Abstract
C1q/TNF-related protein 3 (CTRP3) is a secreted metabolic regulator whose circulating levels are reduced in human and rodent models of obesity and diabetes. Previously, we showed that CTRP3 infusion lowers blood glucose by suppressing gluconeogenesis and that transgenic overexpression of CTRP3 protects mice against diet-induced hepatic steatosis. Here, we used a genetic loss-of-function mouse model to further address whether CTRP3 is indeed required for metabolic homeostasis under normal and obese states. Both male and female mice lacking CTRP3 had similar weight gain when fed a control low-fat (LFD) or high-fat diet (HFD). Regardless of diet, no differences were observed in adiposity, food intake, metabolic rate, energy expenditure, or physical activity levels between wild-type (WT) and Ctrp3-knockout (KO) animals of either sex. Contrary to expectations, loss of CTRP3 in LFD- or HFD-fed male and female mice also had minimal or no impact on whole body glucose metabolism, insulin sensitivity, and fasting-induced hepatic gluconeogenesis. Unexpectedly, the liver sizes of HFD-fed Ctrp3-KO male mice were markedly reduced despite a modest increase in triglyceride content. Furthermore, liver expression of fat oxidation genes was upregulated in the Ctrp3-KO mice. Whereas the liver and adipose expression of profibrotic TGFβ1, as well as its serum levels, was suppressed in HFD-fed KO mice, circulating proinflammatory IL-6 levels were markedly increased; these changes, however, were insufficient to affect systemic metabolic outcome. We conclude that, although it is dispensable for physiological control of energy balance, CTRP3 plays a previously unsuspected role in modulating liver size and circulating cytokine levels in response to obesity.
Keywords: adipokine, C1q/tumor necrosis factor-related protein, C1q/tumor necrosis factor, fatty liver, obesity, diabetes
the c1q/tnf-related protein (CTRP) family comprises 15 secreted plasma proteins of the C1q family, the first seven of which were identified initially on the basis of sequence homology to the globular C1q domain of adiponectin (66); additional members were subsequently described (6, 43, 49, 59–61, 64, 65). Recent functional studies demonstrated important and distinct roles for CTRPs in regulating glucose and/or lipid metabolism in the peripheral tissues (38–42, 57–60) as well as having a central role in modulating food intake (6, 7) and adipocyte differentiation in culture (61). Unlike adiponectin, whose expression is restricted to adipocytes (47), CTRP family members are much more widely expressed (65, 66); all are conserved throughout vertebrate evolution (50).
Multiple in vitro and in vivo approaches have been used to elucidate the biological function of one member, CTRP3, a secreted plasma protein whose circulating levels are decreased in diet-induced obese (DIO) mice (42). Previously, we showed that recombinant CTRP3 infusion lowers blood glucose in wild-type (WT), obese, and diabetic ob/ob mice (42). In cultured hepatocytes and in mouse liver, recombinant CTRP3 administration suppresses gluconeogenic gene (G6Pc and Pck1) expression and glucose output via the Akt signaling pathway (42). We also demonstrated that CTRP3 overexpression strikingly protects the transgenic mice from developing fatty liver in response to high-fat feeding (40). Further, in WT DIO mice that have developed hepatic steatosis, daily administration of recombinant CTRP3 for 5 days is sufficient to markedly reduce liver triglyceride levels (40). We found that CTRP3 acts on hepatocytes to downregulate the expression of genes [glycerol phosphate acyltransferase (Gpat), acylglycerolphosphate acyltransferase (Agpat), and diacylglycerol acyltransferase (Dgat)] involved in triglyceride synthesis (40).
Recent studies by others have shown that CTRP3 expression is reduced in a rat model of obesity and diabetes and that glucagon-like peptide-1 (GLP-1) agonist (extendin-4) treatment upregulates its mRNA and protein levels in visceral adipose tissue (29). Functional insights gained from multiple rodent models have prompted recent investigations of CTRP3 plasma levels in humans; however, conflicting results have been reported for its association with diabetes and the metabolic syndrome (3, 8, 63, 71). The first study showed elevated CTRP3 levels in patients with type 2 diabetes (8), whereas subsequent studies reported lower levels of CTRP3 in individuals with metabolic syndrome (71), newly diagnosed patients with type 2 diabetes (3), obesity (63), or obese patients with hypertension and insulin resistance (9). Furthermore, women with polycystic ovarian syndrome were also found to have lower circulating CTRP3 levels compared with their matched controls, and metformin treatment was found to increase CTRP3 serum levels (54). These studies underscore the relevance of CTRP3 to human physiology in normal and diseased states.
Beyond metabolic control, recent studies have also shown that CTRP3 has anti-inflammatory properties (22, 23, 48) as well as cardioprotective effects in mouse models of ischemic heart attack (69). Interestingly, overexpressing CTRP3 in mice also promotes phosphate-induced vascular smooth muscle cell calcification (73). Although the physiological relevance is unclear, multiple in vitro studies also imply a role for CTRP3 in regulating adipocyte (27, 28), leydig (37), chrondocyte (32), osteocyte (2), endothelial (1), fibroblast (30), and cartilage (70) cell function.
With one exception (36), all of the biological and metabolic effects of CTRP3 in vivo are based on protein infusion or overexpression in mice; collectively, these published studies indicate an important function for CTRP3. Given significant caveats associated with overexpression studies, the present study aimed to address whether CTRP3 is indeed required, in a physiological context, for metabolic homeostasis using a loss-of-function knockout (KO) mouse model.
MATERIALS AND METHODS
Animals.
The Ctrp3-KO mouse strain used for this research project, B6;129S5-C1qtnf3tm1Lex/Mmcd (identification no. 032166-UCD), was obtained from the Mutant Mouse Regional Resource Center (MMRRC), a National Center for Research Resources/National Institutes of Health-funded strain repository, and was donated to the MMRRC by Genentech. The Ctrp3 gene is located on mouse chromosome 15 and comprises six exons. Exon 4 was targeted by homologous recombination. Heterozygous mice were recovered from cryo-preserved embryos. Since the embryonic stem cells were derived from the 129/Sv mouse strain, we back-crossed Ctrp3-KO mice to the C57BL/6J genetic background for six generations. The Ctrp3-KO mice were viable and fertile. Genotyping primers for the Ctrp3 WT allele were forward (primer 50; within exon 4) 5′-CAATCAGAACAGTGGCATTAT-3′ and reverse (primer 51; within intron 4) 5′-CTCTCATTCCACTGCCTTATC-3′. The expected size of the WT band was 182 bp. Genotyping primers for the Ctrp3 null allele were forward (Neo3A; within neomycin-resistant cassette) 5′-GCAGCGCATCGCCTTCTATC-3′ and reverse (primer 53; within intron 4) 5′-TGGGCTCTCTCAGGATTAATTGGT-3′. The expected size of the KO band was 293 bp. To confirm the presence or absence of Ctrp3 mRNA in the adipose tissue of WT and KO mice, we performed standard PCR analysis using the following primer pair: 5′-CTTCAGCATGTACAGCTATG-3′ (forward) and 5′-GTTGCCCATTCTTAGCCAGACT-3′ (reverse). All mice used in this study were generated by intercrossing Ctrp3 heterozygous (+/−) mice. Male and female Ctrp3-KO mice and WT littermate controls were housed in polycarbonate cages on a 12-h light-dark photocycle with ad libitum access to water and food. Littermates were used throughout the study as WT controls. Mice were fed a high-fat diet (HFD; 60% kcal derived from fat, D12492; Research Diets) or the control low-fat diet (LFD; 10% kcal derived from fat, D12450B; Research Diets). Diet was provided for a period of 24 wk, beginning at 4 wk of age. At termination of the study, animals were fasted overnight and euthanized when tissues were collected, snap-frozen in liquid nitrogen, and kept at −80°C until analysis. All animal protocols were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Body composition analysis.
Body composition analyses for fat and lean mass were performed on mice at 25–28 wk using Echo-MRI-100 (Echo Medical Systems, Waco, TX) at the Johns Hopkins University School of Medicine mouse phenotyping core facility. Lean mass was used to normalize the indirect calorimetry data.
Indirect calorimetry.
HFD-fed Ctrp3-WT and -KO mice (n = 10–12/group) at 25–28 wk of age were used for simultaneous assessments of daily body weight change, food intake (corrected for spillage), physical activity, and whole body metabolic profile in the Comprehensive Laboratory Animal Monitoring System (CLAMS) system (Columbus Instruments). Data were collected for 3–4 days to confirm that mice were acclimated to the calorimetry chambers (indicated by stable body weights, food intakes, and diurnal metabolic patterns), and data were analyzed from the 3rd (LFD groups) or 4th day (HFD groups). Rates of oxygen consumption (V̇o2; normalized to ml·lean kg−1·h−1) and carbon dioxide production (V̇co2; ml·lean kg−1·h−1) in each chamber were measured every 24 min throughout the studies. Respiratory exchange ratio (RER = V̇co2/V̇o2) was calculated by CLAMS software (version 4.02) to estimate relative oxidation of carbohydrates (RER = 1.0) vs. fats (RER ∼0.7), not accounting for protein oxidation. Energy expenditure (EE) was calculated as EE = V̇o2 × [3.815 + (1.232 × RER)] (35) and normalized for lean body mass (kcal·lean kg−1·h−1) as recommended (5). Physical activities were measured by infrared beam breaks in the metabolic chamber. Average metabolic values were calculated per subject and averaged across subjects for statistical analysis by Student's t-test.
Glucose tolerance test.
Mice were fasted for 16 h prior to glucose injection. Glucose was injected intraperitoneally (ip) into mice at a dose of 1.5 mg/g body wt. A separate cohort of mice were fasted overnight (∼18 h), and glucose was delivered via gavage into mice at a dose of 1.5 mg/g body wt. Blood glucose was measured at indicated time points using a glucometer (BD Pharmingen, Franklin Lakes, NJ). Serum insulin level during the glucose tolerance test (GTT) was measured using an ELISA kit (Millipore, Billerica, MA).
Insulin tolerance test.
Food was removed 2 h before insulin injection. Insulin was injected ip at a dose of 1.0 U/kg body wt. Blood glucose was measured at the indicated time points using a glucometer (BD Pharmingen).
Isolation of adipose tissue and liver.
Liver, white adipose tissue (perigonadal/visceral and inguinal/subcutaneous), and interscapular brown adipose tissue samples were immediately harvested from euthanized mice and frozen on dry ice. Homogenized cell lysates were prepared in lysis buffer (20 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40, and 10% glycerol) containing protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Protein content was quantified using Bradford protein assay reagent (Bio-Rad).
Histology.
Ctrp3-WT and -KO mouse tissues were fixed overnight in 10% formalin at 4°C. Fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H & E) and Oil Red O at the Histology Reference Laboratory at the Johns Hopkins University School of Medicine. Masson Trichrome Blue staining was performed by AML Laboratories in Baltimore, MD, and Sirius Red staining for fibrotic collagen was performed using the Picro-Sirius Red Stain Kit (Abcam).
Serum and blood chemistry analysis.
Mouse serum was harvested by tail bleed and at time of euthanasia. Samples were separated using microvette CB 300 (Sarstedt, Nümbrecht, Germany) and centrifuged at 10,000 g for 5 min. Glucose concentration was determined at the time of collection with a glucometer (BD Pharmingen). Serum lipid levels were measured by the Mouse Pathology and Phenotyping Core at the Johns Hopkins University School of Medicine. Insulin (Millipore), adiponectin (Millipore), leptin (Millipore), TNFα (Millipore), TGFβ1 (Abcam), and IL-6 (Abcam) were measured using commercially available kits.
Lipid extraction from liver tissue.
Liver (50 mg) was homogenized in 500 μl of distilled water. Two-hundred microliters of the homogenate was collected for lipid extraction, mixed with 1 ml of choloroform-methanol (2:1), and centrifuged at 1,700 rpm for 5 min at 4°C, and the chloroform phase was collected and dried in a speed vacuum. Samples were resuspended in tert-butanol:MeOH:Triton-X100 (3:1:1) before triacylglycerol and cholesterol content were determined using commercially available colorimetric kits (Thermo Scientific).
Quantitative real-time PCR analysis.
Total RNAs were isolated from tissues using Trizol and reverse transcribed using Superscript II RNase H-reverse transcriptase (Invitrogen). Real-time PCR primers for triglyceride synthesis genes (Gpat, Agpat, Dgat) (40) and fibrotic and inflammatory genes (Col1, Col3, Col6, Mmp-12, Tnfα, Mcp-1) (26) have been published previously. Other primer sequences used in this study are listed in Table 1. Quantitative real-time PCR analyses were performed on an Applied Biosystems Prism 7500 sequence detection system (Bio-Rad). Samples were analyzed in 20-μl reactions with SyBR Green PCR Master Mix (Applied Biosystems, Invitrogen) per the manufacturer's directions. Data were normalized to 36B4 and cyclophiln A and expressed as relative mRNA levels using the ΔΔCT method (31).
Table 1.
Real-time PCR primers used in the study
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| Acc1 | TGACAGACTGATCGCAGAGAAAG | TGGAGAGCCCCACACACA |
| Acc2 | GGGCTCCCTGGATGACAAC | GCTCTTCCGGGAGGAGTTCT |
| Fasn | GCTGCGGAAACTTCAGAAAAT | AGAGACGTGTCACTCCTGGACTT |
| Srebp1 | GGAGCCATGGATTGCACATT | GGCCCGGGAAGTCACTGT |
| Srebp2 | GCGTTCTGGAGACCATGGA | ACAAAGTTGCTCTGAAAACAAATCA |
| Lcad | TCTTTTCCTCGGAGCATGACA | GACCTCTCTACTCACTTCTCCAG |
| Mcad | AGGGTTTAGTTTTGAGTTGACGG | CCCCGCTTTTGTCATATTCCG |
| Acadvl | CTCTCCAAGGCTGTATGGACAAAG | CGACTTAACTCTGGGTGGACAATC |
| Acox1 | AGATTGGTAGAAATTGCTGCAAA | ACGCCACTTCCTTGCTCTTC |
| Cpt1 | CACCAACGGGCTCATCTTCTA | CAAAATGACCTAGCCTTCTATCGAA |
| Cpt2 | CAACTCGTATACCCAAACCCAGTC | GTTCCCATCTTGATCGAGGACATC |
| Il-1β | GCCACCTTTTGACAGTGATGAG | GACAGCCCAGGTCAAAGGTT |
| Il-6 | TTCCATCCAGTTGCCTTCTTG | GAAGGCCGTGGTTGTCACC |
| Tgfβ1 | CTCCCGTGGCTTCTAGTGC | GCCTTAGTTTGGACAGGATCTG |
| Adipoq | ACTGCAACTACCCATAGCCCAT | TGTCGACTGTTCCATGATTCTCC |
| Lep | GATGGACCAGACTCTGGCAG | AGAGTGAGGCTTCCAGGACG |
| Retn | AGCAGTGGGTGCTCAGTGC | CGTCATCCGTCACTCCATCC |
| 36B4 | AGATTCGGGATATGCTGTTGGC | TCGGGTCCTAGACCAGTGTTC |
| CypA | AGCACTGGGGAGAAAGGATT | CATGCCTTCTTTCACCTTCC |
| L-PK | GAACATTGCACGACTCAACTTC | CAGTGCGTATCTCGGGACC |
| Gck | TGAGCCGGATGCAGAAGGA | GCAACATCTTTACACTGGCCT |
Acc, acetyl-CoA carboxylase; Fasn, fatty acid synthase; Srebp, sterol regulatory element-binding protein; Lcad, long-chain acyl-CoA dehydrogenase; Mcad, medium-chain acyl-CoA dehydrogenase; Acadvl, acyl-CoA dehydrogenase, very-long; Acox1, acyl-CoA oxidase; Cpt, carnitine palmitoyltransferase; Tgfβ1, transforming growth factor-β; Adipoq, adiponectin; Lep, leptin; Retn, resistin; CypA, cyclophilin A; L-PK, liver pyruvate kinase; Gck, glucokinase.
Statistical analysis.
Comparisons were performed using two-tailed Student's t-tests with 95% confidence intervals. Values were considered significant at P < 0.05. For all data, P values are <0.05 and <0.01. All data are presented as means ± SE.
RESULTS
Generation of Ctrp3-KO mice.
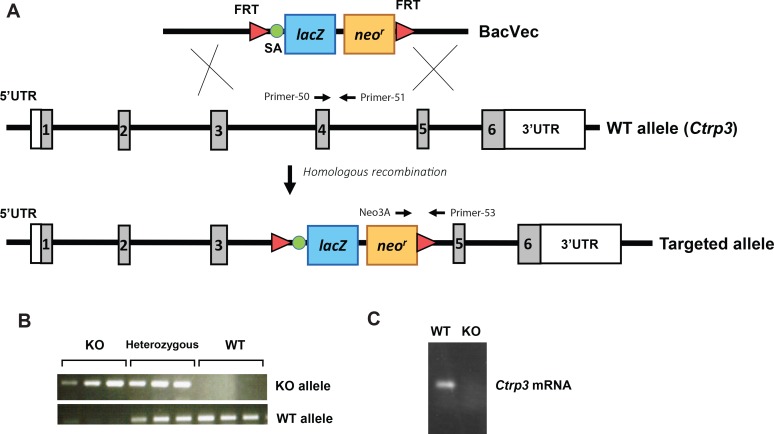
To obtain a null allele for Ctrp3, a 355-bp region of the Ctrp3 gene that spans exon 4 and part of intron 4 was deleted and replaced with a lacZ reporter and a neomycin resistance cassette (Fig. 1A). Exon 4 encodes 43 amino acids' contribution to a portion of the globular C1q domain. The presence of a splice acceptor upstream of the lacZ reporter ensures truncation and disruption of the Ctrp3 transcript to produce a null allele. Two sets of primers were designed to amplify a sequence spanning the upstream deletion site in lacZ and a sequence within the protein-coding region of WT Ctrp3. This enabled us to confirm the Ctrp3-heterozygous (+/−) and -KO (−/−) alleles (Fig. 1B). Since CTRP3 is robustly expressed in adipose tissue (65), we examined its expression in the epididymal fat pad of WT and Ctrp3-KO mice. No Ctrp3 mRNA was detected in the adipose tissue of Ctrp3-KO mice (Fig. 1C), confirming the loss-of-function mouse model. Among the different fat depots, visceral (epididymal) white adipose tissue expresses the highest levels of Ctrp3 compared with subcutaneous (inguinal) white adipose tissue or interscapular brown adipose tissue (not shown). Ctrp3 mRNA was not detected in any of these fat depots in the KO animals (not shown). The Ctrp3-KO mice were born at the expected Mendelian frequencies and were viable, fertile, and developed normally with no gross phenotype. Although CTRP3 is robustly expressed during development at embryonic day 15 onward (65), it was not required for proper embryonic development. We also did not observe any obvious skeletal abnormality in the Ctrp3-KO mice, although previously, a role for CTRP3 in skeletal and cartilage system was suggested based on its developmental expression patterns (70). Thus, CTRP3 did not appear to be essential for proper skeletal and cartilage development.
Fig. 1.
Generation of Clq/TNF-related protein (Ctrp3)-knockout (KO) mice. A: schematic showing the strategy for generating Ctrp3-KO mice by targeted deletion of exon 4/intron 4 of the mouse Ctrp3 gene and replacement with a lacZ reporter and a neomycin resistance cassette. The green circle represents a splice acceptor (SA) site, and the red triangle represents the Flp recombinase target (FRT) site recognized by Flp recombinase. B: genotyping results indicate the successful generation of Ctrp3-wild-type (WT) (+/+), heterozygous (+/−), and homozygous KO (−/−) alleles using the primer set (→ and ←) indicated in A. C: PCR analysis indicates the absence of detectable Ctrp3 mRNA in KO mice. BacVec, bacterial artificial chromosome vector; 5′-UTR, 5′-untranslated region.
Impact of CTRP3 deficiency on whole body metabolic parameters.
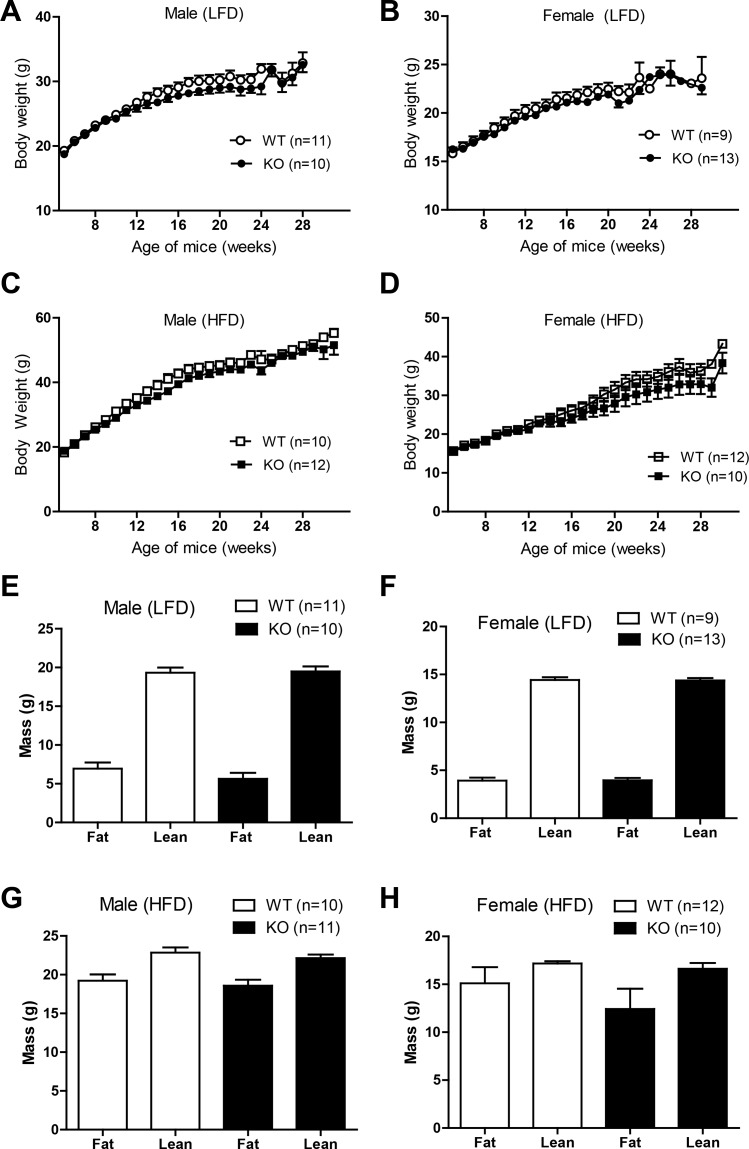
Given the importance and known effects of sex on metabolic outcome (10, 12, 34), we monitored the body weight gain over time of both male and female Ctrp3-WT and -KO mice fed either a control LFD or a HFD. Over a period of 25 wk, the body weights were indistinguishable between Ctrp3-WT and -KO mice irrespective of diet (Fig. 2, A–D); however, a modest decrease in weight gain was observed for the KO female mice fed a HFD compared with WT controls (Fig. 2D). Quantification of fat and lean mass using NMR (EchoMRI) also showed no differences between Ctrp3-WT and -KO male or female mice irrespective of diet (Fig. 2, E–H). Food intake was not different between LFD- and HFD-fed Ctrp3-WT or -KO male and female mice (Table 2). Indirect calorimetry analyses of metabolic rate (V̇o2), RER, EE, and physical activity levels over a 24-h period were similar between LFD- or HFD-fed Ctrp3-WT and -KO male and female mice (Table 2). These measurements also did not differ between WT and KO mice during the light or dark phases of the photocycle (not shown).
Fig. 2.
Body weight and body composition of Ctrp3-WT and -KO mice. A and B: body weight gain over time of WT and KO male and female mice fed a control low-fat diet (LFD). C and D: body weight gain over time of WT and KO male and female mice fed a high-fat diet (HFD). E and F: body composition analyses of fat and lean mass of LFD-fed WT and KO male and female mice. G and H: body composition analyses of fat and lean mass of HFD-fed WT and KO male and female mice. All data are expressed as means ± SE. Sample sizes are indicated.
Table 2.
Whole body metabolic parameters of LFD- and HFD-fed Ctrp3-WT and -KO mice over a 24-h period
| Male |
Female |
|||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| LFD | ||||
| Food intake, g | 4.64 ± 0.19 | 4.52 ± 0.29 | 4.29 ± 0.27 | 3.57 ± 0.29 |
| V̇o2, ml·kg−1·h−1 | 4,579 ± 128 | 4,632 ± 114 | 8,150 ± 318 | 8,563 ± 626 |
| V̇co2, ml·kg−1·h−1 | 4,477 ± 112 | 4,472 ± 110 | 8,039 ± 369 | 8,086 ± 535 |
| Respiratory exchange ratio | 0.9750 ± 0.007 | 0.9603 ± 0.009 | 0.9802 ± 0.015 | 0.9424 ± 0.017 |
| Energy expenditure, kcal·kg−1·h−1 | 22.9 ± 0.62 | 23.2 ± 0.56 | 26.83 ± 1.08 | 40.9 ± 1.65 |
| Physical activity (beam breaks) | 42,890 ± 3,986 | 48,660 ± 5,874 | 62,540 ± 3,951 | 47,890 ± 4,874 |
| HFD | ||||
| Food intake, g | 2.26 ± 0.07 | 2.27 ± 0.12 | 2.19 ± 0.14 | 2.84 ± 0.26 |
| V̇o2, ml·kg−1·h−1 | 4,432 ± 168 | 4,421 ± 104 | 5,675 ± 147 | 5,502 ± 193 |
| V̇co2, ml·kg−1·h−1 | 3,500 ± 125 | 3,349 ± 84 | 4,419 ± 129 | 4,369 ± 169 |
| Respiratory exchange ratio | 0.789 ± 0.004 | 0.7818 ± 0.004 | 0.7769 ± 0.005 | 0.7924 ± 0.009 |
| Energy expenditure, kcal·kg−1·h−1 | 21.2 ± 0.79 | 20.2 ± 0.50 | 27.1 ± 0.72 | 26.3 ± 0.93 |
| Physical activity (beam breaks) | 22,420 ± 1872 | 28,750 ± 1,586* | 36,730 ± 5,600 | 43,190 ± 6,489 |
All data are expressed as means ± SE. Ctrp3, C1q/tumor necrosis factor-related protein 3; WT, wild type; KO, knockout; LFD, low-fat diet; HFD, high-fat diet. Food intake in WT and KO male and female mice fed a LFD or HFD. Sample sizes were as follows: for the LFD-fed group, WT (n = 9) and KO (n = 9) male mice were used, and WT (n = 8) and KO (n = 8) female mice were used; for the HFD group, WT (n = 7) and KO (n = 7) male mice were used, and WT (n = 6) and KO (n = 5) female mice were used.
P < 0.05.
Impact of CTRP3 deficiency on glucose homeostasis.
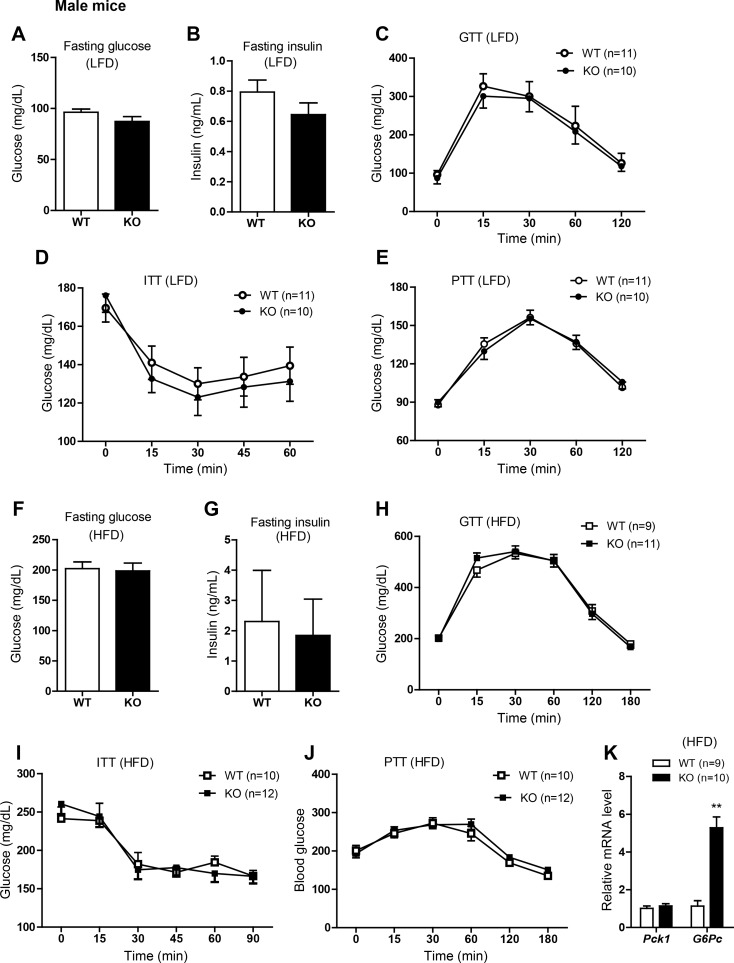
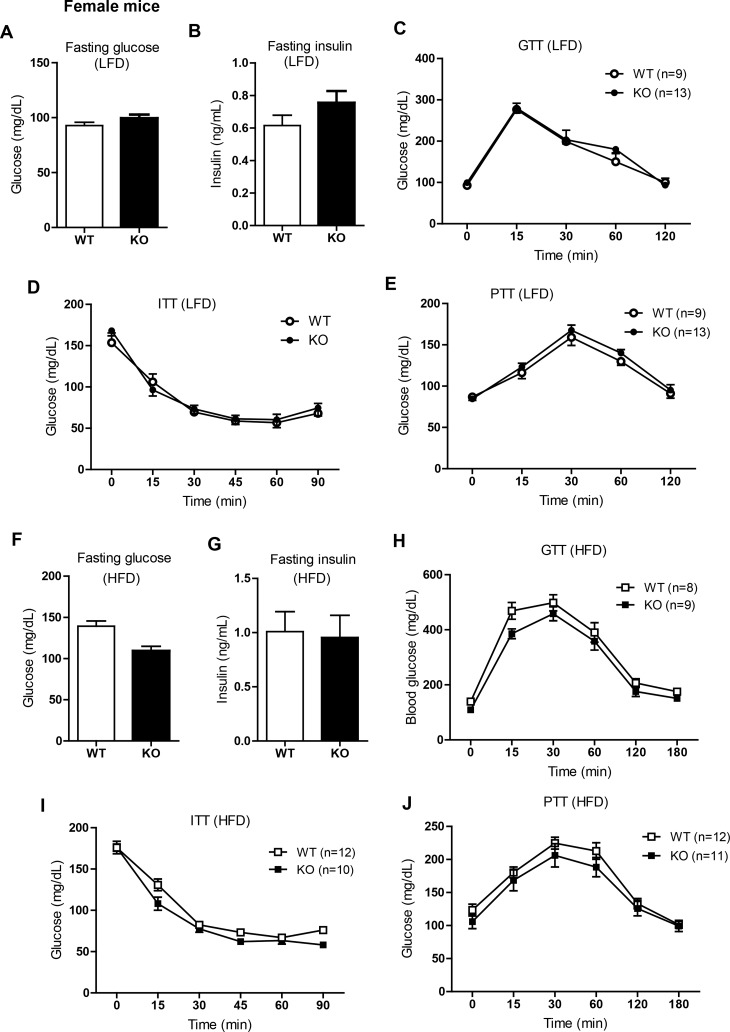
Next we examined the consequences of Ctrp3 deletion on glucose homeostasis. Fasting (16 h) glucose and insulin levels did not differ between WT and Ctrp3-KO male mice fed a LFD (Fig. 3, A and B) or HFD (Fig. 3, F and G). Assessment of whole body insulin sensitivity by intraperitoneal glucose and insulin tolerance tests revealed no differences between Ctrp3-WT and -KO male mice fed a LFD (Fig. 3, C and D) or HFD (Fig. 3, H and I). Insulin secretion in response to glucose injection was comparable between WT and KO male mice (not shown). Previously, a GLP-1 agonist (extendin-4) was shown to increase CTRP3 expression in visceral adipose tissue (29). To address whether gut-derived incretin hormones (e.g., GLP-1) influence glucose handling in Ctrp3-KO animals, we performed a GTT in which glucose was delivered via gavage in a separate cohort of male and female mice. No differences were observed in oral GTTs between genotypes in either sex (not shown). The magnitude of insulin secretion in response to glucose gavage was also not different between genotypes in either sex (not shown). We also performed a pyruvate tolerance test to assess hepatic insulin sensitivity. Overnight-fasted male mice were injected ip with pyruvate, which is a gluconeogenic substrate that can be converted into glucose in liver via gluconeogenesis. Since insulin suppresses hepatic glucose output, the degree of hepatic insulin resistance correlates with the magnitude of glucose production. The pyruvate tolerance test did not reveal any differences between Ctrp3-WT and -KO male mice fed a LFD (Fig. 3E) or HFD (Fig. 3J). Despite comparable hepatic glucose output in the fasted state, the mRNA expression of glucose-6-phosphatase (G6Pase), a gluconeogenic enzyme, was markedly upregulated in the liver of HFD-fed Ctrp3-KO male mice (Fig. 3K). Similar to male mice, female WT and KO mice consuming either LFD or HFD had comparable fasting blood glucose and insulin levels as well as glucose, insulin, and pyruvate tolerance (Fig. 4).
Fig. 3.
Glucose homeostasis in LFD- or HFD-fed Ctrp3-KO male mice. A and B: fasting blood glucose (A) and serum insulin levels (B) of LFD-fed WT and KO male mice. C: blood glucose levels of LFD-fed WT and KO male mice during the glucose tolerance test (GTT). Glucose was delivered by intraperitoneal injection. D: blood glucose levels of LFD-fed WT and KO male mice during the insulin tolerance test (ITT). E: blood glucose levels of LFD-fed WT and KO male mice during the pyruvate tolerance test (PTT). F and G: fasting blood glucose (F) and serum insulin levels (G) of HFD-fed WT and KO male mice. H: blood glucose levels of HFD-fed WT and KO male mice during the GTT. I: blood glucose levels of HFD-fed WT and KO male mice during the ITT. J: blood glucose levels of HFD-fed WT and KO male mice during the PTT. K: quantitative real-time PCR analysis of gluconeogenic gene (Pck1 and G6Pase) expression in the livers of WT and KO male mice. Expression levels were normalized to 36B4 [also known as ribosomal phosphoprotein P0 (RPLP0)]. All data are expressed as means ± SE. Sample sizes are indicated. **P < 0.01.
Fig. 4.
Glucose homeostasis in LFD- or HFD-fed Ctrp3-KO female mice. A and B: fasting blood glucose (A) and serum insulin levels (B) of LFD-fed WT and KO female mice. C: blood glucose levels of LFD-fed WT and KO female mice during the GTT. Glucose was delivered by intraperitoneal injection. D: blood glucose levels of LFD-fed WT and KO female mice during the ITT. Smaller sample size for ITT was due to hypoglycemia in some mice that had to be removed from the test. E: blood glucose levels of LFD-fed WT and KO female mice during the PTT. F and G: fasting blood glucose (F) and serum insulin levels (G) of HFD-fed WT and KO female mice. H: blood glucose levels of HFD-fed WT and KO female mice during the GTT. I: blood glucose levels of HFD-fed WT and KO female mice during the ITT. J: blood glucose levels of HFD-fed WT and KO female mice during the PTT. All data are expressed as means ± SE. Sample sizes are indicated.
CTRP3 deficiency alters liver size and triglyceride levels in HFD-fed mice.
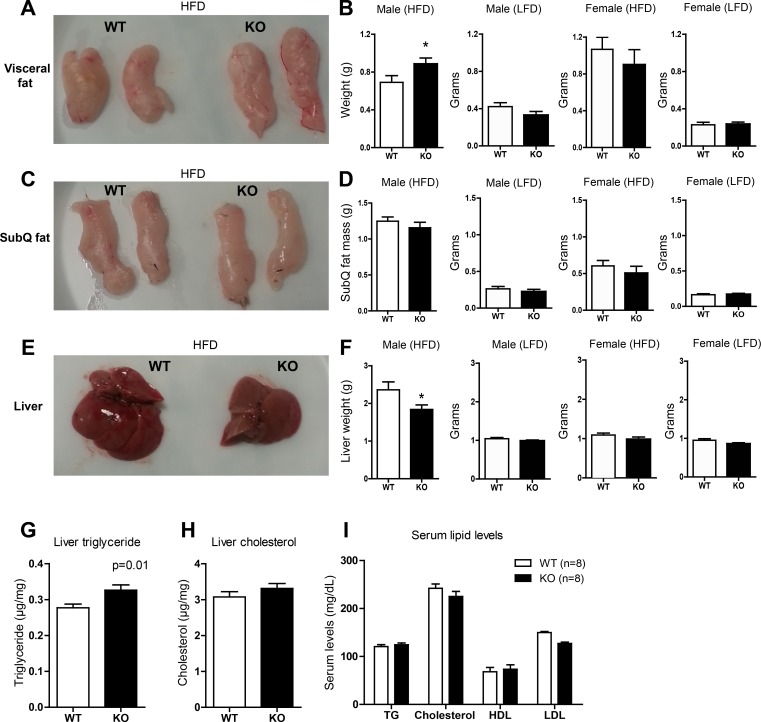
We next examined tissues isolated from Ctrp3-WT and -KO mice. In male and female mice that consumed a LFD, liver and visceral (epididymal) and subcutaneous (inguinal) adipose weights were not different between WT and KO mice (Fig. 5, B, D, and F). For the HFD-fed group, visceral but not subcutaneous fat depots were found to be larger in Ctrp3-KO mice compared with WT controls (Fig. 5, A–D). Adipose tissue histology revealed no apparent difference in adipocyte number and size between HFD-fed WT and KO male mice (not shown). However, liver size and weight was reduced significantly, by 28%, in CTRP3-deficient male mice (Fig. 5, E and F). Liver histology (with H & E stain) did not reveal obvious differences between WT and KO animals (not shown). Analysis of hepatic lipid contents, however, revealed a 13% increase in triglyceride (27.7 ± 3.7 vs. 32.6 ± 5.0 mg/g tissue), but not cholesterol, levels in Ctrp3-KO mice (Fig. 5, G and H). Serum cholesterol, triglycerides, and HDL and LDL levels did not differ significantly between the two groups of male mice (Fig. 5I).
Fig. 5.
Tissue weight, liver lipid content, and serum lipid profiles of LFD- and HFD-fed Ctrp3-WT and -KO mice. A: representative image of visceral (epididymal) fat pads isolated from HFD-fed WT and KO male mice. B: quantification of visceral fat pad weight in WT and KO male and female mice fed HFD and LFD. C: representative image of subcutaneous (SubQ; inguinal) fat pads isolated from HFD-fed WT and KO male mice. D: quantification of SubQ fat pad weight in WT and KO male and female mice fed HFD and LFD. E: representative image of liver isolated from HFD-fed WT and KO male mice. F: quantification of liver weight in WT and KO male and female mice fed a HFD or LFD. G and H: quantification of liver triglyceride (G) and cholesterol (H) levels in HFD-fed WT and KO male mice. I: quantification of serum cholesterol, triglyceride (TG), HDL, and LDL levels in HFD-fed WT and KO male mice. All data are expressed as means ± SE. *P < 0.05. Sample size: for the LFD-fed group, WT (n = 11) and KO (n = 10) male mice and WT (n = 9) and KO (n = 13) female mice were used; for the HFD group, WT (n = 10) and KO (n = 12) male mice and WT (n = 12) and KO (n = 10) female mice were used.
CTRP3 deficiency alters hepatic expression of lipid synthesis and oxidation genes in HFD-fed mice.
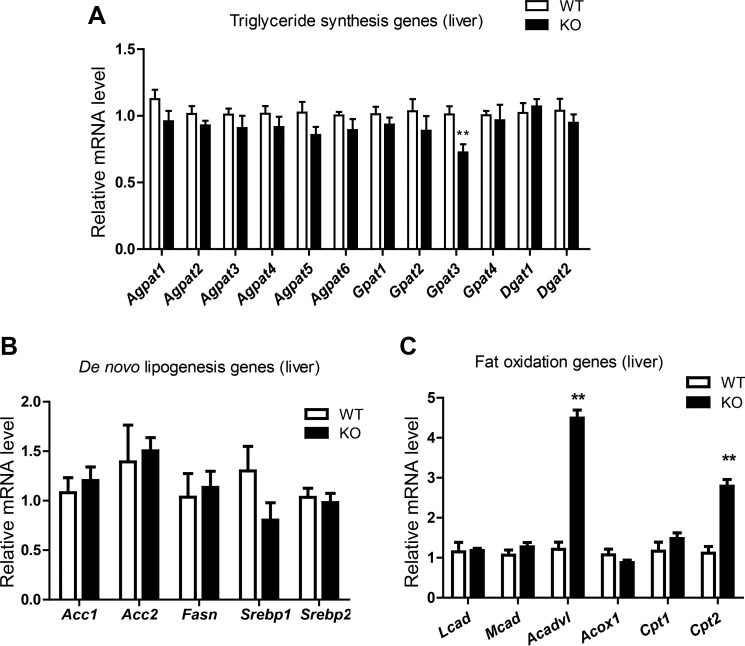
Because liver size is different between WT and KO male mice, and hepatic triglyceride levels were higher in Ctrp3-KO male mice relative to WT controls, we chose to measure the expression of lipid synthesis and oxidation genes in livers of male mice only. In liver, triglyceride is synthesized via the glycerol-phosphate pathway (4) through sequential acylation of glycerol-3-phosphate, lysophosphatidic acid, and diacylglycerol by multiple isoforms of GPAT, AGPAT, and DGAT enzymes (53, 68). Of all the Gpat, Agpat, and Dgat genes involved in triglyceride synthesis, only Gpat3 expression was found to be slightly reduced in Ctrp3-KO mice relative to WT controls (Fig. 6A). Hepatic expression of genes involved in de novo fatty acid synthesis [fatty acid synthase (Fasn), sterol regulatory element-binding protein 1 (Srebp1), acetyl-CoA carboxylase 1 (Acc1), and Acc2] and cholesterol synthesis (Srebp2) was not different between WT and KO animals (Fig. 6B). In contrast, hepatic expression of two genes involved in fat oxidation (Acadvl and Cpt2) was found to be significantly upregulated in Ctrp3-KO male mice relative to WT controls (Fig. 6C). These results were obtained from overnight-fasted mouse livers. Since lipid synthesis is markedly increased in the fed state, we performed fasting/refeeding on a different cohort of male and female mice. In this group, mice were fasted overnight (∼18 h) and refed for 4 h before liver was harvested for gene expression analysis. Expression of glycolytic (L-PK, Gck) and several key lipid synthesis genes (Srebp1, Srebp2, Fasn, Acc1, and Gpat3) was not different between genotypes in either sex (not shown). The exception was Acc2, in which Ctrp3-KO male but not female mice had significantly higher expression in the fed state compared with WT controls (not shown).
Fig. 6.
Altered hepatic expression of lipid synthesis and fat oxidation genes in HFD-fed Ctrp3-KO mice. Quantitative real-time PCR analysis of triglyceride synthesis (A), de novo lipid synthesis (B), and fat oxidation genes (C) in the livers of HFD-fed WT (n = 10) and KO (n = 10) male mice. Tissues were harvested from overnight-fasted mice. Expression levels were normalized to 36B4 (also known as RPLP0). All data are expressed as means ± SE. **P < 0.01. Agpat, Acyl glycerol phosphate acyltransferase; Gpat, glycerol phosphate acyltransferase; Dgat, diacylglycerol acyltransferase; Acc, acetyl-CoA carboxylase; Fasn, fatty acid synthase; Srebp, sterol responsive element-binding protein; Lcad, long-chain acyl-CoA dehydrogenase; Mcad, medium-chain acyl-CoA dehydrogenase; Acox, acyl-CoA oxidase; Cpt, carnitine palmitoyl transferase.
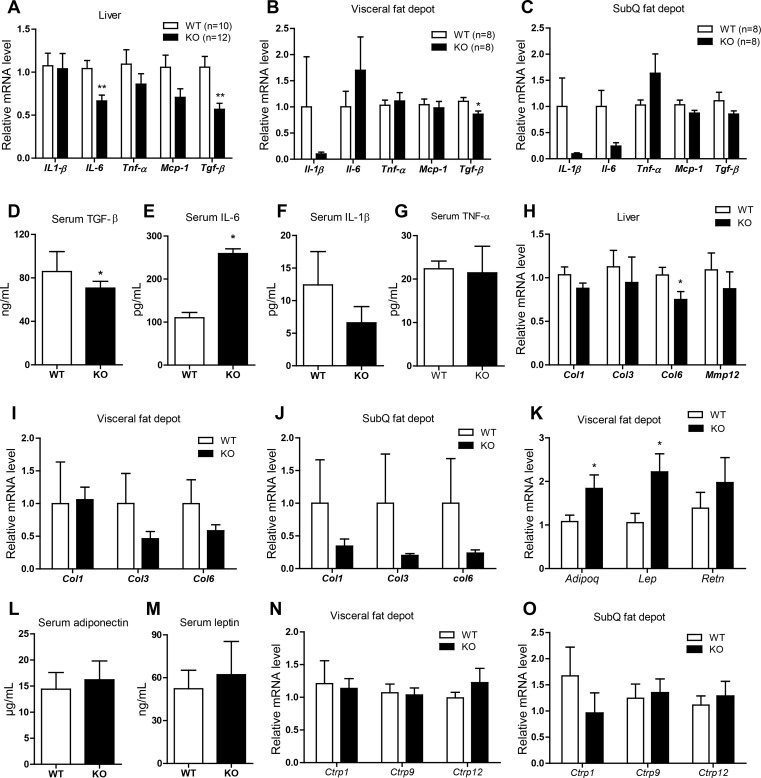
CTRP3 deficiency alters the expression and circulating levels of cytokines in HFD-fed mice.
In the obese state, adipose tissue and the infiltrating macrophages secrete a variety of cytokines that promote a state of low-grade inflammation (16). CTRP3 has been shown to have anti-inflammatory and antifibrotic properties (22, 23, 48), and therefore, we sought to examine whether CTRP3 deficiency alters the expression and circulating levels of cytokines. In liver, the mRNA expression of Il-6 (a proinflammatory cytokine) and Tgfβ1 (a profibrotic cytokine) was reduced in Ctrp3-KO male mice relative to WT controls (Fig. 7A). In adipose tissue, the mRNA expression of Tgfβ1, but not Il-1β, Il-6, Mcp-1, or Tnfα, was reduced in the visceral (epididymal) fat depot of Ctrp3-KO male mice relative to WT controls (Fig. 7B). In the subcutaneous (inguinal) fat depot, expression of Il-1β and Il-6 was decreased (Fig. 7C). Consistent with the mRNA data, the serum level of TGFβ1 was significantly reduced in Ctrp3-KO male mice (Fig. 7D). However, in contrast to the mRNA data in liver and subcutaneous fat depot, the circulating level of IL-6 was increased in Ctrp3-KO male mice (Fig. 7E). Serum levels of IL-β and TNFα were not different between the two groups (Fig. 7, F and G). Because TGFβ is a profibrotic cytokine, we also assessed the mRNA expression of several important fibrotic genes (Col1, Col3, Col3, and Mmp12) in the liver and adipose tissue. We found that Col6 expression was significantly reduced in the liver (Fig. 7H). Expression of collagen genes also appeared to be lower in adipose tissue (Fig. 7, I and J), although the data did not reach statistical significance due to large variations in expression values. Despite reduced expression and circulating levels of TGFβ1, liver tissue sections stained for fibrotic collagen with Masson Trichrome Blue and Sirius Red did not reveal apparent differences in the degree of fibrosis between WT and KO mice that consumed an HFD (not shown).
Fig. 7.
Altered expression and circulating levels of cytokines and adipokines in HFD-fed Ctrp3-KO mice. A–C: quantitative real-time PCR analysis of cytokine gene expression in liver (A), visceral (epididymal) fat depot (B), and SubQ (inguinal) fat depot (C) of WT (n = 8–10) and KO (n = 8–12) male mice. Expression levels were normalized to 36B4 (also known as RPLP0). D–G: quantification of serum transforming growth factor-β1 (TGFβ1; D), IL-6 (E), IL-1β (F), and TNFα (G) levels in WT (n = 8–9) and KO (n = 8–11) male mice. H–J: quantitative real-time PCR analysis of fibrotic gene expression in the liver (H), visceral (epididymal; I), and SubQ (inguinal; J) of WT (n = 10) and KO (n = 10) male mice. K: quantitative real-time PCR analysis of adipokine gene expression in the visceral (epididymal) fat depot of WT (n = 8) and KO (n = 8) male mice. Expression levels were normalized to cyclophilin A. L and M: quantification of serum adiponectin (L) and leptin (M) levels in WT (n = 9) and KO (n = 10) male mice. N and O: quantitative real-time PCR analysis of Ctrp1, Ctrp9, and Ctrp12 gene expression in the visceral (epididymal; N) and SubQ (inguinal; O) fat depot of WT (n = 10) and KO (n = 10) male mice. All data are expressed as means ± SE. *P < 0.05; **P < 0.01. Mcp-1, monocyte chemotactic protein 1; Col, collagen; Mmp12, matrix metalloprotease 12; Adipoq, adiponectin; Lep, leptin; Retn, resistin.
CTRP3 deficiency alters the expression, but not circulating levels, of adipokines in HFD-fed mice.
In addition to cytokines, we also measured the expression and circulating levels of leptin, adiponectin, and resistin, well-known adipokines that play important roles in metabolic homeostasis (11, 17, 24). Although the mRNA expression of leptin and adiponectin was upregulated in the visceral (epididymal) fat depot of Ctrp3-KO male mice relative to WT controls (Fig. 7K), their circulating serum levels were not different between the two groups (Fig. 7, L and M). To further rule out potential compensation by other related CTRP family members in CTRP3-deficient mice, we analyzed the adipose expression of Ctrp1, Ctrp9, and Ctrp12, which we have shown previously to have positive metabolic functions in vivo (38, 41, 57, 59). In both visceral (epididymal) and subcutaneous (inguinal) fat depots, the expression of Ctrp1, Ctrp9, and Ctrp12 was not different between WT and Ctrp3-KO mice (Fig. 7, N and O).
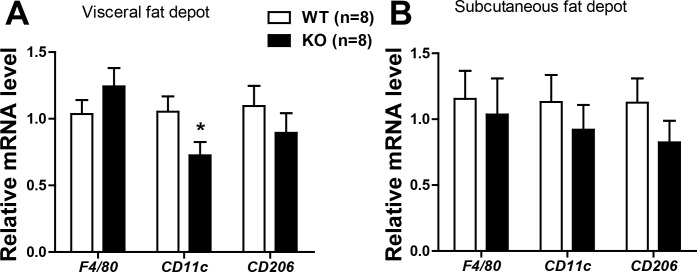
CTRP3 deficiency reduces M1-type macrophage infiltration into white adipose tissue of HFD-fed mice.
Low-grade inflammation within the fat pad is frequently observed in obesity due to macrophage infiltration (14, 16, 62, 67). We did not observe any difference in visceral (epididymal) and subcutaneous (inguinal) adipose tissue macrophages, as judged by the pan-macrophage marker F4/80 levels (Fig. 8). However, the proinflammatory M1-type macrophages were reduced in the visceral fat pad of Ctrp3-KO mice, as judged by the expression of CD11c (Fig. 8). The expression of CD206, an M2-type macrophage marker, was not different between the two groups of mice (Fig. 8).
Fig. 8.
Expression of macrophage markers in the fat pads of HFD-fed Ctrp3-KO mice. A and B: quantitative real-time PCR analysis of pan-macrophage marker (F4/80), M1-type macrophage marker (Cd11c), and M2-type macrophage marker (CD206) in the visceral (epididymal; A) and subcutaneous (inguinal; B) fat depots of WT (n = 8) and KO (n = 8) male mice. Expression levels were normalized to β-actin. All data are expressed as means ± SE. *P < 0.05.
DISCUSSION
This is the first study seeking to address the physiological requirement of CTRP3 for normal metabolic homeostasis and in response to metabolic stress induced by high-fat feeding. In light of the myriad functions demonstrated for CTRP3 based on recombinant protein supplementation and overexpression in rodents, our current loss-of-function studies using Ctrp3-KO mice help provide critical insights into the functional significance of this secreted plasma protein that exhibits reduced circulating levels in rodent models of obesity and diabetes (29, 42) as well as in obese and diabetic humans (3, 9, 63, 71). Based on our previous studies using recombinant protein infusion and transgenic overexpression in mice (40, 42), we expected the Ctrp3-KO mice to exhibit impaired energy balance. Contrary to expectations, loss of CTRP3 in either male or female mice appears to have minimal or no impact on body weight, total adiposity, food intake, metabolic rate, energy expenditure, or physical activity levels regardless of whether mice consumed a control LFD or were challenged with a calorie-dense HFD.
Previously, we showed that recombinant CTRP3 administration lowers blood glucose by suppressing hepatic gluconeogenic gene expression and glucose output (42). Thus, we expected the Ctrp3-KO mice to have impaired glucose homeostasis. Contrary to our expectations, loss of CTRP3 in male or female mice did not alter whole body insulin sensitivity or the capacity to handle a glucose challenge compared with WT littermate controls. Also, a pyruvate tolerance test did not reveal any differences in hepatic insulin sensitivity and liver capacity to carry out gluconeogenesis in response to an overnight fast in WT and CTRP3-deficient mice. Although glucose metabolism in liver was not different, hepatic expression of glucose-6-phosphatase (G6Pc), a critical gluconeogenic gene, was significantly upregulated (∼5-fold) in the Ctrp3-KO mice relative to WT controls. In contrast, liver expression of G6Pc was profoundly suppressed in WT male mice injected with recombinant CTRP3 (42) or in transgenic animals overexpressing CTRP3 (40); reduced G6Pc expression in these previously published mouse models also correlated with decreased fasting blood glucose. Despite strikingly upregulated hepatic G6Pc expression in the HFD-fed Ctrp3-KO male mice, fasting-induced gluconeogenesis (as judged by fasting blood glucose levels) did not differ from WT controls. Although endogenous glucose production and hepatic gluconeogenic gene expression are generally linked and both are upregulated in diabetic state (13, 33, 72), additional mechanisms, besides transcriptional control of gluconeogenic genes, may also be involved in regulating hepatic glucose output in response to fasting or diabetes (46).
One of the most striking phenotypes in HFD-fed Ctrp3-KO male mice was the marked reduction (∼28%) in liver size compared with WT controls. Although the overall size of the liver was reduced significantly, histological analysis of paraffin-embedded liver tissue sections (stained with H & E or Sirius Red) did not reveal any apparent difference between WT and KO mice in terms of overall tissue architecture and the degree of steatosis or fibrosis in response to HFD. In many other knockout mouse models studied in the context of diet-induced obesity, differences in liver size generally resulted from differences in hepatic lipid content and the severity of fatty liver. Contrary to expectations, when liver triglyceride content was quantified and normalized to tissue weight, the Ctrp3-KO mice were found to have 13% higher hepatic triglyceride content compared with WT controls. Thus, the reduced liver size seen in the KO mice cannot be attributed to reduced hepatic triglyceride levels. Liver cholesterol levels were not different between the two groups, nor were the serum levels of triglycerides, cholesterol, HDL, or LDL. The increase in hepatic triglyceride content is not due to increased triglyceride synthesis, as none of the enzymes or their isoforms (GPATs, AGPATs, DGATs), except for Gpat3, were altered in the KO animals. Expression of genes involved in de novo lipogenesis (e.g., Acc1, Fasn, and Srebp) was also not different between the two groups in either the fasted or fed states. Some, but not all, fat oxidation genes (e.g., Cpt2 and Acadvl) were found to be significantly upregulated in the overnight-fasted Ctrp3 KO mice. Since V̇o2 and RER were not different between WT and KO mice, upregulation of fat oxidation genes in the liver of Ctrp3-KO mice was not sufficient to alter whole body substrate utilization and metabolism.
Chronic consumption of an HFD disrupts the proper function of liver and adipose tissue by promoting low-grade inflammation (14, 16, 62, 67). The recruitment of proinflammatory macrophages into adipose tissue in the obese state results in local production and systemic elevation of inflammatory cytokines such as IL-1β, IL-6, and TNFα, which contributes to and exacerbates peripheral tissue insulin resistance and dysfunction (16). Previous studies by others have demonstrated an anti-inflammatory role for CTRP3 (22, 23, 48). This prompted us to examine more closely the inflammatory profiles of the HFD-fed Ctrp3-KO mice. In obese Ctrp3-KO mice, we observed reduced mRNA expression of Il-6, but not Il-1β and Tnfα, in the liver and subcutaneous (inguinal) fat depot relative to WT controls. Surprisingly, serum IL-6 levels were significantly elevated (∼2.7-fold) in the Ctrp3-KO animals compared with WT controls, in striking contrast to IL-6's reduced mRNA expression in the liver and adipose tissue. We presumed that the visceral fat depot, with higher expression of Il-6, and/or circulating immune cells may be the source of elevated serum IL-6 levels in Ctrp3-KO mice compared with WT controls. Although IL-6 is classically considered to be an acute-phase protein and inflammatory cytokine in the context of immunity (52), both beneficial and adverse metabolic effects of IL-6 have also been demonstrated in various mouse models (19–21, 45, 56). Regardless, the elevated circulating IL-6 levels we observed in the Ctrp3-KO mice were not sufficient to have an impact on insulin sensitivity and systemic energy metabolism.
The ability of adipose tissue to expand in response to excess caloric intake is crucial for the proper storage of triglycerides, the failure of which can result in aberrant lipid deposition in nonadipose tissues (e.g., liver and skeletal muscle), leading to insulin resistance (55). Obesity-linked fibrotic response diminishes adipose tissue expandability and its capacity to manage excess dietary lipids (18, 51). TGFβ1 is a major secreted cytokine that modulates inflammation and tissue fibrosis (25, 44). Recent in vitro studies showed that CTRP3 can inhibit TGFβ1 expression and action and thus may have antifibrotic properties (15, 30). However, in a physiological context, we observed the opposite result. Both the mRNA expression of TGFβ1 in liver and the visceral fat depot, as well as its corresponding serum levels, were reduced in the Ctrp3-KO mice relative to WT controls. Since TGFβ1 is a potent inducer of the fibrotic program, expression of fibrotic collagen genes (e.g., Col1, Col3, and Col6) in liver and adipose tissue was also reduced in the Ctrp3-KO mice. None of these changes at the local and systemic level appear to be sufficient to alter the degree of fibrosis in adipose and liver induced by HFD, as judged by histological analysis and gene expression analysis. Furthermore, these changes were also not sufficient to have an impact on whole body glucose or lipid metabolism.
The surprising lack of overt metabolic phenotypes in the absence of CTRP3 raises the possibility of physiological compensation. To rule out whether the mild phenotype of Ctrp3-KO mice could be the result of metabolic compensation by other adipokines, we examined the expression and serum levels of adiponectin, leptin, and resistin, three well-studied adipose-derived hormones with important metabolic functions in the liver and skeletal muscle (11, 17, 24). Although the mRNA expression of adiponectin and leptin was upregulated in the visceral fat depots of Ctrp3-KO mice, circulating levels of these adipokines were not different between WT and KO animals. When we examined the adipose expression of related CTRP family members (Ctrp1, Ctrp9, and Ctrp12) with known metabolic function in vivo (38, 41, 57, 59), we did not observe any differences between WT and Ctrp3-KO mice. Our results thus suggest a general lack of compensation by other secreted metabolic regulators when CTRP3 is absent. It should be noted that an absence of overt metabolic phenotypes seen in the Ctrp3-KO mice does not, in principle, negate its potential beneficial metabolic properties when recombinant protein is administered in vivo, as illustrated by its ability to markedly reduce hepatic triglyceride levels in obese WT mice with existing liver steatosis (40). Elevating plasma levels of CTRP3 can also have beneficial cardiac effects in conditions of ischemic heart attack (69). There are obvious limitations in our current study in which we employ a whole body KO mouse model deficient in CTRP3 protein throughout development and in adult animals. A conditional KO mouse model in which the Ctrp3 gene can be deleted in specific tissue or only in adult animals may reveal additional metabolic phenotypes.
In summary, we conclude that CTRP3 is largely dispensable for physiological control of metabolic homeostasis under normal and pathophysiological states of obesity. Instead, our findings reveal a previously unsuspected sex-dependent role for CTRP3 in modulating liver size and circulating proinflammatory IL-6 and profibrotic TGFβ1 levels in response to HFD-induced obesity.
GRANTS
This work was supported by grants from the National Institutes of Health (NIH; DK-084171 to G. W. Wong). R. M. Wolf was supported by a Lily Endocrine Scholars Award and the Pediatric Endocrine Society Research Fellowship Award. Z. -C. Yang was supported by a visiting scientist fellowship from the China Scholarship Council. X. Lei was supported by a postdoctoral award from the American Heart Association. S. Y. Tan was supported by an NIH predoctoral training grant (T32-GM-007445) and a predoctoral fellowship from the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.W. and G.W.W. conception and design of research; R.M.W., X.L., Z.-C.Y., M.N., and S.Y.T. performed experiments; R.M.W., X.L., Z.-C.Y., M.N., and G.W.W. analyzed data; R.M.W., X.L., and G.W.W. interpreted results of experiments; R.M.W. and X.L. prepared figures; R.M.W. and G.W.W. drafted manuscript; R.M.W., X.L., S.Y.T., and G.W.W. edited and revised manuscript; R.M.W., X.L., Z.-C.Y., S.Y.T., and G.W.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Susan Aja for help with indirect calorimetry.
REFERENCES
- 1.Akiyama H, Furukawa S, Wakisaka S, Maeda T. CTRP3/cartducin promotes proliferation and migration of endothelial cells. Mol Cell Biochem 304: 243–248, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H, Furukawa S, Wakisaka S, Maeda T. Elevated expression of CTRP3/cartducin contributes to promotion of osteosarcoma cell proliferation. Oncol Rep 21: 1477–1481, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Ban B, Bai B, Zhang M, Hu J, Ramanjaneya M, Tan BK, Chen J. Low serum cartonectin/CTRP3 concentrations in newly diagnosed type 2 diabetes mellitus: in vivo regulation of cartonectin by glucose. PLoS One 9: e112931, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem 49: 459–487, 1980. [DOI] [PubMed] [Google Scholar]
- 5.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59: 323–329, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byerly MS, Petersen PS, Ramamurthy S, Seldin MM, Lei X, Provost E, Wei Z, Ronnett GV, Wong GW. C1q/TNF-related protein 4 (CTRP4) is a unique secreted protein with two tandem C1q domains that functions in the hypothalamus to modulate food intake and body weight. J Biol Chem 289: 4055–4069, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byerly MS, Swanson R, Wei Z, Seldin MM, McCulloh PS, Wong GW. A central role for C1q/TNF-related protein 13 (CTRP13) in modulating food intake and body weight. PLoS One 8: e62862, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, Yoo HJ, Lee KW, Nam MS, Park YS, Woo JT, Kim YS, Choi DS, Youn BS, Baik SH. C1q/TNF-related protein-3 (CTRP-3) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes 61: 2932–2936, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng W, Li C, Zhang Y, Zhao J, Yang M, Tian M, Li L, Zheng Y, Chen B, Yang G. Serum C1q/TNF-related protein-3 (CTRP3) levels are decreased in obesity and hypertension and are negatively correlated with parameters of insulin resistance. Diabetol Metab Syndr 7: 33, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 361: 1152–1163, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Gui Y, Silha JV, Murphy LJ. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes Res 12: 1481–1491, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Haber BA, Chin S, Chuang E, Buikhuisen W, Naji A, Taub R. High levels of glucose-6-phosphatase gene and protein expression reflect an adaptive response in proliferating liver and diabetes. J Clin Invest 95: 832–841, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab 92: 2240–2247, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann C, Chen N, Obermeier F, Paul G, Buchler C, Kopp A, Falk W, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis 17: 2462–2471, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS. Inflammation and metabolic disorders. Nature 444: 860–867, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol 29: 1575–1591, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, Kim JK. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53: 1060–1067, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology 146: 3417–3427, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes 52: 2784–2789, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kopp A, Bala M, Buechler C, Falk W, Gross P, Neumeier M, Scholmerich J, Schaffler A. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 151: 5267–5278, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Kopp A, Bala M, Weigert J, Buchler C, Neumeier M, Aslanidis C, Scholmerich J, Schaffler A. Effects of the new adiponectin paralogous protein CTRP-3 and of LPS on cytokine release from monocytes of patients with type 2 diabetes mellitus. Cytokine 49: 51–57, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Lazar MA. Resistin- and Obesity-associated metabolic diseases. Horm Metab Res 39: 710–716, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Lei X, Li Q, Rodriguez S, Tan SY, Seldin MM, McLenithan JC, Jia W, Wong GW. Thromboxane synthase deficiency improves insulin action and attenuates adipose tissue fibrosis. Am J Physiol Endocrinol Metab 308: E792–E804, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Jiang L, Yang M, Wu YW, Sun JZ, Sun SX. CTRP3 improves the insulin sensitivity of 3T3-L1 adipocytes by inhibiting inflammation and ameliorating insulin signalling transduction. Endokrynologia Polska 65: 252–258, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Jiang L, Yang M, Wu YW, Sun SX, Sun JZ. CTRP3 modulates the expression and secretion of adipokines in 3T3-L1 adipocytes. Endocr J J 61: 1153–1162, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Jiang L, Yang M, Wu YW, Sun SX, Sun JZ. Expression of CTRP3, a novel adipokine, in rats at different pathogenic stages of type 2 diabetes mellitus and the impacts of GLP-1 receptor agonist on it. J Diabetes Res 2014: 398518, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin S, Ma S, Lu P, Cai W, Chen Y, Sheng J. Effect of CTRP3 on activation of adventitial fibroblasts induced by TGF-beta1 from rat aorta in vitro. Int J Clin Exp Pathol 7: 2199–2208, 2014. [PMC free article] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Maeda T, Abe M, Kurisu K, Jikko A, Furukawa S. Molecular cloning and characterization of a novel gene, CORS26, encoding a putative secretory protein and its possible involvement in skeletal development. J Biol Chem 276: 3628–3634, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest 90: 1323–1327, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev 5: 197–216, 2004. [DOI] [PubMed] [Google Scholar]
- 35.McLean JA, Tobin G. Animal and Human Calorimetry. New York: Cambridge University, 1987. [Google Scholar]
- 36.Murayama MA, Kakuta S, Maruhashi T, Shimizu K, Seno A, Kubo S, Sato N, Saijo S, Hattori M, Iwakura Y. CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun 443: 42–48, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Otani M, Kogo M, Furukawa S, Wakisaka S, Maeda T. The adiponectin paralog C1q/TNF-related protein 3 (CTRP3) stimulates testosterone production through the cAMP/PKA signaling pathway. Cytokine 58: 238–244, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Peterson JM, Aja S, Wei Z, Wong GW. C1q/TNF-related protein-1 (CTRP1) enhances fatty acid oxidation via AMPK activation and ACC inhibition. J Biol Chem 287: 1576–1587, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson JM, Seldin MM, Tan SY, Wong GW. CTRP2 overexpression improves insulin and lipid tolerance in diet-induced obese mice. PLoS One 9: e88535, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson JM, Seldin MM, Wei Z, Aja S, Wong GW. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am J Physiol Gastrointest Liver Physiol 305: G214–G224, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson JM, Wei Z, Seldin MM, Byerly MS, Aja S, Wong GW. CTRP9 transgenic mice are protected from diet-induced obesity and metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 305: R522–R533, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson JM, Wei Z, Wong GW. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J Biol Chem 285: 39691–39701 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson JM, Wei Z, Wong GW. CTRP8 and CTRP9B are novel proteins that hetero-oligomerize with C1q/TNF family members. Biochem Biophys Res Commun 388: 360–365, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Pohlers D, Brenmoehl J, Loffler I, Muller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G. TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta 1792: 746–756, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Ruderman NB, Keller C, Richard AM, Saha AK, Luo Z, Xiang X, Giralt M, Ritov VB, Menshikova EV, Kelley DE, Hidalgo J, Pedersen BK, Kelly M. Interleukin-6 regulation of AMP-activated protein kinase. Potential role in the systemic response to exercise and prevention of the metabolic syndrome. Diabetes 55, Suppl 2: S48–S54, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, Gerhard GS, Shulman GI. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc Natl Acad Sci USA 106: 12121–12126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270: 26746–26749, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Schmid A, Kopp A, Hanses F, Karrasch T, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem Biophys Res Commun 452: 8–13, 2014. [DOI] [PubMed] [Google Scholar]
- 49.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287: 11968–11980 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seldin MM, Tan SY, Wong GW. Metabolic function of the CTRP family of hormones. Rev Endocr Metab Disord 15: 111–123, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 18: 470–477, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 15: 797–819, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab 296: E1195–E1209, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan BK, Chen J, Hu J, Amar O, Mattu HS, Adya R, Patel V, Ramanjaneya M, Lehnert H, Randeva HS. Metformin increases the novel adipokine cartonectin/CTRP3 in women with polycystic ovary syndrome. J Clin Endocrinol Metab 98: E1891–E1900, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801: 209–214, 2010. [DOI] [PubMed] [Google Scholar]
- 56.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8: 75–79, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Wei Z, Lei X, Petersen PS, Aja S, Wong GW. Targeted deletion of C1q/TNF-related protein 9 increases food intake, decreases insulin sensitivity, and promotes hepatic steatosis in mice. Am J Physiol Endocrinol Metab 306: E779–E790, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei Z, Lei X, Seldin MM, Wong GW. Endopeptidase cleavage generates a functionally distinct isoform of C1q/tumor necrosis factor-related protein-12 (CTRP12) with an altered oligomeric state and signaling specificity. J Biol Chem 287: 35804–35814, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei Z, Peterson JM, Lei X, Cebotaru L, Wolfgang MJ, Baldeviano GC, Wong GW. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem 287: 10301–10315, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei Z, Peterson JM, Wong GW. Metabolic regulation by C1q/TNF-related protein-13 (CTRP13): activation OF AMP-activated protein kinase and suppression of fatty acid-induced JNK signaling. J Biol Chem 286: 15652–15665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei Z, Seldin MM, Natarajan N, Djemal DC, Peterson JM, Wong GW. C1q/tumor necrosis factor-related protein 11 (CTRP11), a novel adipose stroma-derived regulator of adipogenesis. J Biol Chem 288: 10214–10229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolf RM, Steele KE, Peterson LA, Magnuson TH, Schweitzer MA, Wong GW. Lower Circulating C1q/TNF-Related Protein-3 (CTRP3) Levels Are Associated with Obesity: A Cross-Sectional Study. PLoS One 10: e0133955, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J 23: 241–258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 416: 161–177, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci USA 101: 10302–10307, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49: 2283–2301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, Wang Y, Shang X, Gao E, Koch WJ, Ma XL. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation 125: 3159–3169, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yokohama-Tamaki T, Maeda T, Tanaka TS, Shibata S. Functional analysis of CTRP3/cartducin in Meckel's cartilage and developing condylar cartilage in the fetal mouse mandible. J Anat 218: 517–533, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoo HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Choi DS, Baik SH, Bluher M, Youn BS, Choi KM. Implication of progranulin and C1q/TNF-related protein-3 (CTRP3) on inflammation and atherosclerosis in subjects with or without metabolic syndrome. PLoS One 8: e55744, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413: 131–138, 2001. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Y, Wang JY, Feng H, Wang C, Li L, Wu D, Lei H, Li H, Wu LL. Overexpression of c1q/tumor necrosis factor-related protein-3 promotes phosphate-induced vascular smooth muscle cell calcification both in vivo and in vitro. Arterioscler Thromb Vasc Biol 34: 1002–1010, 2014. [DOI] [PubMed] [Google Scholar]