Abstract
Aims/Introduction
Chemokine ligand 5 (CCL5) is a member of the CC‐chemokine family expressed in various organs. It contributes to the migration of monocytes/macrophages into injured vascular walls by binding with its receptor chemokine receptor 5 (CCR5). Many studies have accessed the association between CCL5/CCR5 gene promoter polymorphisms and diabetic microvascular complications (DMI). However, the results are conflicting and inconclusive. The aim of the present study was to evaluate the association more precisely.
Materials and Methods
Trials were retrieved through PubMed, Embase, Medline, China National Knowledge Infrastructure, Web of Science and Cochrane database without restrictions on language. The pooled odds ratio (OR) and 95% confidence interval (CI) were used to describe the strength of association with DMI.
Results
Data were obtained from 11 case–control studies that included 2,737 DMI patients and 2,435 diabetic control subjects. In the overall analysis, the CCL5‐403 G/A and CCL5‐28 C/G gene polymorphisms were not significantly associated with the risk of DMI. However, CCR5‐59029 G/A was an independent risk factor of DMI in a dominant model (OR 1.77, 95% CI 1.06–2.97). Subgroup analysis showed that the risk of the CCR5 59029A‐positive genotype was significant in Asians (OR 2.08, 95% CI 1.68–2.57). In addition, the CCR5 59029A‐positive genotype was associated with increased risk of albuminuria.
Conclusions
There were no associations of CCL5 gene promoter polymorphism with the risk of DMI. However, the 59029A polymorphism in CCR5 might affect individual susceptibility for DMI.
Keywords: Chemokine ligand 5, Chemokine receptor 5, Diabetes mellitus
Introduction
The prevalence of diabetes mellitus has increased rapidly in recent years. Diabetic microvascular complications (DMI), such as diabetic nephropathy (DN) and diabetic retinopathy (DR), are the most devastating clinical manifestations of diabetes. The risk factors of DMI include hyperglycemia, hypertension and genetic factors. Although hypoglycemic and antihypertensive treatments were inversely related to DMI in some cases, many patients still developed DMI even when their blood glucose and blood pressure reached normal levels1, 2. These patients might have genetic risk factors associated with DMI. In addition, DMI can be considered as a cytokine‐associated innate immune response2, and downregulation of specific cytokines could reduce the incidence of DMI3, 4. Identification of cytokines with higher risks will be useful for early preventive care of DMI.
Chemokine ligand 5 (CCL5), also known as regulated on activation normal T expressed and secreted (RANTES), is a member of the C‐C chemokine family, and is mainly expressed on CD8 + T cells3. Chemokine ligand 5 has the ability to recruit, activate and costimulate T cells, and thus mediate innate and adaptive immune responses5. The cognate receptor of CCL5, chemokine receptor 5 (CCR5), a G‐coupled seven‐transmembrane chemokine receptor, is a member of the β‐chemokine receptor family, and mainly expressed on T cells and macrophages3, 5, 6, 7. Genetic inactivation of CCR5 is associated with the reduction of monocytes/macrophages into the injured vessels, which is one of the earliest events in the pathogenesis of DMI1. In addition, several studies have shown that CCL5/CCR5 expression was increased in patients with DN or DR, and their aberrant expression was associated with renal inherent injury or the formation of intraocular neovascularization8, 9. Thus, the mutations in the CCL5 or CCR5 gene might be the pathogenic determinant of DMI.
A screening of the CCL5/CCR5 gene for sequence variants identified several genetic variants. The most common polymorphisms are CCL5‐403 G/A (G to A alteration), CCL5‐28 C/G (C to G alteration) and CCR5 59029 G/A (G to A alteration). However, studies regarding CCL5‐403 G/A, CCL5‐28 C/G, CCR5 59029 G/A and DMI risk are controversial. Some researchers have shown that the CCL5 and CCR5 genes variants could be susceptibility factors for DMI1, 10, 11, 12, 13, 14, 15, 16. Yet, the results have not been verified in other studies3, 9, 17. The primary aim of the present study was to derive a more precise evaluation of the associations between the CCL5/CCR5 genes variants and the risk of DMI. The secondary analysis was to identify factors that might affect the association strength between the CCL5/CCR5 gene polymorphism and the risk of DMI.
Materials and Methods
Search strategy
We searched PubMed, Embase, Medline, China National Knowledge Infrastructure (CNKI), Web of Science, Cochrane database and reference lists of relevant studies. Using the terms ‘CCR5’, ‘CCL5’ and ‘RANTES’ in combination with ‘diabetes mellitus’ or ‘DMI’ or ‘DN’ or ‘diabetes kidney disease’ or ‘DR’ or ‘diabetic foot’ or ‘variant’ or ‘mutation’ or ‘polymorphism’. No restriction was set on language or whether the articles had been published. We also checked the references of these articles to screen out other relevant publications. In addition, we carried out this meta‐analysis in accordance with the Preferred Reporting Items for Reviews and Meta‐Analyses (PRISMA) statement.
Study selection criteria
Eligible articles met the following criteria: (i) case–control studies: patients with DN and/or DR as the case group, diabetic patients without complications as the control group; (ii) prospective cohort studies; (iii) studies related to the CCL5‐403 G/A, CCL5‐28 C/G, and CCR5 59029G/A polymorphism and DMI risk. Exclusion criteria included: (i) non case–control studies; (ii) the control group including non‐diabetic patients; (iii) diabetic patients combined with serious complications, such as malignancies and severe cardiac dysfunction.
Data extraction
Two investigators (ZZ and JD) reviewed the eligibility of the studies and extracted frequency difference of genetic variants in DMI. When more than one study by the same author was included, only the most complete study was included in our research. The data extracted from articles including the author name, year of article, ethnic origin, numbers of genotype cases and control subjects, age, sex, glycated hemoglobin levels and subtype of diabetes mellitus.
Data synthesis and statistical analysis
The Hardy–Weinberg equilibrium was tested by the χ2‐test. The strength of genetic variants and DMI association were evaluated by odds ratio (OR) and 95% confidence interval (CI). If the association showed heterogeneity (I 2 > 25%), the random effects models were merged. Furthermore, sensitivity analysis was carried out to determine the source of heterogeneity, thereby evaluating whether the heterogeneity markedly influenced the results. The association between CCL5/CCR5 gene mutation and DMI were analyzed by the following methods: CCL5‐403 G/A (GA + AA vs GG; AA vs GA + GG), CCL5‐28 C/G (GC + CC vs GG; CC vs GC + GG) and CCR5‐59029G/A (GA + AA vs GG, AA vs GA + GG). The risk frequency of A allele (CCL5‐403 G/A and CCR5‐59029G/A) and G allele (CCL5‐28 C/G) were also calculated in these case–control groups. The subtype of diabetes mellitus (type 1 diabetes or type 2 diabetes), the specific type of DMI (DN or DR), the stage of DN (microalbuminuria, macroalbuminuria or end‐stage renal disease [ESRD]) and ethnicity (Caucasians or Asians) were selected for stratified analysis. The Egger's test and Rosenthal's fail‐safe number (N fs) were used to test the publication bias. Values of P < 0.05 were considered as significant differences. The significance of N fs was set at 0.05 for each meta‐comparison. If the calculated N fs value was smaller than the number of studies, the meta‐analysis results might show a risk of publication bias. The formula N fs0.05 = (ΣZ/1.64)2 − k (k is the number of articles included in this research). All analyses were carried out using two statistical software programs: Review Manager 5.2 (Cochrane Collaboration, Oxford, UK) and stata 10.0 (StataCorp, College Station, TX, USA). This meta‐analysis was carried out using the Mantel–Haenszel statistical method and sample size comparing the weighted mean difference.
Results
Study characteristics
Our research yielded 601 reports of potentially relevant studies. After screening, 11 case–control studies and two conference abstracts were eligible, including nine articles in English and two articles in Chinese. The study selection process is shown in Figure 1. A total of 2,737 DMI patients and 2,435 diabetic controls were enrolled, ten studies focused on the CCR5‐59029G/A polymorphism, three on CCL5‐28 C/G polymorphism and four focused on CCL5‐403 G/A polymorphism. The main characteristics of the included studies are listed in Table S1. Among these studies, ten evaluated albuminuria, three evaluated microalbuminuria, five evaluated macroalbuminuria and two evaluated ESRD (Table S2).
Figure 1.

Flow chart of the systematic search process. RCTs, randomized controlled trials.
Main meta‐analysis results
Risk factor analysis of chemokine ligand 5‐403G/A, chemokine ligand 5‐28 C/G and diabetic microvascular complications
The fixed effects model was used because the heterogeneity of data was less than 30%. It showed that CCL5‐403 G/A, CCL5‐28 C/G genetic polymorphisms had no significant correlation with DMI (OR 1.00, 95% CI 0.82–1.21 for CCL5‐403 G/A; OR 1.02 95% CI 0.79–1.33 for CCL5‐28 C/G). Subgroup analysis was carried out for the subtype of diabetes mellitus and ethnicity. However, exclusion of the two variables individually did not generate significant changes for the pooled association (Table 1).
Table 1.
Meta‐analysis of chemokine ligand 5 (CCL5) polymorphism and the risk of diabetic microvascular complications with a dominant model
| Category | n | Participants, n (cases/controls) | Heterogeneity | OR (95% CI) | Z‐test | |
|---|---|---|---|---|---|---|
| P h | I 2 (%) | |||||
| Four studies for the CCL5‐403 G/A polymorphism | ||||||
| Overall | 4 | 797/1064 | 0.26 | 26 | 1.00 (0.82–1.21)a | Z = 0.02; P Z = 0.98 |
| Adjustment by subtypes of diabetes | ||||||
| Type 1 diabetes mellitus | 1 | 267/440 | NA | NA | 1.21 (0.87–1.68) | Z = 1.14; P Z = 0.26 |
| Type 2 diabetes mellitus | 3 | 530/624 | 0.36 | 1 | 0.90 (0.71–1.15)a | Z = 0.85; P Z = 0.39 |
| Adjustment by ethnicity | ||||||
| Caucasian | 1 | 267/440 | NA | NA | 1.21 (0.87–1.68) | Z = 1.14; P Z = 0.26 |
| Asian | 3 | 530/624 | 0.36 | 1 | 0.90 (0.71–1.15)a | Z = 0.85; P Z = 0.39 |
| Three studies for the CCL5‐28C/G polymorphism | ||||||
| Overall (all T2DM) | 3 | 508/659 | 0.88 | 0 | 1.02 (0.79–1.33)a | Z = 0.17; P Z = 0.87 |
Fixed‐effects model. P Z < 0.05, shows a significant association. CI, confidence interval; NA, not available; OR, odds ratio; P h, P‐values for heterogeneity of Q‐test; T2DM, type 2 diabetes mellitus.
Risk factor analysis of chemokine receptor 5‐59029G/A and diabetic microvascular complications
When CCR5‐59029 GA + AA genotypes were set as dominant risk factors, the result showed that CCR5 59029 GA + AA genotypes were associated with increased DMI risk (OR 1.77, 95% CI 1.06–2.97, P = 0.03). When the AA genotype was set as the recessive risk factor, no significant correlation was found between the AA genotype and DMI risk (OR 1.28, 95% CI 0.75–2.17, P = 0.36). These results suggested that the CCR5 59029A‐positive genotype (G/A or A/A) was an independent risk factor of DMI.
We found significant heterogeneity of the CCR5 59029A‐positive genotype study (I 2 = 91%). Quantitative analysis showed that heterogeneity arose from the trials of Ahluwalia et al.13, Buraczynska et al.1, Pettigrew et al.3, Mlynarski et al.11, Yang et al.9 and Cheng et al.16 (Figure 2). The heterogeneity disappeared (I 2 = 0%) after excluding these studies. The trials published by Pettigrew et al.3, Mlynarski et al.11 and Yang et al.9 evaluated the risk of microvascular complications in type 1 diabetes mellitus, while the trails published by Ahluwalia et al.13, Buraczynska et al.1 and Cheng et al.16 analyzed the risk of microvascular complications in type 2 diabetes mellitus. Thus, the type of diabetes mellitus was selected for stratification analysis. The result showed that the CCR5 59029A‐positive genotype was significantly associated with microvascular complications in type 2 diabetes mellitus (OR 2.61, 95% CI 1.57–4.36, P < 0.05), but not in type 1 diabetes mellitus (OR 0.82, 95% CI 0.56–1.20, P > 0.05; Figure 3). Both the Cochran Q‐test and estimate of I 2 showed significant heterogeneity among these studies. The frequencies of the CCR5 variants from each study are listed in Table S3.
Figure 2.
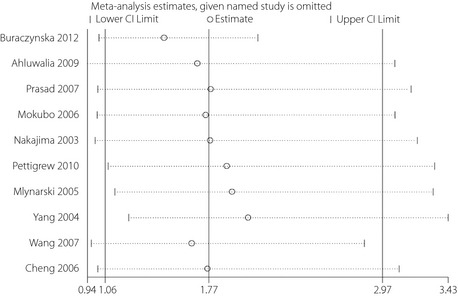
The sensitivity analysis for the association of the CCR5‐59029 G/A polymorphism with the risk of diabetic microvascular complications. CI, confidence interval.
Figure 3.
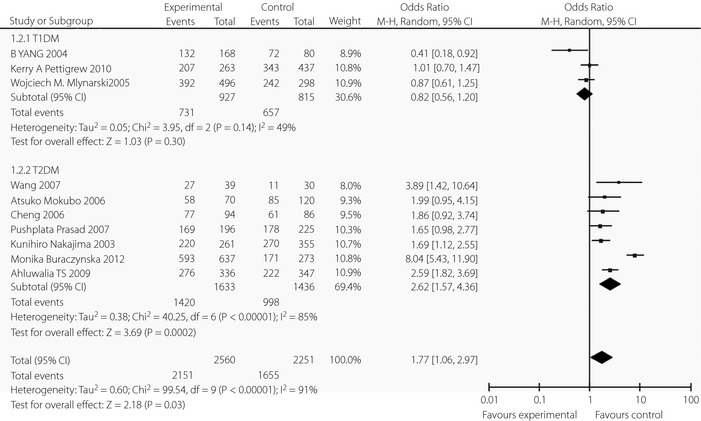
Forest plots of the meta‐analysis for CCR5‐59029G/A polymorphism associated with the subtypes of diabetes mellitus. CI, confidence interval; df, degrees of freedom; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
To further evaluate the cause of heterogeneity in microvascular complications of type 2 diabetes mellitus (I 2 = 85%), the ethnicity, the specific type of DMI and the stage of DN were selected for subgroup analysis. The results showed that ethnicity and the stage of DN were correlated with heterogeneity. After excluding the Caucasian population, the heterogeneity disappeared (I 2 = 0%), and a significant association was found in Asians (OR 2.07, 95% CI 1.68–2.56, P < 0.00001). The CCR5 59029A‐positive genotype significantly increased the risk of albuminuria (OR 1.68, 95% CI 1.15‐2.44, P < 0.05 for microalbuminuria; OR 2.70, 95% CI 1.07–6.83, P < 0.05 for macroalbuminuria). As only one trial was on ESRD, we were unable to explore the relationship between CCR5 59029A‐positive genotype and risk of ESRD. The details are listed in Table 2.
Table 2.
Meta‐analysis of chemokine ligand 5 (CCL5) promoter polymorphism and the risk of diabetic microvascular complications with a dominant model
| Category | n | Participants, n (cases/controls) | Heterogeneity | OR (95% CI) | Z‐test | |
|---|---|---|---|---|---|---|
| P h | I 2 (%) | |||||
| Overall | 10 | 2560/2251 | 0.00 | 91 | 1.77 (1.06–2.97) | Z = 2.18; P Z = 0.02 |
| Adjustment by subtypes of DMI | ||||||
| Diabetic nephropathy | 8 | 2323/2251 | 0.00 | 91 | 1.90 (1.10–3.27) | Z = 2.31; P Z = 0.02 |
| Diabetic retinopathy | 2 | 237/353 | 0.00 | 91 | 1.10 (0.16–7.35) | Z = 0.10; P Z = 0.91 |
| Adjustment by stage of DN | ||||||
| Microalbuminuria | 3 | 281/471 | 0.95 | 0 | 1.68 (1.15–2.44)a | Z = 2.71; P Z = 0.007 |
| Macroalbuminuria | 5 | 1420/1085 | 0.00 | 93 | 2.70 (1.07–6.83) | Z = 2.70; P Z = 0.04 |
| ESRD | 1 | 196/225 | NA | NA | 1.65 (0.98–2.77) | Z = 1.90; P Z = 0.06 |
| Adjustment by subtypes of diabetes mellitus | ||||||
| Type 1 diabetes mellitus | 3 | 927/815 | 0.14 | 49 | 0.82 (0.56–1.20) | Z = 1.02; P Z = 0.31 |
| Type 2 diabetes mellitus | 7 | 1633/1436 | 0.00 | 85 | 2.61 (1.57–4.36) | Z = 5.05; P Z < 0.00 |
| Adjustment by ethnicity | ||||||
| Caucasian | 4 | 1564/1088 | 0.00 | 96 | 1.33 (0.39–4.47) | Z = 0.47; P Z = 0.63 |
| Asian | 6 | 996/1163 | 0.44 | 0 | 2.08 (1.68–2.57)a | Z = 6.78; P Z = 0.00 |
Fixed‐effects model; P Z < 0.05, shows a significant association. DMI, diabetic microvascular complications; DN, Diabetic nephropathy; ESRD, end‐stage renal disease; NA, not available; P h, P‐values for heterogeneity of Q‐test.
Publication bias
Publication biases were tested for all the outcomes of the included studies. From the funnel plot, no significant publication bias was found among these studies (Figures S1–S3). We also calculated the N fs 0.05 for CCL5‐403 G/A, CCL5‐28 C/G and CCR5‐59029G/A. The N fs 0.05 value of each comparison was greater than the number of studies included in our research.
Discussion
Genetic epidemiological studies of single nucleotide polymorphisms can explore the association between the candidate gene and disease risks. In the present meta‐analysis, we found no positive association of the CCL5 gene polymorphism and the risk of DMI. However, the 59029A polymorphism in CCR5 might affect individual susceptibility for DMI, and the CCR5 59029A‐positive genotype was associated with increased DMI risk in a subgroup (‘type 2 diabetes mellitus’ or ‘Asians’, respectively). In addition, the CCR5 59029 A‐positive genotype was associated with increased risk of microalbuminuria and macroalbuminuria.
Ethnic differences might partly attribute to the interaction between the genetic and geographical environment7, 18. Considering that DMI is a complex etiology involving the combined effects of environmental and genetic factors, different ethnic populations have different frequencies of alleles and genetic backgrounds, which could affect the risk of DMI18. In our research, the frequency of the ‘A’ allele in the Caucasian population (case group: mean 0.0035, range 0.002–0.006; control group: mean 0.0057, range 0.002–0.015) was lower than that in the Asian population (case group: mean 0.0123, range 0.004–0.029; control group: mean 0.008, range 0.002–0.0105), which could be one of the sources for variety in genetic susceptibility between different ethnic groups. In addition, ethnicity might correlate with the curative activities, such as drug use or target of blood glucose18. Mokubo et al.10 found that the CCR559029 A (+) genotype and 10‐year mean glycated hemoglobin value were positively correlated with DMI risk among the Asian patients. However, Mlynarski et al.11 failed to find such a correlation with Caucasians. Thus, curative activities might account for the inconsistency of the studies.
There was no significant association of the CCL5 gene polymorphism and the risk of DMI. Some studies pointed out that their relationship might correlate with the stage of DN or sex (male)11, 12. As only a few studies are available, we were not able to confirm these conclusions. In addition, the present study only focused on DMI, and did not evaluate the association of the CCL5 gene polymorphism and other disease, such as atherosclerosis, multiple sclerosis and atopic asthma19, 20, 21. The potential role of the CCL5 gene polymorphism might be masked by other gene–gene or gene–environment interactions.
Diabetic microvascular complications is caused by a combination of the effects of multiple genetic and environmental risk factors. Comprehensive research of the multiple loci will explore a novel biological insight for us22, 23, 24, 25. In the present study, we found that, in Asians, the CCR5 59029A‐positive genotype is a significant susceptibility factor for DMI, which implies that genetic variant rs1799987 in CCR5 could increase the risk of DMI in Asians. Therefore, in the Asian population, CCR5 might present a new target for the early preventive detection of DMI.
Although Nazir et al.26 reported that CCR5 genetic variants showed a significant association with DN, the role of CCR5 in DMI remains unclear. Compared with the study by Nazir et al.26, the present study used a higher number of articles for this meta‐analysis, and carried out subtype and sensitivity analysis to evaluate the causes of heterogeneity. The association between the CCR5 gene polymorphism and the stage of DN were also analyzed in the present study. In addition, the publication biases were tested for all the outcomes of the included studies in the present study. The previous study26 was unable to calculate the publication bias because of a lack of studies for CCL5 C‐28G genetic variants (i.e., less than three studies).
Several limitations to the present study should be considered. First, the sample size of the CCL5 polymorphism is relatively small, and additional studies are required to confirm the conclusion. Second, we focused only on the single nucleotide polymorphisms on CCL5/CCR5 promoter, while the other single nucleotide polymorphisms in the CCL5/CCR5 gene should be studied to clarify the role of CCL5/CCR5 in DMI. Third, in this meta‐analysis, most of the Caucasian studies involved type 1 but not type 2 diabetes. A lack of association between the CCR5 polymorphism and the risk of DMI in Caucasians might partially result from the type of diabetes, rather than ethnicity.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 ¦ Funnel plot of publication bias for the association of the CCL5‐403 G/A polymorphism with the risk of diabetic microvascular complications.
Figure S2 ¦ Funnel plot of publication bias for the association of the CCL5‐28C/G polymorphism with the risk of diabetic microvascular complications.
Figure S3 ¦ Funnel plot of publication bias for the association of the CCR5‐59029G/A polymorphism with the risk of diabetic microvascular complications.
Table S1 ¦ Main characteristics of the studies included in the meta‐analysis.
Table S2 ¦ The description of diabetic nephropathy staging studies included in the subgroup‐analyses.
Table S3 ¦ The frequencies of the CCR5‐59029 A positive genotype from each study in the subgroup analyses.
Acknowledgments
This work was funded by National Natural Science Foundation of China Grants (81070637), Shandong Provincial Natural Science Foundation of China Grants (Nos. Y2006C76 and ZR2010HM044), Shandong Provincial Science & Technology Development Program, China (2009GGB14001), Fund for the Returned Oversea Scholars Sponsored by National Ministry of Personnel (2008, No. 102), and Grant for Excellent Young and Middle‐aged Scientists of Shandong Province (No. 2004BS02016). We are grateful for the support from the Shandong Taishan Scholarship (Ju Liu).
J Diabetes Investig 2016; 7: 212–218
References
- 1. Buraczynska M, Zukowski P, Wacinski P, et al Chemotactic cytokine receptor 5 gene polymorphism: relevance to microvascular complications in type 2 diabetes. Cytokine 2012; 58: 213–217. [DOI] [PubMed] [Google Scholar]
- 2. Su X, Chen X, Liu L, et al Intracellular adhesion molecule‐1 K469E gene polymorphism and risk of diabetic microvascular complications: a meta‐analysis. PLoS ONE 2013; 8: e69940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pettigrew KA, McKnight AJ, Patterson CC, et al Resequencing of the CCL5 and CCR5 genes and investigation of variants for association with diabetic nephropathy. J Hum Genet 2010; 55: 248–251. [DOI] [PubMed] [Google Scholar]
- 4. Syreeni A, El‐Osta A, Forsblom C, et al Genetic examination of SETD7 and SUV39H1/H2 methyltransferases and the risk of diabetes complications in patients with type 1 diabetes. Diabetes 2011; 60: 3073–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006; 354: 610–621. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Z, Dong J, Lobe CG, et al CCR5 facilitates endothelial progenitor cells recruitment and promotes the stabilization of atherosclerotic plaques in ApoE mice. Stem Cell Res Ther 2015; 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Z, Liu J, Wang H, et al Association between chemokine receptor 5 (CCR5) delta32 gene variant and atherosclerosis: a meta‐analysis of 13 studies. Int J Clin Exp Med 2015; 8: 658–665. [PMC free article] [PubMed] [Google Scholar]
- 8. Kawashima M, Shoji J, Kamura Y, et al Role of chemokines in the vitreous of proliferative diabetic retinopathy. Nihon Ganka Gakkai Zasshi 2005; 109: 596–602. [PubMed] [Google Scholar]
- 9. Yang B, Houlberg K, Millward A, et al Polymorphisms of chemokine and chemokine receptor genes in Type 1 diabetes mellitus and its complications. Cytokine 2004; 26: 114–121. [DOI] [PubMed] [Google Scholar]
- 10. Mokubo A, Tanaka Y, Nakajima K, et al Chemotactic cytokine receptor 5 (CCR5) gene promoter polymorphism (59029A/G) is associated with diabetic nephropathy in Japanese patients with type 2 diabetes: a 10‐year longitudinal study. Diabetes Res Clin Pract 2006; 73: 89–94. [DOI] [PubMed] [Google Scholar]
- 11. Mlynarski WM, Placha GP, Wolkow PP, et al Risk of diabetic nephropathy in type 1 diabetes is associated with functional polymorphisms in RANTES receptor gene (CCR5): a sex‐specific effect. Diabetes 2005; 54: 3331–3335. [DOI] [PubMed] [Google Scholar]
- 12. Nakajima K, Tanaka Y, Nomiyama T, et al RANTES promoter genotype is associated with diabetic nephropathy in type 2 diabetic subjects. Diabetes Care 2003; 26: 892–898. [DOI] [PubMed] [Google Scholar]
- 13. Ahluwalia TS, Khullar M, Ahuja M, et al Common variants of inflammatory cytokine genes are associated with risk of nephropathy in type 2 diabetes among Asian Indians. PLoS ONE 2009; 4: e5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prasad P, Tiwari AK, Kumar KM, et al Association of TGFbeta1, TNFalpha, CCR2 and CCR5 gene polymorphisms in type‐2 diabetes and renal insufficiency among Asian Indians. BMC Med Genet 2007; 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang R, Zhang S, Shi LX. Association between polymorphism of CCR5 gene with diabetic nephropathy in type 2 diabetes mellitus in Chinese. Guizhou Med J 2007; 31: 500–503. [Google Scholar]
- 16. Cheng J, Liu RH, Liu FY, et al Impact of gene polymorphism of RANTES and CCR5 on type 2 diabetic nephropathy. Chin J Nephrol 2009; 25: 481–482. [Google Scholar]
- 17. Joo KW, Hwang YH, Kim JH, et al MCP‐1 and RANTES polymorphisms in Korean diabetic end‐stage renal disease. J Korean Med Sci 2007; 22: 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mooyaart AL, Valk EJ, van Es LA, et al Genetic associations in diabetic nephropathy: a meta‐analysis. Diabetologia 2011; 54: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang L, Hu X, Zhang S, et al Association of the CCR5Delta32 polymorphism and its ligand RANTES‐403G/A polymorphism with coronary artery disease: a meta‐analysis. Thromb Res 2013; 131: e77–e84. [DOI] [PubMed] [Google Scholar]
- 20. Al‐Abdulhadi SA, Helms PJ, Main M, et al Preferential transmission and association of the ‐403 G → A promoter RANTES polymorphism with atopic asthma. Genes Immun 2005; 6: 24–30. [DOI] [PubMed] [Google Scholar]
- 21. Gade‐Andavolu R, Comings DE, MacMurray J, et al RANTES: a genetic risk marker for multiple sclerosis. Mult Scler 2004; 10: 536–539. [DOI] [PubMed] [Google Scholar]
- 22. Hosseini SM, Boright AP, Sun L, et al The association of previously reported polymorphisms for microvascular complications in a meta‐analysis of diabetic retinopathy. Hum Genet 2015; 134: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bazzaz JT, Amoli MM, Taheri Z, et al TGF‐beta1 and IGF‐I gene variations in type 1 diabetes microangiopathic complications. J Diabetes Metab Disord 2014; 13: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tong Z, Yang Z, Patel S, et al Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci U S A 2008; 105: 6998–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buchbinder S, Rudofsky G Jr, Humpert PM, et al The DG10S478 variant in the TCF7L2 gene is not associated with microvascular complications in type 2 diabetes. Exp Clin Endocrinol Diabetes 2008; 116: 211–214. [DOI] [PubMed] [Google Scholar]
- 26. Nazir N, Siddiqui K, Al‐Qasim S, et al Meta‐analysis of diabetic nephropathy associated genetic variants in inflammation and angiogenesis involved in different biochemical pathways. BMC Med Genet 2014; 15: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ¦ Funnel plot of publication bias for the association of the CCL5‐403 G/A polymorphism with the risk of diabetic microvascular complications.
Figure S2 ¦ Funnel plot of publication bias for the association of the CCL5‐28C/G polymorphism with the risk of diabetic microvascular complications.
Figure S3 ¦ Funnel plot of publication bias for the association of the CCR5‐59029G/A polymorphism with the risk of diabetic microvascular complications.
Table S1 ¦ Main characteristics of the studies included in the meta‐analysis.
Table S2 ¦ The description of diabetic nephropathy staging studies included in the subgroup‐analyses.
Table S3 ¦ The frequencies of the CCR5‐59029 A positive genotype from each study in the subgroup analyses.


