and respond differently to aging and exercise training (11, 32, 35, 45). The soleus and vastus lateralis were chosen as surrogates for type I and type II fibers, respectively, because numerous studies have shown that homogenate measurements from these two muscles compared with isolated fiber type specific measurements consistently yield similar results (6–8, 10, 14, 21, 22). These comparisons include measurements at the protein, enzymatic activity, protein metabolism, and energy substrate levels. Thus the current results likely reflect the PGE2/COX pathway in type I and II muscle fibers, although the differences found in the current study may be even greater in a “pure” comparison. The higher COX-1 and mPGES-2 levels in the soleus compared with the vastus lateralis suggest an increased capacity to generate PGE2 via enhanced PGH2 generation from arachidonic acid and PGE2 synthesis from PGH2. As PGE2 is an important mediator of protein turnover (49), this increased PGE2 generation capacity in type I compared with type II fibers may be involved in the regulation of fiber type-specific adaptations to exercise training. Additionally, these data further suggest the possibility of a fiber type-specific impact of COX inhibiting drugs on protein metabolism-related adaptations to exercise training. Indeed, it has recently been shown that chronic COX inhibition during resistance exercise training in older adults, likely working through a PGE2-related mechanism, enhances the typical muscle mass gains in a fiber type-specific fashion (49–51). That is, the enhanced myocellular hypertrophy induced by COX inhibition was more pronounced in type I muscle fibers. These findings support further investigation into the effects of common COX inhibitors and future PGES inhibitors (19, 36) on skeletal muscle.
The fiber type-specific differences in PGE2 receptor levels (higher EP3 and lower EP4 in the soleus compared with vastus lateralis) are somewhat more difficult to interpret, primarily due to the limited number of EP receptor-related studies in skeletal muscle and the complexity of EP receptor biology (4, 28, 41). From other tissue types it is clear that different EP receptor subtypes can have similar and opposing intracellular effects, which can be modified by changing cofactors such as the cytokine environment (4, 23, 28, 41, 42, 52). The limited available skeletal muscle information shows that homogenate vastus lateralis mRNA levels of EP4 increase with resistance exercise training (51), suggesting this subtype and its resultant downstream signaling effects are involved in the normal adaptive response to resistance exercise. In addition, EP4 mRNA is higher in isolated type IIa compared with type I human muscle fibers (33), which corresponds with the notion that type II fibers are typically more responsive to resistance exercise. These isolated muscle fiber findings also correspond to the current findings of a higher EP4 protein level in vastus lateralis compared with the primarily type I soleus muscle.
The initial PGE2 receptor data presented in the current investigation provide a basis to develop ex vivo studies of human skeletal muscle EP signaling and extend those studies to in vivo investigations in humans. These studies are possible because there is a well-developed line of EP1-4 receptor agonists and antagonists and specific downstream inhibitors of the known EP-activated pathways (4, 28, 41, 52, 55). Some of these same pathways have been shown to regulate skeletal muscle signaling and adaptations (2, 24, 40). These tools can also be incorporated with the commonly available line of COX-inhibiting drugs for use in humans.
The influence of aging on the PGE2/COX pathway profile in skeletal muscle was another focus of this investigation because of the significance of sarcopenia in the aging population (9, 12) and the apparent role of this pathway in the etiology and exercise-related treatment of sarcopenia (49). The higher level of cPGES in old muscle suggests an increased capacity for PGE2 production. Although there is no information regarding skeletal muscle PGE2 levels with aging in humans, data from aging monkeys show higher PG levels in skeletal muscle (54). Furthermore, data from aging humans show higher rates of skeletal muscle proteolysis (46), to which PGE2 may be linked. Although speculative, the age-related PGE2 receptor downregulation in the current study (Fig. 4) may be the result of chronic elevations in PGE2. This potential adaptation is important considering the likelihood of elevated skeletal muscle PGE2 levels creating an environment that is not ideal for growth or may even contribute to atrophy (40, 47, 51). Interestingly, cPGES mRNA levels in skeletal muscle are reduced ∼20% with resistance exercise training that induces hypertrophy in older individuals (51). Finally, the similar levels of the COX enzymes in young and old skeletal muscle suggest that a given COX inhibitor dose, notwithstanding gastrointestinal absorption or metabolic clearance differences, should be equally efficacious at blocking PGH2 production (see Fig. 1) in young and old individuals. However, the higher cPGES level in old muscle suggests more PGE2 production for a given amount of PGH2; thus a higher dose of COX inhibitor may be needed in an older individual to control intramuscular PGE2 levels.
In summary, the current investigation provides the first comprehensive profile of the PGE2/COX pathway in human skeletal muscle. PGE2 production capacity and receptor levels are different in skeletal muscles with markedly different type I and II muscle fiber composition and are altered in aging skeletal muscle. The current findings coupled with the existing literature provide a platform for investigations into the PGE2/COX pathway as it relates to 1) the understanding and treatment of the age-related loss of skeletal muscle mass and function, and 2) the regulation of fiber type specific adaptations to exercise in young and old individuals. This information will also add to our understanding of the role of COX inhibitors, as well as potential PGE2 synthase and EP receptor modulators in the adaptations of skeletal muscle to exercise training and aging.
GRANTS
This study was funded by National Institute of Aging Grants AG-020532 and AG-038576.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.Z.L., B.J., and T.A.T. conception and design of research; S.Z.L., B.J., K.M.L., B.E.L., and T.A.T. performed experiments; S.Z.L., B.J., K.M.L., and T.A.T. analyzed data; S.Z.L., B.J., K.M.L., B.E.L., S.W.T., and T.A.T. interpreted results of experiments; S.Z.L., K.M.L., and T.A.T. prepared figures; S.Z.L. and T.A.T. drafted manuscript; S.Z.L., B.J., K.M.L., B.E.L., S.W.T., and T.A.T. edited and revised manuscript; S.Z.L., B.J., K.M.L., B.E.L., S.W.T., and T.A.T. approved final version of manuscript.
REFERENCES
- 1.Beaulieu D, Thebault P, Pelletier R, Chapdelaine P, Tarnopolsky M, Furling D, Puymirat J. Abnormal prostaglandin E2 production blocks myogenic differentiation in myotonic dystrophy. Neurobiol Dis 45: 122–129, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Berdeaux R, Stewart R. cAMP signaling in skeletal muscle adaptation: hypertrophy, metabolism, and regeneration. Am J Physiol Endocrinol Metab 303: E1–E17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest 14: 7–110, 1962.13862378 [Google Scholar]
- 4.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Ann Rev Pharmacol Toxicol 41: 661–690, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Burd NA, Dickinson JM, Lemoine JK, Carroll CC, Sullivan BE, Haus JM, Jemiolo B, Trappe SW, Hughes GM, Sanders CE Jr, Trappe TA. Effect of a cyclooxygenase-2 inhibitor on postexercise muscle protein synthesis in humans. Am J Physiol Endocrinol Metab 298: E354–E361, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll CC, Carrithers JA, Trappe TA. Contractile protein concentrations in human single muscle fibers. J Muscle Res Cell Motil 25: 55–59, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Chi MM, Hintz CS, Coyle EF, Martin WH 3rd, Ivy JL, Nemeth PM, Holloszy JO, Lowry OH. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol Cell Physiol 244: C276–C287, 1983. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson JM, Lee JD, Sullivan BE, Harber MP, Trappe SW, Trappe TA. A new method to study in vivo protein synthesis in slow- and fast-twitch muscle fibers and initial measurements in humans. J Appl Physiol 108: 1410–1416, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol 95: 1717–1727, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Essen B, Jansson E, Henriksson J, Taylor AW, Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand 95: 153–165, 1975. [DOI] [PubMed] [Google Scholar]
- 11.Essen-Gustavsson B, Henriksson J. Enzyme levels in pools of microdissected human muscle fibres of identified type. Adaptive response to exercise. Acta Physiol Scand 120: 505–515, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences International working group on sarcopenia. J Am Med Dir Assoc 12: 249–256, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giannico G, Mendez M, LaPointe MC. Regulation of the membrane-localized prostaglandin E synthases mPGES-1 and mPGES-2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 288: H165–H174, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Greenhaff PL, Nevill ME, Soderlund K, Bodin K, Boobis LH, Williams C, Hultman E. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol 478: 149–155, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudis K, Tatsuguchi A, Wada K, Futagami S, Nagata K, Hiratsuka T, Shinji Y, Miyake K, Tsukui T, Fukuda Y, Sakamoto C. Microsomal prostaglandin E synthase (mPGES)-1, mPGES-2 and cytosolic PGES expression in human gastritis and gastric ulcer tissue. Lab Invest 85: 225–236, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103: 2068–2076, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Tai HH. Prostaglandin E2 receptor EP3alpha subtype: the role of N-glycosylation in ligand binding as revealed by site-directed mutagenesis. Prostaglandins Leukot Essent Fatty Acids 59: 265–271, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Korotkova M, Helmers SB, Loell I, Alexanderson H, Grundtman C, Dorph C, Lundberg IE, Jakobsson PJ. Effects of immunosuppressive treatment on microsomal prostaglandin E synthase 1 and cyclooxygenases expression in muscle tissue of patients with polymyositis or dermatomyositis. Ann Rheum Dis 67: 1596–1602, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korotkova M, Jakobsson PJ. Characterization of microsomal prostaglandin E synthase 1 inhibitors. Basic Clin Pharmocol Toxicol 114: 64–69, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Korotkova M, Lundberg IE. The skeletal muscle arachidonic acid cascade in health and inflammatory disease. Nat Rev Rheumatol 10: 295–303, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Lemoine JK, Haus JM, Trappe SW, Trappe TA. Muscle proteins during 60-day bedrest in women: impact of exercise or nutrition. Muscle Nerve 39: 463–471, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Lester BE, Standley RA, Lee JD, Fink WJ, Trappe SW, Trappe TA. Muscle-specific substrate use during cycle exercise at 1 G: implications for astronaut muscle health. Aviat Space Environ Med 84: 789–796, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Ellman M, Muddasani P, Wang JH, Cs-Szabo G, van Wijnen AJ, Im HJ. Prostaglandin E2 and its cognate EP receptors control human adult articular cartilage homeostasis and are linked to the pathophysiology of osteoarthritis. Arthritis Rheum 60: 513–523, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88: 729–767, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Melin E, Lindroos E, Lundberg IE, Borg K, Korotkova M. Elevated expression of prostaglandin E2 synthetic pathway in skeletal muscle of prior polio patients. J Rehabil Med 46: 67–72, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Morath R, Klein T, Seyberth HW, Nusing RM. Immunolocalization of the four prostaglandin E2 receptor proteins EP1, EP2, EP3, and EP4 in human kidney. J Am Soc Nephrol 10: 1851–1860, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Murakami M, Nakatani Y, Tanioka T, Kudo I. Prostaglandin E synthase. Prostaglandins Lipid Mediat 68–69: 383–399, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Negishi M, Sugimoto Y, Ichikawa A. Prostaglandin E receptors. J Lipid Mediat Cell Signal 12: 379–391, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Park JY, Pillinger MH, Abramson SB. Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol 119: 229–240, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Rabuel C, Renaud E, Brealey D, Ratajczak P, Damy T, Alves A, Habib A, Singer M, Payen D, Mebazaa A. Human septic myopathy: induction of cyclooxygenase, heme oxygenase and activation of the ubiquitin proteolytic pathway. Anesthesiology 101: 583–590, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Rahnama'i MS, van Koeveringe GA, Essers PB, de Wachter SG, de Vente J, van Kerrebroeck PE, Gillespie JI. Prostaglandin receptor EP1 and EP2 site in guinea pig bladder urothelium and lamina propria. J Urol 183: 1241–1247, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol 106: 1611–1617, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol 112: 1625–1636, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodemann HP, Goldberg AL. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem 257: 1632–1638, 1982. [PubMed] [Google Scholar]
- 35.Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. In: Handbook of Physiology. Skeletal Muscle, edited by Peachey LD, Adrian RH, Geiger SR. Bethesda, MD: Am. Physiol. Soc, 1983, sect. 10, p. 555–631. [Google Scholar]
- 36.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev 59: 207–224, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Shinmura K, Tamaki K, Sato T, Ishida H, Bolli R. Prostacyclin attenuates oxidative damage of myocytes by opening mitochondrial ATP-sensitive K+ channels via the EP3 receptor. Am J Physiol Heart Circ Physiol 288: H2093–H2101, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev 56: 387–437, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Smith WL, Urade Y, Jakobsson PJ. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem Rev 111: 5821–5865, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Standley RA, Liu SZ, Jemiolo B, Trappe SW, Trappe TA. Prostaglandin E2 induces transcription of skeletal muscle mass regulators interleukin-6 and muscle RING finger-1 in humans. Prostaglandins Leukot Essent Fatty Acids 88: 361–364, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 282: 11613–11617, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Tang EH, Libby P, Vanhoutte PM, Xu A. Anti-inflammation therapy by activation of prostaglandin EP4 receptor in cardiovascular and other inflammatory diseases. J Cardiovasc Pharmacol 59: 116–123, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Testa M, Rocca B, Spath L, Ranelletti FO, Petrucci G, Ciabattoni G, Naro F, Schiaffino S, Volpe M, Reggiani C. Expression and activity of cyclooxygenase isoforms in skeletal muscles and myocardium of humans and rodents. J Appl Physiol 103: 1412–1418, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trappe S, Harber M, Creer A, Gallagher P, Slivka D, Minchev K, Whitsett D. Single muscle fiber adaptations with marathon training. J Appl Physiol 101: 721–727, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Trappe T, Williams R, Carrithers J, Raue U, Esmarck B, Kjaer M, Hickner R. Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. J Physiol 554: 803–813, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol 90: 2070–2074, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Trappe TA, Liu SZ. Effects of prostaglandins and COX-inhibiting drugs on skeletal muscle adaptations to exercise. J Appl Physiol 115: 909–919, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trappe TA, Ratchford SM, Brower BE, Liu SZ, Lavin KM, Carroll CC, Jemiolo B, Trappe SW. COX inhibitor influence on skeletal muscle fiber size and metabolic adaptations to resistance exercise in older adults. J Gerontol A Biol Sci Med Sci, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trappe TA, Standley RA, Jemiolo B, Carroll CC, Trappe SW. Prostaglandin and myokine involvement in the cyclooxygenase-inhibiting drug enhancement of skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 304: R198–R205, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang P, Zhu F, Lee NH, Konstantopoulos K. Shear-induced interleukin-6 synthesis in chondrocytes: roles of E prostanoid (EP) 2 and EP3 in cAMP/protein kinase A- and PI3-K/Akt-dependent NF-kappaB activation. J Biol Chem 285: 24793–24804, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinheimer EM, Jemiolo B, Carroll CC, Harber MP, Haus JM, Burd NA, LeMoine JK, Trappe SW, Trappe TA. Resistance exercise and cyclooxygenase (COX) expression in human skeletal muscle: implications for COX-inhibiting drugs and protein synthesis. Am J Physiol Regul Integr Comp Physiol 292: R2241–R2248, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Young MK, Bocek RM, Herrington PT, Beatty CH. Aging: effects on the prostaglandin production by skeletal muscle of male rhesus monkeys (Macaca mulatta). Mech Ageing Dev 16: 345–353, 1981. [DOI] [PubMed] [Google Scholar]
- 55.Zhu Z, Fu C, Li X, Song Y, Li C, Zou M, Guan Y, Zhu Y. Prostaglandin E2 promotes endothelial differentiation from bone marrow-derived cells through AMPK activation. PLoS One 6: e23554, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]