Abstract
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by inflammation and joint destruction that causes significant morbidity and mortality. However, the combined use of methotrexate, a synthetic disease-modifying antirheumatic drug (DMARD), and biologic DMARD has revolutionized treatment of RA. Clinical remission is now realistic targets, achieved by a large proportion of RA patients, and rapid and appropriate induction of remission by intensive treatment with biological DMARD and methotrexate is prerequisite to halt joint damage and functional disabilities. However, biological DMARD is limited to intravenous or subcutaneous uses and orally available small but strong molecules have been developed. Oral administration of tofacitinib targeting the Janus kinase (JAK) is significantly effective than placebo in active patients with methotrexatenaïve, inadequately responsive to methotrexate or tumor necrosis factor (TNF)-inhibitors. The efficacy was rapid and as strong as adalimumab, a TNF-inhibitor. Meanwhile, association of tofacitinib on carcinogenicity and malignancy is under debate and further investigation on post-marketing survey would be warranted. On the other hand, discontinuation of a biological DMARD without disease flare is our next goal and desirable from the standpoint of risk reduction and cost effectiveness, especially for patients with clinical remission. Recent reports indicate that more than half of early RA patients could discontinue TNF-targeted biological DMARD without clinical flare and functional impairment after obtaining clinical remission. Contrarily, for established RA, fewer patients sustained remission after the discontinuation of biological DMARD and “deep remission” at the discontinuation was a key factor to keep the treatment holiday of biological DMARD.
Keywords: Arthritis, rheumatoid; Antirheumatic agents; Biological antirheumatic agents; Remission; Janus kinase inhibitor
INTRODUCTION
Rheumatoid arthritis (RA) is a representative systemic autoimmune disease characterized with chronic and destructive inflammatory synovitis and multiple organ manifestations that causes severe disability and mortality. It affects from 0.5% to 1.0% of adults with a 4-fold higher frequency in women than in men. Auto-reactive T cells and inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin 6 (IL-6) play a pivotal role in the pathological processes of RA through the accumulation of inflammatory cells, the self-perpetuation of inflammation, and production of matrix metalloproteinase and induction and/or activation of osteoclasts, leading to destruction of cartilage and bone [1-3]. It is noteworthy that such a joint damage derived from synovial inflammation is apparent in the early stage of the disease. It is required to treat patients at a stage when the evolution of joint destruction can still be prevented.
However, the combined use of methotrexate, a standard synthetic disease-modifying anti-rheumatic drug (DMARD) and a biological DMARD targeting TNF, IL-6, and T cells has revolutionized treatment of RA. Currently, clinical remission or low disease activity are now realistic targets for the treatments, achieved by a large proportion of RA patients. Also, it has been recognized that early therapeutic intervention targeting remission improves clinical outcomes and reduces the accrual and progress of joint damage and disability. Furthermore, the maintenance of remission, especially with biological DMARD, leads to long-term structural and functional remission. Thus, the combinational application of methotrexate and biological DMARD has brought about a paradigm shift in the management of RA and the treatment target of RA has evolved to not only clinical remission but also structural remission and functional remission.
THE CURRENT CONCEPT OF TREATMENT OF RA AND ITS PARADIGM SHIFT BY BIOLOGICAL DMARD
Recent progress in the treatment of RA has changed diagnosis of RA [4]. The 2010 Rheumatoid Arthritis Classification Criteria was published by collaborative initiative of an American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR). The new classification system redefines the current paradigm of RA as arthritis at high risk of chronicity and erosive damage by focusing on features at earlier stages of disease that are associated with the definition. By the new diagnostic system, it has become possible to treat patients with synthetic DMARD such as methotrexate at an early stage when evolution of joint destruction can still be prevented or minimized. Actually it has proven that early therapeutic intervention using synthetic DMARD and biological DMARD not only improves clinical outcomes but also reduces the occurrence of joint damage and disability [1-5].
From the global evidence, the treatment with methotrexate and TNF inhibitors including infliximab, etanercept, adalimumab, golimumab, and certolizumab or a IL-6-receptor inhibitor such as tocilizumab leads to clinical remission in approximately 30% to 60% of RA patients. Effective treatments using TNF-inhibitors have led to more stringent criteria for the clinical remission. Furthermore, induction and/or maintenance of clinical remission with TNF inhibitors and methotrexate can potentially lead to reduction of radiographic progress in joint destruction and improvement and keep of physical functions and abilities. For instance, structural remission defined with yearly changes of modified total Sharp score (ΔmTSS) can be achieved in approximately 60% to 90% of patients treated with any TNF-inhibitors and methotrexate. Furthermore, recent evidence shown by clinical studies such as OPTIMA (Optimal Protocol for Treatment Initiation with Methotrexate and Adalimumab) and HOPEFUL (adalimumab, a human anti- TNF monoclonal antibody, outcome study for the persistent efficacy under allocation to treatment strategies in early RA), indicate that the delayed addition of TNF-inhibitors to methotrexate-naïve, early RA patients did not impact clinical and functional outcomes at week 52, compared to the earlier addition of TNF-inhibitors [6,7]. However, the occurrence of significant structural damage during methotrexate mono-therapy period (the first 26 weeks) contributed to the persistence of differences between the treatment strategies. These results underscore the irreversible nature of erosive bone and cartilage loss present in RA patients. From these results, RA patients at risk for aggressive disease should benefit from the early and intensive intervention with combination therapy of methotrexate and TNF inhibitors.
Thus, clinical remission has become a goal to be targeted in the treatment of RA. A committee of ACR/EULAR also provided new remission criteria that is stringent but achievable and can be applied using outcome composite measures to predict later good radiographic and functional outcomes [8]. The new remission criteria are defined by simplified disease activity index, clinical disease activity index, and Boolean definition. Furthermore, a ‘treat to target’ approach making good outcomes and remission as the target for treatment have been published based on accumulated background information in terms of management of RA by an international task force (Fig. 1) [5]. Thus, clinical remission has recently become an achievable goal in many patients and rapid and appropriate induction of remission is prerequisite to halt joint damage and functional disabilities, which revealed improved outcomes with strategic therapeutic approaches.
Figure 1.
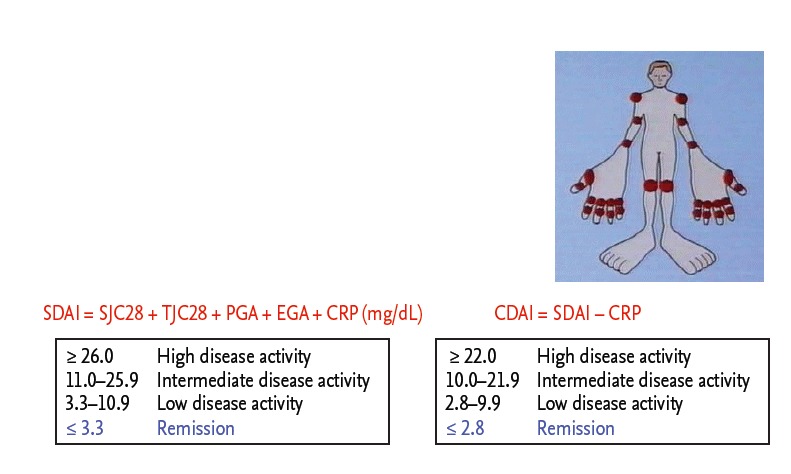
Composite measures of disease activity: simplified disease activity index (SDAI) and clinical disease activity index (CDAI) and remission criteria using SDAI or CDAI. SJC, swollen joint count; TJC, tender joint count; PGA, patient’s global health assessment (0–10.0 cm visual analogue scale [VAS]); EGA, evaluator’s global health assessment (0–10.0 cm VAS); CRP, C-reactive protein.
Once clinical remission is obtained, the remission has to be maintained. The maintenance of the remission is also applied to the treatment with biological DMARD. The most important study regarding maintenance of TNF-inhibitors was reported by Weinblatt et al. [9]. Etanercept, a TNF inhibitor, maintained disease activity and functional abilities beyond 10 years of therapy in both early RA and longstanding RA patients; a total of 163 of 558 early RA patients and 264 of 714 longstanding RA patients followed through year 10. Improvements in ACR20, ACR50, and ACR70 responses, ratio of achieving remission defined as a disease activity score 28 (DAS28) < 2.6 and reduction in Health Assessment Questionnaire disability index score were maintained during the 10- year period in the early RA patients as well as the longstanding RA patients. During 11 years, five opportunistic infections and 21 cases of sepsis were reported and occurrence of all malignancies was similar to that expected in the general population, but the occurrence of lymphomas (n = 14) was higher than expected in the general population. Thus, maintenance of functional ability and inhibition of structural changes in joints have become a long-term outcome of the treatment of RA using biological DMARD and methotrexate.
THE NEXT DEVELOPMENT OF TREATMENT OF RA: ORAL KINASE INHIBITORS
Although biological DMARDs have brought about paradigm shift in the treatment of RA, only subcutaneous or intravenous administration is allowed for their use because of their size the agents, which is reported 90,000 to 150,000 Dalton. Due to the size, these agents only inhibit or activate intracellular interaction by binding to cytokines or cell surface functional molecules such as cytokine receptor or co-stimulatory molecules. Orally available low molecular weight products, targeting key molecules during the disease processes; therefore, currently attract particular attention because they enter into cytoplasm and directly regulate intracellular signals. Among them, products targeting kinase proteins have been emerging because multiple signaling kinases are involved in the pathological processes. The multiple cytokines and cell surface molecules bind to receptors, resulting in the activation of various signaling, including phosphorylation of kinase proteins. Five hundred and eighteen genes encoding kinase proteins have been identified from human genome-wide studies. Among them, the tyrosine kinase is phosphorylated following receptor binding in a cytokine response and is involved in cell proliferation, differentiation and adhesion during pathological processes including those of RA. Therefore, many investigators have shed light on tyrosine kinases as the target of the treatment of various diseases. More than 90 genes encoding tyrosine kinases have been identified from human genome-wide studies and 14 tyrosine kinases are known to be involved in synovial membrane in patients with RA, compared to those with osteoarthritis [10].
Among them, members of Janus kinase (JAK) family are essential for the signaling pathways of various cytokines and are implicated in the pathogenesis of RA. Molecules in a JAK family consist of homodimer or heterodimer of Jak1, Jak2, Jak3, and tyrosine kinase 2 (Tyk2). After the engagement of cytokines receptors constitutively bound to JAK, JAK is activated by a conformational change and phosphorylated (Fig. 2). These in turn phosphorylate the cytokine receptors, which leads to phosphorylation of the signal transducers and activators of transcription that subsequently translocate into the nucleus, where they regulate gene expression. Among members of a JAK family, the expression of JAK3 is limited to lymphocytes and constitutively binds to the commonγchain which is a common receptor subunit for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 [11]. Therefore, the deficiency or dysfunction of Jak3 is synonymous with impairment in these cytokines which impaired lymphocyte development and function and leads to immunodeficiency in mice. However, because of its limited expression on hematopoietic cells, the lack of Jak3 is not known to affect other organs, whereas the deficiency of Jak1 or Jak2 results in fetal death. Thus, selective inhibition of Jak3 was considered as a potential target in the treatment of RA without affecting other organ systems [12-14].
Figure 2.
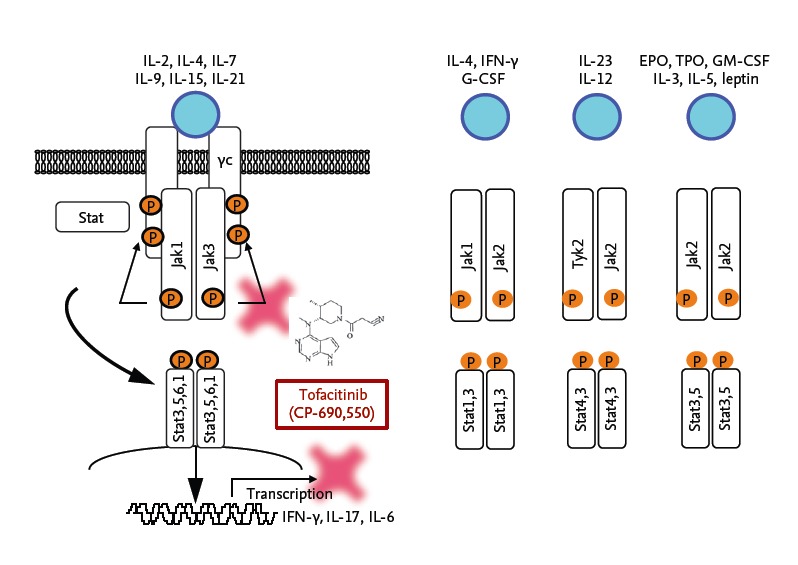
Signaling mechanisms through the Janus kinase (JAK)/STAT pathway and action point of a JAK inhibitor tofacitinib. IL, interleukin; IFN, interferon; G-CSF, granulocyte colony stimulating factor; EPO, erythropoietin; TPO, thrombopoietin; GM-CSF, granulocyte-macrophage colonystimulating factor.
Based on these backgrounds, an orally available Jak inhibitor tofacitinib was developed with expectations to be a new immunosuppressant with a few side effects. Tofacitinib was found by screening for inhibitors of in vitro Jak3 kinase activity from the Pfizer chemical library and extensive chemical modification by Changelian et al. [15], Flanagan et al. [16]. Thereafter, multiple clinical trials using an orally available JAK inhibitor tofacitinib for patients with RA have been globally undertaken. Subsequently to multiple phase 2 trials, 6 phase 3 studies were performed to investigate the efficacy and safety of tofacitinib [17-23]. Briefly, oral administration of 5 or 10 mg twice a day of tofacitinib was significantly effective than placebo with or without methotrexate in RA patients with methotrexate-naïve, inadequately responsive to methotrexate or inadequately responsive to TNF-inhibitors. The efficacy occurred rapidly and strongly and there was not significant difference between tofacitinib and adalimumab, a representative TNF-inhibitor. Also, it is worth noting that significant improvement in 6 months-changes of ΔmTSS, erosion score, joint space narrowing score was observed in patients treated with 10 mg of tofacitinib, compared to placebo, indicating that tofacitinib has a potential to inhibit progress in joint destruction in patients with RA.
The most commonly reported adverse events were infections such as nasopharyngitis, increases in total cholesterol, elevation of transaminase and serum creatinine, decreases in neutrophil counts and anemia [17-23]. Although the majority of the adverse events have been tolerable and managed, opportunistic infections such as herpes zoster disseminated, pulmonary tuberculosis, cryptococcal pneumonia and pneumocystis pneumonitis were reported. In our in vitro studies, proliferation of CD4+ T cells in patients with RA stimulated with anti-CD3 and anti-CD28 antibodies was significantly reduced at week 52 after the tofacitinib treatment, compared to that at the baseline, although no significant decrease in CD4+ T cell count was observed, indicating the possible relevance of the impairment in T cell responsiveness by tofacitinib to the serious infectious events [24]. Thus, although tofacitinib is approved in United States, Japan, Switzerland and many countries except for the European Union, careful post-marketing surveillances would be required to pay special attention on infections and malignancies and the accumulation of evidence regarding long-term safety would be warranted.
Although the precise action of tofacitinib on JAK pathway in mice has been investigated, the exact mechanism of action in patients with RA remained unclear. Ghoreschi et al. [25,26] reported that tofacitinib potently inhibited Jak3 and Jak1 and to a lesser extent Jak2 with little effects on Tyk2 and that it, thereby, inhibited signaling by interferon γ (IFN-γ), IL-6 and to a lesser extent IL-12 and IL-23, indicating that Th1 cell differentiation is therefore blocked, as is the generation of pathogenic Th17 cells. We also assessed the in vivo effects of tofacitinib using the severe combined immune deficiency (SCID)-human rheumatoid arthritis-transgenic (HuRAg) mice, an RA animal model utilizing SCID mice implanted with synovium and cartilage from patients with RA and tofacitinib was continuously given to the mice by the osmotic mini-pump [27]. Treatment of SCID-HuRAg mice with tofacitinib suppressed IL-17 and IFN-γ production and proliferation of CD4+ T cells, resulting in inhibition of IL-6 and IL-8 production by synovial fibroblasts and CD14+ cells as well as cartilage destruction. We also clarified that tofacitinib inhibited differentiation and antibody production of B cells as well as antigen-presentation activity of dendritic cells by inhibiting type I IFN-mediated signaling and subsequently reducing expression of costimulatory molecules such as CD80 and CD86 [28,29]. Taken together, primary targets of tofacitinib appear dendritic cells, CD4+ T cells and activated B cells which leads to multi-cytokine targeting beyond simply a JAK3 inhibitor.
According to the launch of tofacitinib, although there are long-term safety concerns, multiple low molecular weight products targeting JAK are emerging for the development (Fig. 3). The JAK3 inhibitors decernotinib and peficitinib showed strong and rapid efficacy and similar adverse events to tofacitinib in their phase 2 trials. Phase 2 clinical trials were over regarding baricitinib targeting JAK1/2 and filgotinib targeting JAK1 and similar efficacy were reported. Thus, orally available small molecules targeting specific kinase could represent a valuable addition to the current DMARD and biologic agents and these kinase inhibitors such as “Jakinibs” would take in the therapeutic armamentarium in RA and multiple autoimmune diseases.
Figure 3.
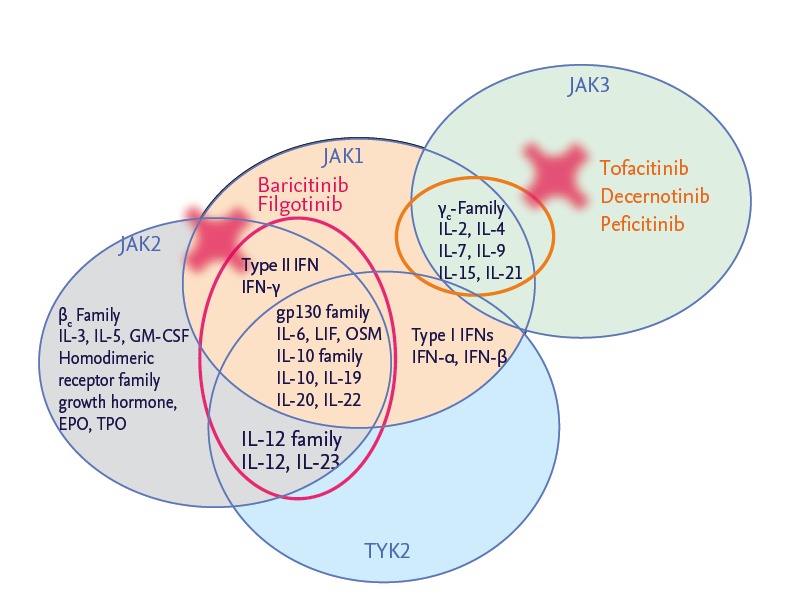
Development of Janus kinase (JAK) inhibitors. IL, interleukin; IFN, interferon; LIF, leukemia inhibitory factor; OSM, oncostatin M; EPO, erythropoetin; TPO, thrombopoetin; TYK, tyrosine kinase.
THE NEXT DEVELOPMENT OF TREATMENT OF RA: DRUG HOLIDAY
After clinical remission is obtained by DMARD and/or biological DMARD, the remission has to be maintained. The 8th recommendation in the treat-to-target is that “the desired treatment target should be maintained throughout the remaining course of the disease” [5]. However, biological DMARDs are also associated with short and long-term adverse effects including injection site reactions, increased risk of infection and high costs. Optimal use of these drugs is therefore warranted, including the right dose for the right patient. Effective dose reduction in the context of low disease activity is; however, up to recently very uncommon in daily clinical practice. Currently, discontinuation of a biological DMARD without disease flare is our next goal and desirable from the standpoint of risk reduction and cost effectiveness, especially for patients with clinical remission. Thus, how and when biological DMARDs are reescalated without disease flare is an emerging theme to strategically treat RA. We are now in a position to evaluate what is possible in terms of maintaining remission while at the same time reducing the burden of treatment on the patient and health care system. Currently, data are emerging from large, well-conducted studies designed to answer this question, shedding light on which patient populations and treatment strategies can survive treatment discontinuation or tapering with low risk of disease flare without functional and radiographic damage progression [30-32].
Data emerging from large, well-conducted studies indicate that approximately half of early RA patients could discontinue biological DMARDs targeting TNF without clinical flare and functional impairment after obtaining reduction of disease activity to remission by biological DMARDs in combination with methotrexate. For instance, a multinational, double-blinded, randomized controlled study, OPTIMA study, was performed to determine the optimal protocol for treatment initiation with adalimumab plus methotrexate in patients with RA [6]. In this study, the withdrawal of adalimumab in early RA patients with a mean RA duration of 3.9 months was also assessed. Outcomes of withdrawal or continuation of adalimumab were assessed in patients who achieved a stable low disease activity target after 26 weeks of initially assigned treatment with adalimumab and methotrexate. Of the 466 RA patients treated with adalimumab and methotrexate, 207 achieved the stable low disease activity measured by DAS28-C-reactive protein at weeks 22 and 26 and were re-randomized to placebo plus methotrexate or adalimumab plus methotrexate during the second study period for 52 weeks. After 52 weeks, 91% and 86% of patients who continued adalimumab treatment maintained low disease activity and remission, respectively, compared with 81% and 66% of patients who withdrew from adalimumab treatment. Saleem et al. [33] also reported that a TNF-inhibitor-free sustained remission rate was 60% after acquiring DAS28 remission in methotrexate-naïve early RA patients. Within the initial treatment group, the only clinical predictor of the successful discontinuation was shorter symptom duration prior to receiving therapy (median, 5.5 months vs. 9.0 months, p = 0.008). No other clinical features including activity measured by power Doppler were associated with the discontinuation of biological DMARD.
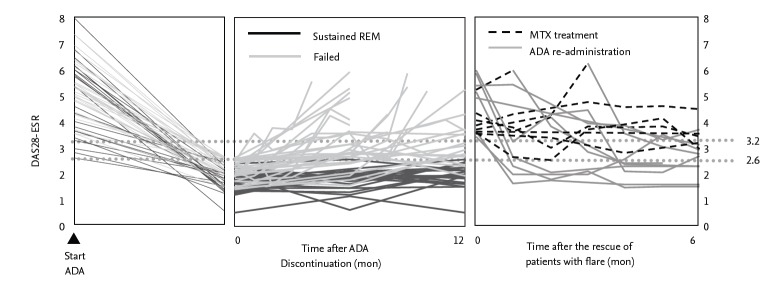
However, fewer patients sustained remission or low disease activity after the discontinuation of biological DMARDs for patients with established RA, compared to early RA. It is often difficult to successfully discontinue biological DMARDs and the results were controversial among studies. For instance, we carried HONOR study to investigate the possibility of discontinuing adalimumab for 1 year without flaring in RA patients [34]. Prior to the study, 197 RA patients with inadequate response to methotrexate were treated with methotrexate and adalimumab and 75 patients met the adalimumab-free criteria, steroid-free, and sustained DAS28-erythrocyte sedimentation rate (ESR) remission for more than 6 months (Fig. 4). The mean disease duration and DAS28- ESR score in 75 patients was 7.5 years and 5.1 at baseline, respectively. Of the 75 patients, 52 agreed to adalimumab discontinuation and 23 patients continued to use adalimumab for 1 year. The remission rate (83%) and the rates of low disease activity (91%) measured by DAS28- ESR in the adalimumab continuation group were significantly higher than those (48% and 62%, respectively) in the adalimumab discontinuation group 1 year after the continuation or discontinuation decision was made. In the analysis of predictive factors related to sustaining remission for 1 year, DAS28-ESR at the discontinuation and disease duration had a marked correlation with sustained remission in multivariate analyses. Patients whose disease duration was less than 2 years, higher ratio of remission and low disease activity were kept at 1 year after the discontinuation than those with longer than 2 years (Fig. 5). Subsequent receiver operating characteristic analysis for high estimation of sustained remission indicated a cut-off value for the adalimumab-free remission of 1.98, indicating that “deep remission” would be a key for successful discontinuation of adalimumab in established patients with RA. The RRR (remission induction by Remicade in RA) study was also undertaken to assess the possibility of discontinuation of infliximab DMARDs in established RA patients. This study also indicated that “deep remission” is required to successfully discontinue biological; DAS28-ESR cut-off point at discontinuation was 2.22 for achieving low disease activity at week 52 in the infliximab-free group [35]. Thus, the mild remission is insufficient for the discontinuation and biological DMARDs should be continued in such patients even under DAS28 remission.
Figure 4.
Clinical course of rheumatoid arthritis patients who discontinued adalimumab after obtaining clinical remission by methotrexate and adalimumab. Adapted from Tanaka et al. [34]. DAS28, disease activity score 28; ESR, erythrocyte sedimentation rate; ADA, adalimumab; REM, remission; MTX, methotrexate.
Figure 5.
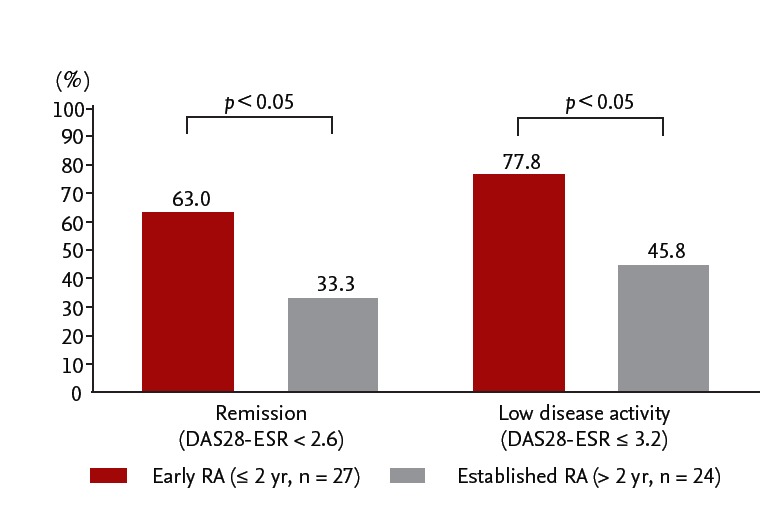
%Remission and %low disease activity at 1 year after discontinuation of adalimumab in patients with early rheumatoid arthritis (RA) versus established RA. Adapted from Tanaka et al. [34]. DAS28, disease activity score 28; ESR, erythrocyte sedimentation rate.
Thus, “treatment holiday” of biological DMARDs is now feasible in some patients with RA with long-standing RA, but “deep remission” at the discontinuation is a key factor to keep the treatment holiday of biological DMARDs (Fig. 6) [30-32]. However, such intensive treatment would have the potential of reducing drug-induced adverse effects and reducing long-terms medical costs, although the risks of worsening clinical, structural, and functional outcomes should be considered with careful monitoring.
Figure 6.
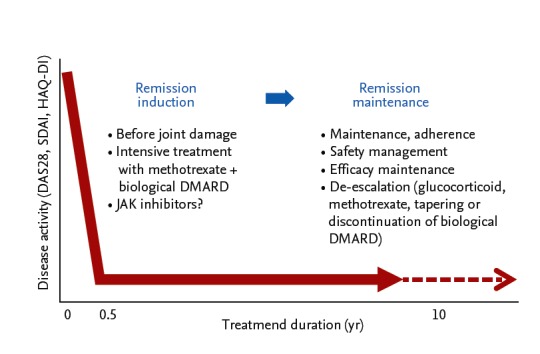
Treatment strategy for rheumatoid arthritis to stop joint damage. DAS28, disease activity score 28; SDAI, simplif ied disease activity index; HAQ-DI, Health Assessment Questionnaire disability index; DMARD, disease-modifying anti-rheumatic drug; JAK, Janus kinase.
CONCLUSIONS
RA is a systemic autoimmune disease, leading to synovial hypertrophy and adjacent bone and cartilage destruction that causes significant morbidity and mortality. However, the combined use of synthetic DMARD such as methotrexate and a biological DMARD targeting TNF and IL-6 have revolutionized treatment of RA and clinical remission or low disease activity are now realistic targets, achieved by a large proportion of RA patients. Furthermore, the maintenance of remission or low disease activity has produced significant improvements in radiographic and function outcomes.
However, biological DAMRD requires special handling and transport and parenteral administration. Even with this outstanding effective drugs, there are portion of patients that are still refractory to all existing biologics. Tofacitinib targets the JAK which plays pivotal roles in the beginning of the intracellular cytokine signaling pathway thereby inhibits multiple pathways. The multiple phase 3 studies revealed that and oral administration of 5 or 10 mg tofacitinib was significantly effective than placebo with or without methotrexate in active RA patients with methotrexate-naïve, inadequately responsive to methotrexate or TNF-inhibitors. Therapeutic efficacy of tofacitinib was observed in a short term after administration in patients and was as strong as adalimumab, a TNF-inhibitor. The most commonly observed adverse events were related to infection, hematologic, hepatic, and renal disorders. Meanwhile, association of tofacitinib on carcinogenicity is under debate. Further investigation on post-marketing survey will greatly help us understand the positioning of this drug.
We also need to create the therapeutic strategies for the long-term maintenance after obtaining remission in order to keep structural remission, functional remission, social remission and comprehensive remission. However, there are concerns regarding long-term safety by using synthetic DMARDs as well as economic burden associated with expensive biological DMARDs. Thus, de-escalation of synthetic and/or biological DMARDs attracts attention from the contexts. How and when DMARDs are de-escalated without disease flare is an emerging theme to strategically treat RA. Recent reports indicate that more than half of early RA patients could discontinue biological DMARD targeting TNF after obtaining reduction of disease activity remission. However, fewer patients sustained remission after the discontinuation of biological DMARDs for patients with established RA, but “deep remission” at the discontinuation is a key factor to keep the treatment holiday of biological DMARD. However, such a treatment strategy would have the potential of reducing drug-induced adverse effects and reducing long-terms medical costs, although the risks of worsening clinical, structural and functional outcomes should be considered with careful monitoring. Since we have obtained strong weapons to treat RA, a new strategy rather than a new target should be required for the advanced therapy of RA.
Acknowledgments
The authors thank all medical staff in all institutions for providing the data. This work was supported in part by a Grant-In-Aid for Scientific Research from the Ministry of Health, Labor and Welfare of Japan, the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the University of Occupational and Environmental Health, Japan, through UOEH Grant for Advanced Research.
Footnotes
Y.T. has received consulting fees, speaking fees, and/or honoraria from Abbvie, Chugai, Astellas, Takeda, Santen, Mitsubishi-Tanabe, Pfizer, Janssen, Eisai, Daiichi-Sankyo, UCB, GlaxoSmithKline, Bristol-Myersand has received research grants from Mitsubishi-Tanabe, Chugai, MSD, Astellas, Novartis. No funding was used to support the writing of the manuscript.
REFERENCES
- 1.Burmester GR, Kivitz AJ, Kupper H, et al. Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis. 2015;74:1037–1044. doi: 10.1136/annrheumdis-2013-204769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furst DE, Emery P. Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatology (Oxford) 2014;53:1560–1569. doi: 10.1093/rheumatology/ket414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 4.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 5.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet. 2014;383:321–332. doi: 10.1016/S0140-6736(13)61751-1. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi T, Yamanaka H, Ishiguro N, et al. Adalimumab, a human anti-TNF monoclonal antibody, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis. 2014;73:536–543. doi: 10.1136/annrheumdis-2012-202433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404–413. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 9.Weinblatt ME, Bathon JM, Kremer JM, et al. Safety and efficacy of etanercept beyond 10 years of therapy in North American patients with early and longstanding rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:373–382. doi: 10.1002/acr.20372. [DOI] [PubMed] [Google Scholar]
- 10.D’Aura Swanson C, Paniagua RT, Lindstrom TM, Robinson WH. Tyrosine kinases as targets for the treatment of rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:317–324. doi: 10.1038/nrrheum.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macchi P, Villa A, Giliani S, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 12.Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O’Shea JJ. Therapeutic targeting of Janus kinases. Immunol Rev. 2008;223:132–142. doi: 10.1111/j.1600-065X.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y, Yamaoka K. JAK inhibitor tofacitinib for treating rheumatoid arthritis: from basic to clinical. Mod Rheumatol. 2013;23:415–424. doi: 10.1007/s10165-012-0799-2. [DOI] [PubMed] [Google Scholar]
- 14.O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72 Suppl 2:ii111–ii115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Changelian PS, Flanagan ME, Ball DJ, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 16.Flanagan ME, Blumenkopf TA, Brissette WH, et al. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem. 2010;53:8468–8484. doi: 10.1021/jm1004286. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study Investigators Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 2011;63:1150–1158. doi: 10.1002/acr.20494. [DOI] [PubMed] [Google Scholar]
- 18.Fleischmann R, Cutolo M, Genovese MC, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64:617–629. doi: 10.1002/art.33383. [DOI] [PubMed] [Google Scholar]
- 19.Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 20.Kremer J, Li ZG, Hall S, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159:253–261. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 21.Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 22.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367:508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 23.van der Heijde D, Tanaka Y, Fleischmann R, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65:559–570. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 24.Sonomoto K, Yamaoka K, Kubo S, et al. Effects of tofacitinib on lymphocytes in rheumatoid arthritis: relation to efficacy and infectious adverse events. Rheumatology (Oxford) 2014;53:914–918. doi: 10.1093/rheumatology/ket466. [DOI] [PubMed] [Google Scholar]
- 25.Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550) J Immunol. 2011;186:4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maeshima K, Yamaoka K, Kubo S, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-gamma and interleukin-17 production by human CD4+ T cells. Arthritis Rheum. 2012;64:1790–1798. doi: 10.1002/art.34329. [DOI] [PubMed] [Google Scholar]
- 28.Wang SP, Iwata S, Nakayamada S, et al. Amplification of IL-21 signalling pathway through Bruton’s tyrosine kinase in human B cell activation. Rheumatology (Oxford) 2015;54:1488–1497. doi: 10.1093/rheumatology/keu532. [DOI] [PubMed] [Google Scholar]
- 29.Kubo S, Yamaoka K, Kondo M, et al. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann Rheum Dis. 2014;73:2192–2198. doi: 10.1136/annrheumdis-2013-203756. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka Y, Hirata S, Saleem B, Emery P. Discontinuation of biologics in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2013;31(4 Suppl 78):S22–S27. [PubMed] [Google Scholar]
- 31.Tanaka Y. Intensive treatment and treatment holiday of TNF-inhibitors in rheumatoid arthritis. Curr Opin Rheumatol. 2012;24:319–326. doi: 10.1097/BOR.0b013e3283524e4c. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y. Next stage of RA treatment: is TNF inhibitor-free remission a possible treatment goal? Ann Rheum Dis. 2013;72 Suppl 2:ii124–ii127. doi: 10.1136/annrheumdis-2012-202350. [DOI] [PubMed] [Google Scholar]
- 33.Saleem B, Keen H, Goeb V, et al. Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped? Ann Rheum Dis. 2010;69:1636–1642. doi: 10.1136/ard.2009.117341. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka Y, Hirata S, Kubo S, et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann Rheum Dis. 2015;74:389–395. doi: 10.1136/annrheumdis-2013-204016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka Y, Takeuchi T, Mimori T, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis. 2010;69:1286–1291. doi: 10.1136/ard.2009.121491. [DOI] [PMC free article] [PubMed] [Google Scholar]