Abstract
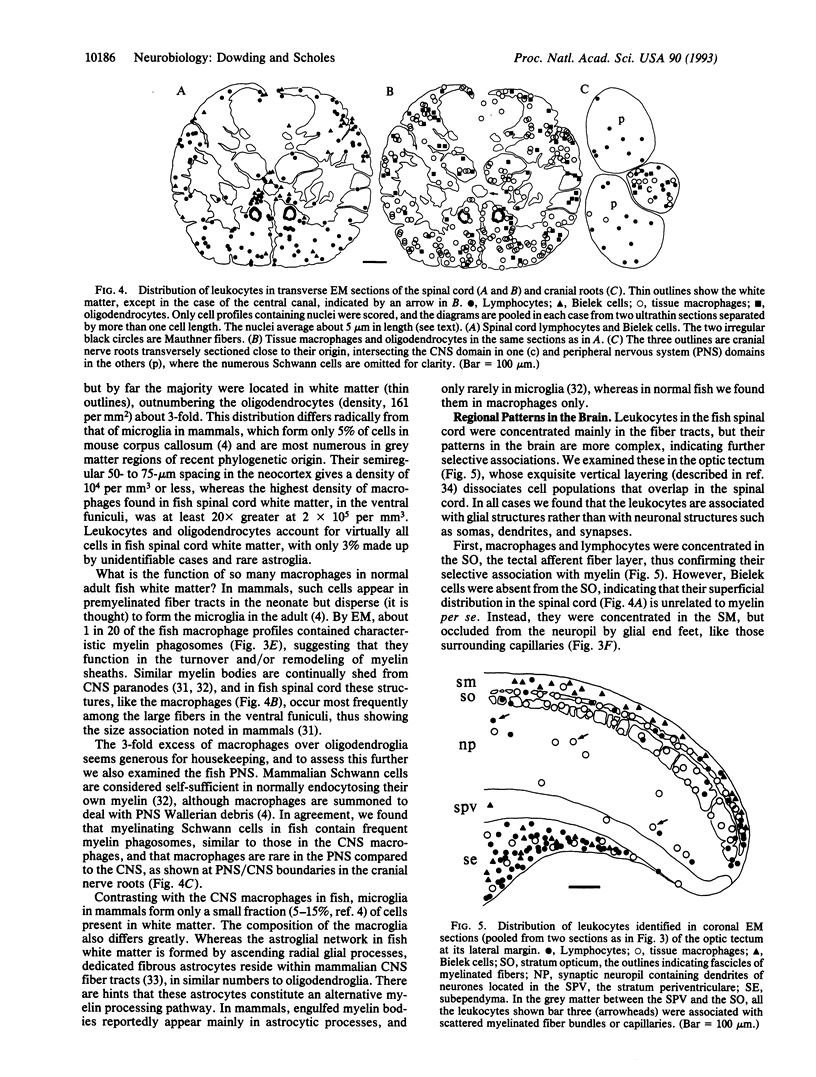
As shown by staining with a monoclonal antibody against fish CD45, leukocytes are present in very large numbers in the fish central nervous system. Their subtypes were distinguished by electron microscopy and found to include all major hematogenous forms except thrombocytes, the most numerous being tissue macrophages and lymphocytes. As a population, they differ fundamentally from ramified microglia, the restricted form of myeloid cells present in the central nervous system in mammals. They are rare in most grey matter regions but are concentrated in myelinated fiber tracts as well as in certain strata of the radial glial network. The macrophages engulf discarded myelin and outnumber the oligodendrocytes in normal spinal cord white matter, where the density of lymphocytes is > 5000-fold greater than reported in rat.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsteindottir K., Brennan A., Jessen K. R., Mirsky R. In the presence of dexamethasone, gamma interferon induces rat oligodendrocytes to express major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9054–9058. doi: 10.1073/pnas.89.19.9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore W. F., Summers B. A., Sedgwick J. Lymphocyte-myelin sheath interactions in acute experimental allergic encephalomyelitis. J Neuroimmunol. 1989 Jun;23(1):19–24. doi: 10.1016/0165-5728(89)90067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano B., Gonzalez B., Dalmau I., Vela J. M. Identification and distribution of microglial cells in the cerebral cortex of the lizard: a histochemical study. J Comp Neurol. 1991 Sep 15;311(3):434–444. doi: 10.1002/cne.903110312. [DOI] [PubMed] [Google Scholar]
- Corneliuson O., Berthold C. H., Fabricius C., Gatzinsky K., Carlstedt T. Marchi-positive myelinoid bodies at the transition between the central and the peripheral nervous system in some vertebrates. J Anat. 1989 Apr;163:17–31. [PMC free article] [PubMed] [Google Scholar]
- David S., Bouchard C., Tsatas O., Giftochristos N. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron. 1990 Oct;5(4):463–469. doi: 10.1016/0896-6273(90)90085-t. [DOI] [PubMed] [Google Scholar]
- Dowding A. J. FL.1, an anti-fish leukocyte mAb, reacts with CD45 in Oreochromine fish. Biochem Soc Trans. 1992 Nov;20(4):350S–350S. doi: 10.1042/bst020350s. [DOI] [PubMed] [Google Scholar]
- Dowding A. J., Maggs A., Scholes J. Diversity amongst the microglia in growing and regenerating fish CNS: immunohistochemical characterization using FL.1, an anti-macrophage monoclonal antibody. Glia. 1991;4(4):345–364. doi: 10.1002/glia.440040403. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L. Origin and evolution of the vertebrate immune system. APMIS. 1992 May;100(5):383–392. doi: 10.1111/j.1699-0463.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- Fierz W., Endler B., Reske K., Wekerle H., Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985 Jun;134(6):3785–3793. [PubMed] [Google Scholar]
- Goodbrand I. A., Gaze R. M. Microglia in tadpoles of Xenopus laevis: normal distribution and the response to optic nerve injury. Anat Embryol (Berl) 1991;184(1):71–82. doi: 10.1007/BF01744263. [DOI] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Kiefer R., Schoen S. W., Kreutzberg G. W. New expression of myelomonocytic antigens by microglia and perivascular cells following lethal motor neuron injury. J Neuroimmunol. 1990 May;27(2-3):121–132. doi: 10.1016/0165-5728(90)90061-q. [DOI] [PubMed] [Google Scholar]
- Griffin J. W., George R., Lobato C., Tyor W. R., Yan L. C., Glass J. D. Macrophage responses and myelin clearance during Wallerian degeneration: relevance to immune-mediated demyelination. J Neuroimmunol. 1992 Oct;40(2-3):153–165. doi: 10.1016/0165-5728(92)90129-9. [DOI] [PubMed] [Google Scholar]
- Harel A., Fainaru M., Shafer Z., Hernandez M., Cohen A., Schwartz M. Optic nerve regeneration in adult fish and apolipoprotein A-I. J Neurochem. 1989 Apr;52(4):1218–1228. doi: 10.1111/j.1471-4159.1989.tb01869.x. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981 Aug 1;154(2):347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey W. F., Hsu B. L., Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991 Feb;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. Presence of Marchi-positive myelinoid bodies in the spinal cord white matter of some vertebrate species. J Morphol. 1977 Jul;153(1):1–21. doi: 10.1002/jmor.1051530102. [DOI] [PubMed] [Google Scholar]
- Hintzen R. Q., Polman C. H., Lucas C. J., van Lier R. A. Multiple sclerosis: immunological findings and possible implications for therapy. J Neuroimmunol. 1992 Jul;39(1-2):1–10. doi: 10.1016/0165-5728(92)90169-l. [DOI] [PubMed] [Google Scholar]
- Janzer R. C., Raff M. C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987 Jan 15;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Joly E., Mucke L., Oldstone M. B. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991 Sep 13;253(5025):1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- Maggs A., Scholes J. Reticular astrocytes in the fish optic nerve: macroglia with epithelial characteristics form an axially repeated lacework pattern, to which nodes of Ranvier are apposed. J Neurosci. 1990 May;10(5):1600–1614. doi: 10.1523/JNEUROSCI.10-05-01600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male D., Pryce G., Linke A., Rahman J. Lymphocyte migration into the CNS modelled in vitro. J Neuroimmunol. 1992 Oct;40(2-3):167–171. doi: 10.1016/0165-5728(92)90130-d. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen-bearing microglia in the rat central nervous system of radiation bone marrow chimeras. J Neuroimmunol. 1987 Dec;17(1):71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Macrophages and the nervous system. Int Rev Cytol. 1991;125:203–244. doi: 10.1016/s0074-7696(08)61220-6. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Glial cell diversification in the rat optic nerve. Science. 1989 Mar 17;243(4897):1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Secombes C. J., van Groningen J. J., Egberts E. Separation of lymphocyte subpopulations in carp Cyprinus carpio L. by monoclonal antibodies: immunohistochemical studies. Immunology. 1983 Jan;48(1):165–175. [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D., Schwender S., Imrich H., Dörries R., Butcher G. W., ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J. A., Yoon M. G. Morphology of radial glia, ependymal cells, and periventricular neurons in the optic tectum of goldfish (Carassius auratus). J Comp Neurol. 1982 Feb 20;205(2):128–138. doi: 10.1002/cne.902050204. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Functional plasticity of microglia: a review. Glia. 1988;1(5):301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S. CD45. A prototype for transmembrane protein tyrosine phosphatases. J Biol Chem. 1991 Dec 15;266(35):23517–23520. [PubMed] [Google Scholar]
- Vanegas H., Laufer M., Amat J. The optic tectum of a perciform teleost. I. General configuration and cytoarchitecture. J Comp Neurol. 1974 Mar 1;154(1):43–60. doi: 10.1002/cne.901540104. [DOI] [PubMed] [Google Scholar]
- Wilson M., Hsu E., Marcuz A., Courtet M., Du Pasquier L., Steinberg C. What limits affinity maturation of antibodies in Xenopus--the rate of somatic mutation or the ability to select mutants? EMBO J. 1992 Dec;11(12):4337–4347. doi: 10.1002/j.1460-2075.1992.tb05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]