Abstract
Renovascular hypertension (RVH) is a common cause of both cardiovascular and renal morbidity and mortality. In renal artery stenosis (RAS), atrophy in the stenotic kidney is associated with an influx of macrophages and other mononuclear cells. We tested the hypothesis that chemokine receptor 2 (CCR2) inhibition would reduce chronic renal injury by reducing macrophage influx in the stenotic kidney of mice with RAS. We employed a well-established murine model of RVH to define the relationship between macrophage infiltration and development of renal atrophy in the stenotic kidney. To determine the role of chemokine ligand 2 (CCL2)/CCR2 signaling in the development of renal atrophy, mice were treated with the CCR2 inhibitor RS-102895 at the time of RAS surgery and followed for 4 wk. Renal tubular epithelial cells expressed CCL2 by 3 days following surgery, a time at which no significant light microscopic alterations, including interstitial inflammation, were identified. Macrophage influx increased with time following surgery. At 4 wk, the development of severe renal atrophy was accompanied by an influx of inducible nitric oxide synthase (iNOS)+ and CD206+ macrophages that coexpressed F4/80, with a modest increase in macrophages coexpressing arginase 1 and F4/80. The CCR2 inhibitor RS-102895 attenuated renal atrophy and significantly reduced the number of dual-stained F4/80+ iNOS+ and F4/80+ CD206+ but not F4/80+ arginase 1+ macrophages. CCR2 inhibition reduces iNOS+ and CD206+ macrophage accumulation that coexpress F4/80 and renal atrophy in experimental renal artery stenosis. CCR2 blockade may provide a novel therapeutic approach to humans with RVH.
Keywords: CCL2, CCR2, hypertension, macrophages, parenchymal cells, renal artery stenosis
renal artery stenosis (RAS) has been identified in 6.8% of individuals over 65 and in up to 40% in individuals with coronary or peripheral vascular disease (8, 10). Management of patients is challenging: a recent study has shown that stenting of the renal artery fails to improve renal or cardiovascular outcomes in patients with renal artery stenosis (2). Current medical therapies are inadequate, as mortality of patients with renovascular disease is as high as 16% per year, mainly due to cardiovascular events (11). Novel therapeutic interventions are clearly needed to address this common and important problem.
In addition to interstitial fibrosis and tubular atrophy, the development of chronic renal disease in RAS is characterized by the accumulation of mononuclear cells, including macrophages (12). It is now recognized that macrophages may be polarized to phenotypes that carry out distinct functions: the proinflammatory “M1” macrophages express inducible nitric oxide synthase (iNOS) and are an abundant source of inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and reactive oxygen species (ROS), whereas the reparative “M2” macrophages express arginase or CD206 and secrete chemokines associated with tissue repair and matrix remodeling (25).
Of the inflammatory mediators, chemokine (C-C motif) ligand 2 (CCL2; also known as monocyte chemoattractant protein or MCP-1) has been shown to play a crucial role in the accumulation of proinflammatory macrophages to sites of tissue injury (21, 30). We have previously demonstrated that CCL2 is rapidly induced in the stenotic kidney (STK) of mice with RVH (1). The primary receptor for CCL2 is chemokine receptor 2 (CCR2), which is expressed on monocytes, myeloid precursor cells and T cells, dendritic cells, and a variety of tissue parenchymal cells (16, 23). Blockade of CCL2/CCR2 signaling reduces chronic tissue injury in a variety of experimental models (7, 13–15, 22, 28, 34). However, the specific cell type(s) expressing CCL2 and/or CCR2 during the development of chronic renal damage in RAS have not yet been defined.
Our present study investigates the early role of CCL2- CCR2 signaling in the development of atrophy and renal injury in the STK of mice subjected to RAS. Utilizing the 2-kidney-1 clip (2K1C) murine model of unilateral RAS, we demonstrate that CCL2-CCR2 signaling plays a critical role in the initiation and development of renal injury. Following RAS, the initial CCL2 signal originates in the renal proximal tubular epithelial cells before the accumulation of inflammatory cells in the STK. The progression of chronic renal injury is characterized by amplification of CCL2/CCR2 signaling and accumulation primarily of iNOS+ and CD206+ macrophages. Employing a CCR2b-specific inhibitor (RS-102895) (5, 19, 28), we demonstrate that CCR2 inhibition appears to prevent accumulation of F4/80+ coexpressing iNOS+ and CD206+ but not arginase+ macrophages. We propose that the CCL2/CCR2 axis may provide a novel therapeutic target for preventing the progression of chronic renal damage in RVH.
MATERIALS AND METHODS
Animal model.
Male C57BLKS/J and Ccl2-RFP reporter mice (Ccl2-RFPflox) (29) (6–7 wk old; Jackson Laboratory, Bar Harbor, ME) were used for the studies. The 2K1C model of renovascular hypertension was used, and the animals were subjected to renal artery stenosis (RAS) or sham surgery as previously described (32). All mice strains were housed at Mayo Clinic animal facilities, and animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee before performance of the experiments.
Blood pressure measurements.
Blood pressure measurements were taken 3 days before the surgery and at 3, 7, 14, and 28 days following surgery using noninvasive tail blood volume determination (CODA 6, Kent Scientific, Torrington, CT).
Biochemical analysis.
Blood was collected via the inferior vena cava at the time of harvest. The plasma was separated by centrifugation upon blood collection and stored at −80°C until the time of assay. Quantitative determination of plasma renin content was done by the radioimmunoassay of generated angiotensin I from angiotensinogen using a commercially available GammaCoat Plasma Renin Activity 125I RIA kit (DiaSorin, Stillwater, MN). Porcine angiotensinogen (A2283; Sigma-Aldrich, St. Louis, MO) substrate was used for the assay.
In vivo neutralization of CCR2.
CCR2b inhibitor RS-102895 (Sigma-Aldrich) or vehicle (water) was administered to mice via drinking water at 10 mg·kg−1·day−1 concentration 2 days before RAS or sham surgery up to a 28-day period following surgery.
Immunohistochemistry and immunofluorescence.
Kidneys were fixed with 10% neutral buffered formalin and processed for histology or immunostaining using standard techniques. Five-micrometer-thick histological sections were stained with hematoxylin-eosin (H&E), picro sirius red (Sigma-Aldrich), anti-F4/80 (1:200, Abd Serotec, Raleigh, NC), and collagen III (1:20, Southern Biotech, Birmingham, AL). Histological sections from CCL2 reporter mice were also stained with anti-red fluorescent protein (1:100, Life Technologies, Eugene, OR) antibody. Atrophy was determined semiquantitatively by measuring percent area of atrophic tubules over the whole cortical surface. F4/80-positive percent area was measured over the whole cortical surface, leaving the perivascular regions of large vessels and medulla. All measurements and quantifications were performed in a random blinded fashion using an Olympus Bx50 microscope (Olympus Optical, Buffalo Grove, IL) and a Micropublisher 3.3 RTV camera (QImaging, Surrey, BC). Quantitative analysis of fibrosis on picro sirius red- and collagen III and also CD3-stained sections was performed using the NIS elements BR 4.13.00 64-bit image analysis system (Nikon Instruments, Melville, NY).
Immunofluorescence staining was performed on deparaffinized kidney tissues. Antigen retrieval was done using Dako antigen retrieval solution (Dako North America, Carpinteria, CA). Primary antibodies for F4/80 (1:200, Abcam, Cambridge, MA), iNOS (1:800, Abcam), arginase 1 (1:100, Santa Cruz Biotechnology, Dallas TX), and CD206 (1:800, Abcam) were incubated overnight. The following day, a cocktail of secondary antibodies, Alexa Fluor 488 goat anti-rat IgG (h+l) and Alexa Fluor 594 goat anti-rabbit IgG (Life Technologies, Grand Island, NY) was incubated for 30 min. The slides were coverslipped with mounting media containing 4′,6-diamidino-2-phenylindole (DAPI). The confirmation of macrophages showing staining for DAPI, F4/80, and iNOS or arginase 1 or CD206 was done by using a Zeiss axio observer Z1, and 10–20 fields/slide were captured at ×400 magnification with water. The double-positive cells for F4/80 and iNOS or F4/80 and arginase 1 or F4/80 and CD206 were then counted in the whole cortical region using these images, avoiding the perivascular region and represented as the number of cells per high-power field (HPF).
Fractionation of macrophages and parenchymal cells.
Kidneys for fractionation study were obtained from mice at 3 and 7 days following RAS or sham surgery. Kidneys were homogenized to obtain single-cell suspensions as described previously (9). Briefly, the renal cortex was minced using a surgical blade in a sterile petri dish and digested with collagenase type IV (CLS4, Worthington, WA) and DNase I (Roche, Indianapolis, IN) at 37°C for 30–40 min. The suspension was then filtered through 40-μm strainers, and cells were counted. Approximately 107 cells were incubated with CD11b antibody-conjugated magnetic microbeads, and CD11b+ or CD11b− fractions were obtained after running through the MACS separation columns in the octoMACS System following the manufacturer's protocol (Miltenyi Biotec, Auburn, CA). The CD11b− fraction was then incubated with CD90 antibody-conjugated magnetic microbeads, and CD90+ or CD11b−/CD90− fractions were collected using the same method as for CD11b+ or CD11b− fractions.
PCR.
Total RNA was extracted with an RNeasy Mini Plus kit (Qiagen, Valencia, CA). RNA quality was assessed using the Agilent 2100 Bioanalyzer (Santa Clara, CA), and cDNA was made using an iScript kit (Bio-Rad, Hercules, CA). The quantitative real-time polymerase reaction was performed using SYBR mix (Roche Diagostics) with specific primers in Bio-Rad IQ5 (Bio-Rad). Primers for Tnfa, Ren1, Agt, and 18S have been published previously (9). Sequences for primers were the following: Ccl2 Forward: 5′-AGC ACC AGC ACC AGC CAA CTC-3′, Reverse: 5′-TGG ATG CTC CAG CCG GCA ACT-3′; Ccl5 Forward: 5′-CGC CAA GTG TGT GCC AAC CCA-3′, Reverse: 5′-GTG GCA TCC CCA AGC TGG CTA-3′; Ccr2 Forward: 5′-TCA GCT GCC TGC AAA GAC CAG A-3′, Reverse: 5′-CAT ACG GTG TGG TGG CCC CT -3′; iNOS Forward: 5′-TGG CTC GCT TTG CCA CGG AC-3′, Reverse: 5′-GCT GCG ACA GCA GGA AGG CA-3′; Ccl8 Forward: 5′-AGG CTC CAG TCA CCT GCT GCT-3′, Reverse: 5′-ACC ACA GCT TCC ATG GGG CAC-3′; Il6 Forward: 5′-TGG TGA CAA CCA CGG CCT TCC-3′, Reverse: 5′-TAA GCC TCC GAC TTG TGA AGT GGT-3′; Il10 Forward: 5′-TCC ATC ATG CCT GGC TCA GCA C-3′, Reverse: 5′-GGC CGA CTG GGA AGT GGG TG -3′, Il12 Forward: 5′ - CAT CGA TGA GCT GAT GCA GT -3′ Reverse: 5′- GCA GAG CTT CAT TTT CAC TCT GT-3′; Tgfb1 Forward: 5′-TTG CCG AGG GTT CCC GCT CT T-3′, Reverse: 5′-CCT CCC GGG CGT CAG CAC TA-3′; and Cd206 Forward: 5′-CCA GCT CGG ATA TGA GCC AA-3′, Reverse: 5′-CTG GGG TTC CAT CAC TCC AC-3′.
Statistical analysis.
Data are presented as means ± SE. Pairwise comparisons were done using Student's t-test for parametric and a Mann-Whitney test for nonparametric data. To analyze the difference between groups, ANOVA or a χ2 test was used when appropriate. A Bonferroni adjustment was used for post hoc comparison of the measurements. A statistically significant difference was accepted when P < 0.05. Statistical analyses were performed with Graphpad Prism 6 (GraphPad Software, La Jolla, CA).
RESULTS
Development of renal atrophy is associated with interstitial inflammation.
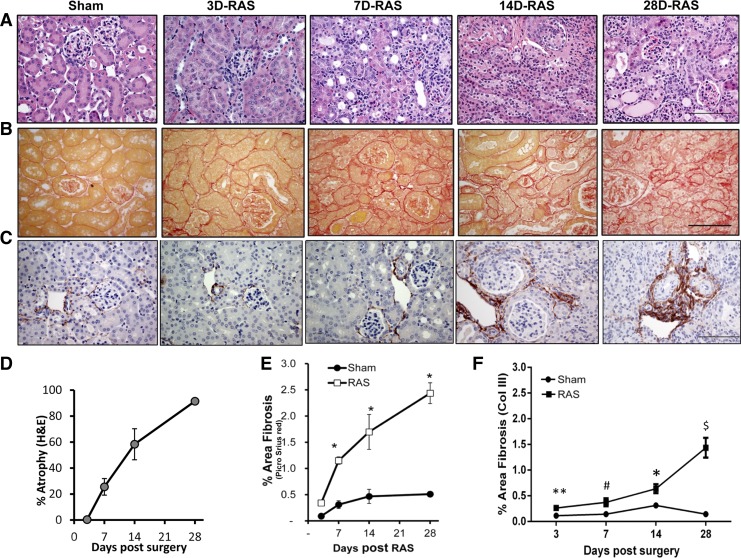
We have earlier reported that systolic blood pressure is significantly elevated within 3 days following RAS surgery and remains elevated at all later time points (9). In the present studies, we sought to define the relationship between macrophage-mediated inflammation and the development of tubular atrophy in the STK of mice with RAS. The characteristics of the mice at different time points following RAS or sham surgery are represented in Table 1. Although the weight of the STK was significantly decreased at 3 days following RAS surgery (147 ± 5 RAS vs. 209 ± 3 mg sham control, P < 0.05, Table 1), microscopic analysis revealed no significant histopathological alterations (Fig. 1A). In particular, there was no evidence of acute kidney injury (tubular dilation or tubular epithelial cell necrosis), tubular atrophy, or interstitial inflammation (Fig. 1, A and D). Also, we did not observe any interstitial fibrosis at day 3 (Fig. 1, B, C, E, and F). By day 7, the STK had lost approximately half its weight (110 ± 8 RAS vs. 223 ± 3 mg sham control, P < 0.001, Table 1). Histological examination revealed moderate tubular atrophy involving ∼30% of the cortical surface area (Fig. 1, A and D); the amount of interstitial fibrous tissue was mildly increased (Fig. 1, B, C, E, and F). By day 14, >50% of the cortex had developed histopathological evidence of severe tubular atrophy (Fig. 1, A and D) with a further increase in interstitial fibrous tissue (Fig. 1, B, C, E, and F). Histological evaluation showed that the development of severe tubular atrophy was associated with an interstitial accumulation of mononuclear leukocytes (Fig. 1A). The contralateral kidney (CLK) showed compensatory enlargement by day 28 (Table 1) without any significant abnormalities (data not shown).
Table 1.
Characteristics of mice at different time points following RAS or sham surgery
|
Day 3 |
Day 7 |
Day 14 |
Day 28 |
|||||
|---|---|---|---|---|---|---|---|---|
| Sham (n = 9) | RAS (n = 20) | Sham (n = 10) | RAS (n = 17) | Sham (n = 5) | RAS (n = 9) | Sham (n = 5) | RAS (n = 7) | |
| Body weight, g | 21 ± 0.2 | 20 ± 0.4 | 22 ± 0.0 | 20 ± 0.4 | 23 ± 0.7 | 20 ± 0.4 | 24 ± 0.4 | 24 ± 0.3 |
| STK weight, mg | 209 ± 3.0 | 147 ± 5.0* | 223 ± 3.0 | 110 ± 8.0† | 226 ± 10.0 | 98 ± 12.0† | 204 ± 2.0 | 69 ± 9.0† |
| CLK weight, mg | 187 ± 3.0 | 175 ± 8.0 | 194 ± 3.0 | 179 ± 3.0 | 198 ± 7.0 | 193 ± 4.0 | 194 ± 7.0 | 211 ± 6.0 |
Values are means ± SE.
RAS, renal artery stenosis; STK, stenotic kidney; CLK, contralateral kidney.
P < 0.05,
P < 0.001 compared with respective sham for each time point.
Fig. 1.
Stenotic kidney of renal artery stenosis (RAS) mice did not develop acute injury but experienced progressive atrophy and fibrosis. A: representative histological images of stenotic kidney of mice with RAS surgery at various time points as stained with hematoxylin and eosin (H&E) at ×400 magnification showing absence of acute injury and progressive atrophy over time compared with 7-day sham. B: representative histological images of stenotic kidney of mice with RAS surgery at various time points as stained with picro sirius red at ×400 magnification showing progressive fibrosis over time compared with 7-day sham. Scale bar = 100 μm. C: representative histological images of stenotic kidney of mice with RAS surgery at various time points as stained with collagen III at ×400 magnification showing progressive fibrosis over time compared with 7-day sham. Scale bar = 100 μm. D: percentage of tubular atrophy over the whole cortical surface as measured semiquantitatively using H&E sections at ×200 magnification. E: percentage of fibrosis over the whole cortical surface as measured by percent area positive for picro sirius red at ×200 magnification. F: percentage of fibrosis over the whole cortical surface as measured by percent area positive for collagen III at ×200 magnification. *P < 0.001, **P = 0.03, $P = 0.003, and #P = 0.004 compared with respective sham.
Development of renal atrophy in the STK is associated with accumulation of macrophages.
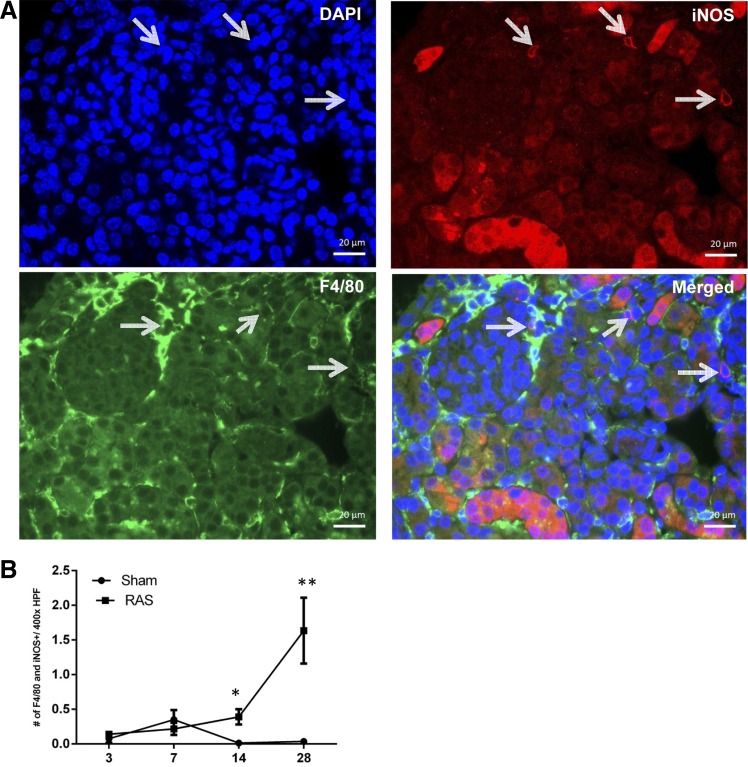
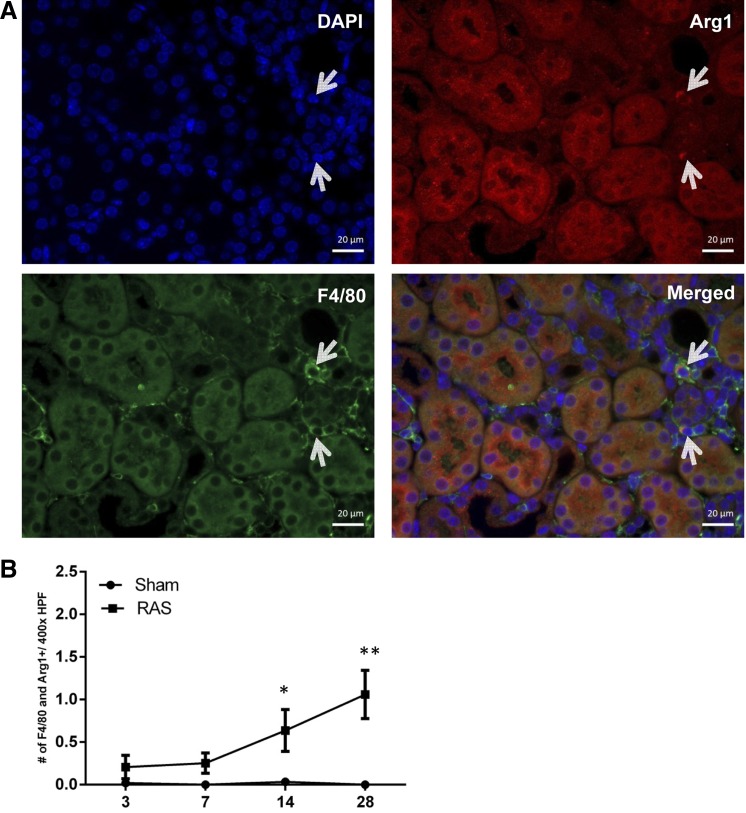
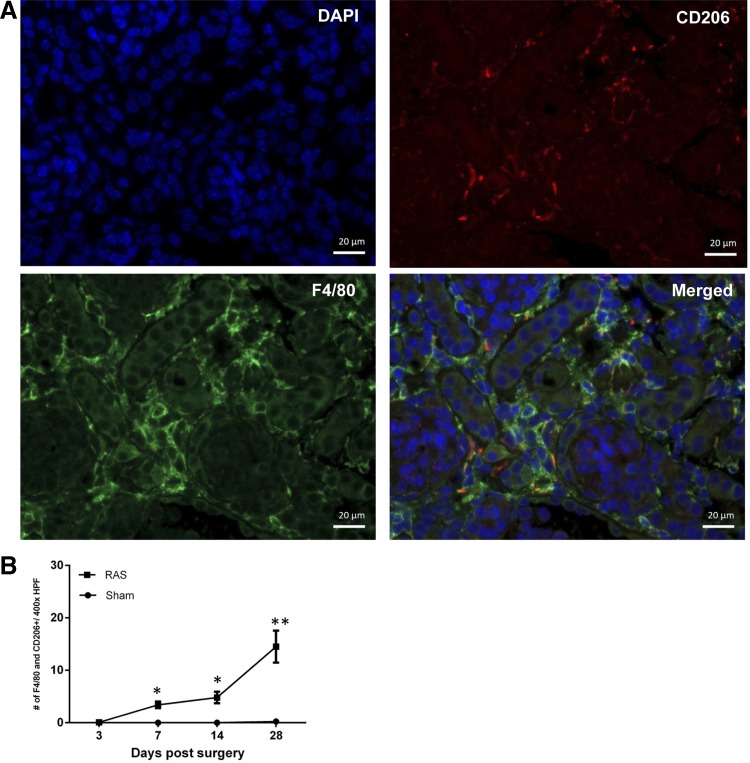
A potential role for macrophage accumulation in the initial stages of chronic renal injury in RAS has not been defined previously. Therefore, our initial studies focused on infiltration of mononuclear cells at early time points following RAS surgery. Immunofluorescence staining was done for iNOS and F4/80 (Fig. 2), or arginase 1 and F4/80 (Fig. 3), or CD206 and F4/80 (Fig. 4) to confirm the presence of double-positive macrophages, and images were obtained. Captured images were then analyzed for the presence of these double-stained cells, and iNOS and F4/80, arginase 1 and F4/80, or CD206 and F4/80 were counted in cortical region of kidney tissues (Figs. 2, 3, and 4, respectively).
Fig. 2.
Immunofluorescence staining for double-positive macrophages showing expression of F4/80 and inducible nitric oxide synthase (iNOS). A: immunofluorescence image showing staining for 4′,6-diamidino-2-phenylindole (DAPI; blue), F4/80 (green), and iNOS (red). Double-positive macrophages expressing F4/80 and iNOS are pointed out with white arrows. B: number of F4/80 and iNOS double-positive cells at ×400 magnification. *P = 0.05. **P = 0.001.
Fig. 3.
Immunofluorescence staining for double-positive macrophages showing expression of F4/80 and arginase 1. A: immunofluorescence image showing staining for DAPI (blue), F4/80 (green), and arginase 1 (red). Double-positive macrophages expressing F4/80 and arginase 1 are pointed out with white arrows. B: number of F4/80 and arginase 1 double-positive cells at ×400 magnification. *P = 0.008. **P = 0.01
Fig. 4.
Immunofluorescence staining for double-positive macrophages showing expression of F4/80 and CD206. A: immunofluorescence image showing staining for DAPI (blue), F4/80 (green), and CD206 (red). Double-positive macrophages expressing F4/80 and CD206 are pointed out with white arrows. B: number of F4/80 and CD206 double-positive cells at ×400 magnification. *P = 0.001. **P = 0.0176
Ccl2 expression by renal tubular epithelial cells precedes macrophage influx.
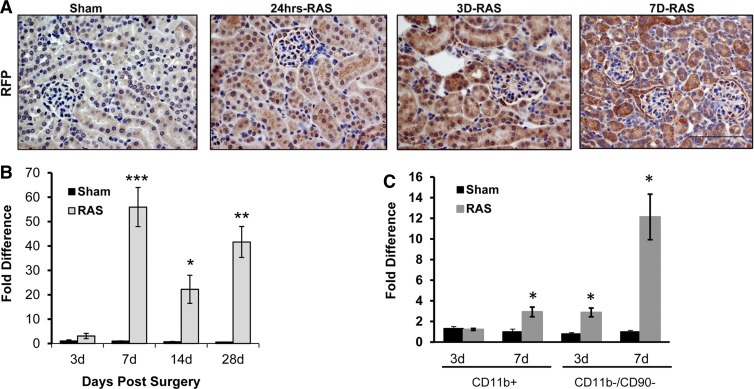
CCL2 is recognized as a critical chemokine that directs macrophage influx and activation in chronic tissue injury. We sought to test the hypothesis that CCL2 expression by renal parenchymal cells preceded accumulation of macrophages in the STK of mice with RVH. To identify the cellular site(s) of CCL2 production, we performed RAS in CCL2-RFP reporter mice, which express red fluorescent protein (RFP) under the control of the CCL2 promoter (29). We detected RFP expression in tubular epithelial cells (both proximal and distal) at 24 h following RAS surgery (Fig. 5A). The intensity of RFP staining within proximal tubular cells increased further at 3 and 7 days (Fig. 5A).
Fig. 5.
Renal parenchymal cells were responsible for early Ccl2 expression post-RAS. A: representative histological images of stenotic kidneys of CCL2-RFP reporter mice with RAS surgery as stained with antibody against red fluorescent protein (RFP) at 24 h, 3 days, and 7 days postsurgery compared with mice 7 days post-sham surgery. All images were taken at ×400 magnification. Scale bar = 100 μm. B: expression of Ccl2 as assessed by RT-PCR in the stenotic kidneys of mice with RAS (n = 13–20 mice for each time point) or sham surgery (n = 10 mice for each time point). C: gene expression of Ccl2 in CD11b+- and CD11b−/CD90− (kidney parenchymal cells)-enriched fractions isolated from the stenotic kidney of mice with RAS (n = 4–5 mice for each time point) or sham surgery (n = 4–5 mice for each time point) at 3 and 7 days postsurgery. Values are means ± SE normalized against 18S transcript and expressed as fold-increase relative to the 7-day sham expression. *P < 0.05, **P = 0.001, ***P = 0.0001 compared with respective sham.
In renal homogenates, we found that Ccl2 expression in RAS mice was significantly elevated at 7 days and persisted at high levels over 28 days (Fig. 5B). To confirm that renal parenchymal cells were the source of early Ccl2 expression, we fractionated renal homogenates by antibody-conjugated magnetic beads to select for renal macrophages (CD11b+ cells) and depleted T cells (CD90+ cells) from the remaining fractions to create a single-cell fraction that was enriched in parenchymal cells (CD11b−/CD90− cells). RT-PCR of the different fractions indicated that the increase in Ccl2 expression at both 3 and 7 days can be attributed mainly to the renal parenchymal cells (Fig. 5C), while the CD11b+ fraction showed only a modest increase at 7 days post-RAS.
CCR2 inhibition reduces renal atrophy by limiting accumulation of macrophages in the STK.
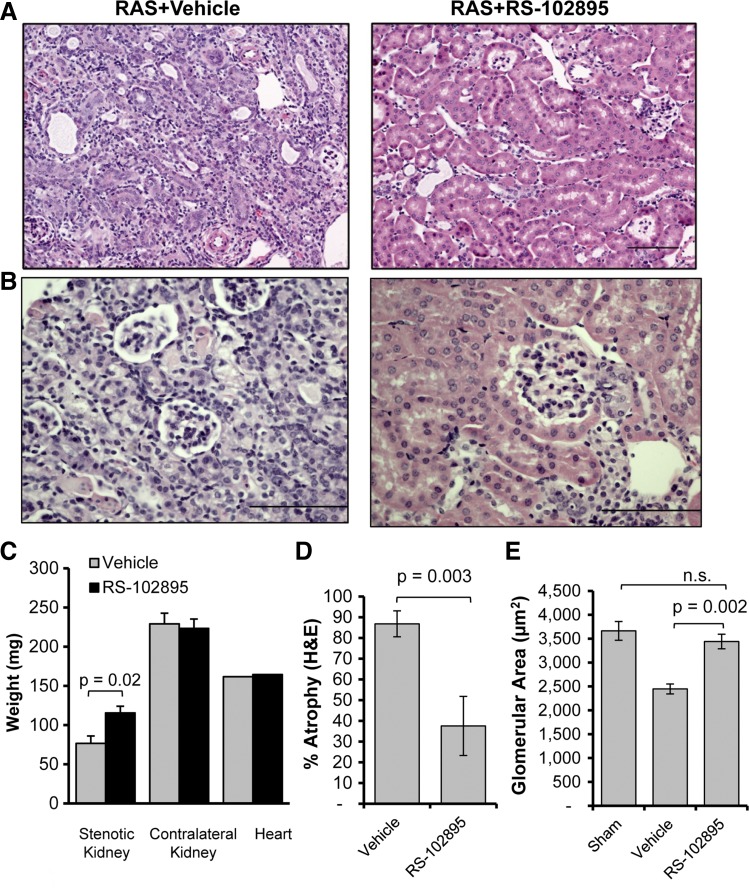
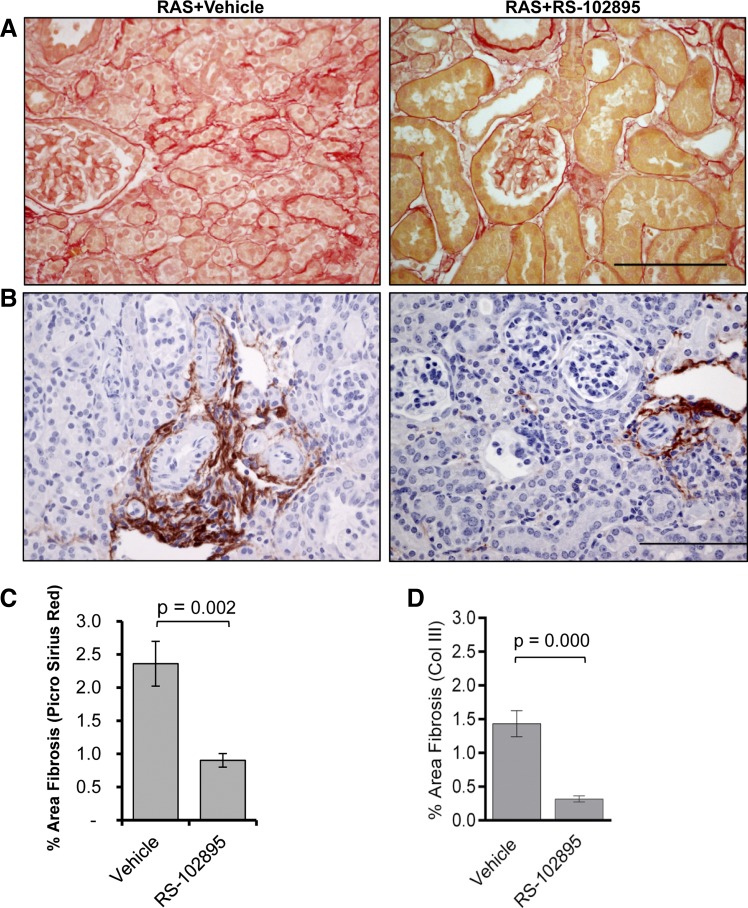
Given that CCR2 is the predominant receptor for CCL2, we sought to test the hypothesis that blockade of CCL2 signaling via CCR2 reduces chronic renal injury in the STK of mice with RVH. We administered the CCR2b-specific inhibitor RS-102895 to mice at the time of RAS surgery and assessed renal histopathological alterations at 28 days. Inhibition of CCL2 signaling through CCR2 attenuated development of atrophy in the STK (Fig. 6, A and D). The CLK of RS-102895-treated mice showed isolated foci of tubular atrophy compared with vehicle-treated RAS mice (atrophy 1.7 ± 0.6% vs. 0.1 ± 0.04 control, P = 0.03). The hearts of RAS mice showed mild fibrosis (mean 2.12% surface area) and did not differ significantly between drug- and vehicle-treated controls (Fig. 6C). Despite the loss of 43% of kidney weight compared with the age-matched sham (116 ± 14 vs. 204 ± 2 mg, P < 0.001), mice with treatment retained their normal glomerular size (Fig. 6, B and E). The RS-102895-treated mice developed significantly less fibrosis compared with vehicle as assessed by picro sirius red (Fig. 7, A and C) and collagen III stains (Fig. 7, B and D).
Fig. 6.
Abrogration of CCL2 signaling by CCR2 inhibitor attenuates development of atrophy in the stenotic kidney. A: representative histological images from RAS mice treated with CCR2 inhibitor (RS-102895) or vehicle (water) as stained with H&E at ×200, showing reduced atrophy in the stenotic kidneys of mice treated with CCR2 inhibitor at 4 wk following RAS. Scale bar = 100 μm. B: representative histological images from RAS mice treated with CCR2 inhibitor (RS-102895) or vehicle (water) as stained with H&E at ×200, showing reduced glomerular area in the stenotic kidneys of mice with vehicle whereas CCR2 inhibitor-treated mice have normal glomerular area at 4 wk following RAS. Scale bar = 100 μm. C: kidney and heart weights of RAS mice treated with CCR2 inhibitor or vehicle. D: comparison of percentage of tubular atrophy over the whole cortical surface between RAS mice treated with CCR2 inhibitor (RS-102895; n = 9 mice) or vehicle (water) (n = 12 mice). Tubular atrophy was measured semiquantitatively using H&E sections. E: comparison of glomerular area between mice with sham surgery (n = 5 mice) and mice with RAS surgery that were either treated with CCR2 inhibitor or vehicle. Glomerular area was obtained from all (20–40) glomeruli with vascular pole in that plane of section at ×200 magnification.
Fig. 7.
CCR2 inhibitor showed reduced fibrosis in the stenotic kidney. A: representative histological images from RAS mice treated with CCR2 inhibitor (RS-102895) or vehicle (water) as stained with picro sirius red at ×400, showing reduced fibrosis in the stenotic kidneys of mice treated with CCR2 inhibitor. Scale bar = 100 μm. B: representative histological images from RAS mice treated with CCR2 inhibitor (RS-102895) or vehicle (water) as stained with collagen III at ×400, showing reduced fibrosis in the stenotic kidneys of mice treated with CCR2 inhibitor. Scale bar = 100 μm. C: comparison of fibrosis between RAS mice treated with CCR2 inhibitor or vehicle. Percentage of fibrosis was measured over the whole cortical surface by percent area positive for picro sirius red at ×200 magnification. D: comparison of fibrosis between RAS mice treated with CCR2 inhibitor or vehicle. Percentage of fibrosis was measured over the whole cortical surface by percent area positive for collagen III at ×200 magnification. Values are means ± SE.
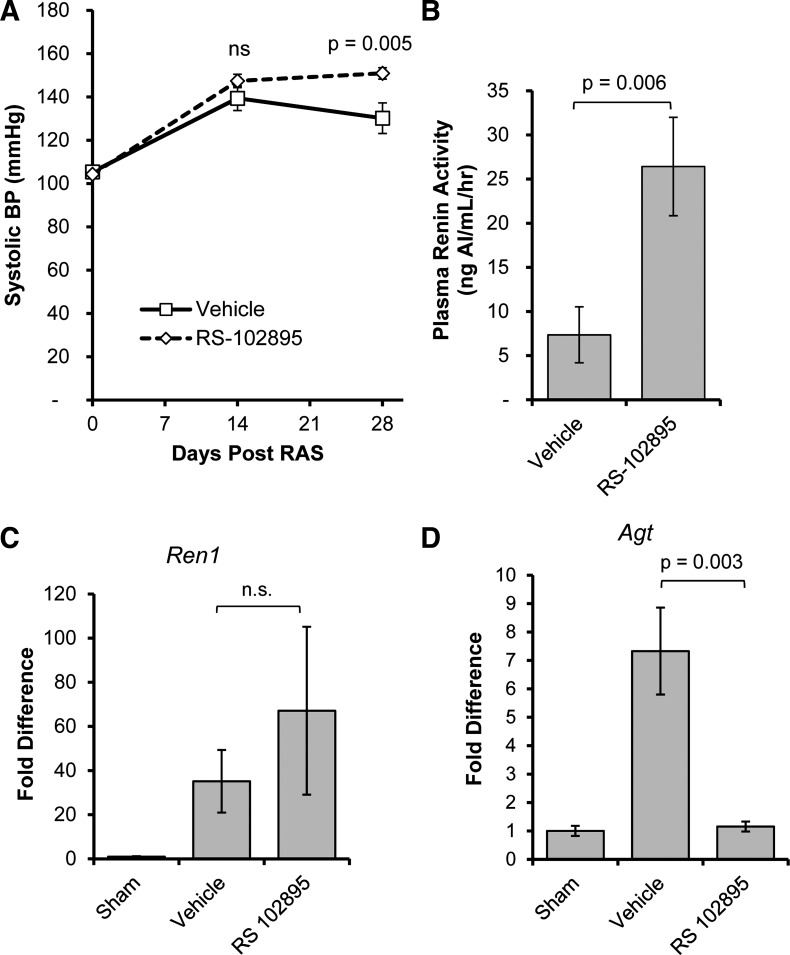
In contrast to reports indicating that CCR2 inhibition reduces blood pressure (5), we found that treated mice had higher blood pressure than RAS mice treated with vehicle (Fig. 8A). Plasma renin activity was significantly higher in RS-102895-treated than vehicle-treated RAS mice (Fig. 8B). RS-102895 did not significantly alter the elevated renal expression of renin mRNA in the STK of mice with RVH (Fig. 8C). However, renal expression of angiotensinogen was significantly reduced by RS-102895 (Fig. 8D).
Fig. 8.
Mice treated with CCR2 inhibitor experienced sustained hypertension despite attenuation of renal injury. Systolic tail-cuff blood pressure measurements (A) and plasma renin activity (B) of RAS mice treated with CCR2 inhibitor (RS-102895; n = 9 mice) or vehicle (water; n = 12 mice) are shown. Plasma renin activity was assessed by production of angiotensin 1. C and D: gene expression changes in renin (Ren1; C) and angiotensinogen (Agt; D) in whole kidney homogenates from the stenotic kidneys of mice with sham surgery (n = 5 mice) or mice with RAS surgery that were treated with either CCR2 inhibitor or vehicle. Values are means ± SE normalized against 18S transcript and expressed as fold-increase relative to the 28-day sham expression.
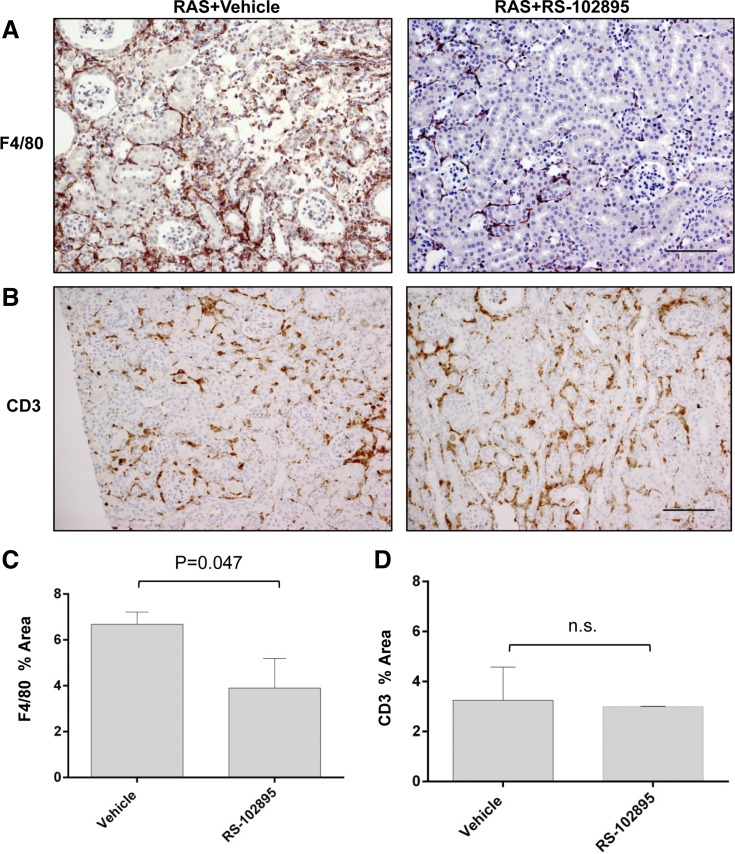
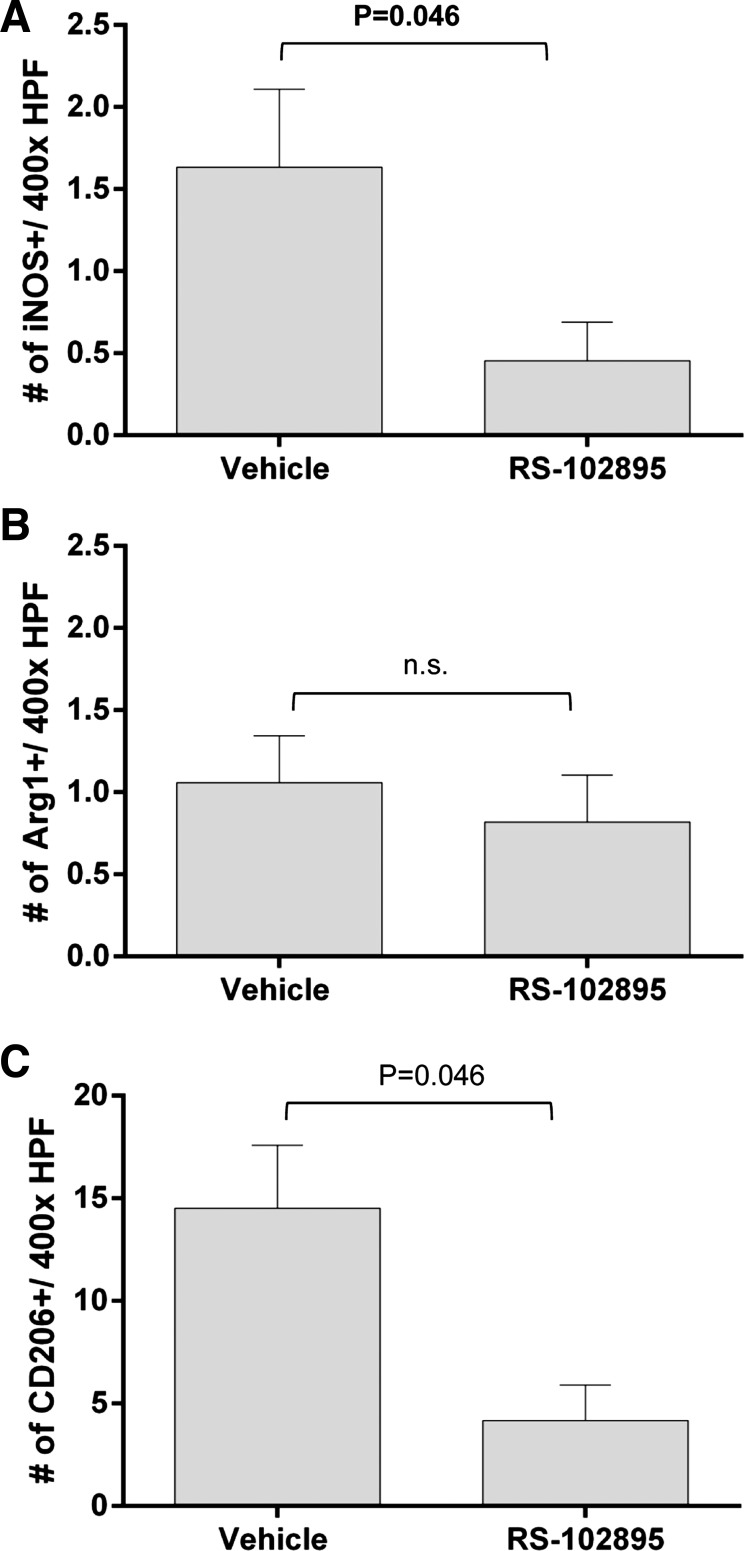
We tested the hypothesis that the reduction in renal atrophy through abrogation of CCL2 signaling is associated with a reduction in macrophage accumulation. RS-102895 significantly reduced the percentage of F4/80+ area in the renal cortex following RAS (Fig. 9, A and C). There was no difference in the percentage of CD3 area between RS-102895- and vehicle-treated mice (Fig. 9, B and D). The number of iNOS+ and CD206+ macrophages that were also coexpressing F4/80 was significantly decreased in RS-102895-treated mice subjected to RAS (Fig. 10, A and C). In contrast, we did not observe any changes in the number of F4/80 and arginase 1 dual-positive macrophages (Fig. 10B).
Fig. 9.
CCR2 inhibition decreased F4/80 percent area in the stenotic kidney with no change in CD3 percent area. A: representative histological images of stenotic kidney of RAS mice treated with CCR2 inhibitor (RS-102895) or vehicle (water) as stained with anti-F4/80 at ×200. B: representative histological images of stenotic kidney of RAS mice treated with CCR2 inhibitor (RS-102895) or vehicle (water) as stained with CD3. Images were taken at ×200. C: extent of macrophage infiltration as assessed by quantifying percent area positive for F4/80 stains. Assessments were done at ×200 magnification over the whole cortex using color-assisted image analysis. D: extent of T cell infiltration assessed by CD3 stains at ×200 magnification. Scale bar = 100 μm. Values are means ± SE.
Fig. 10.
CCR2 inhibition decreased the number of macrophages that were double positive for iNOS and F4/80 and CD206 and F4/80 in the stenotic kidney with no change in F4/80 and arginase 1 double-positive macrophages. The extent of macrophage infiltration is shown as assessed by counting the number of double-positive macrophages for F4/80 and iNOS (A), F4/80 and arginase 1 (B), and F4/80 and CD206 (C) stain in 10–15 random fields over the whole cortical region.
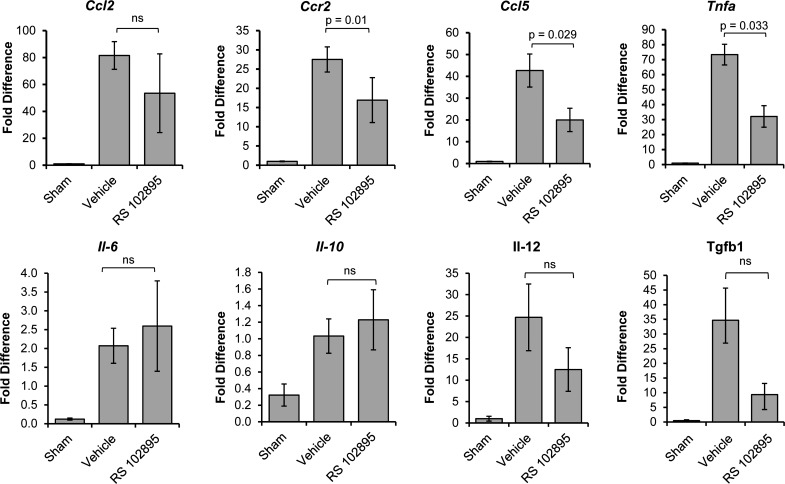
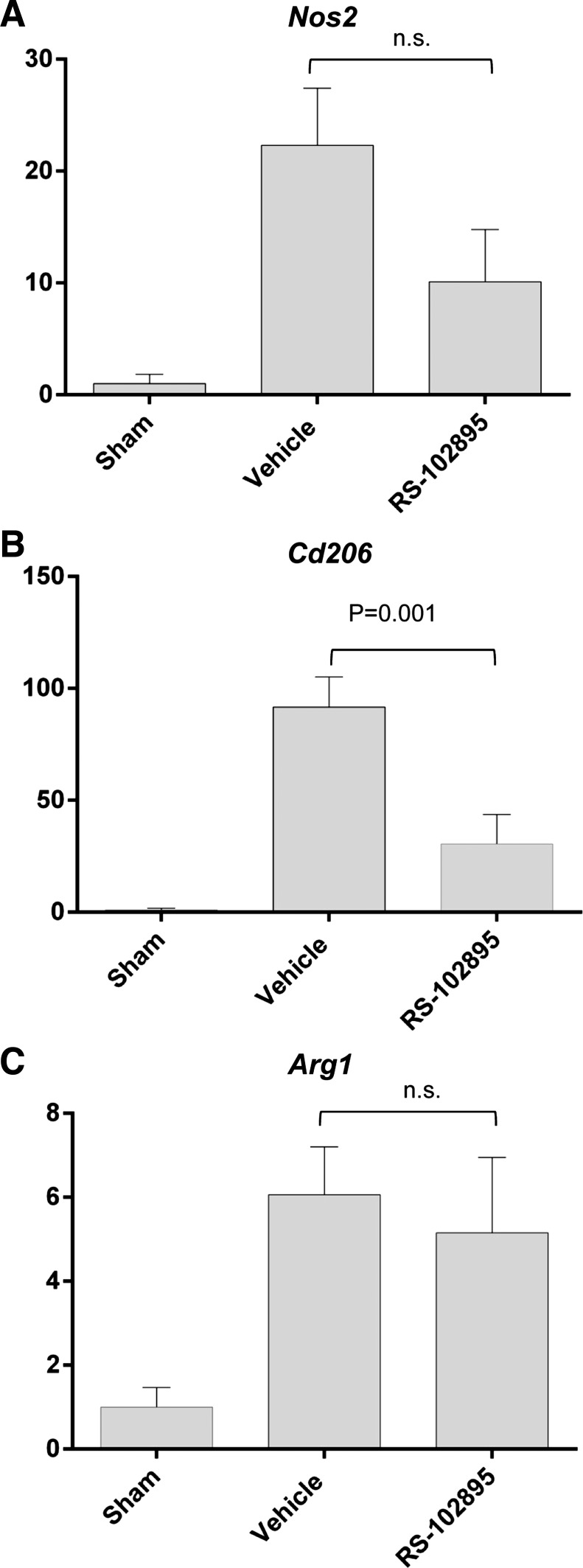
RS-102895 had no effect on renal Ccl2, Il10, IL6, Il12, and TGF-β1 expression in renal homogenates. However, RS-102895 reduced Ccr2, Ccl5 (RANTES), and Tnfa mRNA expression in the STK of RAS mice (Fig. 11). Gene expression studies showed that RS-102895 had no significant effect on Nos2 and arg1 whereas it reduced CD206 expression, as assessed by PCR analysis of total renal homogenates (Fig. 12).
Fig. 11.
CCR2 inhibition decreased production of inflammatory chemokines. Expression is shown of Ccl2, Ccr2, RANTES (Ccl5), Tnfα, Il-6, Il-10, Il-12, and Tgfβ1 as assessed by RT-PCR in the stenotic kidney of mice with sham surgery (n = 5 mice) or mice with RAS surgery that were either treated with CCR2 inhibitor (RS-102895; n = 9 mice) or vehicle (water; n = 12 mice). Values are means ± SE normalized against 18S transcript and expressed as fold-increase relative to sham expression.
Fig. 12.
Effect of RS-102895 on the expression of iNOS, arginase 1, and CD206. Expression of iNos (A), CD206 (B), and Arg 1 (C) in stenotic kidneys of mice with RAS or sham surgery. Values are means ± SE normalized against 18S transcript and expressed as fold increase relative to sham expression.
DISCUSSION
Renal artery stenosis is an important cause of morbidity and mortality due to cardiovascular and cerebrovascular disease, as well as renal disease. Recent studies have demonstrated that surgical intervention with stenting does not improve cardiovascular or renal survival in patients with RAS (2). There is evidence that persistent inflammation may predict those that may not respond to stenting with a decrease in serum creatinine. In a recent study, patients with increased levels of the proinflammatory marker CRP identified patients who did not benefit from renal revascularization (31). The relationship between inflammation and development of chronic renal damage in RVH has not been adequately defined. Although previous studies have established a critical role for CCL2 signaling in chronic renal disease (13–15, 22), our studies provide novel insights related to CCL2 signaling in the development of chronic renal disease in RAS. First, our model recapitulates many of the features observed in human renal artery stenosis (12). In particular, following cuff placement, the STK appears to adapt to the reduction in blood flow, showing minimal histopathological alterations for 3 days. This period is followed by progressive atrophy (32). This pattern of chronic injury is quite distinct from that of acute ischemia-reperfusion injury (20). We demonstrate, for the first time, that tubular epithelial cells are the initial source of CCL2 and are likely responsible for the recruitment of macrophages to the kidney. We show that abrogation of CCR2 protects the STK from development of interstitial fibrosis and tubular atrophy despite a persistent reduction in blood flow. Finally, we demonstrate that the CCR2 inhibitor reduces accumulation of F4/80- and iNOS- and F4/80- and CD206-expressing dual-positive macrophages with no significant effects on F4/80- and arginase 1-expressing macrophages (25).
In humans with chronic renal disease, urine CCL2 levels are associated with interstitial macrophage influx and fibrosis (4). Although CCL2 expression correlates with macrophage influx in human kidney disease (6, 24), the temporal relationship between tubular epithelial cell CCL2 expression and macrophage influx has not been previously defined. We found that CCL2 was highly induced in renal cortical homogenates at 7 days after surgery, a time at which there were relatively few macrophages infiltrating the STK. Using a CCL2 reporter mouse, we found that CCL2 was highly expressed within tubular epithelial cells of the STK within 24 h of surgery. Following magnetic separation of renal homogenates, we found that high CCL2 expression at 3 days localized to the CD11b−/CD90− parenchymal cell-enriched fraction; increased CCL2 expression in the CD11b+ macrophage-enriched fraction was not observed until 7 days following surgery. These studies support our hypothesis that renal tubular epithelial cell expression of CCL2 may be a predominant mediator of macrophage influx and chronic renal damage in RVH.
We found that the CCR2 inhibitor RS-102895 significantly reduced the extent of interstitial fibrosis and tubular atrophy in the STK, even though systolic blood pressure was higher in the CCR2 inhibitor-treated than vehicle-treated mice with RVH. In vehicle-treated mice, we observe a transient increase in plasma renin content which peaks at 14 days and returns to baseline levels by 28 days, as the STK develops severe atrophy. However, the CLK undergoes compensatory enlargement, but is without any histopathological abnormalities. RS-102895 did not produce injury in either kidney of sham mice, indicating that this compound is not nephrotoxic. We demonstrated that the CLK of RS-102895-treated mice subjected to RAS showed very mild tubular atrophy (<2%). Our data indicated that blood pressure and plasma renin activity are higher in RS-102895 mice than vehicle-treated mice subjected to RAS. It is certainly possible that the higher plasma renin activity and blood pressure observed in the RS-102895-treated animals may have contributed to the modest chronic injury to the CLK that we observed in drug-treated but not vehicle-treated mice. However, there was no difference in myocardial fibrosis between drug-treated and control RAS mice. The RS-102895-treated mice retained the normal glomerular area compared with the vehicle-treated group. We believe that this effect on glomerular size is a morphological manifestation of reduced atrophy in RS-102895-treated RAS mice compared with vehicle-treated mice. We observed a significant reduction in CCL5 and CCl7 expression in the drug-treated group compared with vehicle. Although TGF-β1 was reduced to a greater extent in the drug-treated group, this difference did not reach statistical significance, perhaps due to the fact that whole kidney homogenates were used to assess TGF-β expression. As blockade of CCR2 with RS-102895 reduces macrophage infiltration, we propose that proinflammatory mediators are downstream of CCL2-CCR2 blockade in that reduced macrophage infiltration leads to reduced chemokine expression.
Recent studies have demonstrated that macrophages are pleiotropic cells which may be polarized to perform a variety of critical functions related to inflammation, clearance of invading organisms, repair, and fibrosis. For example, “M1-like” macrophages produce iNOS and a number of other proinflammatory chemokines and have been shown to directly mediate renal injury (33), whereas “M2-like” macrophages produce arginase, express CD206, and may be involved in resolution of inflammation and/or repair (18). We sought to determine, for the first time, the phenotype of infiltrating macrophages at early time points following induction of RVH. As we previously reported, there were few macrophages identified in the first week following RAS surgery, as assessed by the percentage of cortical surface area staining positively for F4/80, but the number of infiltrating macrophages progressively increased as the STK developed severe atrophy over the following 3 wk. At later time points, there was an increase in dual-positive F4/80 and iNOS+ (M1) and F4/80 and CD206 but not F4/80 and arginase 1+ (M2) macrophages. Expression of the M1 chemokines IL-6 and IL-12 and the M2 chemokine IL-10 were all significantly induced in RAS mice at 28 days after surgery. Although RS-102895 reduced expression of these M1 and M2 chemokines, these differences did not reach statistical significance.
Although both arginase and CD206 are accepted markers of M2 macrophages (26), our studies indicate that more interstitial cells coexpress F4/80 and CD206 than F4/80 and arginase 1. Furthermore, we demonstrate that F4/80- and CD206-positive cell infiltrates, but not F4/80- and arginase 1-positive infiltrates, are reduced in RS-102895-treated mice. This observation raises the possibility that F4/80- and CD206-positive cells identify a different population than F4/80- and arginase 1-positive cells. For example, some subsets of dendritic cells express CD206 (27), raising the possibility that dendritic cells may contribute to the greater number of interstitial cells coexpressing F4/80 and CD206. Further studies are needed to address this issue.
Although this is the first report to document CCL2 expression and macrophage influx during the development of chronic renal damage in RVH, there are several limitations. Although we have shown that renal accumulation of macrophages is associated with renal injury, we have not defined whether they accumulate through recruitment from bone marrow or through in situ proliferation. Furthermore, we have not determined whether macrophages can differentiate along M1 or M2 lines at sites of injury. However, recent studies have called into question the “M1/M2 paradigm” (17). In particular, the M1/M2 polarization phenotype appears to change in response to external signals (3), suggesting that the balance of M1 vs. M2 macrophages in chronic tissue injury may reflect environmental cues rather than intrinsic properties of the infiltrating macrophages. Our studies represent an important first step in characterizing infiltrating cells during the initiation and progression of renal injury. Additional studies are needed to completely characterize the nature and function of infiltrating cells in this model. Despite the specificity of RS-102895 for CCR2b, there are other chemokines that could signal through this (or another) receptor. Specificity for CCR2 signaling in prevention of chronic renal damage needs to be verified by studies employing CCR2 knockout mice. Although we have demonstrated that CCR2 inhibition at the outset of development of chronic renal damage in RVH is remarkably protective, it is not known whether administration of a CCR2 inhibitor at a later time point during progression of renal injury in RVH would have any beneficial effect. This critical issues need to be addressed in future studies.
Based on these considerations, we conclude that signaling through the CCL2/CCR2 axis is an important pathway for the development of chronic renal disease in RVH. Signaling appears to be initiated by tubular epithelial cells, which direct the subsequent influx of macrophages. The CCL2/CCR2 signaling pathway may provide a novel therapeutic target to prevent renal disease progression in patients with RVH.
GRANTS
These studies were supported by National Institutes of Health (NIH) Grants NIAID R01 AI100911-01, NHLBI P01 HL85307, UL1 RR024150 (J. P. Grande), DK73608, and DK104273 (L. O. Lerman). S. P. Hartono is supported by F30 DK102232-01 and NIGMS T32 GM 6584. This paper's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.K., S.P.H., R.B., B.E.K., and K.R.L. performed experiments; S.K., S.P.H., and J.P.G. drafted manuscript; S.K. and J.P.G. edited and revised manuscript; G.M.W., K.A.N., S.C.T., L.O.L., and J.P.G. provided conception and design of research; A.S.Z. and J.P.G. analyzed data; K.A.N., S.C.T., L.O.L., and J.P.G. interpreted results of experiments; J.P.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Hui Tang, John R. Woollard, and Kyra L. Jordan for assistance with experiments. We also acknowledge the Mayo Gene Expression Core for the RNA quality assessment. The secretarial assistance of Jennifer Scott is acknowledged.
REFERENCES
- 1.Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP. Temporal analysis of signaling pathways activated in a murine model of 2-kidney, 1-clip hypertension. Am J Physiol Renal Physiol 297: F1055–F1068, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D'Agostino RB, Dworkin LD. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio 4: e00264–00213, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eardley KS, Zehnder D, Quinkler M, Lepenies J, Bates RL, Savage CO, Howie AJ, Adu D, Cockwell P. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int 69: 1189–1197, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in angiotensin II-salt hypertension. Hypertension 50: 1069–1076, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandaliano G, Gesualdo L, Ranieri E, Monno R, Montinaro V, Marra F, Schena FP. Monocyte chemotactic peptide-1 expression in acute and chronic human nephritides: a pathogenetic role in interstitial monocytes recruitment. J Am Soc Nephrol 7: 906–913, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Grassia G, Maddaluno M, Guglielmotti A, Mangano G, Biondi G, Maffia P, Ialenti A. The anti-inflammatory agent bindarit inhibits neointima formation in both rats and hyperlipidaemic mice. Cardiovasc Res 84: 485–493, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 36: 443–451, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Hartono S, Knudsen B, Zubair A, Nath K, Textor S, Lerman L, Grande J. Redox signaling is an early event in the pathogenesis of renovascular hypertension. Int J Mol Sci 14: 18640–18656, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iglesias JI, Hamburger RJ, Feldman L, Kaufman JS. The natural history of incidental renal artery stenosis in patients with aortoiliac vascular disease. Am J Med 109: 642–647, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Kalra PA, Guo H, Kausz AT, Gilbertson DT, Liu J, Chen SC, Ishani A, Collins AJ, Foley RN. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 68: 293–301, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, Grande JP. Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrol Dial Transplant 25: 3615–3622, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol 165: 237–246, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE Jr, Lobo PI, Okusa MD. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med 185: 1371–1380, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol 166: 4697–4704, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6: 13, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng XMM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol 10: 493–503, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol 302: G1310–G1321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol 301: F436–F442, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro-Gonzalez JF, Mora-Fernandez C, de Fuentes MM, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7: 327–340, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Ninichuk V, Clauss S, Kulkarni O, Schmid H, Segerer S, Radomska E, Eulberg D, Buchner K, Selve N, Klussmann S, Anders HJ. Late onset of Ccl2 blockade with the Spiegelmer mNOX-E36-3′PEG prevents glomerulosclerosis and improves glomerular filtration rate in db/db mice. Am J Pathol 172: 628–637, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, Huffnagle GB, Toews GB. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol 181: 610–620, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prodjosudjadi W, Gerritsma JS, van Es LA, Daha MR, Bruijn JA. Monocyte chemoattractant protein-1 in normal and diseased human kidneys: an immunohistochemical analysis. Clin Nephrol 44: 148–155, 1995. [PubMed] [Google Scholar]
- 25.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015: 816460, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med 209: 653–660, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seok SJ, Lee ES, Kim GT, Hyun M, Lee JH, Chen S, Choi R, Kim HM, Lee EY, Chung CH. Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrol Dial Transplant 28: 1700–1710, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Shi C, Jia T, Mendez-Ferrer S, Hohl Tobias M, Serbina Natalya V, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating Toll-like receptor ligands. Immunity 34: 590–601, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol 294: F697–F701, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Trani C, Porto I, Tommasino A, Giammarinaro M, Burzotta F, Niccoli G, Leone AM, Coroleu SF, Cautilli G, Mazzari MA, Schiavoni G, Crea F. Baseline inflammatory status and long-term changes in renal function after percutaneous renal artery stenting: a prospective study. Int J Cardiol 167: 1006–1011, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Warner GM, Cheng J, Knudsen BE, Gray CE, Deibel A, Juskewitch JE, Lerman LO, Textor SC, Nath KA, Grande JP. Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol 302: F1455–F1464, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You H, Gao T, Cooper TK, Brian Reeves W, Awad AS. Macrophages directly mediate diabetic renal injury. Am J Physiol Renal Physiol 305: F1719–F1727, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu XY, Chade AR, Krier JD, Daghini E, Lavi R, Guglielmotti A, Lerman A, Lerman LO. The chemokine monocyte chemoattractant protein-1 contributes to renal dysfunction in swine renovascular hypertension. J Hypertens 27: 2063–2073, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]