Abstract
It has been proposed that diet-induced obesity at thermoneutrality (TN; 29°C) is reduced by a UCP1-dependent thermogenesis; however, it has not been shown how UCP1-dependent thermogenesis can be activated in the absence of sympathetic activity. A recent study provides such a mechanism by showing that dietary bile acids (BAs) suppress obesity in mice fed a high-fat diet (HFD) by a mechanism dependent on type 2 deiodinase (DIO2); however, neither a role for UCP1 nor the influence of sympathetic activity was properly assessed. To test whether the effects of BAs on adiposity are independent of Ucp1 and cold-activated thermogenesis, obesity phenotypes were determined in C57BL6/J.+/+ (WT) and C57BL6/J.Ucp1.−/− mice (Ucp1-KO) housed at TN and fed a HFD with or without 0.5% (wt/wt) cholic acid (CA) for 9 wk. CA in a HFD reduced adiposity and hepatic lipogenesis and improved glucose tolerance in WT but not in Ucp1-KO mice and was accompanied by increases in food intake and energy expenditure (EE). In iBAT, CA increased Ucp1 mRNA and protein levels 1.5- and twofold, respectively, and increased DIO2 and TGR5 protein levels in WT mice. Despite enhanced Dio2 expression in Ucp1-KO and Ucp1-KO-CA treated mice, this did not enhance the ability of BAs to reduce obesity. By comparing the effects of BAs on WT and Ucp1-KO mice at TN, our study showed that BAs suppress diet-induced obesity by increasing EE through a mechanism dependent on Ucp1 expression, which is likely independent of adrenergic signaling.
Keywords: brown adipose tissue, diet-induced obesity, mitochondrial uncoupling protein 1, bile acids, thermogenesis, type 2 deidodinase
in mice, a major mechanism for maintaining body temperature in a cold environment involves induction of uncoupling protein 1 (UCP1)-dependent thermogenesis in brown adipose tissue (BAT) (7). The consequence of this increase in energy expenditure (EE) is increased oxidation of lipid stores in the body with overall reductions in adipose tissue fat stores. Most of this enhanced thermogenesis in the cold is mediated by sympathetically activated thermogenesis by BAT (4), (10). It has been reported that dietary bile acids (BAs) suppress the development of diet-induced obesity by a mechanism in which BAs activate the type 2 deiodinase (DIO2) pathway through the G-coupled receptor TGR5 (GPR131 or GpBAR1; a G protein-coupled plasma membrane receptor for BAs) (27). DIO2 was implicated in the mechanism through the use of Dio2-KO (Dio2-knockout) mice, which were resistant to the weight-reducing effects of BAs (27). However, since the expression of Ucp1 in the Dio2-KO mouse is nearly normal, consistent with the observation that the Dio2-KO mice show only a slight sensitivity to cold exposure (8, 9), and the study was conducted at an undefined ambient temperature, the role of UCP1 in this thermogenic mechanism of BA actionwas unclear.
In this study, we investigated the effects of BA on diet-induced obesity in the Ucp1-KO mouse. Furthermore, by performing the experiments at thermoneutrality (TN), we were able to assess the involvement of thermogenic mechanisms that are independent of cold-activated adrenergic signaling. We have found that the lack of suppression of diet-induced obesity in the Ucp1-KO mouse by BAs supports a role for UCP1-based thermogenesis in the weight-reducing effects of BAs. Furthermore, the induction of Ucp1 by BAs at TN in wild-type (WT) mice at levels comparable with the β3-adrenegic receptor agonist CL-316243 suggests that the activation of thermogenic mechanism at TN is independent of the sympathetic nervous system.
MATERIALS AND METHODS
Animal experiments.
Breeding colonies of C57BL/6J (B6) were purchased from The Jackson Laboratory (Bar Harbor, ME). Ucp1−/− (Ucp1-KO) mice on a C57BL/6J genetic background were generated from heterozygous breeding pairs maintained in our colony. All Ucp1-KO and Ucp1-WT mice were 8-wk-old males. They were group housed at an ambient temperature of 29°C and fed ad libitum a high-fat diet (HFD; 58% of energy in kcal comes from fat; AIN-76A, Test Diet) or HFD supplemented with 0.5% (wt/wt) cholic acid (CA) for 9 wk. The animal experiments and protocols were approved by the Local Committee for the Ethical Treatment of Experimental Animals of Warmia-Mazury University (NR 38/2011), Olsztyn, Poland.
Phenotype of energy balance.
Body weight (BW) and body composition were measured at different time points, as indicated in the experimental protocol. Body composition was determined by nuclear magnetic resonance (Bruker). Adiposity index (AI) was calculated as the ratio of fat mass (FM) to lean mass (LM). Energy content of FM and LM measured in grams (g) was expressed in kilojoule (kJ) and calculated as 4.18 kJ/kcal × 9 kcal/g of FM or 4 kcal/g of LM, respectively. Food intake was measured on a weekly basis and expressed as the amount of energy in kJ. Total energy expenditure (TEE) was estimated according to Ravussin et al. (21).
Glucose tolerance test.
Glucose tolerance test (GTT) was performed on mice that were fasted for 4 h and injected intraperitoneally with 20% glucose solution (2 mg/g BW). Blood glucose levels from the tail vein were measured at baseline, 20, 40, 60, 90, and 120 min by using an Accu-Chek glucometer (Roche Diagnostics).
Biochemical analysis.
Plasma concentrations of triacylglycerol, total cholesterol, and HDL and the enzyme activity of alanine transaminase were measured by the Pentra C200 Clinical Chemistry Analyzer (Horiba Medical). To calculate the values for non-HDL fraction, HDL was subtracted from total cholesterol (non-HDL = TC − HDL).
RNA isolation and quantitative real-time PCR.
Total RNA was isolated from homogenized interscapular BAT (iBAT), liver, and intestine using TRI reagent per the manufacturer's directions (Molecular Research Centre). Isolated RNA was protected from RNase degradation by treatment with SUPERase-In (Applied Biosystems). Quantity of isolated RNA was assessed by a NanoDrop1000 spectrophotometer (Thermo Scientific). Total RNA (6 ng) was used as a template for quantitative real-time PCR (qRT-PCR) reaction. The analysis was performed using a TaqMan one-step PCR master mix reagents kit on an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems). All samples were run in duplicate and normalized to the reference gene cyclophilin B. Probe and primer sequences are available upon request.
Western blot analysis.
Western blot analysis was performed according to Xue et al. (31). Blots were incubated overnight with primary antibodies against UCP1 (rabbit anti-UCP1, 1:500, sc-6529; Santa Cruz Biotechnology), TGR5 (goat anti-TGR5 1:500, sc-48687; Santa Cruz Biotechnology), DIO2 (rabbit anti-DIO2, 1:250, ab135711; Abcam), voltage-dependent anion channel 1 (VDAC1; goat anti-VDAC1, 1:250, sc-32063; Santa Cruz Biotechnology), cytochrome c oxidase IV (COX-IV; mouse anti-COX-IV, 1:1,000, ab14744; Abcam), cAMP response element-binding protein (CREB; rabbit anti-CREB, 1:250, sc-200; Santa Cruz Biotechnology), phospho-CREB (rabbit anti-p-CREB, 1:500, no. 9198; Cell Signaling Technology), p38 MAPK (rabbit anti-p38 MAPK, 1:500, no. 9212; Cell Signaling Technology), phospho-p38 MAPK (rabbit anti-p-p38 MAPK, 1:500, no. 9211; Cell Signaling Technology), AMP-activated protein kinase (AMPK; rabbit anti-AMPK, 1:1,000, no. 2532; Cell Signaling Technology) phospho-AMPK (rabbit anti-p-AMPK, 1:500, no. 2531; Cell Signaling Technology), PKA RIIβ (mouse anti-PKA RIIβ, 1:1,000, no. 610625; BD Bioscience), phospho-PKA RIIβ (mouse anti-pPKA RIIβ, 1:1000, no. 612550; BD Bioscience), MitoProfile Total OXPHOS Rodent WB Antibody Cocktail (mouse anti-OXPHOS, 1:250, ab110413; Abcam), β-actin (goat anti-β-actin, 1:5,000, ab6276; Abcam). Secondary antibodies [goat anti-rabbit IRDye 800, 611-132-122 (Rockland); goat anti-mouse IRDye 700, 610-730-124 (Rockland); donkey anti-goat IRDye 700, 926-32214 (Li-Cor); donkey anti-mouse IRDye 700, 610-730-124 (Rockland)] were used to detect specific antibody-antigen complexes. Specific bands were visualized and quantified using the Odyssey imaging system (Li-Cor) and normalized against β-actin, which served as an internal loading control.
Histology.
iBAT and inguinal (ING) fat were fixed in 6% formalin. The paraffin-embedded sections were stained with hematoxylin-eosin for morphological examination. Cell size measurement of ING fat was determined using Cell Sens Software with the BX43 microscope (Olympus).
Statistics.
Statistical analyses were performed by two-way ANOVA with Bonferroni's multiple test when two or more groups were compared or by using Student's t-test and Mann-Whitney test for comparisons of two groups, as appropriate. Data are expressed as means ± SE. Differences with P < 0.05 were considered significant. Data analyses were conducted using Graph Pad Prism 6.0 statistical software.
RESULTS
BAs and energy balance phenotypes.
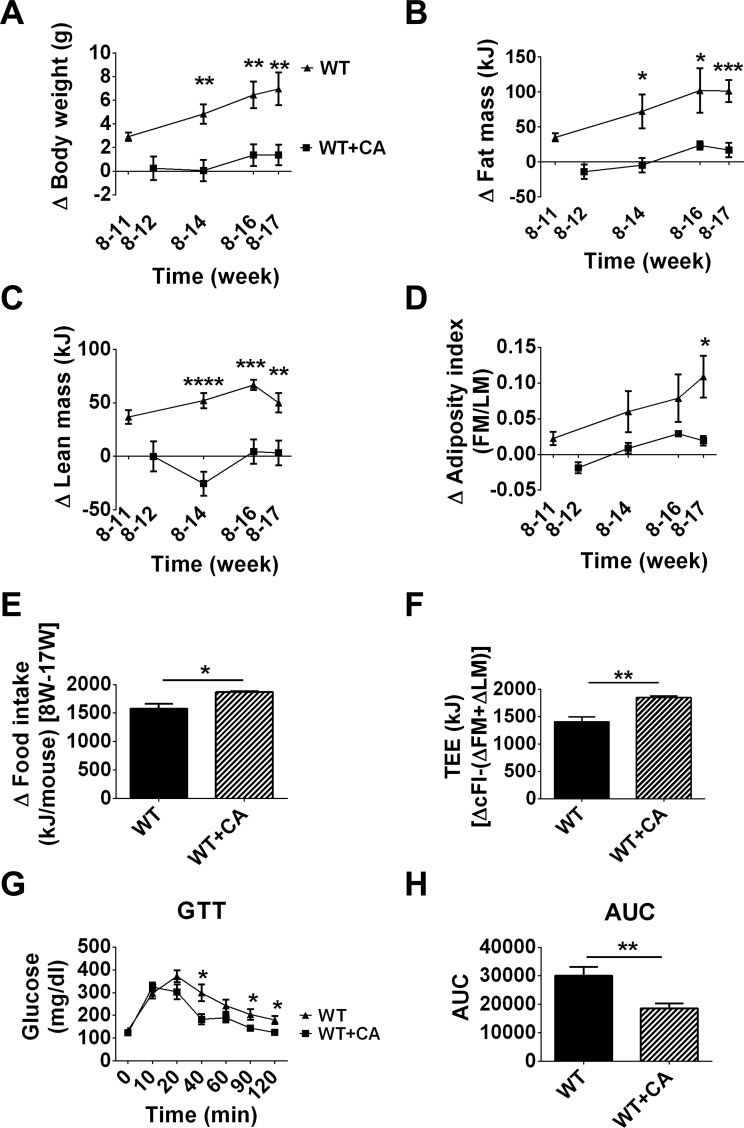
WT mice fed a HFD supplemented with cholic acid (WT + CA vs. WT) showed a significant reduction in BW over a 9-wk period that was due to reduced FM and LM (BW P < 0.01, FM P < 0.001, LM P < 0.01) (Fig. 1, A–C). The AI (Fig. 1D) of mice fed a HFD with CA is comparable with that found in mice fed a chow diet (27). The increase in food intake in WT mice in which dietary CA reduced adiposity (Fig. 1E) translated into increased TEE (Fig. 1F). Consistent with the reduced adiposity and increased EE, dietary CA improved glucose tolerance (Fig. 1, D, F, and G). These results suggest that dietary CA suppressed HFD-induced adiposity in WT mice at TN, and the improved glucose metabolism is consistent with previous results obtained at undefined ambient temperature (14).
Fig. 1.
Cholic acid (CA) prevents diet-induced obesity in wild-type (WT) mice fed a high-fat diet (HFD). A–F: time course of changes in body weight (A), fat mass (FM; B), lean mass (LM; C), adiposity index (D), food intake (E), and total energy expenditure (TEE; F) of WT mice on a HFD and HFD with CA. G and H: glucose tolerance test (GTT; G) and area under the curve (AUC; H). Change in food intake is given as a change between 8 and 17 wk. Data are presented as means ± SE. Differences between groups were analyzed by Student's t-test. *P <0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; n = 8.
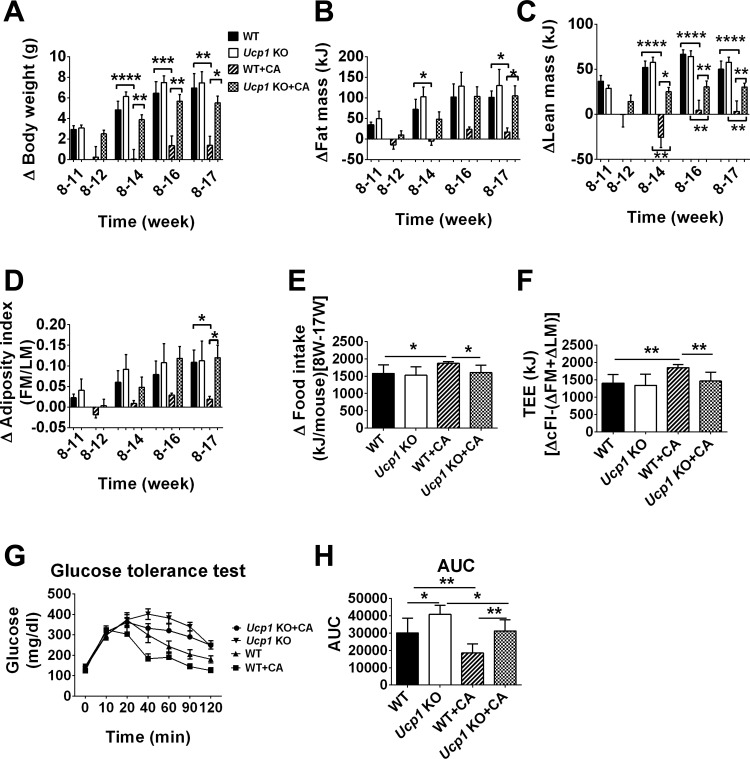
To assess whether Ucp1 is required for the suppression of diet-induced obesity by CA at TN, we determined the effects of CA on the adiposity phenotype in WT and Ucp1-KO mice fed a HFD. CA did not significantly suppress BW, FM, or AI in Ucp1-KO mice (Ucp1-KO vs. Ucp1-KO + CA; Fig. 2, A, B, and D), and their body composition was indistinguishable from that of WT mice fed a HFD (Fig. 2, A, B, and D). On the other hand, CA significantly reduced LM in Ucp1-KO mice, but not as strongly as that which occurred in WT mice fed CA (P < 0.01; Fig. 2C). In summary, Ucp1-KO, Ucp1-KO + CA, and WT mice were indistinguishable from each other with respect to BW, FM, and AI (Fig. 2, A–D). CA treatment led to greater loss in BW (P < 0.05), FM (P < 0.05), LM (P < 0.01), and adiposity (P < 0.05) in WT + CA mice compared with Ucp1-KO + CA mice (Fig. 2, A–D) throughout the experiment. The inability of CA to block diet-induced obesity in Ucp1-KO mice together with lower food intake further supports the interpretation that CA acts by activating UCP1-based nonshivering thermogenesis (Fig. 2E). In parallel with food intake data, TEE was significantly higher in WT + CA than in Ucp1-KO + CA, Ucp1-KO, or WT mice (P < 0.01; Fig. 2F). Glucose tolerance was improved in WT + CA compared with Ucp1-KO + CA mice (Fig. 2G). In addition, CA improved glucose tolerance in Ucp1-KO mice, as indicated by area under the curve (P < 0.05; Fig. 2H).
Fig. 2.
Effect of CA on the regulation of diet-induced obesity in WT and uncoupling protein 1 (Ucp1)-knockout (KO) mice at thermoneutrality. A–F: changes in body weight (A), FM (B), LM (C), adiposity index (D), food intake (E), and TEE (F) in WT, Ucp1-KO mice fed a HFD and WT, and Ucp1-KO mice fed HFD with CA after 9 wk on the diet. G and H: GTT (G) and AUC (H). Change in food intake is given as a change between 8 and 17 wk. Data are presented as means ± SE. Differences between groups were analyzed by 2-way ANOVA with Bonferroni's multiple test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; n = 8.
BAs and the thermogenic program.
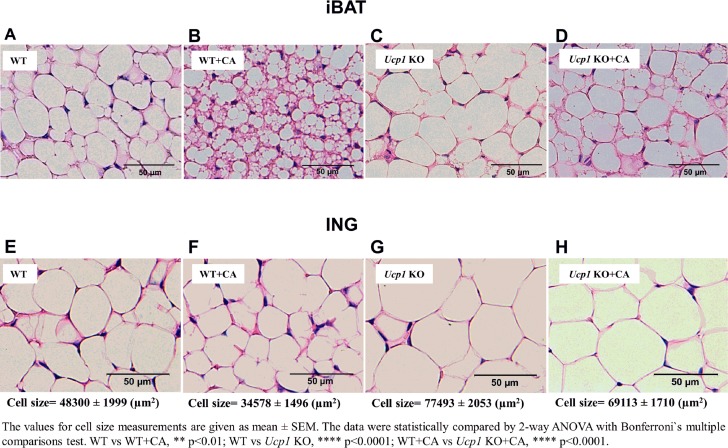
Histology of iBAT depots in WT mice fed a HFD at TN showed adipocyte tissue morphology resembling that of a typical white fat depot (compare Fig. 3, A and E). The increased adipocyte size in iBAT of Ucp1-KO mice fed a HFD is consistent with the reduced EE in iBAT of these mice (Figs. 2F and 3C). WT mice fed a HFD with CA compared with WT and Ucp1-KO mice possessed multilocular adipocytes with small lipid droplets (Fig. 3, A and B). CA treatment in Ucp1-KO mice failed to increase thermogenic activity of iBAT, as was evident from the larger lipid vesicles characteristic of thermogenically quiescent brown fat (Fig. 3, C and D). iBAT morphology of Ucp1-KO + CA resembles that found in Ucp1-KO (10). ING fat of WT and WT + CA, but especially WT + CA mice (Fig. 3, E and F), showed unilocular morphology with smaller lipid droplets than Ucp1-KO and Ucp1-KO + CA mice (Fig. 3, G and H), which was consistent with reduced AI in WT + CA mice (Fig. 2D).
Fig. 3.
The influence of CA on morphology and function of interscapular brown adipose tissue (iBAT) and inguinal (ING) fat of WT and Ucp1-KO mice fed HFD. A–D: hematoxylin and eosin-stained iBAT sections (20×) of WT mice fed HFD (A), WT mice fed HFD + CA (B), Ucp1-KO mice fed HFD (C), and Ucp1-KO fed HFD + CA (D) for 4 wk. E–H: hematoxylin and eosin-stained ING sections (20×) of WT mice fed HFD (E), WT mice fed HFD + CA (F), Ucp1-KO mice fed HFD (G), and Ucp1-KO mice fed HFD + CA (H) for 4 wk.
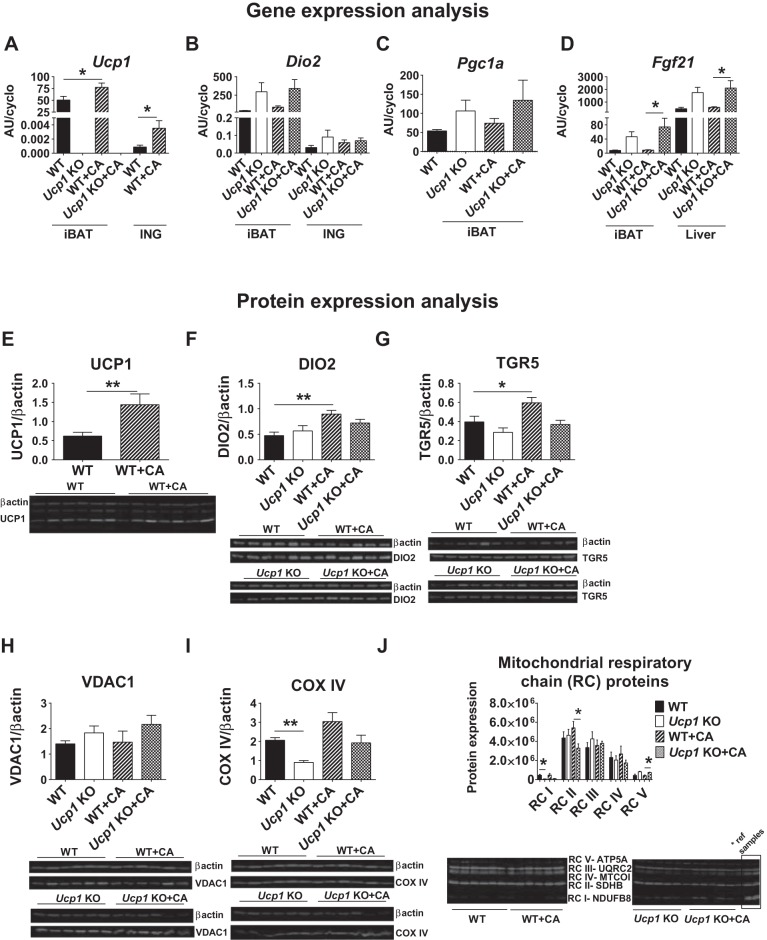
To assess whether BAs are able to induce UCP1-based thermogenesis at TN, functional biomarkers associated with the thermogenic program in iBAT and ING were determined. As expected, Ucp1-KO and Ucp1-KO + CA mice had no detectable Ucp1 mRNA, whereas CA induced Ucp1 mRNA and protein levels in WT mice for iBAT and ING, respectively (Fig. 4A). Dio2 mRNA levels in brown adipocytes were not changed in WT + CA and Ucp1-KO + CA compared with WT and Ucp1-KO mice (Fig. 4B). Despite enhanced expression of Dio2 and peroxisome proliferator-activated receptor-γ coactivator-1α (Pgc1a) in iBAT of Ucp1-KO and Ucp1-KO + CA mice compared with WT and WT + CA, the changes did not reach the statistical significance (Fig. 4, B and C). Expression of fibroblast growth factor 21 (Fgf21) in iBAT was much higher in Ucp1-KO + CA compared with WT + CA mice (P < 0.05; Fig. 4D). Accordingly, the enhanced expression of these regulatory factors of iBAT thermogenesis in Ucp1-KO mice did not lead to any UCP1-independent mechanism of thermogenesis driven by BAs.
Fig. 4.
Thermogenic gene and protein expression in iBAT and ING fat after CA treatment at thermoneutrality. A–D: Ucp1 (A) and type 2 deiodinase (Dio2; B) mRNA levels in iBAT and ING fat. C and D: peroxisome proliferator-activated receptor-γ coactivator-1α (Pgc1a) gene expression in iBAT (C) and expression of fibroblast growth factor 21 (Fgf21) in iBAT and liver (D). E–G: protein analyses of UCP1 (E), DIO2 (F), and TGR5 (GPR131 or GpBAR1; a G protein-coupled plasma membrane receptor for BAs; G) in iBAT. H–J: protein levels of voltage-dependent anion channel 1 (VDAC1; H), cytochrome c oxidase IV (COX-IV; I), and mitochondrial respiratory chain (RC) proteins (J) in iBAT. Samples for RC proteins were normalized to reference samples derived from the WT + CA group. Fields with targeted proteins and β-actin in immunoblots were cropped at appropriate band size. All samples were derived at the same time and processed in parallel. Data are given as means ± SE. Data were compared statistically by 2-way ANOVA with Bonferroni's multiple test [quantitative (q)RT-PCR] or Mann-Whitney test (immunoblots); n = 8 (qRT-PCR) and 6 (immunoblots). *P < 0.05; **P < 0.01. AU, arbitrary units.
Immunoblots showed a twofold increase in UCP1 and DIO2 protein in WT + CA compared with WT mice (P < 0.01; Fig. 4, E and F), but no differences in DIO2 protein levels were observed between Ucp1-KO and Ucp1-KO + CA mice (Fig. 4F). The induction of DIO2 in iBAT was accompanied by increased TGR5 protein levels in WT + CA vs. WT mice, but no induction of TGR5 occurred in Ucp1-KO + CA vs. Ucp1-KO (Fig. 4G). CA did not affect the levels of mitochondrial markers VDAC1 and COX-IV in brown adipocytes; nonetheless, Ucp1-KO mice had decreased COX-IV protein levels compared with WT mice (Fig. 4, H and I). The selected subunits of the mitochondrial respiratory complexes (RCs) in iBAT showed higher levels of RC II (P < 0.05) and RC IV (P > 0.05) in WT + CA than in Ucp1-KO + CA mice, and RC V protein expression was increased for Ucp1-KO mice and Ucp1-KO + CA compared with WT and WT + CA mice (Fig. 4J).
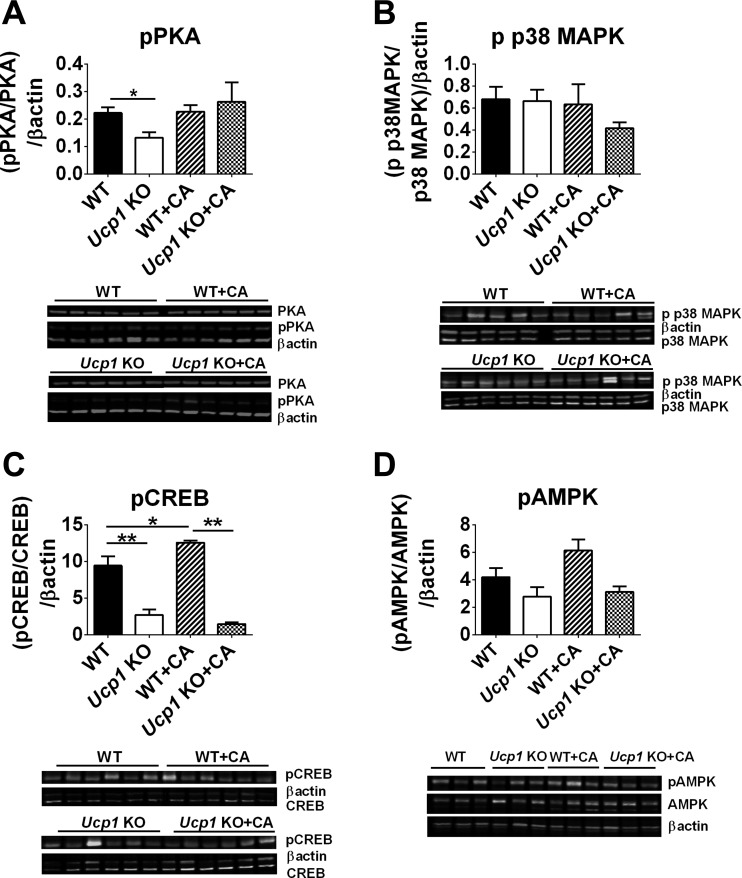
To identify the signaling pathway for induction of Ucp1 expression in response to CA, we assessed the levels of PKA, p38 MAPK, CREB, and AMPK in iBAT. The phosphorylated levels of PKA regulatory subunit RIIβ were unchanged in WT and Ucp1-KO after CA treatment, whereas a modest decrease was observed in Ucp1-KO compared with WT mice (Fig. 5A). Accordingly, the phosphorylation of p38 MAPK protein levels was not affected by CA treatment in WT or Ucp1-KO (Fig. 5B). A significant increase in CREB phosphorylation was observed in WT and WT + CA compared with Ucp1-KO and Ucp1-KO + CA (Fig. 5C). Importantly, CA influenced phosphorylated levels of CREB in WT mice (Fig. 5C). In addition, a slight increase in p-AMPK was observed in WT + CA (Fig. 5D). Previous attempts at detecting changes in this signaling pathway in retroperitoneal fat of C57B6/J vs. A/J mice treated with cold also failed to detect meaningful correlations with the level of UCP1 (30).
Fig. 5.
The modulation of key components of Ucp1 regulation in iBAT in response to CA treatment at thermoneutrality. Protein phosphorylation levels of PKA (RIIβ) (A), p38 MAPK (B), cAMP response element-binding protein (CREB; C), and AMP-activated protein kinase (AMPK; D) in iBAT in WT and Ucp1-KO mice fed a HFD or HFD supplemented with CA. Fields with targeted proteins and β-actin in immunoblots were cropped at appropriate band size. All samples were derived at the same time and processed in parallel. Data are presented as means ± SE. Differences between groups were analyzed by Mann-Whitney test. *P < 0.05 and **P < 0.01; n = 5–6.
BAs and lipid and glucose metabolism.
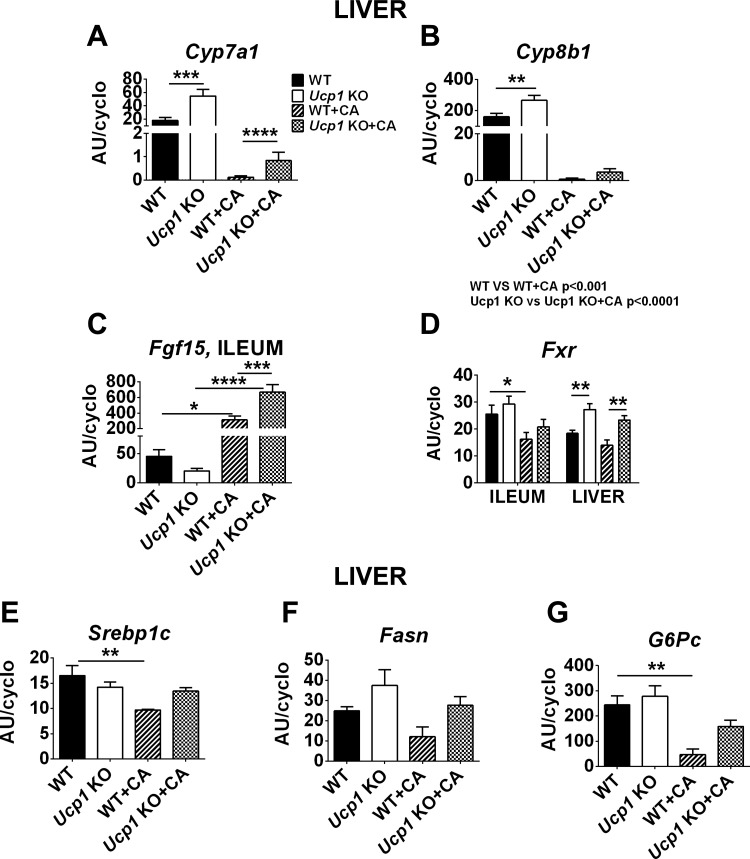
To further probe the effects of BAs and Ucp1 in the regulation of diet-induced obesity, we analyzed genes associated with BA, lipid, and glucose metabolism. CA administration significantly repressed cholesterol 7α-hydroxylase (Cyp7a1) and sterol 12α-hydroxylase (Cyp8b1) mRNA levels (the enzymes involved in BA synthesis; Fig. 6, A and B). Ucp1-KO mice had higher Cyp7a1 and Cyp8b1 gene expression than WT mice (Fig. 6, A and B). Consistent with the fibroblast growth factor (Fgf15) function as a negative regulator of hepatic Cyp7a1 (23), its expression in ileum was higher in CA-treated mice of WT and Ucp1-KO mice, which had low levels of Cyp7a1 and Cyp8b1 (Fig. 6C). In addition, farnesoid X receptor (FXR) signaling constitutes a feedback regulatory mechanism for BAs synthesis. The increased hepatic Fxr gene expression in Ucp1-KO mice vs. WT and Ucp1-KO + CA vs. WT + CA mice corresponded to lower Cyp7a1 and Cyp8b1 gene expression (Fig. 6, A, B, and D). Interestingly, CA-treated WT mice had significantly reduced ileal Fxr mRNA levels compared with WT mice, which is associated with reduced obesity (BW and FM; Figs. 1, B and C, and 6D), as reported by Li et al. (18). Consistent with changes in adiposity phenotype, a key regulator of fatty acid biosynthesis, the sterol response element-binding protein-1c (Srebp1c), was lower after CA treatment in WT (P < 0.01) but not Ucp1-KO mice (Fig. 6E). In addition to Srebp1c, fatty acid synthase (Fasn) mRNA levels were also downregulated in WT + CA vs. WT mice, but the changes did not reach statistical significance (Fig. 6F). BAs also regulate hepatic glucose production via gluconeogenesis (32). CA treatment decreased the hepatic expression of the glucose-6-phosphatase catalytic subunit (G6Pc) in WT and Ucp1-KO mice (Fig. 6G). However, Ucp1-KO + CA had slightly induced G6Pc mRNA levels compared with WT + CA. We have found no changes in plasma levels of triacylglycerol, total cholesterol, HDL, and non-HDL fractions or alanine aminotransferase after CA treatment of either WT or Ucp1-KO mice compared with mice fed a HFD (Table 1).
Fig. 6.
Regulation of energy metabolism by CA treatment. A and B: hepatic gene expression of Cyp7a1 (A) and Cyp8b1 (B). C: intestinal level of Fgf15, the main suppressor of bile acid synthesis. D: farnesoid X receptor (Fxr) mRNA level in the liver and ileum. E–G: transcriptional regulator sterol response element-binding protein-1c (Srebp1c; E) controlling the rate of lipogenesis, fatty acid synthase (Fasn; F), and glucose-6-phosphatase catalytic subunit (G6Pc; G) involved in fatty acid synthesis and gluconeogenesis. Data are given as means ± SE. Data were statistically compared by 2-way ANOVA with Bonferroni's multiple test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; n = 8.
Table 1.
Lipid profile of mice fed a HFD and HFD supplemented with CA
| WT | Ucpl-KO | WT + CA | Ucp1-KO + CA | |
|---|---|---|---|---|
| TAG, mmol/l | 0.77 ± 0.03 | 0.98 ± 0.17 | 0.84 ± 0.04 | 1.08 ± 0.13 |
| TC, mmol/l | 4.66 ± 0.28 | 4.36 ± 0.26 | 3.89 ± 0.28 | 3.80 ± 0.12 |
| HDL, mmol/l | 2.32 ± 0.05 | 2.21 ± 0.09 | 1.28 ± 0.34 | 2.03 ± 0.11 |
| Non-HDL, mmol/l | 2.34 ± 0.26 | 2.15 ± 0.0.18 | 2.59 ± 0.52 | 1.77 ± 0.08 |
| ALT, U/l | 33.48 ± 4.72 | 28.98 ± 5.15 | 171.4 ± 52.54 | 57.62 ± 14.40 |
Data are presented as means ± SE; n = 5–6.
HFD, high-fat diet; CA, cholic acid; WT, wild type; Ucpl, uncoupling protein 1; KO, knockout; TAG, triacylglycerol; TC, total cholesterol; ALT, alanine transaminase.
Plasma levels of TAG, TC, HDL, and non-HDL are the difference between TC and HDL and ALT. Differences between groups were analyzed by 2-way ANOVA with Bonferroni’s multiple test.
DISCUSSION
Dietary BAs have been shown to suppress the induction of adiposity in mice fed a HFD (27). Based upon the phenotype of the Dio2-KO model, it was postulated that BAs reduced adiposity by activating thyroid hormone-dependent thermogenesis in iBAT. However, the characterization of the thermogenic mechanism of EE, presumably by UCP1-based thermogenesis, was inadequate. Most problematic is that the data and methods for determination of EE by indirect calorimetry could not provide valid measurements (6). The problem with the data of Watanabe et al. (27) has been discussed by Teodoro et al. (24), who, parenthetically, did not detect an increase in EE in mice fed BAs. Although Teodoro et al. (24) showed a robust induction of Ucp1 expression in mice treated with BAs, whole body EE, as determined by indirect calorimetry, was not increased in diet-induced obese mice treated with BA. This may mean that the magnitude of the increase in EE caused by iBAT nonshivering thermogenesis over a period of several weeks cannot be detected by indirect calorimetry but can be detected by the energy balance methodology of Ravussin et al. (21). An additional question in the studies by both Watanabe et al. (27) and Teodoro et al. (24) comes from the lack of information on the ambient temperature in the former study, whereas the latter study was conducted at an ambient temperature of 20°C. Accordingly, does the EE/thermogenic mechanism involve an interaction between the BAs, thyroid, and cold-activated adrenergic-mediated signaling pathways of thermogenesis?
It is crucial to realize that no specific thermogenic mechanism has been described for DIO2 or thyroid hormone in BAT, although isoforms of sarcoendoplasmic Ca2+-ATPase regulated by thyroid hormone are expressed in iBAT (25). If it has a thermogenic function, it may be supportive like leptin and insulin but have no direct thermogenic mechanism like UCP1 in BAT or the Ca2+-cycling mechanism in skeletal muscle (2, 16). This is illustrated with the Dio2-KO mouse, since this mouse has normal or even slightly elevated levels of Ucp1 and when exposed to the cold at 4°C for 24 h it loses about 1.5°C of body temperature in 24 h with no change in Ucp1 mRNA levels (9). In contrast, the body temperature of Ucp1-KO mice decreases 10°C after 3 h of exposure (10). Together with PGC-1α and PPARα, DIO2 is part of a group of transcription factors and signaling molecules that support Ucp1 regulation, but in their absence other factors can substitute (30).
Although Watanabe et al. (27) clearly showed that dietary BAs reduce diet-induced obesity and that this suppression is lost in Dio2-KO mice, it is uncertain what thermogenic mechanism is responsible for the reduction in adiposity, because Ucp1 expression in Dio2-KO mice is not reduced. At TN, BAs are not able to suppress diet-induced obesity in Ucp1-KO mice despite the elevated expression of Dio2, indicating that in the absence of a functional Ucp1 gene, BAs are ineffective at reducing diet-induced obesity. Since EE is induced in WT mice upon treatment with BA and also in Ucp1-KO mice treated with BA, but much less so, it would be of value to identify other organ systems in which BA could stimulate thermogenesis. On the basis of reports that the TGR5 is expressed in white fat depots, we also assessed the induction of brown adipocytes in ING fat. Induction of Ucp1 mRNA levels at TN by BAs in ING fat is only 0.0045% of that present in iBAT. In contrast, induction of Ucp1 mRNA levels at TN by the β3-adrenergic agonist CL-316243 in ING fat is 8% of that achieved in iBAT (15). Thus, ING fat can respond to induction of Ucp1 by a β3-adrenergic agonist, but there is no induction of Ucp1 by BAs. The treatment of human brown adipocytes with BAs, specifically CDCA, or TGR5 agonists showed significant enhancement of EE and BAT activity through the UCP1-dependent mechanism, an effect that was absent in WAT (5).
Some studies using Ucp1-KO mice have shown that there is no difference in diet-induced obesity at TN between WT and Ucp1-KO mice (10, 19), whereas others have shown higher adiposity in the Ucp1-KO (11), the interpretation being that there is suppression of diet-induced obesity in WT mice with a capacity to induce Ucp1 expression, but not in the Ucp1-KO mice.
The mechanism described so far for Ucp1 regulation in brown adipocytes proceeds through the β3-adrenergic receptor adenylyl cyclase, the PKA pathway, and activation of the CREB/p38 MAPK and ATF2/PGC1 pathway. BAs stimulate the same pathway, except that they bind to the TGR5 receptor rather the β-adrenergic receptors to initial signaling to induce the brown adipocyte phenotype (BAP). Accordingly, the administration of the β3-adrenergic agonist to C57BL6/J mice housed at 29°C caused a modest induction of Ucp1 in iBAT, which is about 33% compared with control, and its induction in ING fat is very robust (15). Notably, the effectiveness of BAs to stimulate Ucp1 at TN is comparable with the effect of β3-adrenergic agonist, leading to a 35% increase in Ucp1 in iBAT. On the other hand, the slight induction of Ucp1 in ING fat by BAs was insignificant, amounting to 0.0045% of the level expressed in iBAT. Furthermore, the morphology of iBAT showed induction of BAP by BAs, whereas the morphology of ING fat shows no evidence of brown adipocytes, and the reduced adipocyte size in WT mice treated with BAs is consistent with the reduced systemic adiposity of these mice (Figs. 1D and 3B). The significance of these data is that, together with the evidence for the elevation of Cyp7a1 and Cyp8b1 in Ucp1-KO mice, dietary BAs function as a mechanism for induction of thermogenesis that is independent of the sympathetic nervous system. In other words, this is a mechanism of diet-induced thermogenesis that might be independent of cold-activated thermogenesis.
The modulation of Ucp1 expression by the β3-adrenergic receptor agonist, thyroid hormones, the AMPK system, and FGF21 treatment, has been shown to stimulate EE (3, 28). Studies in humans and mice have indicated that FGF21 is a metabolic regulator of energy homeostasis, being expressed in multiple tissues (liver, muscle, pancreas, and adipose tissue) (1). Furthermore, the induction of FGF21 concurrently with the cold activation of the thermogenic program in iBAT has been postulated to mediate the browning of white adipose tissue (12, 13, 17). Obese mice treated pharmacologically with FGF21 have reduced BW with significantly increased EE and improved insulin tolerance (29). The proposed mechanism underlying the weight-reducing effect of FGF21 involves the brain-BAT axis, in which FGF21 acts in the hypothalamus via β-Klotho and stimulates SNS signaling to iBAT thermogenesis (20). In our study, we suggest that Fgf21 does not activate Ucp1 expression in iBAT in the absence of cold-induced sympathetic activity and does not increase thermogenic EE. This is in agreement with Samms et al. (22), who reported significantly higher Fgf21 mRNA levels in iBAT of Ucp1-KO mice than WT mice without EE being increased. Furthermore, the pharmacological treatment with FGF21 of UCP1-deficient mice causes weight reduction to a level comparable with WT mice (22, 26).
In summary, previous studies have shown that dietary BAs suppressed diet-induced obesity in WT mice at room temperature, but not in Dio2-KO mice. Since Ucp1 is still expressed in Dio2-KO mice, it was not clear which thermogenic pathway was activated by the BAs in iBAT (27). This uncertainty was confounded by the fact that the study was performed at a temperature at which the SNS was still activated. Here, we present evidence that at TN, CA suppressed the development of diet-induced obesity of WT mice but not of Ucp1-KO mice, establishing that Ucp1 expression is required for the weight-reducing action of BAs. This action by BAs was confirmed by showing that the induction of Ucp1 mRNA and protein by BAs occurs in WT mice at TN. Accordingly, Ucp1 is essential for the anti-obesity effects of BAs through a mechanism that might be independent of cold-activated sympathetic activity.
GRANTS
This work was supported by a grant to L. P. Kozak from the Foundation for Polish Science, programme WELCOME, no. WELCOME/2010-4/3, entitled “Nutrition and ambient temperature during early development can reduce susceptibility to obesity,” financed by EU Structural Funds in Poland within the Innovative Economy Programme and REFRESH project FP7-REGPOT-2010-1-264103.
DISCLOSURES
The authors declare that they have no conflicts of interest, financial or otherwise, with the contents of this article.
AUTHOR CONTRIBUTIONS
M.Z. and L.P.K. conception and design of research; M.Z. performed experiments; M.Z. and L.P.K. analyzed data; M.Z. and L.P.K. interpreted results of experiments; M.Z. prepared figures; M.Z. and L.P.K. drafted manuscript; M.Z. and L.P.K. edited and revised manuscript; L.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Agnieszka Korytko for support in the management of the mouse colony. Lucja Brzuzan is acknowledged for valuable work regarding lipid profile analysis.
REFERENCES
- 1.Antonellis PJ, Kharitonenkov A, Adams AC. Physiology and Endocrinology Symposium: FGF21: Insights into mechanism of action from preclinical studies. J Anim Sci 92: 407–413, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Anunciado-Koza R, Ukropec J, Koza RA, Kozak LP. Inactivation of UCP1 and the glycerol phosphate cycle synergistically increases energy expenditure to resist diet-induced obesity. J Biol Chem 283: 27688–27697, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arch JR, Trayhurn P. Detection of thermogenesis in rodents in response to anti-obesity drugs and genetic modification. Front Physiol 4: 64, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Broeders EP, Nascimento EB, Havekes B, Brans B, Roumans KH, Tailleux A, Schaart G, Kouach M, Charton J, Deprez B, Bouvy ND, Mottaghy F, Staels B, van Marken Lichtenbelt WD, Schrauwen P. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab 22: 418–426, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59: 323–329, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET, Bianco AC. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes 53: 577–584, 2004. [DOI] [PubMed] [Google Scholar]
- 9.de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108: 1379–1385, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9: 203–209, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271–281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 286: 12983–12990, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Kawanaka K, Tabata I, Higuchi M, Tange T, Yamamoto TT, Ezaki O. Cholate inhibits high-fat diet-induced hyperglycemia and obesity with acyl-CoA synthetase mRNA decrease. Am J Physiol Endocrinol Metab 273: E37–E45, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Jaroslawska J, Chabowska-Kita A, Kaczmarek MM, Kozak LP. Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status. PLoS Genet 11: e1005287, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak LP, Young ME. Heat from calcium cycling melts fat. Nat Med 18: 1458–1459, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 19: 302–309, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Jiang C, Krausz KW, Li Y, Albert I, Hao H, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4: 2384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest 111: 399–407, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 20: 670–677, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravussin Y, Gutman R, LeDuc CA, Leibel RL. Estimating energy expenditure in mice using an energy balance technique. Int J Obes (Lond) 37: 399–403, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samms RJ, Smith DP, Cheng CC, Antonellis PP, Perfield JW 2nd, Kharitonenkov A, Gimeno RE, Adams AC. Discrete Aspects of FGF21 In Vivo Pharmacology Do Not Require UCP1. Cell Rep 11: 991–999, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17: 225–235, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Teodoro JS, Zouhar P, Flachs P, Bardova K, Janovska P, Gomes AP, Duarte FV, Varela AT, Rolo AP, Palmeira CM, Kopecky J. Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice. Int J Obes (Lond) 38: 1027–1034, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J Biol Chem 281: 31894–31908, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Véniant MM, Sivits G, Helmering J, Komorowski R, Lee J, Fan W, Moyer C, Lloyd DJ. Pharmacologic Effects of FGF21 Are Independent of the “Browning” of White Adipose Tissue. Cell Metab 21: 731–738, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Whittle A, Relat-Pardo J, Vidal-Puig A. Pharmacological strategies for targeting BAT thermogenesis. Trends Pharmacol Sci 34: 347–355, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue B, Coulter A, Rim JS, Koza RA, Kozak LP. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol Cell Biol 25: 8311–8322, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 48: 41–51, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J, Fukamizu A. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem 279: 23158–23165, 2004. [DOI] [PubMed] [Google Scholar]