Abstract
Safe, efficient liquid feeding in infant mammals requires the central coordination of oropharyngeal structures innervated by multiple cranial and spinal nerves. The importance of laryngeal sensation and central sensorimotor integration in this system is poorly understood. Recurrent laryngeal nerve lesion (RLN) results in increased aspiration, though the mechanism for this is unclear. This study aimed to determine the effect of unilateral RLN lesion on the motor coordination of infant liquid feeding. We hypothesized that 1) RLN lesion results in modified swallow kinematics, 2) postlesion oropharyngeal kinematics of unsafe swallows differ from those of safe swallows, and 3) nonswallowing phases of the feeding cycle show changed kinematics postlesion. We implanted radio opaque markers in infant pigs and filmed them pre- and postlesion with high-speed videofluoroscopy. Markers locations were digitized, and swallows were assessed for airway protection. RLN lesion resulted in modified kinematics of the tongue relative to the epiglottis in safe swallows. In lesioned animals, safe swallow kinematics differed from unsafe swallows. Unsafe swallow postlesion kinematics resembled prelesion safe swallows. The movement of the tongue was reduced in oral transport postlesion. Between different regions of the tongue, response to lesion was similar, and relative timing within the tongue was unchanged. RLN lesion has a pervasive effect on infant feeding kinematics, related to the efficiency of airway protection. The timing of tongue and hyolaryngeal kinematics in swallows is a crucial locus for swallow disruption. Laryngeal sensation is essential for the central coordination in feeding of oropharyngeal structures receiving motor inputs from different cranial nerves.
Keywords: dysphagia, tongue kinematics, swallow control, infant, mammalian feeding
drinking and swallowing in infant mammals are functionally complex behaviors performed by soft tissue muscular structures such as the tongue, soft palate, pharynx, and larynx. Successful feeding requires transport of milk from the nipple to the valleculae, the pharyngeal space at the base of the tongue, and then safe passage of the milk from the oropharynx into the esophagus. The pathway that liquid takes through the oropharynx crosses the opening to the airway, creating potential problems should that liquid enter the larynx or the trachea. Failure to maintain airway integrity during the swallow, that is allowing liquid into the upper larynx, above the vocal folds (penetration), or lower larynx and trachea (aspiration), can compromise health and even lead to death (25, 39). Successful performance of these behaviors requires the functional, anatomical, and neurological integration of several structures that are innervated by multiple cranial and cervical spinal nerves. Such integration is well documented between the oral cavity and pharynx: disruption or modification of anterior sensation in the oral cavity perturbs pharyngeal function (11, 15).
The muscles that perform the swallow are innervated by nerve branches of trigeminal (V3), vagus (X), and hypoglossal (XII), as well as cervical spinal nerves of ansa cervicalis (C1–C3) (16). The sensory nerves, including the maxillary division of the trigeminal nerve (V2), the mandibular division of the trigeminal nerve (V3), the glossopharyngeal nerve (IX), and especially the branches of the vagus nerve (X), are critical to a successful swallow. In particular, the superior laryngeal nerve (SLN), which innervates the valleculae and the upper portion of the larynx to the level of the vocal folds, triggers the swallow (4, 21, 50). Unilateral and bilateral lesion of the internal branch of the SLN results in significant aspiration (4, 5, 14), and electrical stimulation of the iSLN is sufficient to trigger a pharyngeal swallow (21, 35, 51).
The role of the RLN, another branch of X, however, is less clear. The RLN supplies all the intrinsic laryngeal muscles, except cricothyroid, which is innervated by the external branch of the SLN (34), and provides sensation below the vocal folds, in the lower half of the larynx. Neither these muscles nor the sensory field innervated by the RLN are involved in the pathway of the bolus in safe swallows. Yet clinically, it is well documented that damage to the RLN results in increased incidence of failure of airway protection during swallow, leading to feeding dysfunction and the risk of aspiration pneumonia (1, 3, 23, 33, 37).
The mechanism that causes aspiration resulting from RLN damage is not known. One hypothesis of a passive mechanism, i.e., that the vocal folds, left abducted by lack of efferent stimulus, would leave an open airway for liquid to pour into, has been falsified (13). On the other hand, work showing that palatal anesthesia, which removes afferents from V2, produces kinematic changes in tongue, palate, and pharynx suggests central integration among the several cranial nerves involved in oral transport and swallowing. This in turn suggests that disruptions of vagal nerves may also produce kinematic changes in structures controlled by other cranial and cervical nerves, modifying the spatiotemporal relationships in the movements between these structures.
The aim of this project was to determine the effect of unilateral recurrent laryngeal denervation on the movement of both oral and pharyngeal structures throughout liquid feeding, and to relate these changes to the effectiveness of airway protection in mammalian infant feeding. We hypothesized that sensorimotor integration between the oral cavity, pharynx, and larynx is responsible for RLN-induced disruptions in infant feeding both in oral transport and effective swallowing. We tested the following three hypotheses.
Hypothesis 1.
RLN lesion results in modified kinematics of the tongue and hyolaryngeal structures during infant swallowing. We hypothesize that independent of swallow safety and performance, there will be changes in kinematics of oral, pharyngeal, and laryngeal structures involved in swallowing.
Hypothesis 2.
Because not all postlesion swallows produce aspiration, there will be a functional relationship between changes in kinematics and changes in airway protection performance. The increased aspiration post-RLN lesion is reflected in kinematics. The kinematics of swallows in which the airway is compromised differ from those swallows in which airway protection is maintained postlesion. We hypothesize that there will be a change in swallowing performance, and that the kinematics will be different, postlesion, between swallows that are safe vs. swallows that involve penetration or aspiration.
Hypothesis 3.
Owing to the neurological integration of oropharyngeal control mechanisms, nonswallowing (oral transport) phases of the feeding cycle will also show changed kinematics after RLN lesion. To test the extent of the impact of the lesion, we hypothesize other nonswallow behaviors utilizing the same anatomical structures will also exhibit kinematic differences.
MATERIALS AND METHODS
Experimental procedures.
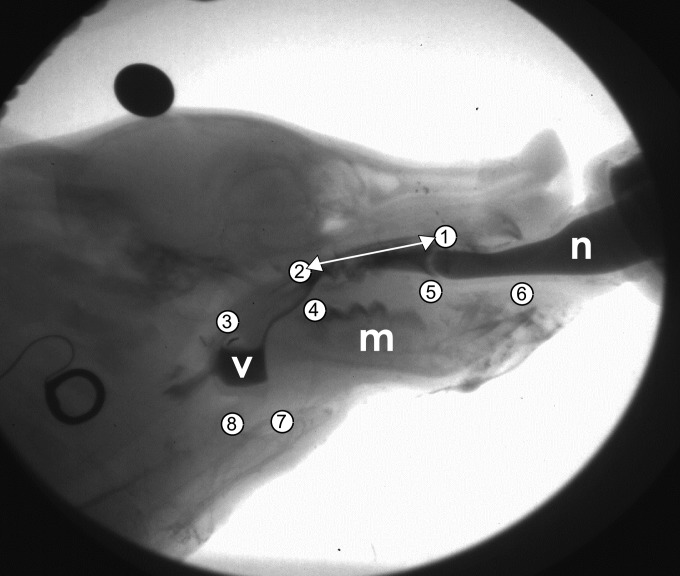
Six infant pigs (1 male, 5 females) aged 5 to 14 days on arrival (Michael Fanning Farm, Howe, IN) were trained to drink milk mixed with barium contrast agent from a modified infant bottle to which a pig nipple (NASCO Farm and Ranch, Fort Atkinson, WI) had been attached. Under anesthesia (2–5% isoflurane), radio opaque markers were placed intraorally into the hard palate, soft palate, and anterior, middle, and posterior tongue (Fig. 1). A radio opaque microvascular clip (Weck Ligation solutions, NC) was placed on the epiglottis. Nine days later, in an intubated surgery, radio opaque markers were sutured to the hyoid bone and thyroid cartilage. During this surgery, the right RLN was identified visually from its entry point into the larynx and marked with a loose suture. Animals were allowed to recover from anesthesia, and then recorded feeding on barium milk in front of a C-arm fluoroscope (GE9400 C-Arm, 80 kV, 4MA) connected to a high-speed digital video camera (XC 1M digital video camera, Xcitex, Cambridge, MA). Videos were recorded at 100 fps. Animals were filmed in a lateral view. At between 9 and 27 days, animals underwent a second surgery in which the right RLN was transected just distal to its entry into the larynx, with a 1 to 2-mm segment removed. The free ends were ligated, crushed, and displaced to prevent reinnervation. The time between hyoid and thyroid marker surgery and nerve lesion surgery was 1 to 2 days. Lesioned individuals were then recorded feeding in front of the fluoroscope 24 to 48 h postlesion surgery, as previous work (13) has shown that a delay of at least 24 h is necessary to see changes in swallowing performance postlesion. All procedures were approved by the NEOMED institutional animal care and use committee (IACUC protocol 13-011).
Fig. 1.
Position of markers implanted in pig, as seen in lateral fluoroscopy view. Markers: 1, anterior hard palate; 2 posterior hard palate; 3, epiglottal tip; 4 posterior tongue; 5, middle tongue; 6, anterior tongue; 7, hyoid; and 8, thyroid. The white double arrow is the axis of rotation, translation, and scaling of each frame. V, valleculae; m, mandible; n, bottle nipple.
Scoring of swallows.
The effectiveness of airway protection in these swallows was assessed by scoring them with the infant mammalian penetration-aspiration scale (IMPAS) (14). The IMPAS scale is an ordinal scale where swallows are ranked from 1 to 7 (Table 1), with 1 indicating a safe swallow with no compromising of the upper or lower airway, and 7 indicating aspiration, liquid descending below the vocal folds into the trachea. Each stage on the scale indicates a specific type of configuration of fluid in the airway that are presumed to represent increasingly more severe stages of airway protection failure. All swallows were scored by the same investigator (J. Ohlemacher) who had been trained on usage of the scale. The investigator was blinded to the treatment condition of all animals during IMPAS scoring.
Table 1.
Summary of the IMPAS scale
| Score | What Happens |
|---|---|
| 1 | Normal swallow |
| 2 | Some penetration that is cleared during the swallow |
| 3 | Some penetration that is not cleared during the swallow |
| 4 | A lot of penetration that is not cleared during the swallow |
| 5 | Aspiration with a successful attempt to clear |
| 6 | Aspiration with an unsuccessful attempt to clear |
| 7 | Aspiration with no attempt to clear |
Penetration: liquid enters the larynx above the vocal folds. Aspiration: liquid passes through the vocal folds and enters the trachea. IMPAS, infant mammalian penetration-aspiration.
Digitizing and isolating sucks and swallows.
Markers were digitized using computerized auto tracking software (ProAnalyst, Xcitex). In the case of the epiglottis, manual tracking had to be used for certain frames, as either the marker was obscured by radio opaque liquid or it had fallen off. Cross-correlation scores for multiple recorders were above 95% for auto tracked markers and around 80% for manually tracked points.
The x and y coordinates obtained from digitizing were translated, rotated, and scaled by using the anterior and posterior palate markers as reference points. This removes all motion of the markers that is due to dorsoventral motion of the whole head, leaving only motion of the structures relative to the hard palate (15). Individual swallows were isolated from continuous sequences manually, by looking at the x-axis (craniocaudal) position of the epiglottis to identify the beginning and end of the epiglottal movement. This period is slightly longer than the period of time taken by the liquid bolus to pass from the valleculae into the esophagus. However, it was chosen as it represents a cyclic biomechanical motion, with the return of the epiglottis to its preswallow resting state.
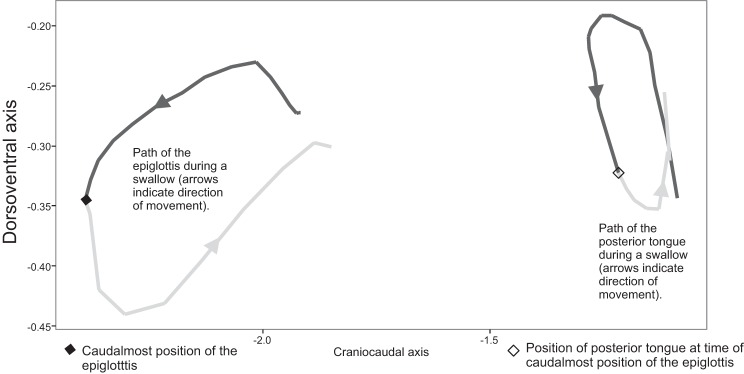
In each isolated swallow, the time at which the epiglottis reached its caudalmost point was calculated. The total distance traveled by the posterior tongue during each swallow was calculated, as was the portion traveled before the epiglottis reaches its maximum caudal point. The ratio of this partial distance traveled to total distance traveled by the posterior tongue in this time interval was then calculated. This metric, referred to as the posterior tongue ratio, measures the relative amount of movement occurring in the first portion of the swallow, during which the bolus is propelled from the valleculae to the esophagus (Fig. 2). We calculated the same ratio for the middle tongue marker to test whether the patterns seen in the posterior tongue resembled those in the middle of the tongue or were localized to the posterior tongue.
Fig. 2.
Division of the posterior tongue cycle by epiglottal movement. The posterior tongue ratio is the ratio of the length of the dark grey segment of the posterior tongue path to the length of the entire path of the posterior tongue.
Sucks were isolated by identifying the most ventral point reached by the posterior tongue marker in each oscillation in the y-axis as the start of each suck cycle. Only suck cycles that occurred outside of swallows were included in the analysis (“pure sucks”). The total distance traveled by the posterior tongue marker was then calculated for each suck cycle, as was the duration of each suck cycle. Scaling, rotation, and translation, as well as the identification of suck and swallow cycles, were done in SYSTAT 13 (Systat Software, San Jose, CA).
We also calculated the difference in the time between when the posterior tongue marker reached its most dorsal position and when the middle tongue marker reached its most dorsal position in each cycle (sucks and swallows). The difference in these times is a reflection of tongue movements responsible for liquid transport (10). We tested whether this difference in timing changed in response to RLN lesion.
Statistical analysis.
As predicted, aspiration was very rare in prelesioned animals, thus we did not have a full balanced design of all IMPAS scores in all treatment conditions. So we approached our hypothesis test with two different statistical models to maximize power from our data.
To test hypothesis 1, that tongue and hyoid kinematics differed pre- and postlesion, we tested kinematics pre- and postlesion by using a two-factor ANOVA with an interaction term. The factors were treatment and IMPAS score, although we limited the analysis to swallows with an IMPAS score of 1 or 2 (safe swallows). The dependent variable was the posterior tongue ratio. We also used a Levene's test to test whether the variation among ratios changed pre- and posttreatment. We also tested the effect of IMPAS and treatment on the middle tongue ratio, and the middle to posterior tongue time difference, to examine the within-tongue dynamics of the system.
To test hypothesis 2, that there is an effect of performance on postlesion kinematics, we tested the effect of IMPAS score on the kinematics of postlesion swallows by using a single-factor ANOVA and looked only within the postlesion swallows. We used user-defined orthogonal contrasts to test the specific hypothesis that the posterior tongue ratio of swallows in which aspiration occurred differed from the kinematics of safe swallows. We also used a Levene's test to determine differences in variation in posterior tongue swallows between safe swallows and swallows with aspiration. We also tested the middle tongue variables, as well as the timing of middle and posterior tongue variables to understand within-tongue changes that occur as a function of RLN lesion.
To test hypothesis 3, that kinematics in nonswallow behaviors would be affected by lesion, we used t-tests to test for pre- and postlesion differences in both duration of suck cycles and total distance traveled by the posterior tongue during suck cycles. We similarly tested the middle tongue variables to determine if within-tongue changes occurred during sucking as a function of RLN lesion. All the statistics were done in R (40). For clarity of graphical representation, dot plots are used where any group has fewer than ten observations. Boxplots are used otherwise.
RESULTS
Hypothesis 1 (pre- and postlesion kinematics were different): the posterior tongue ratio was greater and less variable in safe swallows post lesion.
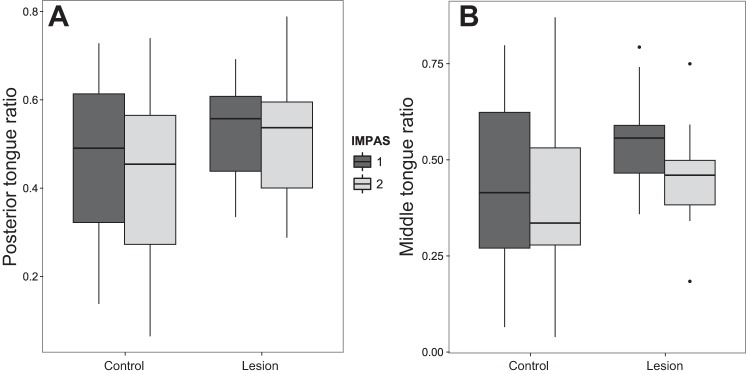
The kinematics of tongue movement differed between prelesion and postlesion swallows. The mean posterior tongue ratio, measuring the distance traveled by the tongue at the time of maximum epiglottal displacement, was greater in swallows without aspiration postlesion. There was no effect of IMPAS score nor a significant interaction between IMPAS score and treatment for posterior tongue ratio (Table 2, Fig. 3A). The magnitude of the difference in mean posterior tongue ratio pre- and postlesion was of medium size (Cohen's d −0.54) (2).
Table 2.
Results of two-factor ANOVA of posterior tongue ratio
| Factor | df | F | P Value |
|---|---|---|---|
| IMPAS | 1 | 0.33 | 0.564 |
| Treatment | 1 | 4.95 | 0.029 |
| IMPAS and treatment | 1 | 0.05 | 0.823 |
| Error | 73 |
Bold text indicates significant result (α = 0.05).
Fig. 3.
Box plot of posterior tongue ratio values (A) and middle tongue ratio values (B) for infant mammalian penetration-aspiration (IMPAS) score of 1 and 2 pre- and postlesion. Only the treatment difference is significant. Boxes are median and interquartile range, and whiskers are maximum range.
For safe swallows, IMPAS scores 1 and 2 combined, the posterior tongue ratio was more variable prelesion (σ2 = 0.44) than postlesion (σ2 = 0.35) {Levene's test, [F(1,75) = 5.83, P = 0.018]}.
The results for the middle tongue ratio were similar for the posterior tongue. The mean middle tongue ratio was significantly changed in the postlesion swallows without aspiration. There was no effect of either IMPAS score or the interaction of IMPAS score and treatment on the mean middle tongue ratio (Table 3, Fig. 3B). The magnitude of the effect of treatment on the mean middle tongue ratio was of medium size (Cohen's d = −0.51).
Table 3.
Results of a two-way ANOVA on the effect of IMPAS and nerve lesion on the middle tongue ratio in each swallow
| Factor | df | F | P Value |
|---|---|---|---|
| IMPAS | 1 | 1.9266 | 0.169 |
| Treatment | 1 | 4.6356 | 0.035 |
| IMPAS and treatment | 1 | 0.5236 | 0.472 |
| Error | 73 |
Bold text indicates significant result (α = 0.05).
As with the posterior tongue ratio, the variation in middle ratio of safe swallows (IMPAS 1 and 2 combined) was significantly less postlesion (σ2 = 0.34) than prelesion (σ2 = 0.47) [Levene's test, F(1,75) = 7.96, P < 0.01]. The difference between the time at which the posterior tongue and the middle tongue reached their maximum dorsal position was affected by neither treatment (P = 0.675), IMPAS (P = 0.675), nor their interaction (P = 0.841) for IMPAS scores of 1 and 2.
Hypothesis 2 (kinematic differences between safe and unsafe swallows postlesion): posterior tongue ratio differed in swallows with aspiration in postlesion animals.
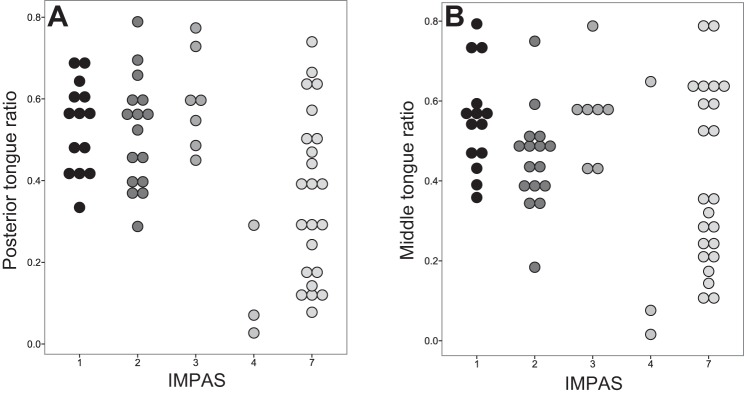
There were significant differences in postlesion kinematics between safe and unsafe swallows (Fig. 4). In postlesion animals, mean posterior tongue ratio differed between swallows with different IMPAS scores [one-way ANOVA, F(58,4) = 7.924, P < 0.01]. Specifically, safe swallows (IMPAS 1 and 2) differed from swallows in which aspiration occurred (post hoc contrast test of means P < 0.01). The magnitude of the difference in mean posterior tongue ratio of safe swallows and swallows with aspiration in postlesion animals was large (Cohen's d = 0.96). In postlesion animals, the variance of the posterior tongue ratio in safe swallows was lower (σ2 = 0.33) than in swallows, where aspiration occurs (σ2 = 0.45) (Levene's test, P = 0.012).
Fig. 4.
Dot plot of posterior tongue ratio (A) and middle tongue ratio (B) in lesioned swallows only by IMPAS.
Again, the pattern observed was the same for the middle tongue ratio as for the posterior tongue ratio (Fig. 4B). The mean middle tongue ratio differed postlesion among swallows with different IMPAS scores [ANOVA, F(4,58) = 3.142, P = 0.021]. However, there was no specific difference in the mean middle tongue ratio of safe swallows (IMPAS 1 and 2) and swallows in which aspiration occurred postlesion (post hoc contrast of means, P = 0.063). Variance in middle tongue ratio of safe swallows was lower (σ2 = 0.36) than the variance in swallows where aspiration occurs (σ2 = 0.47) (Levene's test, P = 0.014). There was no difference in the time between the middle and posterior tongue reaching their maximum dorsal position (P = 0.736).
Hypothesis 3 (effect of lesion on kinematics of suck cycles): total distance moved by the posterior tongue was less in suck cycles in lesioned animals.
Significant differences existed in kinematics between pre- and postlesion suck cycles. Total distance moved by the posterior tongue during suck cycles was less in postlesion than prelesion animals [t-test, t(90.776) = 4.47, P < 0.01] (Fig. 5A). The total distance moved by the middle tongue in suck cycles was also less postlesion than prelesion [t-test, t(86.67) = 4.229, P < 0.01] (Fig. 5B). However, there was no change in duration of suck cycles pre- and postlesion [t-test, t(92.83) = −0.12, P = 0.9] (Fig. 5C). We found no relationship between the time difference between the middle and posterior tongue reaching their maximal dorsal position and RLN lesion in suck cycles [t-test, t(83.15) = −1.038, P = 0.3022].
Fig. 5.
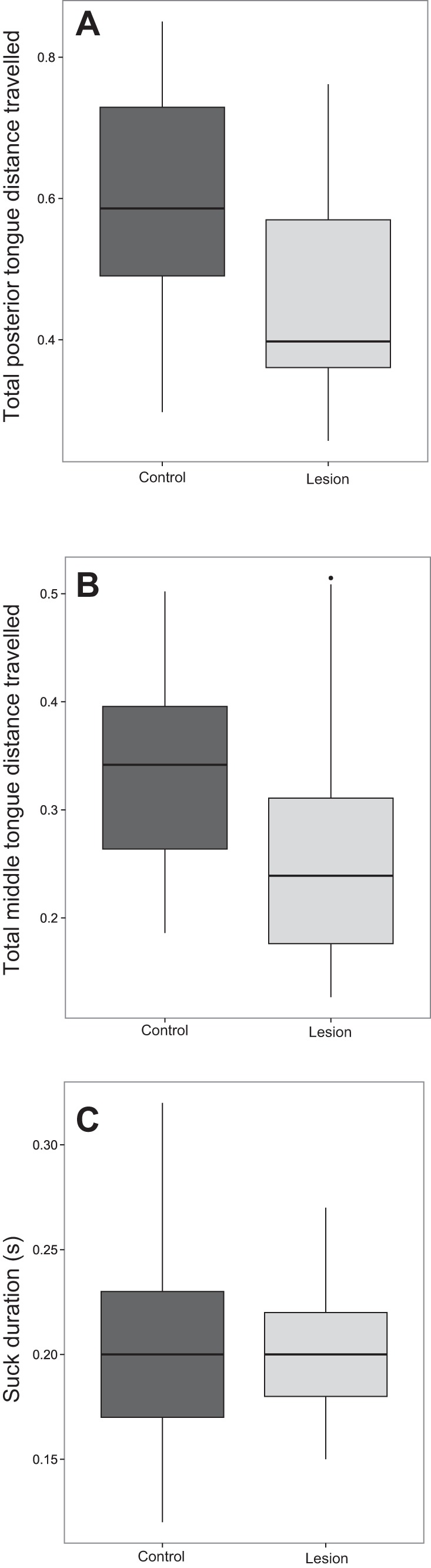
Box plot of total distance traveled by the posterior tongue (A) total distance traveled by the middle tongue (B) and duration in suck cycles (C) pre- and postlesion. Boxes are median and interquartile range, and whiskers are maximum range.
DISCUSSION
Kinematic changes to swallowing following RLN lesion are pervasive.
RLN damage results in a significant, sustained increase in the number of swallows in which aspiration occurs (1, 20, 33, 54), driving a continued effort to understand the functional relationship between RLN lesion and incidence of aspiration (19, 36, 54). However, little to no work exists on the mechanism by which either sensory or motor laryngeal deficits might change swallow kinematics and airway protection. The results of this study indicate that a narrow focus only on the swallows in which the airway is compromised does not capture the entire scope of the pathophysiology, which is much larger. Safe swallows were different in animals postlesion, and indeed the nonswallow portions of infant feeding (intraoral transport) differ post-RLN lesion.
Following lesion, both penetration and aspiration occurred more frequently, consistent with what is known about RLN injury in both humans and animal models. The pattern of kinematic differences underlying these performance differences is more complex than a simple change due to lesion. Both pre- and postlesion animals had safe swallows, but the kinematics of safe postlesion swallows, measured by the posterior tongue ratio, differed from prelesion swallows. After lesion, the kinematics also differed between the safe and unsafe swallows. This interaction between kinematics and performance suggests the existence of a potential compensation mechanism that allows safe swallows despite the deficit due to lesion. If, for example, safe swallows were identical in pre- and postlesion animals, while unsafe swallows in postlesion animals differed from both, then airway protection failure could be ascribed to the pathological kinematics of the unsafe postlesion swallow. Conversely, if all postlesion swallows had been the same kinematically, regardless of IMPAS score, then the mechanism of aspiration would be uncorrelated with kinematic failure per se. However, the data detail a different situation. The kinematics of postlesion safe swallows differ from those of prelesion safe swallows, implying that a different mechanism was necessary to generate a safe swallow postlesion. The kinematics of postlesion unsafe swallows resembled prelesion safe swallows, so that the kinematics that generated safe swallows prior to lesion generated unsafe swallows postlesion. This points to the existence of a possible biomechanical compensatory mechanism allowing lesioned animals to perform safe swallows.
Alternatively, the fact that postlesion kinematics during unsafe swallows resemble prelesion kinematics of safe swallows may indicate that the actual functional deficit is located in a different part of the oropharyngeal system, or at a different time point within the swallow cycle. It is in fact likely that multiple possible failure points may exist in the process of swallowing a liquid bolus, and that no single measure of oropharyngeal kinematics will capture all possible routes by which aspiration may occur. However, the evidence presented in this paper indicates that tongue kinematics are modified by RLN lesion throughout feeding, which implicates tongue function in the failure of airway protection.
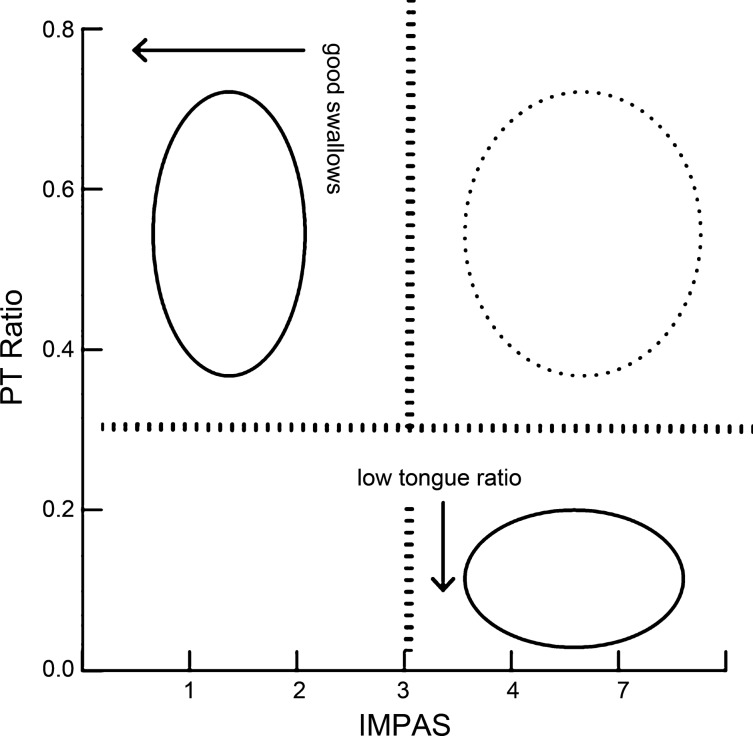
The specific distribution of the posterior tongue ratio in the swallows of lesioned animals indicates that the kinematic changes identified here are only part of the mechanism by which aspiration occurs in infants with unilateral RLN lesion (Fig. 6). The existence of unsafe swallows with high posterior tongue ratio values indicates that this metric is a sufficient but not necessary condition for aspiration. As such, the compensatory mechanism discussed above may be located beyond the valleculae in the swallowing pathway. It may include the muscular elements of the pharynx and thus be related to the timing of laryngeal vestibule closure (26). Alternatively, there may be multiple pathways by which aspiration may be caused, with only a subset of them being the result of the disruption in kinematics engendered here.
Fig. 6.
Interaction between IMPAS score and posterior tongue (PT) ratio in postlesion animals. No swallows exist with a low PT ratio and a low IMPAS score (empty quadrant). Swallows in the two quadrants with solid circles indicate that all good swallows have a high PT score, and that all swallows with a low PT ratio have a high IMPAS score. However, this relationship does not explain all swallows, as some swallows with a high PT ratio have a high IMPAS score.
The RLN also carries afferent fibers from the muscles of the cervical esophagus (53), which have been shown to be crucial in initiating the esophageal phase of swallowing (24). Anatomical work suggests that the location of the lesion in these experiments would have cut these esophageal afferents, and previous work on these animals indicates that esophageal function is compromised (13). That study found that in animals with RLN lesions similar to those done in this study, esophageal swallowing was impaired, and that this impairment was correlated with aspiration severity. Thus esophageal afferents carried by the RLN may be an important part of the central sensory integration by which safe swallow occurs, in addition to laryngeal sensation. This could be tested by isolating the esophageal fibers and selectively lesioning them.
RLN lesion changes kinematics of rhythmic tongue movement.
Sucks are rhythmic tongue movements that transport food from the oral cavity to the valleculae during infant feeding. They are highly characteristic behaviors that, in infant pigs, occur both alone and in conjunction with swallows (12). Individual pigs suckle at a preferred frequency when allowed to feed freely that is independent of swallowing frequency (9). Previous work indicates that, during a swallow, a complex interaction exists between rhythmic tongue motion and the swallow reflex (18). Each component appears to be highly stereotyped at the level of the motor pattern, with relatively variable timing between the two (46). Furthermore, palatal anesthesia can modify the relationship of the suck and swallow components of a suck-swallow cycle (16).
This study indicates that the tongue in infant feeding operates as a functionally integrated structure across the different functions necessary for successful feeding: sucking and swallowing. During sucks, both posterior and middle regions of the tongue showed reduced total motion despite no change in the duration of cycles. Crucially, the relative timing of the middle to posterior tongue, or intratongue coordination, was unaffected by the lesion in both sucks and swallows. This strongly indicates that the timing component of the tongue rhythmic motion is unaffected by this lesion. Thus the entire tongue muscular complex may be under the control of a single functional central nervous system element that is largely independent from the swallowing central pattern generator.
Implication for brainstem connection.
Pharyngeal swallow is a largely reflexive behavior controlled by a CPG (Central Pattern Generator) located in the brainstem (30, 31, 41). Electrical stimulation of the SLN is sufficient to generate a pharyngeal swallow (35, 43, 51), and pharyngeal swallows can be elicited by injecting fluid into the valleculae in decerebrate animals (6, 7, 45). However, extensive investigation has shown that characteristics of the pharyngeal swallows can be modulated (21, 27, 32) by a variety of oral (17, 42) and pharyngeal (44) sensory triggers. The evidence for whether airway protection can be modified by oral stimulation in neurologically compromised subjects is, however, inconclusive (38). The results presented here indicate that a persistent neural deficit outside of the pharynx (in the larynx) can induce chronic, permanent change in movements of structures that are anatomically and neurologically independent of the lesion. The RLN is a branch of the vagus nerve, whereas the tongue muscles are innervated by the hypoglossal nerve. Thus sensory feedback (either visceral or proprioceptive from intrinsic laryngeal muscles) from the larynx, carried by the vagus, is essential for the appropriate swallow motor pattern to be generated by the CPG in the brainstem. Critically, these changes are independent of any actual direct sensory stimulation of the larynx, as the differences in the kinematics of safe swallows show.
Connections between the sensory fibers of the RLN (which synapse in the nucleus tractus solitarius), the motor neurons of RLN (located in the nucleus ambiguous), and the motor nuclei of the hypoglossal nerve (in the hypoglossal nucleus) are essential to the performance of safe swallows. Whether these connections are all located in the brainstem, or whether they involve cortical pathways, is as yet unclear (28, 29). Rhythmic jaw and tongue motion can occur in decerebrate animals and influence the characteristics of the pharyngeal swallow (47), indicating that much of the sensorimotor integration of the oropharynx occurs in the brainstem alone. However, the complex relationship between neural control of swallowing and respiration (49) is also relevant to this discussion, particularly as laryngeal denervation is also likely to influence the respiration centers of the brain. Increased respiratory drive is known to inhibit swallowing (48), and the muscles of the tongue and pharynx that are active during swallowing are also active during respiration (8). Thus it is possible that part of the discoordination documented here may be attributable to changes in the relationship between the breathing and swallowing centers in the brain.
The relationship between the suck and swallow cycles is a fundamental and as yet understudied part of the feeding and airway protection complex. It was already known that modulation of oral sensation through anesthesia (16) and controlled delivery of food (11) could modify the rate of sucking and swallowing. The pervasive kinematic changes resulting from laryngeal denervation indicate that similar sensorimotor integration exists between sensation in the larynx and the sucking and swallowing function. Furthermore, the changes in timing of suck and swallow cycles that follow RLN lesion are associated with significant worsening of airway protection, and changes to the kinematics necessary to produce safe swallows. Based on these results, we hypothesize that differences in the relative timing of various muscle complexes under the control of the rhythmic tongue movement generator and the swallowing CPG underlie these kinematic patterns. Further, these results suggest that any intervention that disrupts or modifies the rhythmic motion of the tongue (in amplitude, frequency, or onset) may have a significant effect on feeding, and in particular swallowing. Recent work has shown that motor signals from the hypoglossal nucleus to the tongue can be modified by a variety of biological factors. Aerobic exercise changes the firing pattern from the hypoglossal motor units of the genioglossus muscles (52). Intrauterine exposure of rats to high doses of nicotine changes the intrinsic spike rate of hypoglossal motor neurons (37a). We hypothesize that interventions like these could be associated with changes in swallowing kinematics, and possibly effectiveness of airway protection.
CONCLUSION
In this study, we have shown that changes in tongue and epiglottis kinematics in response to RLN lesion are pervasive in infant feeding, affecting both oral transport and swallow. Furthermore, we show that change occurs in both safe and unsafe swallows, and that therefore compromising of the airway in individuals with RLN lesion is a manifestation of a more fundamental disordering of the system. Our results indicate that afferent information from the lower larynx is essential to coordinate the central pattern circuits which control the tongue on the one hand and the pharyngeal swallow on the other. The relative timing of rhythmic tongue movements to pharyngeal swallow appears as a key locus of central control in infant feeding airway protection.
GRANTS
This work was supported by National Institutes of Health Grant DC 009980 (to R. German).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.D.H.G. and R.Z.G. conception and design of research; F.D.H.G., J.O., A.R.L., A.G., A.B., L.F., and R.Z.G. performed experiments; F.D.H.G., J.O., and R.Z.G. analyzed data; F.D.H.G. and R.Z.G. interpreted results of experiments; F.D.H.G. and R.Z.G. prepared figures; F.D.H.G., J.O., and R.Z.G. drafted manuscript; F.D.H.G., J.O., A.R.L., and R.Z.G. edited and revised manuscript; F.D.H.G., J.O., A.R.L., A.G., A.B., L.F., and R.Z.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the staff at the Northeast Ohio Medical University Comparative Medicine Unit. The authors acknowledge C. Vinyard and J. Young for useful discussion of these results.
All work was performed in the Department of Anatomy and Neurobiology at Northeast Ohio Medical University.
REFERENCES
- 1.Benjamin JR, Smith PB, Cotten CM, Jaggers J, Goldstein RF, Malcolm WF. Long-term morbidities associated with vocal cord paralysis after surgical closure of a patent ductus arteriosus in extremely low birth weight infants. J Perinatol 30: 408–413, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
- 3.Daya H, Hosni A, Bejar-Solar I, Evans JNG, Bailey CM. Pediatric vocal fold paralysis: a long-term retrospective study. Arch Otolaryngol Head Neck Surg 126: 21–25, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Fukuhara T, Gierbolini-Norat EM, Thexton AJ, German RZ. Unilateral superior laryngeal nerve lesion in an animal model of dysphagia and its effect on sucking and swallowing. Dysphagia 28: 404–412, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding P, Fung GSK, Lin M, Holman SD, German RZ. The effect of bilateral superior laryngeal nerve lesion on swallowing: a novel method to quantitate aspirated volume and pharyngeal threshold in videofluoroscopy. Dysphagia 30: 47–56, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doty R, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol 19: 44–60, 1956. [DOI] [PubMed] [Google Scholar]
- 7.Doty RW, Richmond WH, Storey AT. Effect of medullary lesions on coordination of deglutition. Exp Neurol 17: 91–106, 1967. [DOI] [PubMed] [Google Scholar]
- 8.Fregosi RF, Ludlow CL. Activation of upper airway muscles during breathing and swallowing. J Appl Physiol 116: 291–301, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.German RZ, Crompton AW, Hertweck DW, Thexton AJ. Determinants of rhythm and rate in suckling. J Exp Zool 278: 1–8, 1997. [DOI] [PubMed] [Google Scholar]
- 10.German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J Exp Zool 261: 322–330, 1992. [DOI] [PubMed] [Google Scholar]
- 11.German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and rate of milk delivery as determinants of swallowing in an infant model animal (Sus scrofia). Dysphagia 19: 147–154, 2004. [DOI] [PubMed] [Google Scholar]
- 12.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol 102: 1017–1025, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould FDH, Lammers AR, Ohlemacher J, Ballester A, Fraley L, Gross A, German RZ. The physiologic impact of unilateral recurrent laryngeal nerve (RLN) lesion on infant oropharyngeal and esophageal performance. Dysphagia 30: 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Griffioen AM, Inokuchi H, Lukasik SL, German RZ. Development, reliability, and validation of an infant mammalian penetration-aspiration scale. Dysphagia 28: 178–187, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, Waranch DR, German RZ. Swallowing kinematics and airway protection after palatal local anesthesia in infant pigs: swallowing after palatal anesthesia. Laryngoscope 124: 436–445, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holman SD, Waranch DR, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, German RZ. Sucking and swallowing rates after palatal anesthesia: an electromyographic study in infant pigs. J Neurophysiol 110: 387–396, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humbert IA, Joel S. Tactile, gustatory, and visual biofeedback stimuli modulate neural substrates of deglutition. NeuroImage 59: 1485–1490, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humbert IA, Lokhande A, Christopherson H, German R, Stone A. Adaptation of swallowing hyo-laryngeal kinematics is distinct in oral vs. pharyngeal sensory processing. J Appl Physiol 112: 1698–1705, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishman S, Halum S, Patel N, Kerschner J, Merati A. Management of vocal paralysis: a comparison of adult and pediatric practices. Otolaryngol Head Neck Surg 135: 590–590, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour J, Martin T, Beste D, Robey T. Pediatric vocal fold immobility: Natural history and the need for long-term follow-up. JAMA Otolaryngol Head Neck Surg 140: 428–433, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol 550: 287–304, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupfer RA, Callaghan BC, Hogikyan ND. Neurogenic vocal fold motion impairment after routine intubation for tonsillectomy in a pediatric patient. J Voice 28: 112–114, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Lang IM, Medda BK, Babaei A, Shaker R. Role of peripheral reflexes in the initiation of the esophageal phase of swallowing. Am J Physiol Gastrointest Liver Physiol 306: G728–G737, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logemann JA. Swallowing disorders. Best Pract Res Clin Gastroenterol 21: 563–573, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, Lin S. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol Gastrointest Liver Physiol 262: G338–G344, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J Speech Hear Res 38: 556–563, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg 17: 166–171, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Michou E, Mistry S, Jefferson S, Tyrrell P, Hamdy S. Characterizing the mechanisms of central and peripheral forms of neurostimulation in chronic dysphagic stroke patients. Brain Stimul 7: 66–73, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med 13: 409–425, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Revs 14: 77–86, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Molfenter SM, Steele CM. Physiological variability in the deglutition literature: hyoid and laryngeal kinematics. Dysphagia 26: 67–74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortellaro VE, Pettiford JN, St Peter SD, Fraser JD, Ho B, Wei J. Incidence, diagnosis, and outcomes of vocal fold immobility after esophageal atresia (EA) and/or tracheoesophageal fistula (TEF) repair. Eur J Pediatr Surg 21: 386–388, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Mu L, Sanders I. The human cricothyroid muscle: three muscle bellies and their innervation patterns. J Voice 23: 21–28, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Naganuma K, Inoue M, Yamamura K, Hanada K, Yamada Y. Tongue and jaw muscle activities during chewing and swallowing in freely behaving rabbits. Brain Res 915: 185–194, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Nichols BG, Jabbour J, Hehir DA, Ghanayem NS, Beste D, Martin T, Woods R, Robey T. Recovery of vocal fold immobility following isolated patent ductus arteriosus ligation. Int J Pediatr Otorhinolaryngol 78: 1316–1319, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Pereira da R K, Firpo C, Gasparin M, Teixeira AR, Dornelles S, Bacaltchuk T, Levy DS. Evaluation of swallowing in infants with congenital heart defect. Int Arch Otorhinolaryngol 19: 055–060, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Powell GL, Levine RB, Frazier AM, Fregosi RF. Influence of developmental nicotine exposure on spike-timing precision and reliability in hypoglossal motoneurons. J Neurophysiol 113: 1862–1872, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Power ML, Fraser CH, Hobson A, Singh S, Tyrrell P, Nicholson DA, Turnbull I, Thompson DG, Hamdy S. Evaluating oral stimulation as a treatment for dysphagia after stroke. Dysphagia 21: 49–55, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Prasse JE, Kikano GE. An overview of pediatric dysphagia. Clin Pediatr 48: 247–251, 2008. [DOI] [PubMed] [Google Scholar]
- 40.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2014. [Google Scholar]
- 41.Shaker R, Hogan WJ. Reflex-mediated enhancement of airway protective mechanisms. Am J Med 108: 8–14, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia 25: 323–333, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takagi M, Noda T, Yamada Y. Comparison of SLN-evoked swallows during rest and chewing in the freely behaving rabbit. Brain Res 956: 74–80, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Shingai T, Saito I, Yamamura K, Yamada Y, Kitagawa J. Facilitation of the swallowing reflex with bilateral afferent input from the superior laryngeal nerve. Neurosci Lett 562: 50–53, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol 102: 587–600, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Thexton AJ, Crompton AW, German RZ. EMG activity in hyoid muscles during pig suckling. J Appl Physiol 112: 1512–1519, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol 101: 1386–1393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Timms BJM, DiFiore JM, Martin RJ, Miller MJ. Increased respiratory drive as an inhibitor of oral feeding of preterm infants. J Pediatr 123: 127–131, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Troche MS, Brandimore AE, Godoy J, Hegland KW. A framework for understanding shared substrates of airway protection. J Appl Oral Sci 22: 251–260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsuji K, Tsujimura T, Magara J, Sakai S, Nakamura Y, Inoue M. Changes in the frequency of swallowing during electrical stimulation of superior laryngeal nerve in rats. Brain Res Bull 111: 53–61, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Tsujimura T, Yamada A, Nakamura Y, Fukuhara T, Yamamura K, Inoue M. The digastric muscle is less involved in pharyngeal swallowing in rabbits. Dysphagia 27: 271–276, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Walls CE, Laine CM, Kidder IJ, Bailey EF. Human hypoglossal motor unit activities in exercise. J Physiol 591: 3579–3590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wank M, Neuhuber WL. Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J Comp Neurol 435: 41–59, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Wilson JA, Pryde A, White A, Maher L, Maran AGD. Swallowing performance in patients with vocal fold motion impairment. Dysphagia 10: 149–154, 1995. [DOI] [PubMed] [Google Scholar]