Abstract
We evaluated normal tissue specific radioprotection of the oral cavity in radiosensitive Fanconi Anemia (FA) Fancd2−/−mice with orally established tumors using mitochondrial-targeted GS-nitroxide (JP4-039). Adult (10–12 weeks old) Fancd2+/+, Fancd2+/− and Fancd2−/− mice (C57BL/6 background) and subgroups with orally established TC-1 epithelial cell tumors received a single fraction of 28 Gy or four daily fractions of 8 Gy to the head and neck. Subgroups received JP4-039 in F15 emulsion (F15/JP4-039; 0.4 mg/mouse), 4-amino-Tempo in F15 emulsion (F15/4-amino-Tempo; 0.2 mg/ mouse) or F15 emulsion alone prior to each irradiation. Oral mucosa of Fancd2−/− mice showed baseline elevated RNA transcripts for Sod2, p53, p21 and Rad51 (all P < 0.0012) and suppressed levels of Nfkb and Tgfb, (all P < 0.0020) compared with Fancd2+/+ mice. The oral mucosa in tumor-bearing mice of all genotypes showed decreased levels of p53 and elevated Tgfb and Gadd45a (P ≤ 0.0001 for all three genotypes). Intraoral F15/JP4-039, but not F15/4-amino-Tempo, modulated radiation-induced normal tissue transcript elevation, ameliorated mucosal ulceration and reduced the depletion of antioxidant stores in oral cavity tissue of all genotypes, but did not radioprotect tumors. Mitochondrial targeting makes F15/JP4-039 an effective normal tissue radioprotector for Fancd2−/− mice, as well as wild-type mice.
INTRODUCTION
Patients with epithelial carcinomas of the head and neck often suffer significant toxicity during radiotherapy. In particular, radiotherapy of epithelial cancer in radiosensitive Fanconi Anemia (FA) patients can induce severe normal tissue damage, which often limits effective treatment (1–6). There are an estimated 1,000 FA patients in the U.S. and 3,000 FA patients world-wide, many of whom are at risk of developing epithelial cancers, including squamous cell carcinomas of the head and neck and esophagus (5). While FA patients with head and neck cancer represent a small population, the radiosensitivity of their normal tissue makes them ideal candidates for evaluating the safety as well as the degree of success of a new radioprotective agent. Successful reduction of radiation toxicity to the oral cavity/oropharynx in FA patients by a new agent would suggest that this radioprotectant would be appropriate for the estimated 45,000 new patients per year with head and neck cancer, who may require radiotherapy (7). Prior studies have shown that intraoral administration of mitochondrial localized transgene protein manganese superoxide dismutase (SOD2) (8) prior to each fraction of radiation improved oral cavity/oropharynx dose tolerance and reduced normal tissue apoptosis, ulceration and fibrosis (8–12) without tumor protection (9). Since radiation-induced damage to normal tissue involves cell nucleus to mitochondrial mechanisms of apoptosis (13–17), we broadened this approach by developing new mitochondrial-targeted small molecule mimics of MnSOD. It is, however, imperative to determine whether radioprotection using mitochondrial-targeted drugs will benefit FA patients with epithelial tumors.
A major concern in the development of radiation protector agents for clinical radiotherapy is the possibility of simultaneous radiation protection of tumors within the treatment field (8, 9, 13). Previous studies with mitochondrial-targeted SOD2 demonstrated normal tissue-specific radiation protection of the oral cavity/oropharynx in mice with orthotopic head and neck tumors (9). SOD2 dismutates superoxide radicals to yield hydrogen peroxide, which is still a very potent oxidizing, as well as signaling species (10, 12) and may be even more toxic than superoxide (11, 14, 15). In contrast, recently designed nitroxide conjugates act as electron scavengers preventing superoxide formation (16). This important difference led to the development of mitochondrial-targeted GS-nitroxides (17), which block normal cellular apoptosis (16) and have shown potential as clinical radioprotectors (17–20).
If the mitochondrial mechanism of normal tissue damage by radiation exposure is a dominant event in cells and tissues, and can supersede a higher level of nuclear radiation-induced damage, then the defective oxidative stress response in tissues of FA patients might be compensated by localized delivery of JP4-039. In a previous study of one radiosensitive Fancd2−/− mouse strain, it was reported that mitochondrial-targeted GS-nitroxide, JP4-039, ameliorated radiation-induced normal tissue ulceration and depletion of antioxidant stores in normal oral cavity tissue (18). However, normal tissue radioprotection in tumor-bearing FA mice has not been reported.
In this study of tumor-bearing head- and neck-irradiated Fancd2−/− mice of a different background mouse strain (C57BL/6) (21), we compared radioprotection by intraorally administered JP4-039 nitroxide to nonmitochondrial-targeted 4-amino-Tempo in an F15 liposomal emulsion (17), which localizes drug to the local tissues in the irradiated oral cavity.
The results from this study show that orally established tumors in Fancd2−/− mice can be effectively irradiated while providing normal tissue specific radioprotection based on mitochondrial targeting of GS-nitroxide.
MATERIALS AND METHODS
Irradiation (Machines and Dose Rate)
Mice of each genotype were irradiated with a single fraction dose (24, 26 and 28 Gy), or fractionated doses of 8 Gy × 4 and were scored for percentage ulceration of the tongue on day 5 postirradiation (8). Both Fancd2−/− and control mice received oral cavity irradiation, using a Varian linear accelerator (Varian Medical Systems Inc., Palo Alto, CA), at a dose rate of 2 Gy/min. The mice were anesthetized using Nembutal® and shielded appropriately ensuring that only the head and neck region, above the cervical spine, received exposure (8, 9).
Animal Models
Fancd2−/−, Fancd2+/− and Fancd2+/+ (C57BL/6 background), obtained from the Dana Farber Cancer Institute (21), were derived from breeding pairs of Fancd2+/− mice. Fancd2+/+ mice resulting from these breeding pairs were used as control mice. Mice were housed 4/ cage according to Institutional IACUC regulations and fed standard Purina® laboratory chow.
Fancd2+/+, Fancd2+/− and Fancd2−/− mice were injected intraorally with 2 × 106 cells of the murine epithelial cell carcinoma cell line TC-1 (22) in the center of the right cheek. Tongue tissue was used for representation of normal oral tissue response and tumor samples for tumor response. Palpable tumors formed by day 4 and 5 in all mice, and tumor growth was measured daily using calipers until tumor size reached 1.5–2 cm in the greatest dimension. A 100 μl volume of F15 formulation containing either F15/JP4-039, F15/4-amino-Tempo and F15 alone was administered intraorally and mice were irradiated 15 min later. In preliminary experiments, mice of each genotype received head and neck irradiation (0, 24, 26 and 28 Gy) and the percentage normal tissue ulceration was determined on day 5 after irradiation. While Fancd2−/− mice showed detectable normal tissue ulceration on day 5 after each dose, and a direct dose-response curve, control Fancd2+/+ mice exhibited no ulceration at day 5 after 24 or 26 Gy dose. A dose of 28 Gy to the head and neck was chosen, as it caused a reproducible significant normal tissue increased percentage ulceration great enough to quantitate any protective effect of JP4-039. Therefore, to reduce animal numbers and minimize suffering, we used the 28 Gy dose for all single fraction experiments. Mice of each genotype with tumors were then divided into the following groups to receive a 28 Gy single radiation dose: 1. nonirradiated (control); 2. radiation alone; 3. radiation with F15/JP4-039; 4. radiation with F15/4-amino-Tempo and 5. radiation with F15 emulsion treatment.
Animals in the fractionated doses group (8 Gy × 4) were divided as follows: 1. radiation alone; 2. radiation with F15/JP4-039; 3. radiation with F15/4-amino-Tempo and 4. F15 emulsion alone. F15/JP4-039 and F15/4-amino-Tempo were administered prior to each radiation fraction. This fractionation schedule was calculated to have a biological effective dose (BED) of 46.8 compared with 84 for 28 Gy × 1 (23, 24), While the BED for 8 Gy × 4 was less than the BED for 28 Gy × 1, these treatment groups produced equivalent normal tissue damage, measured as percentage ulceration of the oral cavity. Furthermore, as we have previously reported, these treatment paradigms produced equivalent normal tissue damage levels in another animal model in our laboratory (18).
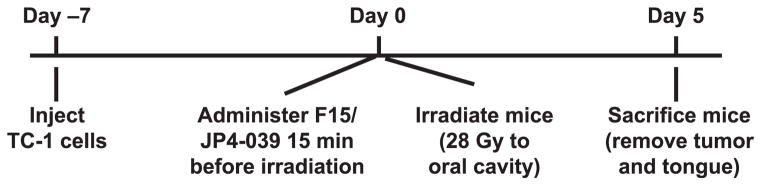
Tumor cell implantation, tumor explant and cell preparations were as follows; TC-1 tumor cells (22) were grown in vitro, trypsinized and prepared as a single cell solution at 2 × 107 cells/ml in phosphate buffered saline (PBS). Mice were lightly anesthetized using Nembutal, and 2 × 106 TC-1 cells in a volume of 100 μl were injected into the mucosal layer of the center of the right cheek. Figure 1 shows the timeline for the single-fraction irradiation experimental protocol.
FIG. 1.
Timeline for the single fraction irradiation experimental protocol.
For fractionated irradiation, 8 Gy × 4 was delivered starting on day 0 (day 0, 1, 2 and 3) and F15/JP4-039 or control drugs were administered before each radiation fraction. Mice were sacrificed 5 days after the final radiation exposure on day 8.
Histopathologic measurements of radiation-induced damage for tongue tissue from irradiated Fancd2+/+, Fancd2+/− and Fancd2−/−mice, as well as control mice, were performed five days after the final irradiation and fixed in 10% formalin.
We chose 5 days after the final irradiation fraction for histopathology for the following reasons: 1. Irradiated mice die beginning on day 6 after irradiation; 2. The University of Pittsburgh’s Institutional Animal Use and Care Committee (IACUC) regulations specify that mice must be euthanized after 20% body weight loss and prior to death. To successfully comply with these regulations and perform measurements on irradiated mice, all mice were sacrificed on day 5.
The tongue was removed, fixed in 2% paraformaldehyde for 2 h and then stored in 30% sucrose. The blocks were sectioned (5 μm) and stained with hematoxylin and eosin (H&E). Slides were then scored for percentage ulceration, blinded, by two observers, according to published methods (9). For each genotype, at least three mice were sacrificed at each time point.
Details of the scoring of ulceration are as follows. To be consistent among animals, three histopathologic slides with three sections per slide were prepared for each tongue (600 microscopic fields per tongue were scored per condition). The three slides were taken from the: 1. dorsal; 2. ventral; and 3. lateral edge of each tongue and each was scored for ulceration (200 fields per slide = 600 fields). In representative mice, oral mucosa was also quantitated for ulceration. Results were consistent among animals. The results among the three tongue slides and oral mucosa were similar for quantitation of drug effect. The data for tongue tissue were calculated and presented. Ulceration was defined as loss of thickness of the epithelial layer of the tongue, as previously reported (8). Mucositis was quantitated as percentage ulceration using LabWorks Image Acquisition and Analysis Software (UVP® LLC, Upland, CA). Data are presented as mean percentage ulceration ± standard deviation (8). Comparisons among groups were made with the two-sided two-sample t test. Significance was indicated as P < 0.05.
In Vitro Studies
In vitro survival curves were determined with TC-1 cells (22). Cells were incubated in 10 μM JP4-039 for 1 h before irradiation (preirradiation group), or JP4-039 was added immediately after irradiation (postirradiation group) as described by Rwigema et al. (16). Control groups were cultured without JP4-039. TC1 cell lines were irradiated in suspension to doses between 0 and 8 Gy at 70 cGy/ min using a Shepherd Mark 1 137Cs gamma-ray source (JL Shepherd, San Fernando, CA). Cells were plated in quadruplicate in 4-well Linbro® plates (Fisher Scientific, Pittsburgh, PA) and incubated at 37°C, 5% CO2 for 9–11 days, stained with crystal violet and colonies of ≥50 cells were counted using a GelCount™ colony counter (Oxford Optronix Ltd, Abingdon, UK). Data were analyzed with linear-quadratic and single-hit multitarget models according to previously published methods (13, 16).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed for analysis of gene expression levels. Tongue tissue was harvested at 5 days after the final irradiation from Fancd2+/+, Fancd2+/− and Fancd2−/− mice that received either no radiation, radiation alone, F15/JP4-039 or F15/4-amino-Tempo pretreatment with radiation, and F15 treatment alone. Tissue from each mouse was individually homogenized and RNA extracted using TRIzol® reagent (Invitrogen™, Carlsbad, CA). A total of 2 μg of RNA was used to synthesize cDNA in a 20 μl reaction system, according to the instructions of the High-Capacity cDNA Reverse Transcription Kit (cat. no. 4368814; Applied Biosystems®, Carlsbad, CA) with reaction conditions of 40 cycles of 95°C (denaturation) for 15 s and 60°C (annealing and elongation) for 1 min using the Eppendorf Realplex2 Mastercycler® (Westbury, NY) (25).
The real time polymerase chain reaction was used to measure radiation-inducible transcripts for: transcription factors Nfkb, Ap1, Sp1 and Nrf2; cytokines including Tgfb1 and IL1a; oxidative stress response enzyme MnSOD (Sod2); and radiation response-related transcripts Gadd45, Rad51, p21 and p53, using previously published methods (25). Results were standardized by comparison to Gapdh (glyceraldehyde-3-phosphate dehydrogenase). The results were shown as fold increase or decrease in gene transcript expression above baseline level, which was adjusted to that for nonirradiated wild-type Fancd2+/+ mouse tongue tissue from nontumor-bearing mice. Significance was determined to be that of a twofold or greater difference or less than 0.5-fold decreased difference.
The RT-PCR conditions were as follows: 96-well plates were prepared with 10 μl of TaqMan® Gene Expression Master mix, 5 μl of RNase-free water, 1 μl of the corresponding TaqMan Gene Expression probe, and 4 μl of cDNA (totaling 2 μg of cDNA) using the Eppendorf epMotion® 5070 automated pipetting system. PCR amplification of the Gapdh gene was used as the housekeeping gene. Expression of Nrf2, Sod2, Nfkb, Tgfb1, Gadd45, Il1a, Sp1, Ap1, Rad51, p21, p53 and Gapdh was determined by RT-PCR. Gene bank numbers for each gene are shown in Supplementary Table S1 (http://dx.doi.org/10.1667/ RR14035.1.S1) (25).
For assay for mitochondrial localization of nitroxide by electron spin trap, Fancd2+/+ and Fancd2−/− mice were administered 100 μl of F15/JP4-039 or F15/4-amino-Tempo intraorally and sacrificed 2 h later. The tongue was removed and placed on ice, homogenized in 1 ml of PBS and centrifuged at 15,000g for 10 min. The supernatant was transferred to an Eppendorf tube, centrifuged again at 12,000g for 45 min and the pellet containing the mitochondria was frozen. The separated mitochondria or unseparated whole cells were mixed with dimethyl sulfoxide (DMSO) (1:1 v/v) and 2 mM potassium ferricyanide to convert reduced nitroxide (hydroxylamine) to EPR-detectable nitroxide radicals. An aliquot of 70 μl of the homogenate was loaded into Teflon™ tubing (0.8 mm internal diameter) which was folded in half and placed into an open EPR quartz tube (3.0 mm diameter). EPR measurements were performed in triplicate using a JEOL-RE1X EPR spectrometer (JEOL Ltd., Tokyo, Japan) under the following conditions: 334.7 mT center field, 5 mT sweep width, 0.079 mT field modulation, 20 mW microwave power, 0.1 s time constant and 2 min scan time at 22.5°C. Utilizing signal magnitude and isolated volumes, the amount and concentration of nitroxide from JP4-039 or 4-amino-Tempo were calculated for each sample.
Western blot analysis of mitochondrial proteins was performed on tissues and cell lines. Tissue from the normal oral cavity of Fancd2+/+, Fancd2+/− and Fancd2−/− mice or explanted right-sided orally-established tumors were homogenized, and electrophoresized on 15% polyacrylamide gels. The protein was transferred to a nitrocellulose membrane treated with anti-Sod2, anti-Cox4 and anti-mitochondrial transcription factor A (Tfam) antibodies and quantitated as standardized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh).
Assay for cellular antioxidant stores was performed with tissues excised from tumor-bearing Fancd2+/+, Fancd2+/− and Fancd2−/−mice exposed to single dose (28 Gy) or fractionated doses (8 Gy × 4) to the oral cavity. Subgroups were administered F15/JP4-039 or F15/ 4-amino-Tempo 10 min before each radiation fraction. At 24 h after the last fraction, subgroups of mice were sacrificed and tumor and normal oral cavity tissue opposite the tumor was removed and homogenized. Protein concentrations were standardized by a Bradford assay and antioxidant reductive capacity and antioxidant status were measured using a commercial kit (Northwest Life Science Specialties LLC, Vancouver, WA). This assay measures the antioxidant capacity of cells based on the ability of cellular antioxidants to reduce Cu++ to Cu+, which reacts with bathocuproine to form a colored complex with absorbance at 480–490 nm. The antioxidant activity was compared to a standard curve generated using Trolox units (milliequivalents) and all data were expressed in Trolox units (9, 19, 20).
Drug Delivery
Intraoral administration of F15 alone, F15/JP4-039, F15/4-amino-Tempo or fluorophore-tagged F15/BODIPY-JP4-039 was performed on nonanesthetized mice. Mice were administered 100 μl volumes containing: F15/JP4-039, fluorophore-labeled F15/BODIPY-JP4-039 (19) or F15/4-amino-Tempo (0.4 mg of JP4-039, 0.6 mg/ml BODIPY-JP4-039 or 0.2 mg/mouse, respectively). We used drugs encapsulated in a detergent-containing F15 emulsion prepared as previously described (17). The components of the F15 emulsion have been described in detail elsewhere (17, 18). Each drug was administered intraorally 15 min prior to irradiation. The boron-dipyrromethene-labeled, fluorescent BODIPY-JP4-039 has been described previously (19). Fancd2+/+, Fancd2+/− and Fancd2−/− mice with orally established tumors were administered F15/BODIPY-JP4-039 intraorally [100 μl of F15 containing 6 mg/ml of BODIPY-JP4-039 (equal in molarity to JP4-039) normalized to its nearly 1.5 higher molecular weight of 598.56 g/mol compared with 424.32 g/mol for JP4-039], and sacrificed 2 h later. Tumor and oral tissue were removed, frozen in optimal cutting temperature (OCT) compound (Fisher Scientific) sectioned and examined microscopically for mitochondrial uptake of BODIPY-JP4-039 by co-staining with an antibody to the mitochon-drial protein TOM 20 (translocase of the outer membrane) (19).
Preparation of BODIPY-JP4-039
For the synthesis of a fluorophore-tagged JP4-039 analog, we used a more direct fluorescence-based visualization and selected a BODIPY-FL® fluorophore for this purpose (26). An analog of JP4-039 with a BODIPY-FL label in place of the N-Boc group was synthesized using a de novo synthesis approach (27). The direct synthesis of this labeled derivative from JP4-039 failed, since, after the Boc group of JP4-039 was successfully removed with HCI, treatment of the free amine with the N-hydroxysuccinimide activated ester of BODIPY-FL (BODIPY-FL-NHS) provided an 83:17 mixture of the undesired bis-coupling product and the desired EMF338-008.
To avoid this unwanted side product, we intended to attach the BODIPY-FL label to the alkene peptide isostere scaffold prior to addition of the TEMPO group. Accordingly, the carboxylic acid was protected as the methyl ester. Removal of the Boc-protecting group with trifluoroacetic acid (TFA) followed by coupling to BODIPY-FL-NHS provided the conjugate in 82% yield. Saponification of the methyl ester under standard basic conditions resulted in extensive decomposition; therefore, pig liver esterase (PLE) in acetone/pH 7 phosphate buffer was used to effect a chemoselective transformation. Coupling of the resulting acid to 4-amino-tempo (4-AT) provided the desired BODIPY-FL-labeled compound BODIPY-JP4-039 (EMF338-008) in 56% yield over two steps (27).
Statistical Analysis
The histologic evaluation of percentage tissue ulceration and qRT-PCR data were summarized for each group using mean ± standard deviation. Comparisons between any two groups were made with the two-sided two-sample t test. P < 0.05 was considered significant. P values were not adjusted for multiple comparisons.
The in vitro radiation survival curves were analyzed by both the linear-quadratic model and the single-hit multitarget model, and were compared using D0 (final slope representing multiple-event killing) and ñ (extrapolation number measuring width of the shoulder on the radiation-survival curve) (13, 16). Results for D0 and ñ were shown as the mean ± standard error from multiple measurements and compared with the two-sided two-sample t test. For the other continuous end points, comparisons were also performed using a t test if they were normally distributed, or otherwise, with a Wilcoxon rank-sum test.
Data for gene expression in tongue tissue from mice with oral cavity tumors were summarized as mean ± standard deviation for each group. For each of multiple gene transcripts, each comparison was performed with the two-sided two-sample t test. P < 0.05 was considered significant. As this was an exploratory study, P values were not adjusted for multiple comparisons. Western blot analysis for proteins was quantitated by densitometry as published elsewhere (28).
RESULTS
F15/JP4-039 Reduces Oral Mucositis in Radiosensitive Fancd2−/− Mice
The sensitivity of Fancd2−/− C57BL/6 mice to total-body irradiation has been previously established (21). In the current study, we first evaluated the radiation sensitivity of head- and neck-irradiated mice. Radiation doses and fractionation were based on previous experience where doses of less than 24 Gy resulted in no detectable normal tissue ulceration in wild-type mice (8, 9, 18). The oral cavity of mice exposed to 28 Gy developed severe mucositis around day 7, which resulted in loss of 20% of body weight, requiring euthanasia as mandated by the IACUC of the University of Pittsburgh. All mice except those in the F15/ JP4-039 treatment groups were sacrificed at day 5 after the final radiation fraction due to over 20% body weight loss and IACUC rules to limit animal suffering. For F15/JP4-039-treated mice, but not for other groups of mice, survivors were observed beyond 30 days and showed no delayed development of deleterious effects.
We quantitated the magnitude of radiation-induced ulceration of the tongue as follows. The percentage ulceration was calculated by determining the area of squamous epithelium denuded (absent) on each section divided by the total area of the tongue. This area was defined as percentage ulceration. This result was in contrast to other reported publications where doses as low as 12 Gy resulted in detectable mucositis (29). Since the radiation doses required to detect a specific effect are known to be mouse strain dependent and vary by institutional animal location and also are based on the radiation source, dose rate and animal health status, we standardized the conditions to our location.
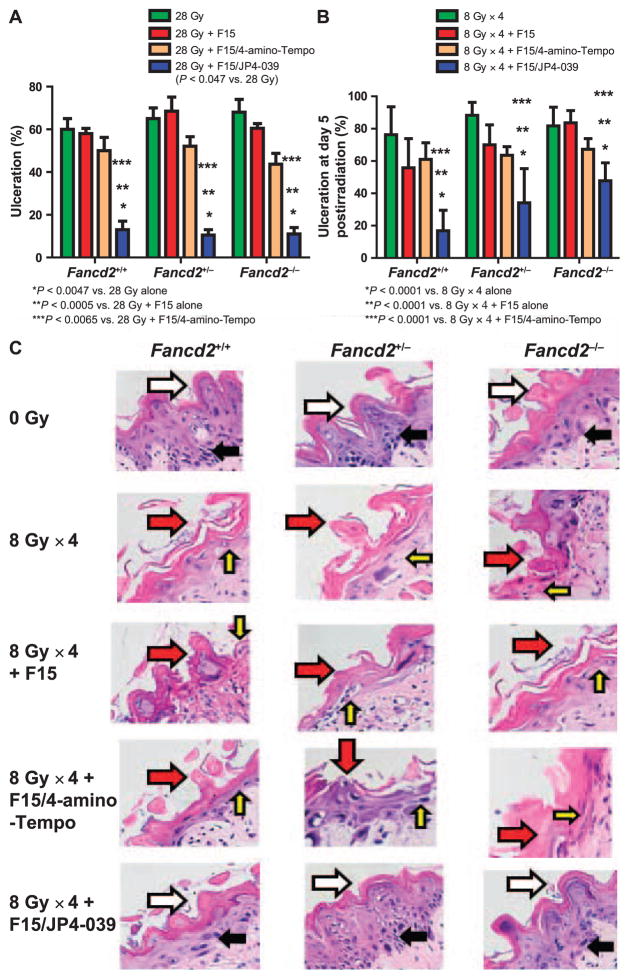
In our animal facility, Fancd2−/− mice showed a higher percentage oral cavity tissue ulceration 5 days after each single fraction dose: 24 Gy (56.1 ± 13.5%) and 26 Gy (59.4 ± 8.2%) compared with control Fancd2+/+ (C57BL/6), which had no detectable ulceration at day 5. All control groups received either intraoral administration of F15 alone, F15-4-amino-Tempo or no added drug. F15 alone and F15/4-amino-Tempo had a modest anti-inflammatory and radiation-protective effect determined by a detectable, but a nonsig-nificant decrease in percentage ulceration in all three mouse strains after a single fraction of 28 Gy (Fig. 2A) and in Fancd2+/+ and Fancd2+/− mice after fractionated doses (Fig. 2B). These data establish that Fancd2−/− (C57BL/6) mice were relatively radiosensitive to head and neck single fraction irradiation, measured at day 5. With the higher dose of 28 Gy (Fig. 2A) control, heterozygote and Fancd2−/− had significant ulceration at day 5. Therefore, the 28 Gy dose to the head and neck was detectably toxic in all three genotypes, Fancd2+/+, Fancd2+/− and Fancd2−/− at day 5 (n = 6) (Fig. 2A). Three mice per genotype next received F15 alone, F15/4-amino-Tempo (0.2 mg/mouse in 100 μl) and F15/JP4-039 (0.4 mg/ mouse in 100 μl) intraorally 15 min before irradiation. As shown in Fig. 2A, there was an equivalent level of ulceration in all three genotypes, which may be explained as follows. The entire head and neck of the mice were irradiated, causing mucosal ulceration and resulting in xerostomia (8, 10). The lack of salvia from xerostomia can exacerbate ulceration. In human patients receiving radiation therapy to the head and neck region, treatment planning (not possible in our animal facility) can facilitate parotid gland shielding, which can reduce radiation dose to the salivary glands and decrease the contribution of xerostomia to ulceration. Intraoral administration of F15/ JP4-039 has been shown to protect the mouse oral cavity and esophagus and decrease ulceration (17, 18). However, the inability to shield the salivary glands may have allowed a uniform level of xerostomia and in part may explain why all three mouse genotypes had a similar level of postirradiation ulceration. All groups that received 28 Gy irradiation were pretreated with intraoral F15/ JP4-039, but not F15/4-amino-Tempo or F15 alone, had a reduction in ulceration to less than 20% at day 5 (P < 0.0047).
FIG. 2.
F15/JP4-039 protects against mucositis induced by single fraction (28 Gy) and fractionated (8 Gy × 4) doses in Fancd2−/− mice. Nonirradiated tongue tissues were examined from Fancd2+/+, Fancd2+/− and Fancd2−/−mice as well as tongue tissues irradiated with a single fraction (28 Gy, panel A) or fractionated dose (8 Gy × 4, panel B), with or without F15/JP4-039 pre-treatment (*P < 0.0047 compared with 28 Gy; *P < 0.0001 compared with 8 Gy × 4). Tongues were dissected on day 5 after the final fraction of 8 Gy. F15/JP4-039-treated mice were irradiated 15 min after drug administration. Mice were sacrificed at day 5 postirradiation (last fraction) and tongues were subsequently sectioned into 5 μm slices and mounted on glass slides. Ulceration of the tongue and oral cavity at day 5 after the final radiation fraction was measured in H&E-fixed tissue sections. Ulceration was defined as loss of thickness of the epithelial layer of the tongue. Panel C: H&E-stained dorsal tongue tissue from Fancd2+/+, Fancd2+/− and Fancd2−/− mice 5 days after irradiation (8 Gy × 4) or daily F15 alone, F15/4-amino-Tempo or F15/JP4-039 intraoral pretreatment. Irradiated tissue is compared with the control, nonirradiated tissue. Photos are from day 5 after the final 8 Gy fraction, which is the same day for groups receiving F15, F15/4-amino-Tempo or F15/JP4-039 intraoral daily pretreatment. The control irradiated only (8 Gy × 4) tissue was compared with control nonirradiated tissue. White arrows identify normal papillae while red arrows indicate radiation-induced damage to the papillae. Black arrows indicate the normal epithelial layer while the yellow arrows indicate a loss or thinning of the epithelial layer in ulcerated areas of the epithelial layer. (H&E stained; all images 10× magnification)
The effect of daily F15/JP4-039 administration during 8 Gy × 4 fractionated irradiation was evaluated next. F15/ JP4-039, but not F15/4-amino-Tempo or F15 emulsion alone (Fig. 2B), when delivered daily prior to each radiation fraction, provided significant radioprotection compared to radiation alone. These results establish that Fancd2−/− mice were sensitive to fractionated head and neck irradiation displaying a percentage tongue ulceration comparable to Fancd2+/+ or heterozygote Fancd2+/− mice. The results also establish that there was effective amelioration of radiation-induced tissue ulceration by F15/JP4-039 pretreatment in all mouse genotypes (Fig. 2B and C). These data confirm prior results showing that intraoral F15/JP4-039 radioprotects oral cavity tissues in Fancd2−/− mice of a different background strain (18) and extend the results to Fancd2−/−mice on the C57BL/6 background (21).
Distinct Constitutive Gene Expression Patterns in Oral Tissue of Nonirradiated Fancd2−/− (C57BL/6) Mice
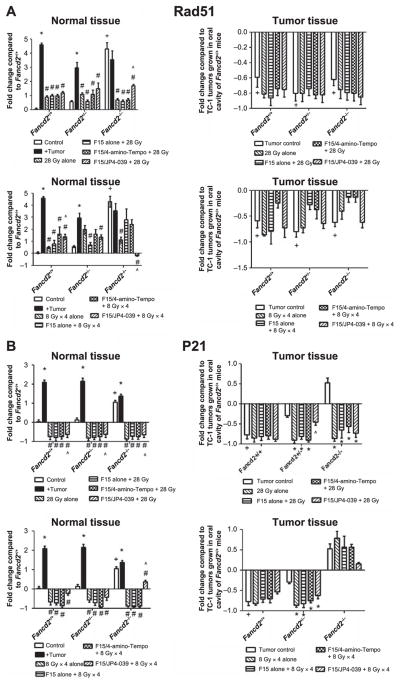
Prior studies showed a constitutive increase in expression of some RNA transcripts in the oral tissues of Fancd2−/−mice of another background strain (18). We next evaluated baseline expression levels of gene transcripts for DNA repair gene Rad51 (Fig. 3A), pro-inflammatory and oxidative stress response genes including: p21 (Fig. 3B), Gadd45 (Fig. 3C), Nrf2 (Fig. 3D), cytokines, I1a (Fig. 3E) and Tgfb (Fig. 3F) in the oral cavity of Fancd2−/− (C57BL/6) mice. Some gene transcript levels were different in the oral cavity of nonirradiated Fancd2−/− compared with Fancd2+/+ wild-type mice (Fig. 3 and Table 1; Supplementary Table S2; http://dx.doi.org/10.1667/RR14035.1.S1). Fancd2−/−mice showed elevated RNA transcript levels for Nrf2 (P = 0.0002), Sod2 (P < 0.0005), p53 (P = 0.0002), p21 (P = 0.0009) and Rad51 (P = 0.0031) compared to wild-type C57BL/6 mice. In contrast, Fancd2−/− mouse oral cavity tissue showed suppressed transcript levels for Nfkb, and Tgfb (P < 0.0020 for both), (Fig. 3 and Table 1; Supplementary Table S2C).
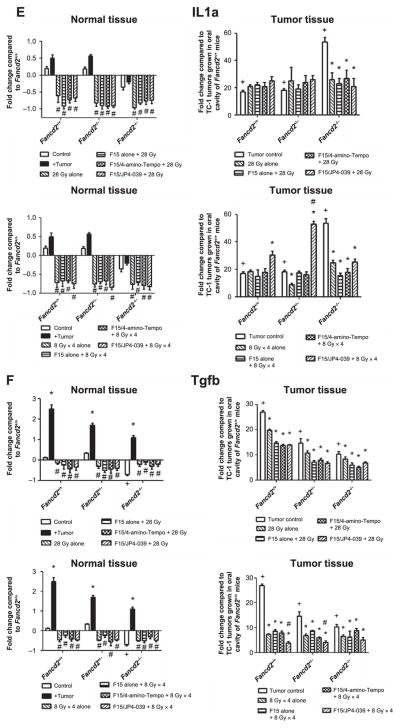
FIG. 3.
Effect of F15/JP4-039 intraoral administration on modulation of head and neck radiation-induced RNA transcript changes in normal oral cavity tissue and TC-1 tumors in Fancd2−/− (C57BL/6) mice. The effect of radiation and JP4-039/F15 administration on the levels of: Rad51 (panel A); p21 (panel B); Gadd45 (panel C); Nrf2 (panel D); Il1a (panel E); and Tgfb (panel F) gene transcripts in normal oral cavity tissue and TC-1 tumors in Fancd2+/+, Fancd2+/− and Fancd2−/− (C57BL/6) mice was measured. Upper panels show 28 Gy single-fraction data and lower panels show fractionated doses (8 Gy × 4) data. Results for normal tissue are standardized to C57BL/6J Fancd2+/+ mouse control oral cavity tissue gene transcript levels. Results for tumor tissue are standardized to untreated control TC-1 tumors in wild-type mice (n = 3–4 per group in all panels). For normal tissue, the “+” symbol indicates gene expression in Fancd2−/− or Fancd2+/− oral cavity to Fancd2+/+ oral cavity. The “*” symbol indicates gene expression in tumor-bearing mouse oral cavity with mice of each genotype with no tumors. The “#” symbol indicates expression in oral cavity tissue of irradiated mice with TC-1 tumors to irradiated oral cavity tissue in mice of the same genotype with no tumors.. The “^” symbol indicates gene expression in oral cavity tissue of F15/JP4-039/F15 treated mice with irradiated TC-1 tumors to mice of the same genotype with control irradiated alone tumors. For tumor tissues, the “+” symbol indicates gene expression in nonirradiated tumors to irradiated tumors in the same genotype mouse. The “*” symbol indicates gene expression in irradiated tumors in Fancd2+/− or Fancd2−/− to irradiated tumors in control Fancd2+/+ mice. The “#” symbol indicates the effect of intraoral F15/JP4-039 treatment on irradiated tumors with nondrug-treated control mice with irradiated tumors in that genotype.
TABLE 1.
Gene Transcripts in Oral Cavity of Nonirradiated Control Mice and Tumor-Bearing Fancd2−/− Mice (C57BL/6)
| Tongue tissue from control or tumor-bearing mice
|
||||||
|---|---|---|---|---|---|---|
| Gene | Fancd2+/+ | Fancd2+/+ plus tumor | Fancd2+/− | Fancd2+/− plus tumor | Fancd2−/− | Fancd2−/− plus tumor |
| Nfkb | 1.09 ± 0.01 | 1.12 ± 0.02 | 1.28 ± 0.03 | 0.98 ± 0.21 | 0.49 ± 0.08 (P1 = 0.0015) | 0.56 ± 0.04 |
| Tgfb | 1.12 ± 0.01 | 3.5 ± 0.18 (P = 0.0004) | 1.33 ± 0.03 | 2.70 ± 0.10 (P = 0.0002) | 0.29 ± 0.06 (P1 = 0.0002) | 2.14 ± 0.08 (P < 0.0001) |
| Sp1 | 1.11 ± 0.03 | 1.65 ± 0.24 | 1.63 ± 0.03 | 0.83 ± 0.02 | 0.89 ± 0.02 | 1.38 ± 0.11 |
| Ap1 | 1.06 ± 0.03 | 0.34 ± 0.01 (P < 0.0001) | 0.34 ± 0.04 (P1 < 0.0001) | 0.51 ± 0.05 | 0.85 ± 0.11 | 0.51 ± 0.12 |
| Il1a | 1.23 ± 0.02 | 1.50 ± 0.10 | 1.19 ± 0.03 | 1.57 ± 0.04 | 0.65 ± 0.08 | 0.81 ± 0.05 |
| Gadd45 | 1.14 ± 0.03 | 2.75 ± 0.12 (P = 0.0005) | 1.46 ± 0.04 | 2.63 ± 0.11 | 0.71 ± 0.05 | 4.72 ± 0.08 (P < 0.0001) |
| Sod2 | 1.11 ± 0.04 | 3.28 ± 0.15 (P = 0.0004) | 2.45 ± 0.08 (P1 = 0.0001) | 3.18 ± 0.32 | 2.79 ± 0.16 (P1 = 0.0005) | 4.50 ± 0.89 |
| Nrf2 | 1.06 ± 0.01 | 2.10 ± 0.11 (P = 0.0041) | 2.24 ± 0.08 (P1 = 0.0001) | 1.51 ± 0.10 | 2.07 ± 0.07 (P1 = 0.0002)a | 1.63 ± 0.11 |
| p53 | 1.08 ± 0.05 | 0.08 ± 0.017 (P < 0.0001) | 2.02 ± 0.15 (P1 = 0.0005) | 0.40 ± 0.08 (P < 0.0001) | 4.70 ± 0.22 (P1 < 0.0001) | 0.21 ± 0.09 (P < 0.0001) |
| p21 | 1.04 ± 0.01 | 3.10 ± 0.12 (P = 0.0002) | 1.13 ± 0.01 | 3.16 ± 0.15 (P = 0.0002) | 2.06 ± 0.09 (P1 = 0.0009) | 2.37 ± 0.11 |
| Rad51 | 1.05 ± 0.07 | 5.60 ± 0.13 (P < 0.0001) | 1.56 ± 0.08 | 3.96 ± 0.38 (P = 0.0233) | 5.29 ± 0.44 (P1 = 0.0031) | 4.55 ± 0.61 |
Notes. RT-PCR was used to quantify gene transcript levels in tongue tissue obtained from control, nonirradiated nontumor-bearing, Fancd2+/+, Fancd2+/− and Fancd2−/− mice. Similar analysis was performed in tongue tissue obtained from tumor-bearing mice; TC1 tumor cells were inoculated in the cheek of Fancd2+/+, Fancd2+/− and Fancd2−/− mice, at 2 × 106 cells per mouse. Once tumors reached 0.3 cm in diameter (usually by day 5), mice were sacrificed and their tongue tissues were analyzed to evaluate if the presence of an orthotopic tumor altered their baseline gene expression. P1 compares the respective Fancd2+/− or Fancd2−/− transcript level to Fancd2+/+. For tumor-bearing mice, P values compare the level of transcripts in tumor-bearing mice to nontumor-bearing mice within the same genotype. Values were not assessed for significance if the fold change differences were not at least a factor of 2 (either a fold change <0.5 or >2) compared to their respective baselines, P1: Fancd2+/+; P: same genotype without tumor implantation. All significant P < 0.05 are indicated with bold text.
Different experiment from those in Supplementary Table S2 (http://dx.doi.org/10.1667/RR14035.1.S1).
The tongue tissue of Fancd2+/− heterozygote mice showed a pattern of gene transcript expression levels that were intermediate between Fancd2−/− and wild-type mice (Table 1 and Supplementary Table S2B; http://dx.doi.org/10.1667/RR14035.1.S1). There was elevation of Nfkb, Tgfb and Gadd45 compared to the homozygote Fancd2−/− mice and decreased levels compared to wild-type mice (Fig. 3). These data confirm and extend a prior publication, which showed elevated baseline levels of p53, p21, Nrf2 and Sod2 gene transcripts in heterozygote Fancd2+/− mice on a different genetic background (18).
The Presence of Orally Established Tumors in the Cheek Alters Gene Transcript Levels in Tongue Tissue of Fancd2−/−Mice
We next evaluated whether the presence of a tumor in one area of the oral cavity changed the level of expression of stress response or inflammatory gene transcripts in the normal tissue of the oral cavity using tissue from the tongue that was at least 0.5 cm distant from the tumor location in each mouse genotype. The presence of a TC-1 tumor clearly altered levels of some RNA transcripts in normal tissue of mice of all three genotypes (Fig. 3 and Table 1; Supplementary Table S2, http://dx.doi.org/10.1667/RR14035.1.S1) compared to levels in the same genotype tissues with no tumor present. Detectable differences common to multiple genotypes included elevated levels of Gadd45a and Tgfb (P < 0.0005) and suppressed levels of p53 transcripts (P < 0.0001). The presence of tumor in the oral cavity did not significantly alter transcript levels for Nfkb or Il1a (all P ≥ 0.087).
These results establish that gene transcript levels in tumor-free areas of distant oral cavity tissue of Fancd2−/−, heterozygote and control mice were altered by the presence of a tumor in the oral cavity.
Radiation Effects on Gene Transcript Levels in Oral Tissue Of Tumor-Bearing Fancd2−/− Mice
The effect of head and neck irradiation on RNA transcript levels in normal oral cavity tissues in tumor-bearing mice of each genotype was evaluated. As shown in Fig. 3 and Supplementary Table S2 (http://dx.doi.org/10.1667/RR14035.1.S1), single fraction (28 Gy) and fractionated (8 Gy × 4) irradiation of the head and neck of tumor-bearing mice of all genotypes altered the level of normal tissue gene transcripts. All genotypes showed decreased levels of most gene transcripts, but elevation of Rad51 and Gadd45 in Fancd2−/− normal tissue.
Intraoral F15/JP4-039 Effects on Radiation-Induced Alterations in Gene Transcript Levels in Oral Cavity Tissue of Irradiated, Tumor-Bearing Fancd2−/− Mice
The effect of F15/JP4-039 on the radiation-induced changes in oral cavity tissues of tumor-bearing mice of each genotype was measured next. We first quantitated the effect of intraoral administration of F15/JP4-039 to the oral cavity on baseline gene transcript levels in nontumor-bearing mice. No significant changes were seen. We then tested the effect of F15/JP4-039 in single fraction (28 Gy) and fractionated (8 Gy × 4) irradiated Fancd2−/− mice with established tumors. As shown in Fig. 3 and Supplementary Table S2 (http://dx.doi.org/10.1667/RR14035.1.S1), intraoral administration of F15/ JP4-039 further elevated Gadd45 gene transcripts in oral cavity tissue in all mouse genotypes (Fig. 3C) and reduced Rad51 in irradiated Fancd2−/− mouse tissue (Fig. 3A). Tumor-bearing Fancd2−/− mice showed a more dramatic elevation of Gadd45 (Fig. 3C) levels than tumor-bearing Fancd2+/− or Fancd2+/+ mice. F14/JP4-039 had no significant effect on Nrf2, IL1a and Tgfb gene expression after either the single fraction or fractionated irradiation (Fig. 3D–F).
Irradiated TC-1 Tumors Show Gene Transcript Level Alterations Independent of the Genotype of the Microenvironment
We investigated the effect of mouse genotype on levels of gene transcripts in cells from explanted TC-1 tumors in mice that were nonirradiated, irradiated alone and pretreated with F15/JP4-039 prior to irradiation. For most gene transcripts, including: Rad51, p21, Gadd45 and Nrf2 (Fig. 3A–D), orthotopic tumors had decreased levels of gene expression. Tumors had high levels of IL1a and Tgfb (Fig. 3E and F). There was no significant effect of radiation (Fig. 3) or specifically F15/JP4-039 treated prior to irradiation (Fig. 3) on the transcript levels of Tgfb or IL1 in explanted tumor cells from the irradiated mouse oral cavity, but there was an increased expression of Gadd45 and Nrf2 in Fancd2−/− mice in both radiation paradigms (Figs. 3C and D). The data indicate less of an effect of either irradiation or F15/JP4-039 treatment on TC-1 tumors compared to normal tissues with respect to explanted tumor RNA transcript levels. There was no detectable effect of F15/JP4-039 on the in vitro clonogenic radiation survival curve for TC-1 tumor cells (Supplementary Table S3; http://dx.doi.org/10.1667/RR14035.1.S1).
Intraoral F15/JP4-039 Administration Does Not Radioprotect Established Oral Cavity Tumors
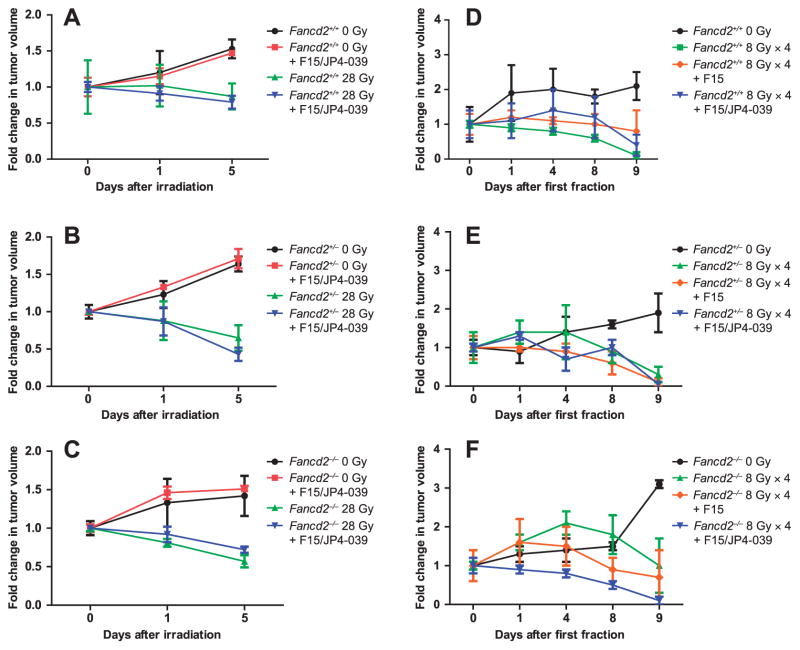
We quantitated the effect of intraoral F15/JP4-039 on radiation control of TC-1 cell line-established oral tumors. Mice with tumors received intraoral F15/JP4-039 (0.4 mg/ mouse in 100 μl of F15), alone or prior to each radiation fraction, while other groups of control tumor-bearing mice received F15 alone. F15/JP4-039 did not increase tumor growth in nonirradiated mice (Fig. 4A–C). There was no significant difference among the three genotypes in radiation-induced tumor growth delay (Fig. 4). F15/JP4-039-treated mice showed no tumor radiation protection compared with control irradiated mice [P = 0.6807 in the Fancd2+/+ group, P =0.6851 in the Fancd2+/− group and P = 0.7559 in the Fancd2−/− group (Fig. 4)]. F15/JP4-039 administration to mice in both single fraction 28 Gy (Fig. 4A–C) and in the fractionated head- and neck-irradiated groups (8 Gy × 4) (Fig. 4D–F) showed no significant alteration of radiation exposure on tumor growth inhibition. In the fractionated radiation experiments, F15 alone had no tumor-protective effect (Fig. 4D–F). Therefore, radiation exposure effectively reduced tumor volume in mice of all genotypes with no detectable tumor radioprotection by intraoral administration of F15/JP4-039 (Fig. 4).
FIG. 4.
F15/JP4-039 does not radioprotect TC-1 tumors in the oral cavity of fractionated head- and neck-irradiated Fancd2−/− mice. Mice with palpable tumors were divided into the following groups: nonirradiation; nonirradiation with F15/JP4-039 treatment; and irradiation with F15/JP4-039 treatment. Mice were irradiated with a single dose (28 Gy) or fractionated doses (8 Gy × 4), with or without F15/JP4-039 or F15 treatment. Drugs were administered intraorally at 0.4 mg/mouse in 100 μl and mice were irradiated 15 min later. Mice were sacrificed 5 days after the final radiation exposure. Panels A–C: Single-fraction dose. Panels D–F: Fractionated doses. For tumor growth measurements in the 28 Gy experiment, P values compare 0 Gy to 0 Gy with JP4-039 for: Fancd2+/+ (0.6807); Fancd2+/− (0.6851); and Fancd2−/− (0.7559). P values also compared 28 Gy to 28 Gy with JP4-039 for: Fancd2+/+ (0.7520); Fancd2+/− (0.4106); and Fancd2−/− (0.1843). For tumor growth measurements in the 8 Gy × 4 experiment, P values compare 8 Gy × 4 to 8 Gy × 4 with F15/JP4-039 for: Fancd2+/+ (0.4012); Fancd2+/− (0.2930); and Fancd2−/− (0.5885).
Specifically, in wild-type Fancd2+/+ mice after 28 Gy irradiation to the head and neck, there was no radioprotective effect of F15/JP4-039 on tumor regrowth (P = 0.7520) (Fig. 4). Tumor-bearing Fancd2−/− (P = 0.1843) and Fancd2+/− mice also showed no tumor protection (P = 0.4106) by intraoral F15/JP4-039. There was equivalent tumor regression after 28 Gy irradiation in mice pretreated with F15/JP4-039 compared with each control irradiated group (Fig. 4). These data establish that the normal oral cavity tissue radioprotective effect of F15/ JP4-039 did not alter radiation-induced growth inhibition of TC-1 cell line-derived established tumors in the treatment field of tumor-bearing Fancd2+/+, Fancd2+/–and Fancd2−/− mice.
Mitochondrial Localization of JP4-039 Mediates Radioprotection of Oral Cavity Tissue in Fancd2−/− Mice
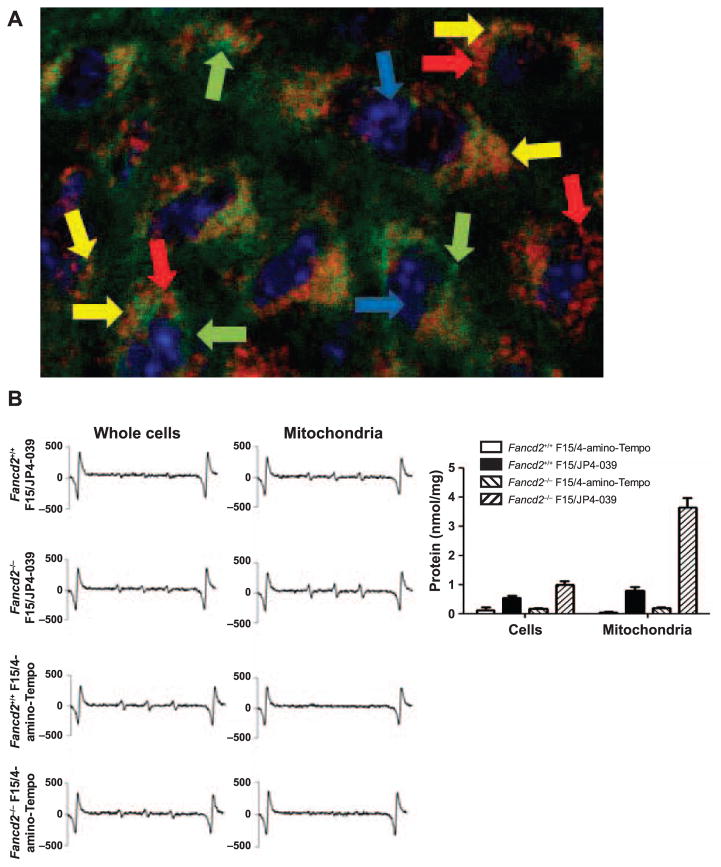
We evaluated whether the mechanism of normal tissue radioprotection by F15/JP4-039 was mediated by mitochondrial localization of drug. We measured the subcellular mitochondrial localization of fluorochrome-labeled BODI-PY-JP4-039 in oral cavity tissue cells from tumor-bearing mice. Fluorescent microscopy revealed mitochondrial targeting of BODIPY-JP4-039 in explanted oral cavity cells after in vivo intraoral delivery of F15/BODIPY-JP4-039 (Fig. 5A). In another assay based on cell explant, mitochondrial separation and EPR detection of the nitroxide signal, it was found that JP4-039, but not 4-amino-Tempo was localized to the mitochondria of oral cavity tissue cells in vivo in both Fancd2+/+ and Fancd2−/− mice (Fig. 5B; and Supplementary Tables S4–5, http://dx.doi.org/10.1667/RR14035.1.S1).
FIG. 5.
Mitochondrial mechanism of action of JP4-039 in radiation protection of the oral cavity of Fancd2−/−mice. Panel A: Mice were administered F15/BODIPY-JP4-039 intraorally and sacrificed 2 h later. Oral cavity tissue was removed, frozen in OCT and sectioned. The sections were fixed, then stained with an anti-TOM 20 antibody and observed with a fluorescent microscope. Fancd2−/− mouse cells demonstrated co-localization of BODIPY-JP4-039 with TOM 20 in the mitochondria. Similar results were seen in Fancd2+/+ and Fancd2+/−cells. Blue arrows point to cell nucleus. Green arrows point to BODIPY-JP-039. Red arrows point to mitochondria stained with anti-TOM. Yellow arrows point to mitochondria stained with anti-TOM and BODIPY-JP4-039. Panel B: Fancd2+/+ and Fancd2−/− mice were administered F15/JP4-039 or F15/4-amino-Tempo intraorally and sacrificed 2 h later. Oral cavity tissue was removed and homogenized and mitochondria were isolated. EPR assays demonstrated increased uptake of JP4-039, but not 4-amino-Tempo in the mitochondria of the Fancd2−/− and Fancd2+/+ mouse cells (nmol/nitroxide/mg protein).
The data confirmed that the nitroxide 4-amino-Tempo delivered by F15/4-amino-Tempo was indeed reaching cells and was biologically active. We detected the 4-amino-Tempo nitroxide signal in explanted oral cavity tissue whole cells (Fig. 5B). If 4-amino-Tempo had not reached the oral cavity cells or had become biochemically inactive, we would not have detected the nitroxide signal by EPR in whole cells.
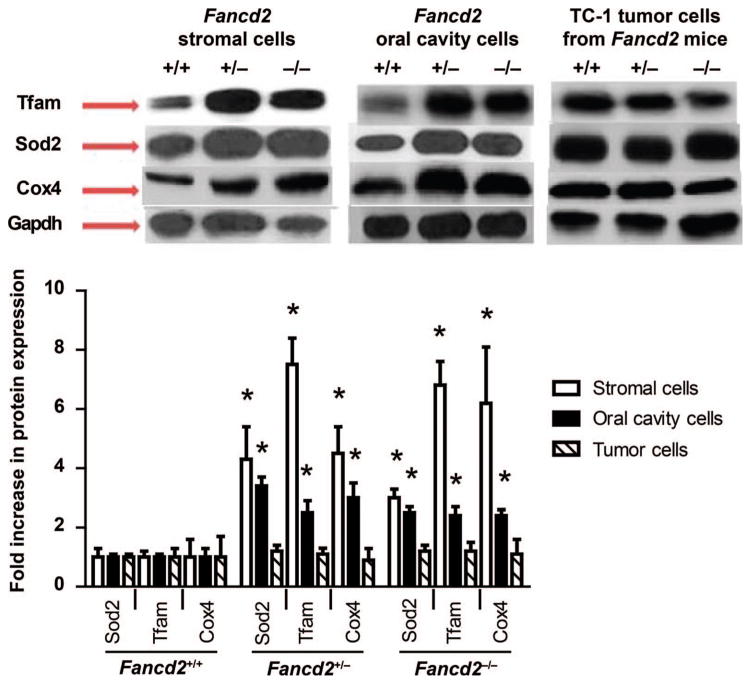
We next questioned whether the JP4-039-mediated radiation protection of normal tissue cells in Fancd2−/− mice might be attributable to increased mitochondria in those cells, perhaps reflecting a compensatory mechanism for their defective baseline nuclear DNA stress response to radiation. We measured mitochondrial protein levels in freshly removed Fancd2−/− compared with Fancd2+/+ mouse oral cavity cells and also in permanent bone marrow stromal cell lines derived from continuous bone marrow cultures of mice of each genotype (20) (Fig. 6). Freshly removed oral cavity tissue and permanent bone marrow stromal cell lines from Fancd2−/− mice compared with Fancd2+/+ had more mitochondria by weight, measuring Tfam, Sod2 and Cox4 (Fig. 6). Levels were also higher in heterozygote Fancd2+/–cells (Fig. 6). Electron microscopy confirmed this observation at the single cell level (not shown). TC-1 tumor cells explanted from tumors in each mouse genotype showed no change in mitochondrial signals (Fig. 6).
FIG. 6.
Quantitation of increased mitochondria in Fancd2−/− mouse oral cavity tissue and in cell lines. Western blot analysis for mitochondrial proteins Sod2, Tfam and Cox4 was performed with oral cavity cells, TC-1 tumors or bone marrow stromal cells (19). Freshly removed oral cavity cells from Fancd2+/+, Fancd2+/−and Fancd2−/− mice as well as explanted TC-1 tumors from each genotype were lysed and electrophoresed, and proteins were transferred to nitrocellulose membrane and probed for protein expression using antibodies to Sod2, Tfam and Cox4. Densitometry was performed to quantitate protein expression using LabWorks Image Acquisition and Analysis Software. Protein expression was standardized by comparing the densitometry results for Sod2, Tfam and Cox4 to Gapdh. Protein expression is shown as fold increase compared to oral cavity Fancd2+/+ cell expression, which was set as 1.0. There was a significant increase in Fancd2−/− mitochondrial proteins (P < 0.05) in the bone marrow stromal cells and oral cavity cells compared with corresponding Fancd2+/+ cells (*). Protein expression for explanted TC-1 tumor cells was independent of mouse genotype of the oral cavity microenvironment.
F15/JP4-039 Preserves Antioxidant Levels in Irradiated Normal Oral Tissue, but Not Tumors
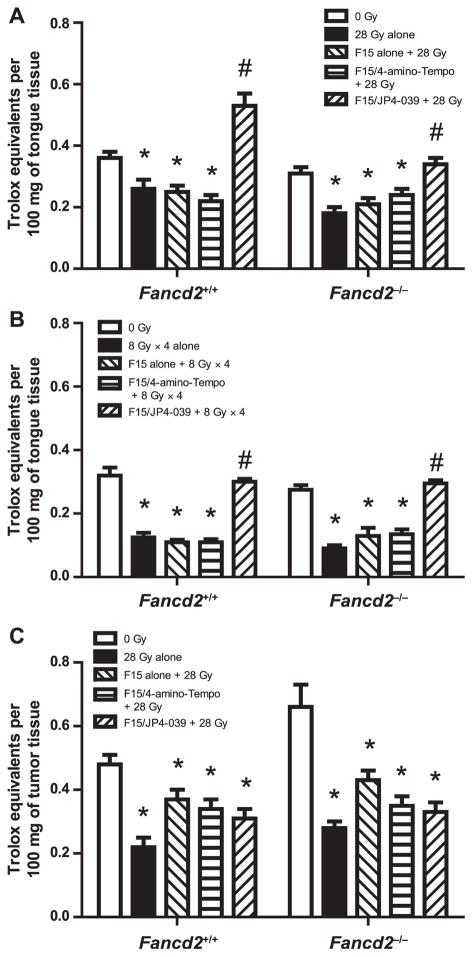
We measured antioxidant levels in mouse oral cavity tissues from Fancd2−/− compared with Fancd2+/+ experimental groups. Treatment with F15/JP4-039, but not F15/4-amino-Tempo or F15 alone, preserved levels of antioxidant stores in oral cavity cells from mice of both Fancd2+/+ and Fancd2−/− genotypes after single fraction (28 Gy) and fractionated (8 Gy × 4) irradiation (Fig. 7A and B). In contrast, F15/JP4-039 did not preserve antioxidant levels in irradiated TC-1 tumor xenografts explanted from the oral cavity of Fancd2+/+ and Fancd2−/− mice after a 28 Gy dose to the head and neck (Fig. 7C). The results establish that mitochondrial localization of nitroxide in JP4-039 but not 4-amino-Tempo-treated mice radioprotected Fancd2−/−, as well as heterozygote and wild-type mouse oral cavity cells and tissues. Furthermore, these data establish that mito-chondrial targeting of nitroxide in JP4-039-treated Fancd2−/−, as well as wild-type mice did not protect established tumors from therapeutic radiation exposure.
FIG. 7.
Pretreatment with F15/JP4-039 preserves levels of cellular antioxidants in irradiated oral cavity tissue in tumor-bearing mice. Cellular antioxidant levels (Trolox units) were determined in tissue from the oral cavity of Fancd2+/+ and Fancd2−/− mice 24 h after: 28 Gy single fraction (panel A), 8 Gy × 4 irradiation (panel B) or in TC-1 orthotopic tumors 28 Gy irradiated (panel C). Mice were pretreated with F15 alone, F15/4-amino-Tempo and F15/JP4-039, 15 min before each radiation fraction.
DISCUSSION
Radiotherapy of patients with head and neck cancer can result in significant normal tissue mucositis. We reasoned that testing of new radioprotectant agents might be effectively performed using a model system representative of radiosensitive normal oral cavity tissues.
Bone marrow transplanted, as well as nontransplanted FA patients are at high risk for solid tumors, in particular, head and neck cancer (1–5, 30–32). There is a significantly increased risk of squamous cell tumors of the head and neck, as well as esophageal cancer among FA patients (standardized incidence ratio, 500; 95% confidence interval, 300–781, P < 0.001) (3, 5, 21, 32). Treatment difficulties associated with many of these patients are due to the observed normal tissue toxicity at lower radiation doses (6).
We tested a mitochondrial-targeted GS-nitroxide, JP4-039, for radioprotection of oral cavity established tumors in radiosensitive, Fancd2−/− mice. When delivered in F15 emulsion, JP4-039, but not 4-amino-Tempo, was an effective radioprotector for Fancd2−/− mouse normal oral cavity mucosa, as measured by reduced tongue ulceration. TC-1 cell line derived and established tumors in the radiation field were not protected by mitochondrial-targeted JP4-039, consistent with the lack of radioprotection of other squamous cell tumors and tumor cell lines by mitochondrial-targeted SOD2-PL (8, 9). The oral cavity tumor response to fractionated irradiation was not altered by daily administration of F15/JP4-039. Irradiated tumors regressed as effectively in F15/JP4-039-treated Fancd2−/− mice compared with control irradiated mice. These results establish that JP4-039 is a safe and therapeutic radioprotector in radiosensitive Fancd2−/− mice under conditions that do not alter tumor response to radiation.
We observed an altered baseline expression pattern of gene transcripts in the oral cavity tissue of Fancd2−/−, as well as control mice by the presence of an established intraoral tumor, and this pattern was modified after irradiation. Both Fancd2−/− and heterozygote Fancd2+/− mice showed constitutive upregulation of several gene transcripts involved in the oxidative stress response and inflammation compared to levels observed with wild-type mice. The current observations on baseline gene transcript levels in the oral cavity of Fancd2−/− (C57BL/6) mice confirm and extend prior data with freshly removed tissue from Fancd2−/− mice on another genetic background mouse strain (18).
In Fancd2−/−, as well as control mice, with established oral cavity tumors, there were alterations in RNA transcript levels in the normal oral cavity tissue. Mouse tissues responded to the presence of a tumor by elevating transcript levels of Tgfb and Gadd45a. Levels of Nfkb and Il1a gene transcripts were not elevated by the presence of a tumor (all P ≥ 0.087). These data establish that gene transcript levels in the oral cavity tissue microenvironment at distant sites from a tumor respond to the presence of an established tumor, perhaps reflecting activity of specific resident T cells or other immunocytes (33). Further studies will be required to test this hypothesis.
Radiation-induced normal oral tissue toxicity was not altered by the presence of a tumor. Furthermore, the presence of tumor did not alter JP4-039 amelioration of radiation toxicity. It is currently not known whether JP4-039 (or 4-amino-Tempo) may have altered the kinetics of the tissue response to radiation, or whether the therapeutic effect of JP4-039 was mediated by reducing the level of toxicity, by improved healing or by a combination of mechanisms. With Fancd2−/− mice, JP4-039 had the effect of returning the magnitude of damage recovery to the level observed in irradiated wild-type mice. There are several possible mechanisms for the superior activity of JP4-039 compared to 4-amino-Tempo, which include mitochondrial targeting, decreased mitochondrial free radical production, decreased mitochondrial cytochrome C leakage and decreased cytoplasmic caspase activation. Other strategies to achieve the same therapeutic level of reduced toxicity may include other new agents with mitochondrial-targeting properties. Additional approaches to improve normal tissue toxicity may also involve delivery of a single drug at multiple times or adding combinations of radiation protectors and mitigators.
The mechanism of oral cavity radioprotection by JP4-039 was shown to be at the level of the mitochondria. Mitochondrial targeting of the nitroxide delivered by JP4-039, but not 4-amino-Tempo, was documented by both fluorophore-labeled BODIPY-JP4-039 localization to mitochondria in vivo and by electron spin trap detection of the nitroxide signal in explanted, then separated mitochondria from JP4-039 treated, but not 4-amino-Tempo treated mice. Treatment with JP4-039, but not 4-amino-Tempo, reduced the magnitude of radiation-induced depletion of antioxidant stores (by Trolox assay), and reduced the degree of radiation-induced ulceration of oral cavity tissue by histopathology. In prior radiobiology studies with Fancd2−/−mice on a different genetic background (18), mitochondrial localization of JP4-039 was also associated with reduction in radiation-induced DNA double-strand breaks.
The mechanism of the superior radioprotective effect of mitochondrial-targeted nitroxide over nonmitochondrial 4-amino-Tempo with no detectable tumor protection may be explained by the presence of more mitochondria in normal cells compared to tumor cells. Alternatively, the presence of tumor deep in the cheek may have reduced orally delivered drug uptake beyond the normal tissue. There was a uniform beneficial effect of JP4-039, in Fancd2−/−, as well as in heterozygote and wild-type mice. There may have been a threshold for the positive radioprotective effects that achieved an optimum protection in all genotypes. Radiation-induced oxidative stress and the effect of JP4-039 may have involved initial Sod2 or Nrf2 effects on downstream targets. Gene transcripts that were initially upregulated by radiation exposure, and then modulated by JP4-039, may have led to a decreased level of apoptosis. Whether Nrf2 was responsible for Sod2 changes or the reverse sequence is not known. The effect of radiation and JP4-039 on detailed elements of mitochondrial-nuclear cross talk is also not yet identified.
Radioprotection of the oral cavity of Fancd2−/− mice by F15/JP4-039 was achieved to a degree comparable to that of wild-type mice. This result may reflect a dominant role of the mitochondria in the mechanism of radiation-induced apoptosis and other mechanisms of cell death in oral cavity tissues, independent of the heightened nuclear DNA stress response to radiation in Fancd2−/− mice (10–12). The results are also consistent with a prior report showing “normalization” of the radiosensitivity of Fancd2−/−tissues (18) and cell lines (20) by JP4-039 through inhibition of the apoptotic pathway at the mitochondrial level. Alternatively, the radioprotection by JP4-039 in Fancd2−/− oral cavity tissue may be correlated to the increased number of mitochondria in these cells. This explanation is supported by our data showing greater mitochondrial Tfam, Cox4 and Sod2 levels by Western blot in Fancd2−/− compared with Fancd2+/+ tissues and cell lines. Previous studies showing normalization of the radiosensitive phenotype of Fancd2−/− cells in bone marrow stromal cell lines by JP4-039 are consistent with either model (19). FA cells may have more mitochondria but a defective mitochondrial pathway unrelated to cellular or tissue radiosensitivity (34, 35). Experiments are underway to quantitate the uptake of JP4-039 per cell relative to the number and viability of mitochondria in Fancd2−/− cells, as well as in cells from Fancg−/− and Fanca−/− mice. Taken together, these data support potential use of JP4-039 in clinical trials of radiotherapy in FA patients, as well as in the general population of head and neck cancer patients.
Supplementary Material
Table S1. Gene Bank number for gene expression analyzed by RT-PCR.
Table S2. Effect of F15/JP4-039 intraoral administration on modulation of head and neck radiation-induced RNA transcript changes in normal oral cavity tissue and TC-1 tumors in Fancd2−/− (C57BL/6) mice.
Table S3. F15/JP4-039 does not protect TC-1 tumor cells in vitro from ionizing radiation treatment.
Table S4. Distribution of 4-amino-Tempo nitroxide in subcellular fractions isolated from Fancd2+/+ and Fancd2−/−mouse oral cavity cells.
Table S5. Distribution of JP4-039 nitroxide in subcellular fractions isolated from Fancd2+/+ and Fancd2−/− mouse oral cavity cells.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases/National Institutes of Health (no. U19-A1068021) and the Fanconi Anemia Research Foundation. The UPCI Animal Facility used for these studies is supported in part by the NIH (grant no. P30CA047904).
Footnotes
Editor’s note. The online version of this article (DOI: 10.1667/ RR14035.1) contains supplementary information that is available to all authorized users.
Conflict of interest: Drs. Greenberger, Wipf, Kagan and Epperly are inventors on patents issued for the use of JP4-039 and MMS350 as radiation protectors or mitigators.
References
- 1.Soulier J. Fanconi anemia. Hematology Am Soc. 2011;2011:492–7. doi: 10.1182/asheducation-2011.1.492. [DOI] [PubMed] [Google Scholar]
- 2.Mehta PA, Harris RE, Davies SM, Kim MO, Mueller R, Lampkin B, et al. Numerical chromosomal changes and risk of development of myelodysplastic syndrome–acute myeloid leukemia in patients with Fanconi anemia. Cancer Genet Cytogenet. 2010;203:180–6. doi: 10.1016/j.cancergencyto.2010.07.127. [DOI] [PubMed] [Google Scholar]
- 3.Sareen A, Chaudhury I, Adams N, Sobeck A. Fanconi anemia proteins FANCD2 and FANCI exhibit different DNA damage responses during S-phase. Nucleic Acids Res. 2012;40:8425–39. doi: 10.1093/nar/gks638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Higuera I, Tangiguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001;7:249–62. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg PS, Socie G, Alter BP, Gluckman E. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 6.Birkeland AC, Auerbach AD, Sanborn E, Parashar B, Kuhel WI, Chandrasekharappa SC, et al. Postoperative clinical radiosensitivity in patients with Fanconi Anemia and head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137:930–4. doi: 10.1001/archoto.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Facts and Figures. American Cancer Society; 2015. ( http://bit.ly/1UFsomc) [Google Scholar]
- 8.Guo H, Seixas-Silva JA, Epperly MW, Gretton JE, Shin DM, Bar-Sagi D, et al. Prevention of radiation-induced oral cavity mucositis by plasmid/liposome delivery of the human manganese superoxide dismutase (SOD2) transgene. Radiat Res. 2003;159:361–70. doi: 10.1667/0033-7587(2003)159[0361:porioc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Epperly MW, Wegner R, Kanai AJ, Kagan V, Greenberger EE, Nie S, et al. Irradiated murine oral cavity orthotopic tumor antioxidant pool destabilization by MnSOD-plasmid liposome gene therapy mediates tumor radiosensitization. Radiat Res. 2007;267:289–97. doi: 10.1667/RR0761.1. [DOI] [PubMed] [Google Scholar]
- 10.Epperly MW, Gretton JE, Bernarding M, Nie S, Rasul B, Greenberger JS. Mitochondrial localization of copper/zinc superoxide dismutase (Cu/ZnSOD) confers radioprotective functions in vitro and in vivo. Radiat Res. 2003;160:568–78. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 11.Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, et al. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med. 2005;39:567–83. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Epperly MW, Gretton JE, Bernarding M, Nie S, Rasul B, Greenberger JS. Mitochondrial localization of copper/zinc superoxide dismutase (Cu/ZnSOD) confers radioprotective functions in vitro and in vivo. Radiat Res. 2003;160:568–78. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 13.Hall E, Giaccia A. Radiobiology for the radiologist. 6. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 14.Tyurin VA, Tyurina YY, Ritov VB, Lysytsya A, Amoscato AA, Kochanek PM, et al. Oxidative lipidomics of apoptosis: Quantitative assessment of phospholipid hydroperoxides in cells and tissues. Methods Mol Biol. 2010;610:353–74. doi: 10.1007/978-1-60327-029-8_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoyanovsky DA, Huang Z, Jiang J, Belikova NA, Tyurin V, Epperly MW, et al. A manganese-porphyrin complex decomposes hydrogen peroxide, compartmentalizes into mitochondria, inhibits apoptosis, and acts as a radiation mitigator in vivo. ACS Med Chem Lett. 2011;362:21–34. doi: 10.1021/ml200142x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rwigema JCM, Beck B, Wang W, Doemling A, Epperly MW, Shields D, et al. Two strategies for the development of mitochondrial-targeted small molecule radiation damage mitigators. Int J Radiat Oncol Biol Phys. 2011;80:860–8. doi: 10.1016/j.ijrobp.2011.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epperly MW, Rwigema J-CM, Li S, Gao X, Wipf P, Goff J, et al. Intraesophageal administration of GS-nitroxide (JP4-039) protects against ionizing irradiation-induced esophagitis. In Vivo. 2010;24:811–21. [PMC free article] [PubMed] [Google Scholar]
- 18.Berhane H, Shinde A, Kalash R, Xu K, Epperly MW, Goff J, et al. Amelioration of irradiation induced oral cavity mucositis and distant bone marrow suppression in Fancd2−/− (FVB/N) mice by intraoral JP4-039/F15. Radiat Res. 2014;182:35–49. doi: 10.1667/RR13633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Zhang X, et al. GS-nitroxide (JP4-039)-mediated radioprotection of human Fanconi Anemia cell lines. Radiat Res. 2011;176:603–12. doi: 10.1667/rr2624.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berhane H, Epperly MW, Goff J, Kalash R, Cao S, Franicola D, et al. Radiobiologic differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi Anemia (Fancd2−/−) mice. Radiat Res. 2014;181:76–89. doi: 10.1667/RR13405.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parmar K, Kim J, Sykes SM, Shimamura A, Stuckert P, Zhu K, et al. Hematopoietic stem cell defects in mice with deficiency of Fancd2 or Usp1. Stem Cells. 2010;28:1186–95. doi: 10.1002/stem.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 23.Ang KK, Landuyt W, Xu FX, Vanuytsel L, van der Schueren E. The effect of small radiation doses per fraction on mouse lip mucosa assessed using the concept of partial tolerance. Radiother Oncol. 1987;8:79–86. doi: 10.1016/s0167-8140(87)80025-7. [DOI] [PubMed] [Google Scholar]
- 24.Dörr W, Breitner A, Kummermehr J. Capacity and kinetics of SLD repair in mouse tongue epithelium. Radiother Oncol. 27:36–45. doi: 10.1016/0167-8140(93)90042-7. 199. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopalan MS, Stone B, Rwigema J-C, Salimi U, Epperly MW, Goff J, et al. Intraesophageal manganese superoxide dismutase-plasmid liposomes ameliorates novel total body and thoracic irradiation sensitivity of homologous deletion recombinant negative nitric oxide synthase-1 (NOS1−/−) mice. Radiat Res. 2010;174:297–312. doi: 10.1667/RR2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulrich G, Ziessel R, Hartman A. The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew Chem Int Ed. 2008;47:1184–201. doi: 10.1002/anie.200702070. [DOI] [PubMed] [Google Scholar]
- 27.Frantz MC, Forbeck EM, Davoren JE, Wang Z, Epperly MW, Greenberger JS, et al. Synthesis and biological analysis of mitochondria-targeted nitroxide conjugates based on Gramicidin S. J Am Chem Soc. 2015 in press. [Google Scholar]
- 28.Kalash R, Epperly MW, Goff J, Dixon T, Sprachman MM, Zhang X, et al. Amelioration of irradiation pulmonary fibrosis by a water-soluble bi-functional sulfoxide radiation mitigator (MMS350) Radiat Res. 2013;180:474–90. doi: 10.1667/RR3233.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dörr W, Dörr E, Haagen J, Hartmann JT, Riesenbeck D, Schmidt M, et al. Side effects of radiotherapy in the oral cavity. MMW Fortschritte der Medizin. 2010;152:37–9. German. [PubMed] [Google Scholar]
- 30.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JW, Pitot HC, Strati K, Spardy N, Duensing S, Grompe M, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70:9959–68. doi: 10.1158/0008-5472.CAN-10-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutler DI, Auerbach AD, Satagopan J, Giampietro PF, Batish SD, Huvos AG, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi Anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–12. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 33.Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song J-Y, et al. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–5. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- 34.Kumari U, Jun WY, Bay BH, Lyakhovich A. Evidence of mitochondrial dysfunction and impaired ROS detoxifying machinery in Fanconi Anemia cells. Oncogene. 2014;33:165–72. doi: 10.1038/onc.2012.583. [DOI] [PubMed] [Google Scholar]
- 35.Stoepker C, Ameziane N, van der Lelij P, Kooi IE, Oostra AB, Rooimans MA, et al. Defects in the Fanconi Anemia pathway and chromatid cohesion in head and neck cancer. Cancer Res. 2015;75:3543–53. doi: 10.1158/0008-5472.CAN-15-0528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Gene Bank number for gene expression analyzed by RT-PCR.
Table S2. Effect of F15/JP4-039 intraoral administration on modulation of head and neck radiation-induced RNA transcript changes in normal oral cavity tissue and TC-1 tumors in Fancd2−/− (C57BL/6) mice.
Table S3. F15/JP4-039 does not protect TC-1 tumor cells in vitro from ionizing radiation treatment.
Table S4. Distribution of 4-amino-Tempo nitroxide in subcellular fractions isolated from Fancd2+/+ and Fancd2−/−mouse oral cavity cells.
Table S5. Distribution of JP4-039 nitroxide in subcellular fractions isolated from Fancd2+/+ and Fancd2−/− mouse oral cavity cells.