Abstract
Aims/Introduction
Visfatin is a newly discovered adipocytokine hormone, which exerts an insulin‐like effect by binding to the insulin receptor‐1. However, the role of visfatin in human gestational diabetes mellitus (GDM) remains controversial. The purpose of the present study was to investigate the correlation between serum visfatin and metabolism of glucose and lipid in GDM.
Materials and Methods
This was a prospective study. A total of 38 GDM patients and 35 age‐ and body mass index‐matched controls were studied between January 2012 and October 2013. Fasting serum levels of visfatin, fasting plasma glucose, hemoglobin A1c and lipid profile were measured. Two‐tailed t‐tests and Pearson's correlation coefficient were used to analyze the data.
Results
Perinatal visfatin levels were negatively correlated with fasting plasma glucose, insulin resistance index and triglycerides in controls (r = −0.47, −0.51, −0.57, respectively; P < 0.05), and positively correlated with high‐density lipoprotein cholesterol (r = 0.32, P < 0.05). A positive correlation with visfatin level only appeared in weight gain and body mass index in women with GDM (r = 0.36, 0.45, respectively; P < 0.05).
Conclusions
Visfatin appears to be involved in glucose and lipid metabolism regulation and insulin resistance, suggesting a role in GDM pathogenesis.
Keywords: Gestational diabetes mellitus, Glucose and lipid metabolism, Visfatin
Introduction
Adipocytokines, the bioactive proteins produced by adipose tissue, have recently been implicated in mediating insulin resistance (IR)1. It has been suggested that hormones secreted by the placenta and cytokines secreted by adipose tissues are related to the development of IR during pregnancy, possibly playing an important role in the pathogenesis of gestational diabetes mellitus (GDM)1.
Visfatin is a newly discovered 52 kDa adipocytokine hormone in humans, and is preferentially produced by visceral adipose tissue2, 3, 4, 5, 6. It exerts an insulin‐like effect by binding to the insulin receptor‐1. Thus, visfatin is able to cause hypoglycemia through a combined mechanism involving the reduction of glycogenolysis in hepatocytes, and stimulation of glucose utilization in adipocytes and myocytes through downstream signaling2. A firm correlation has been previously established between plasma visfatin levels and type 2 diabetes mellitus, and recent research also suggests that circulating maternal visfatin levels could play a role in the development GDM3.
Pregnancy presents a unique situation in which transient physiological insulin resistance, approaching levels observed in type 2 diabetes mellitus patients, often forms in order to facilitate nutrient delivery to the developing fetus3. Notably, pregnancy is also associated with the most dramatic increase in adipose tissue observed during adulthood1. Both IR and reduced insulin secretion in gestational diabetes mellitus (GDM) have been linked to genetic traits, though IR is generally considered to play the dominant role7.
Though the role of visfatin in human GDM remains controversial, it is likely that visfatin is involved in the pathogenesis of GDM. In fact, circulating maternal visfatin concentrations of the plasma and serum have been reported to be both higher3, 8, 9, 10 and lower11, 12 in GDM patients compared with healthy pregnant women by different studies, contributing to the controversial role of visfatin in GDM. Interestingly, higher levels of circulating maternal visfatin have been observed in patients with fetal growth restriction than in patients with an appropriate‐for‐gestational‐age neonate, suggesting a possible correlation between insulin resistance associated with GDM and fetal growth3.
The present study investigates the possible association between visfatin and GDM using measurement of visfatin, body mass index (BMI), blood lipids and blood glucose in pregnant females with GDM compared with normal pregnant women. The present analysis was designed to provide further information pertaining to the role of visfatin in GDM.
Materials and Methods
Participants
The protocols in the present study were approved by Women's Hospital Ethics Committee of School of Medicine, Zhejiang University (Hangzhou, China; Approval ID: 20120016). Written informed consent was obtained from patients.
A total of 38 pregnant women with GDM (age 28 ± 4.1 years; BMI 32.1 ± 2.2 kg/m2) and 35 age‐ and body mass index‐matched controls (age 29 ± 3.2 years; BMI 29.4 ± 3.2 kg/m2) were recruited for the present study between gestational weeks 24 and 28. All participants were recruited while undergoing regular prenatal examinations between January 2012 and October 2013. GDM was diagnosed during gestational weeks 24–28 according to the American Diabetes Association diagnostic criteria (2011)13. All participants completed pregnancy in gestational weeks 37–40. Perinatal baseline data for all participants are presented in Table 1.
Table 1.
Perinatal baseline data for all participants
| Parameter | Mean ± SD | t‐statistic | P‐value | |
|---|---|---|---|---|
| GDM group (n = 38) | Control group (n = 35) | |||
| Age (years) | 28 ± 4.1 | 29 ± 3.2 | −0.213 | 0.512 |
| Gravidity | 1.66 ± 0.76 | 1.57 ± 0.77 | 0.198 | 0.840 |
| Gestational weeks | 38.4 ± 2.1 | 38.2 ± 3.2 | −0.234 | 0.232 |
| BMI at childbirth (kg/m2) | 32.1 ± 2.1 | 29.4 ± 3.2 | 2.889 | 0.039a |
| BMI before pregnant (kg/m2) | 23.2 ± 3.6 | 22.9 ± 3.2 | 1.922 | 0.098 |
| Pregnancy weight gain (kg) | 20.3 ± 4.3 | 15.6 ± 2.9 | 2.152 | 0.041a |
Pregnancy weight gain and body mass index (BMI) at childbirth in the gestational diabetes mellitus (GDM) group were significantly higher than that of the control group. SD, standard deviation.
Subjects were excluded from the study in the case of multiple pregnancies; history of or present cardiovascular disease, hypertensive disorders, liver disease, or kidney disease; current or persistent infection; pre‐pregnancy diabetes; and thyroid disease. All women included in the study were at low risk, as indicated by Down's screening and 3‐D ultrasonography during pregnancy. All participants provided written informed consent before participating in the study, and the study design and procedures were approved by our institution.
Sample collection and assessment
Standard clinical procedures were used to measure BMI (kg/m2) before and after pregnancy. Before‐pregnancy data were taken, as necessary, from patient records collected during previous routine prenatal appointments. Total weight gain during pregnancy was similarly calculated using weight measurements collected during routine clinical visits.
Fasting blood samples were taken from all participants on the day of delivery. All samples were kept at room temperature for at least 30 min to allow the blood to clot, and were then centrifuged at 2000 g for 15 min. Serum was collected and stored at −80°C until assay. Serum visfatin concentration was determined with enzyme‐linked immunosorbent assay (EK‐003‐80; Phoenix Pharmaceuticals, Burlingame, CA, USA).
Homeostasis Model of Assessment – Insulin Resistance (HOMA‐IR) was determined using a homeostasis model, as previously described, wherein14: HOMA = fasting plasma glucose × fasting insulin/22.5. Fasting plasma glucose (FPG), fasting insulin (FINS), hemoglobin A1c (HbA1c), total cholesterol (TC), triglyceride (TG), low‐density lipoprotein cholesterol (LDL‐C) and high‐density lipoprotein cholesterol (HDL‐C) were subsequently measured by using routine laboratory methods.
Statistical analysis
The data were presented as means ± standard deviations. Two‐tailed t‐tests were used to analyze differences between the control and GDM group. P‐values less than 0.05 were considered statistically significant (P < 0.05). The magnitude of correlations was determined by Pearson's correlation coefficient. P < 0.05 was considered statistically significant. All the data were analyzed by statistical package Prism 3.0 (GraphPad software, San Diego, CA, USA).
Results
Comparison of clinical data
Age, gestational week, parity, gravidity and pre‐pregnancy BMI were recorded for all participants. A comparison of these parameters showed no significant differences between the GDM and control groups. Notably, the GDM group showed significantly higher weight gain and BMI at childbirth than the control group (P < 0.05; Table 1).
Visfatin was detected in the serum of all participants. Pre‐delivery serum visfatin, FPG, FINS, HbA1c, TG, LDL and HOMA‐IR were significantly higher in the GDM group than in the control group (P < 0.05). Only HDL‐C was significantly lower in the GDM group than in the control group (P < 0.05). No differences were observed in TC levels between two groups (Tables 2 and 3).
Table 2.
Parameters of serum insulin resistance for all participants
| Parameter | Mean ± SD | t‐value | P‐value | |
|---|---|---|---|---|
| GDM group (n = 38) | Control group (n = 35) | |||
| Visfatin (ng/mL) | 189.4 ± 77.3 | 117.9 ± 44.3 | 7.123 | 0.001a |
| FPG (mmol/L) | 5.4 ± 0.5 | 4.4 ± 0.6 | 3.312 | 0.034a |
| FINS (mU/L) | 19.1 ± 4.3 | 12.1 ± 3.3 | 3.845 | 0.021a |
| HbA1c (%) | 6.1 ± 1.9 | 5.2 ± 0.9 | 3.511 | 0.031a |
| HOMA‐IR | 3.1 ± 1.0 | 2.6 ± 0.7 | 4.219 | 0.017a |
P < 0.05, compared with control group. FINS, fasting insulin; FPG, fasting plasma glucose; GDM, gestational diabetes mellitus; HbA1c, haemoglobin A1; HOMA‐IR, Homeostasis Model of Assessment – Insulin resistance; SD, standard deviation.
Table 3.
Parameters of serum lipid metabolism for all participants
| Parameter | Mean ± SD | t‐value | P‐value | |
|---|---|---|---|---|
| GDM group (n = 38) | Control group (n = 35) | |||
| TG (mmol/L) | 3.2 ± 1.3 | 2.4 ± 0.9 | −2.934 | 0.033a |
| HDL (mmol/L) | 1.9 ± 0.4 | 2.4 ± 0.8 | 4.123 | 0.010a |
| LDL (mmol/L) | 3.2 ± 1.1 | 2.2 ± 0.8 | 3.765 | 0.023a |
| TC (mmol/L) | 5.9 ± 2.7 | 4.8 ± 2.0 | −1.56 | 0.075 |
P < 0.05, compared with control group. HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Correlation analyses
Visfatin was negatively correlated with FBG, HOMA‐IR and TG in the control group (r = −0.47, −0.51, −0.57, respectively; P < 0.05; Figure 1a–c). Visfatin was also positively correlated with HDL‐C (r = 0.32; P < 0.05; Figure 1d). No statistically significant correlations were shown between visfatin and any of the indicators in the GDM group; however, visfatin was shown to be positively correlated with pregnancy weight gain and BMI at childbirth in women with GDM (r = 0.36, 0.45 respectively; P < 0.05; Figure 1e,f).
Figure 1.
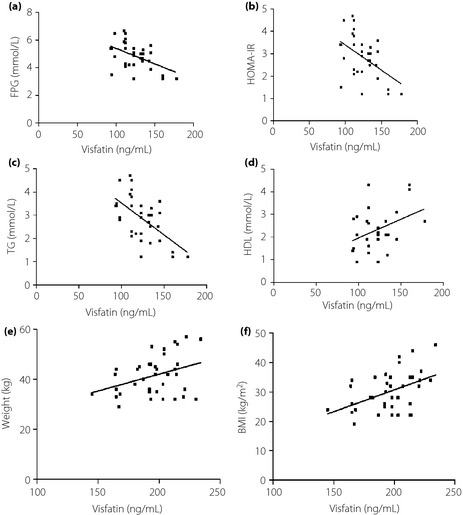
Correlation analyses. (a) Control group: visfatin vs fasting plasma glucose (FPG). (b) Control group: visfatin vs Homeostasis Model of Assessment (HOMA). (c) Control group: visfatin vs triglyceride (TG). (d) Control group: visfatin vs low‐density lipoprotein cholesterol (HDL). (e) Gestational diabetes mellitus (GDM) group: visfatin vs pregnancy weight gain. (f) Control group: visfatin vs body mass index.
Discussion
Elevated serum levels of the adipocytokine visfatin, increased weight gain and higher BMI were observed in GDM patients. These findings suggest that the insulin‐like hypoglycemic effect of visfatin of the GDM patients is damaged, which leads to elevated glucose levels. This might be involved in the pathogenesis and development of GDM. Although the full mechanisms behind this process are not fully understood, these results confirm the link between GDM and increased visfatin levels. These data will aid in resolving the current controversy surrounding visfatin in GDM development and pathogenesis.
While stable visfatin levels are normally observed in the serum of pregnant women, gradually increasing serum visfatin levels are characteristic of gestational weeks 24–28, the period generally referred to as mid‐pregnancy9. This effect is most pronounced in normal pregnant women, with a progressively smaller response observed in women with pregnancy‐impaired glucose tolerance or GDM. Furthermore, mid‐pregnancy visfatin levels generally show a positive correlation with HOMA, fasting insulin levels and blood glucose levels 2 h after dextrose administration9. These observations provided the initial foundation for the hypothesis that visfatin possessed insulin‐like effects that might serve an important function during pregnancy. Coupled with the current findings, these observations suggest that visfatin is likely to play a protective role, providing an additional response to hyperglycemia during pregnancy.
Visfatin could also impact insulin sensitivity, as evidenced by observations that women with GDM show more severe IR than normal pregnant women15. The increase of visfatin in GDM might be a feedback or compensation mechanism that functions in maintenance of the normal metabolic balance. The role of visfatin in insulin sensitivity in GDM patients is supported by previously identified polymorphisms in the visfatin gene that have been clearly associated with insulin resistance in type 2 diabetes mellitus16. Furthermore, in vivo studies in humans have shown that hyperglycemia increases circulating visfatin concentrations17. Therefore, visfatin could act by a similar mechanism during pregnancy. The current study supports these findings, as serum visfatin levels were shown to be associated with increased blood glucose levels and HOMA‐IR scores.
Pregnancy‐induced weight gain and BMI at childbirth were significantly higher in the GDM group than in the control group of the present study, indicating a positive correlation with serum visfatin levels. This finding suggests that excessive pregnancy weight gain in the GDM group might be related to increased visfatin levels. During periods of dramatically increased weight gain, characteristic of pregnancy, abnormal growth and proliferation of adipocytes could result in an abundance of metabolically active adipocytes, leading to hypoxic conditions. It has been proposed that the additional secretion of the adipocytokine visfatin is a response to this stressor, providing a means for prevention of hypoglycemia in this altered environment18. This hypothesis would also explain the substantially increased risk for GDM observed in obese patients19. Thus, high levels of adipose tissue as a result of weight gain or obesity could stimulate overproduction of visfatin, though this mechanism has yet to be characterized.
Circulating maternal visfatin has been shown to exert a wide range of autocrine and paracrine effects during pregnancy. During GDM, failure to maintain normal glucose levels might result in long‐term hyperglycemia, further stimulating visfatin secretion as an attempt to regulate glucose and lipid metabolism. These increased visfatin levels might exert other effects, such as increasing the risk of pre‐eclampsia, and affecting secretion of the related adipocytokines leptin and adiponectin20. Visfatin might also promote differentiation and maturation of preadipocytes, further promoting glucose transport, lipogenesis and accumulation in visceral fat21. Cumulatively, these effects all contribute to excessive pregnancy weight gain, consistent with the observed weight gain increases in women with GDM.
A small increase in blood lipids during normal pregnancy, generally followed by a reduction to levels below the pre‐pregnancy levels during the post‐natal period, might benefit the mother and provide additional energy to the fetus22. Although alterations in lipid levels are considered normal for growth and development during pregnancy, women with GDM show abnormal lipid changes, including chylomicron formation, inhibited degradation of TG in very low‐density lipoprotein and increased very low‐density lipoprotein synthesis23. The result of these factors is an increase in TG, LDL‐C, very low‐density lipoprotein and a decrease in HDL‐C. The current study observed elevated TG and LDL in women with GDM, though HDL levels remained low. While these results are consistent with previous findings, the between‐group difference in TC values was not statistically significant, possibly as a result of measurement error or the relatively small cohort examined in the current study. Similar studies involving larger cohorts will be necessary to confirm these findings.
Akturk et al.24 compared the relationship between visfatin and blood lipid in the GDM group and normal control group in the late‐pregnancy period. They found that plasma visfatin levels were significantly decreased in pregnant women with GDM compared with those with NGT. They did not observe any statistically significant correlation between the plasma visfatin levels and the selected parameters in the GDM group. It is inconsistent with the current study, maybe because of the different races and determination method between the two studies. However, by regression analysis, their study showed that having GDM was found to be the only significant determinant of visfatin concentration. Their study supports the hypothesis that visfatin is involved in the regulation of abnormal glucose and lipid metabolism in the GDM women.
As an adipocytokine, visfatin could promote the deposition and differentiation of TG in the adipocytes of normal pregnant females through the autocrine and paracrine glands of splanchnic tissues21, resulting in a negative correlation with TG levels. Although visfatin secretion is increased in women with GDM, its secretion cannot compensate for the relative deficiency of insulin because of more severe IR than the normal control group. The correlation between visfatin and glucose or lipid levels in the present study is absent, so glucose and lipid metabolic disorder is the final performance. IR can exist in normal pregnancy, but visfatin exerts an insulin‐like function in glucose level regulation, which can maintain stable blood glucose or lipid and normal lipid metabolism function. Therefore, visfatin levels in late pregnancy are related to the underlying glucose and lipid metabolism index, and function to maintain blood glucose lipid metabolism. Visfatin is associated with glucose or lipid metabolic disorder of GDM, but specific regulatory mechanism needs to be further studied.
Although circulating maternal visfatin serum levels, BMI and weight gain were observed to be substantially increased in women with GDM at the time of childbirth, both glucose and lipid metabolism remained abnormal. These observations suggest that visfatin and its mechanistic pathway might be related to the development and progression of GDM. Further investigation will be required to more fully determine the association between visfatin levels, glucose and blood lipids. Additionally, further characterization of the mechanism of visfatin expression, regulation, and secretion in placental tissue and adipocytes will be required in order to develop a complete understanding of the relationship between visfatin and GDM.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of Zhejiang Province (LY12H04010) and the National Natural Science Foundation of China (81170587/81370725).
J Diabetes Investig 2016; 7: 247–252
References
- 1. Harlev A, Wiznitzer A. New insights on glucose pathophysiology in gestational diabetes and insulin resistance. Curr Diab Rep 2010; 10: 242–247. [DOI] [PubMed] [Google Scholar]
- 2. Adeghate E. Visfatin: structure, function and relation to diabetes mellitus and other dysfunctions. Curr Med Chem 2008; 15: 1851–1862. [DOI] [PubMed] [Google Scholar]
- 3. Mazaki‐Tovi S, Romero R, Kusanovic JP, et al Visfatin in human pregnancy: maternal gestational diabetes vis‐a‐vis neonatal birthweight. J Perinat Med 2009; 37: 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karrasch T, Leszczak S, Bala M, et al Short‐term regulation of Visfatin release in vivo by oral lipid ingestion and in vitro by fatty acid stimulation. Exp Clin Endocrinol Diabetes 2014; 122: 126–134. [DOI] [PubMed] [Google Scholar]
- 5. Teplan V Jr, Senolt L, Hulejova H, et al Early changes in serum visfatin after abdominal surgery: a new pro‐inflammatory marker in diagnosis? Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014. doi:10.5507/bp.2014.012. [DOI] [PubMed] [Google Scholar]
- 6. Zhao Y, Liu XZ, Tian WW, et al Extracellular visfatin has nicotinamide phosphoribosyltransferase enzymatic activity and is neuroprotective against ischemic injury. CNS Neurosci Ther 2014; 20: 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yilmaz O, Kucuk M, Ilgin A, et al Assessment of insulin sensitivity/resistance and their relations with leptin concentrations and anthropometric measures in a pregnant population with and without gestational diabetes mellitus. J Diabetes Complications 2010; 24: 109–114. [DOI] [PubMed] [Google Scholar]
- 8. Krzyzanowska K, Krugluger W, Mittermayer F, et al Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci (Lond) 2006; 110: 605–609. [DOI] [PubMed] [Google Scholar]
- 9. Lewandowski KC, Stojanovic N, Press M, et al Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance. Diabetologia 2007; 50: 1033–1037. [DOI] [PubMed] [Google Scholar]
- 10. Zhaoxia L, Ying W, Danqing C. Changes in visfatin levels after oral glucose tolerance test in women with gestational diabetes mellitus. Diabetes Res Clin Pract 2012; 96: e76–e79. [DOI] [PubMed] [Google Scholar]
- 11. Chan TF, Chen YL, Lee CH, et al Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. J Soc Gynecol Investig 2006; 13: 364–367. [DOI] [PubMed] [Google Scholar]
- 12. Haider DG, Handisurya A, Storka A, et al Visfatin response to glucose is reduced in women with gestational diabetes mellitus. Diabetes Care 2007; 30: 1889–1891. [DOI] [PubMed] [Google Scholar]
- 13. Basevi V, Di Mario S, Morciano C, et al Comment on: American Diabetes Association. Standards of medical care in diabetes–2011. Diabetes Care 2011;34(Suppl. 1):S11–S61. Diabetes Care 2011; 34: e53; author reply e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004; 27: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 15. Sun Q, Li L, Li R, et al Overexpression of visfatin/PBEF/Nampt alters whole‐body insulin sensitivity and lipid profile in rats. Ann Med 2009; 41: 311–320. [DOI] [PubMed] [Google Scholar]
- 16. Zhang YY, Gottardo L, Thompson R, et al A visfatin promoter polymorphism is associated with low‐grade inflammation and type 2 diabetes. Obesity (Silver Spring) 2006; 14: 2119–2126. [DOI] [PubMed] [Google Scholar]
- 17. Haider DG, Schaller G, Kapiotis S, et al The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia 2006; 49: 1909–1914. [DOI] [PubMed] [Google Scholar]
- 18. Kabon B, Nagele A, Reddy D, et al Obesity decreases perioperative tissue oxygenation. Anesthesiology 2004; 100: 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chu SY, Callaghan WM, Kim SY, et al Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007; 30: 2070–2076. [DOI] [PubMed] [Google Scholar]
- 20. Tavana Z, Madadi G, Zolghadri J. The relationship between maternal serum visfatin level and hypertensive disorders of pregnancy. Int J Gynecol Obstet 2011; 15: 1528–8439. [Google Scholar]
- 21. Berndt J, Klöting N, Kralisch S, et al Plasma visfatin concentrations and fat depot‐specific mRNA expression in humans. Diabetes 2005; 54: 2911–2916. [DOI] [PubMed] [Google Scholar]
- 22. Van Stiphout WA, Hofman A, de Bruijn AM. Serum lipids in young women before, during, and after pregnancy. Am J Epidemiol 1987; 126: 922–928. [DOI] [PubMed] [Google Scholar]
- 23. Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000; 71: 1256S–1261S. [DOI] [PubMed] [Google Scholar]
- 24. Akturk M, Altinova AE, Mert I, et al Visfatin concentration is decreased in women with gestational diabetes mellitus in the third trimester. J Endocrinol Invest 2008; 31: 610–613. [DOI] [PubMed] [Google Scholar]


