Abstract
It is possible that SGLT2 inhibitors trigger euglycemic diabetic ketoacidosis in some patients. Possible mechanism of euglycemic DKA induced by SGLT2 inhibitors is illustrated.
Diabetic ketoacidosis (DKA) is a serious acute complication of diabetes mellitus that occasionally can become life threatening. It is induced as a result of a profound deficiency of insulin action in the body, often developing in individuals with poorly controlled type 1 diabetes or in those with type 2 diabetes who are subject to external stress such as infection, injury, or surgery. Although DKA is typically associated with marked hyperglycemia and resultant dehydration, it can occur with only a moderate increase in blood glucose (BG) levels or, in rare instances, in the setting of normal glucose concentrations. This latter, uncommon form of DKA, known as euglycemic or normoglycemic DKA, was originally defined as DKA with a BG level of <300 mg/dL, but it is now recognized as that in the presence of a BG concentration of <200 mg/dL. Euglycemic DKA develops mostly in individuals with type 1 diabetes, occurring only rarely in those with type 2 diabetes. Cases of euglycemic DKA have also been reported in pregnant women with diabetes mellitus, possibly as a result of the ketogenic change in metabolism during pregnancy.
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors are newly launched oral hypoglycemic drugs indicated for type 2 diabetes mellitus that prevent the reabsorption of glucose from primary urine at the proximal renal tubules by targeting SGLT2. The drugs have a relatively pronounced glucose‐lowering effect with a low risk of hypoglycemia when administered as monotherapy. Given that they promote excretion of an energy source into urine, treatment with these drugs reduces body weight and has pleiotropic effects attributable to weight loss including amelioration of insulin resistance, dyslipidemia, and nonalcoholic fatty liver disease. Their favorable clinical profile has increased interest in SGLT2 inhibitors by health care providers. The entire picture concerning the humoral and metabolic effects of these drugs in patients has not been recognized, however.
Recent evidence suggests that euglycemic DKA might occur not so infrequently in individuals treated with SGLT2 inhibitors. Peters et al.1 recently reported 13 episodes of DKA associated with mild hyperglycemia or normoglycemia in nine individuals treated with the SGLT2 inhibitor canagliflozin. Seven of these nine individuals, who manifested 11 episodes of DKA, had type 1 diabetes mellitus, which is not an on‐label indication for this class of drug; the BG levels at the onset of DKA were <200 mg/dL in six episodes, 200–250 mg/dL in four episodes, and undetermined in one episode. The remaining two individuals in the report had type 2 diabetes and developed DKA with BG levels of <200 mg/dL;1 both had recently undergone surgery, either 1 week or 12 h earlier. Hine et al.2 reported two cases of euglycemic DKA in individuals who had been diagnosed with type 2 diabetes mellitus and were being treated with the SGLT2 inhibitor dapagliflozin. One of the two subjects had undergone distal pancreatectomy for a mucinous cystadenoma and had been treated with insulin after the surgery, but insulin was switched to the SGLT2 inhibitor during the subsequent stay in intensive care. Within 24 h after the treatment change, the patient developed DKA with a BG level of 106 mg/dL. For the second patient, who developed DKA with a BG level of 187 mg/dL, no direct contributory factor was reported2. However, this individual had a history of pancreatitis and pancreatic atrophy. Hayami et al.3 described euglycemic DKA in a patient with no apparent organic problems with the pancreas who was taking the SGLT2 inhibitor ipragliflozin. This individual, diagnosed with Prader‐Willi syndrome and diabetes mellitus, had been treated with the combination of a sulfonylurea, a DPP4 inhibitor, and a biguanide, but this regimen was switched to monotherapy with the SGLT2 inhibitor. Thirteen days after the change in treatment, she developed DKA with a BG level of 191 mg/dL and an undetectable urinary level of C‐peptide. After treatment of the DKA for 6 days, the excretion of C‐peptide in urine had increased to 40.2 μg/day. Prior to this event, the patient had followed a low‐carbohydrate diet with an estimated carbohydrate intake of 66 g/day.
In Japan, six SGLT2 inhibitors (ipragliflozin, dapagliflozin, luseogliflozin, tofogliflozin, canagliflozin, and empagliflozin) are on the market, and the manufacturers disclose the postmarketing reports of adverse events of the drugs on their websites (Table 1). As of July 2015, a total of 28 cases of DKA or ketoacidosis had been reported. The BG levels at the onset of these events were <200 mg/dL in seven cases (including that described by Hayami et al.3), 200–299 mg/dL in two cases, >300 mg/dL in five cases, and not determined in 14 cases (Table 1). In addition, the U.S. Food and Drug Administration recently made known in a safety announcement that 20 cases of DKA, ketoacidosis, or ketosis associated with SGLT2 inhibitors had been reported from March 2013 (date of approval of the first drug in this class) through 6 June 2014 and that ‘glucose levels were only mildly elevated at less than 200 mg/dL in some reports' (http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm).
Table 1.
Cases of DKA and ketoacidosis in postmarketing reports by drug manufacturers of adverse events in patients treated with SGLT2 inhibitors
| Age (years) | Sex | BMI (kg/m2) | pH | BG (mg/dL) | Possible contributing factors |
|---|---|---|---|---|---|
| Ipragliflozin | |||||
| 30s | F | 28.4 | 7.121 | 185 | Cessation of insulin secretagogue, carbohydrate restriction |
| 70s | M | 21.9 | 7.120 | 619 | Cessation of insulin secretagogue |
| 20s | M | 31.2 | ND | ND | Starvation (for 3 days) |
| 40s | M | 38.5 | ND | 245 | NA |
| 30s | M | 31.8 | ND | ND | Strenuous exercise? |
| 70s | F | 17.4 | ND | 413 | NA |
| 20s | F | 36.7 | ND | 292 | NA |
| 80s | F | 16.7 | ND | 398 | NA |
| 20s | F | 29.4 | 7.268 | 175 | NA |
| 60s | F | 16.9 | ND | ND | Appetite loss due to a flulike condition |
| 60s | M | 35.3 | 7.348 | 157 | NA |
| 50s | F | 20.3 | ND | 140 | NA |
| 60s | M | NA | ND | ND | NA |
| Dapagliflozin | |||||
| 50s | F | ND | ND | ND | Cessation of insulin |
| 60s | M | ND | 7.1 | 198 | Cessation of insulin |
| 70s | F | ND | 7.2 | 450 | NA |
| 40s | M | ND | 7.312 | 188 | Pancreatic cancer |
| 40s | F | ND | ND | ND | NA |
| 30s | F | ND | ND | 90 | Appendicitis |
| Tofogliflozin | |||||
| 30s | M | ND | ND | ND | Sick days(not specified) |
| 40s | M | ND | ND | ND | Carbohydrate restriction |
| 80s | F | ND | ND | ND | NA |
| Luseogliflozin | |||||
| 50s | M | ND | ND | ND | Cerebral infarction |
| Canagliflozin | |||||
| 50s | M | ND | ND | ND | Heavy alcohol use |
| 50s | F | ND | ND | ND | Influenza |
| 40s | M | ND | ND | ND | Cessation of insulin due to appetite loss |
| 60s | F | ND | ND | ND | Cessation of insulin and insulin secretagogue |
| Empagliflozin | |||||
| 30s | M | ND | ND | 798 | NA |
DKA, diabetic ketoacidosis; SGLT2, sodium‐glucose cotransporter 2; BMI, body mass index; pH, arterial blood pH; BG, blood glucose; ND, not determined; NA, information not available. In most cases, it is not known whether blood glucose level was determined with whole blood or plasma. The information described is available on the following websites (in Japanese): ipragliflozin, http://med2.astellas.jp/med/jp/basic/details/SGL/shihanchosa/shchosa-sgl07.pdf; dapagliflozin, http://med2.astrazeneca.co.jp/product/fxg_report201506.pdf and https://www.ononavi1717.jp/contents/pdf/diabetes/forxiga/report12.pdf; tofogliflozin, http://e-mr.sanofi.co.jp/di/information/apw_interim.pdf?date=20150622094749 and http://www.kowa-souyaku.co.jp/file/1506se_dbt.pdf; luseogliflozin; http://medical.nikkeibp.co.jp/all/special2/lusefi/pdf/survey_lusg_01.pdf; canagliflozin, http://medical.mt-pharma.co.jp/intro/can/pdfs/sideeffect_150716.pdf; and empagliflozin, http://www.bij-kusuri.jp/information/jad_t_info_201507.pdf.
Given the limited amount of information available to date, it is difficult to judge precisely whether the reported cases of DKA were directly triggered by SGLT2 inhibitors or were induced independently of these drugs, with the SGLT2 inhibitors simply reducing the BG levels during the events. However, a possible mechanism by which SGLT2 inhibitors might trigger euglycemic DKA is presented in Figure 1. SGLT2 inhibitors lower BG levels by increasing urinary glucose excretion, which in turn reduces insulin secretion from pancreatic β‐cells. The decline in circulating insulin levels results in a lowering of the antilipolytic activity of insulin and consequent stimulation of the production of free fatty acids, which are converted to ketone bodies by β‐oxidation in the liver. Moreover, insulin stimulates the activity of acetyl‐CoA carboxylase, which produces malonyl‐CoA, a potent inhibitor of carnitine palmitoyltransferase–I (CPT‐I). Given that CPT‐I promotes the transport of fatty acids into mitochondria and hence increases the rate of β‐oxidation, the decrease in the circulating level of insulin promotes the production of ketone bodies through activation of CPT‐I. In addition, evidence suggests that the administration of SGLT2 inhibitors stimulates the secretion of glucagon4, which might be either a secondary effect mediated by the decrease in insulin secretion or a direct effect of SGLT2 inhibitors on pancreatic α‐cells.4 Given that glucagon inhibits acetyl‐CoA carboxylase and thereby increases CPT‐I activity in the liver, the up‐regulation of glucagon secretion also likely contributes to the overproduction of ketone bodies.
Figure 1.
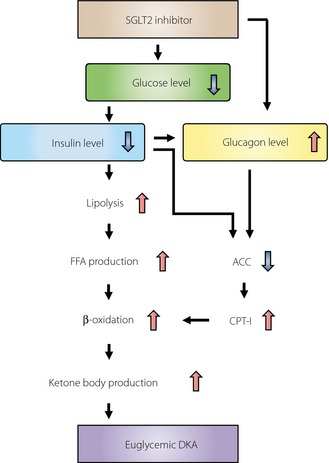
Possible mechanism of euglycemic DKA induced by SGLT2 inhibitors SGLT2, sodium‐glucose cotransporter 2; FFA, free fatty acid; ACC, acetyl‐CoA carboxylase; CPT‐I, carnitine palmitoyltransferase–I; DKA, diabetic ketoacidosis.
Obviously, not all individuals who take SGLT2 inhibitors are at high risk for euglycemic DKA. Eleven episodes reported by Peters et al.1 developed in patients with type 1 diabetes, and the two cases reported by Hine et al.2 were in patients with organic pancreatic insufficiency. The postmarketing reports of the Japanese drug manufacturers do not feature any cases in individuals with type 1 diabetes but include one case in a patient with pancreatic cancer (Table 1). Given that administration of SGLT2 inhibitors can further reduce the circulating level of insulin in individuals with impaired insulin secretion, caution is warranted in dispensing these drugs for such patients. The case reported by Hayami et al.3 occurred in a patient who followed a low‐carbohydrate diet, which also reduces circulating insulin levels and triggers a ketogenic metabolic state. The postmarketing reports of the Japanese drug manufacturers include two cases of DKA associated with carbohydrate restriction (one being that described by Hayami et al.3) and one case associated with long‐term (3 days) starvation (Table 1). The withdrawal of insulin or insulin secretagogues at the onset of treatment with an SGLT2 inhibitor, which occurred in one case described by Hine et al.2 and in the case reported by Hayami et al.3, also appears to be a risk factor for the development of euglycemic DKA. In the postmarketing reports, insulin treatment was terminated in four cases (likely at the instruction of health care providers in three cases and based on the patient's own judgment as a result of appetite loss in one case) and insulin secretagogues were withdrawn (on the instruction of health care providers) in three cases (including the case described by Hayami et al.3) before the development of the events.
The relative frequencies of DKA in individuals taking SGLT2 inhibitors in Japan and in other countries are unknown. However, evidence suggests that individuals with type 2 diabetes in East Asia, including Japan, tend to be leaner and their disease to be more largely attributable to β‐cell insufficiency5. It is thus possible that more caution is warranted in the administration of SGLT2 inhibitors to patients with such characteristics—and, in particular, to those with a long history of diabetes, given that β‐cell function in type 2 diabetes is thought to decline with time. The postmarketing reports of the Japanese drug manufacturers do not include information on insulin secretory capacity for most cases, and body mass index is available for only 12 cases (<22 kg/m2 in five cases, >25 kg/m2 in seven cases) (Table 1).
A point worth emphasizing is that euglycemic DKA might easily be missed based on only clinical signs, given that it is not necessarily associated with typical manifestations of DKA such as dehydration induced by marked hyperglycemia. Severe metabolic acidosis alone has the potential to become a life‐threatening condition, however. Further insight into the metabolic and humoral effects of SGLT2 inhibitors as well as more detailed clinical information on related DKA cases should help to provide a more solid basis for the safe, appropriate, and broad application of this new class of drugs.
Disclosure
W.O. and K.S. have received lecture fees from Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, MSD, Novartis, Ono Pharmaceutical, Sanofi, and Taisho Toyama Pharmaceutical. W.O. has received research support from Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Kowa Pharmaceutical, Mitsubishi Tanabe Pharma, MSD, Novartis, Ono Pharmaceutical, and Taisho Toyama Pharmaceutical, and K.S. has received research support from Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, MSD, Novartis, and Ono Pharmaceutical.
Acknowledgments
We thank Takeshi Ohara, Yushi Hirota, and Genzo Iguchi for discussion and suggestions.
References
- 1. Peters AL, Buschur EO, Buse JB, et al Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium‐glucose cotransporter 2 inhibition. Diabetes Care 2015. doi:10.2337/dc15‐0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hine J, Paterson H, Abrol E, et al SGLT inhibition and euglycaemic diabetic ketoacidosis. Lancet Diabetes Endocrinol 2015; 3: 503–504. [DOI] [PubMed] [Google Scholar]
- 3. Hayami T, Kato Y, Kamiya H, et al Case of ketoacidosis by a sodium‐glucose cotransporter 2 inhibitor in a diabetic patient with a low‐carbohydrate diet. J Diabetes Investig 2015; 6: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kibbey RG. SGLT‐2 inhibition and glucagon: cause for alarm? Trends Endocrinol Metab 2015; 26: 337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Møller JB, Pedersen M, Tanaka H, et al Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 2014; 37: 796–804. [DOI] [PubMed] [Google Scholar]


