Abstract
Kidney disease, a common complication of diabetes, associates with poor prognosis. Our previous animal model studies linked aquaporin (AQP)11 to acute kidney injury, hyperglycemia-induced renal impairment, and kidney disease in diabetes. Here, we report the AQP11 rs2276415 variant as a genetic factor placing type 2 diabetic patients at greater risk for the development of kidney disease. We performed two independent retrospective case-control studies in 1,075 diabetic and 1,619 nondiabetic individuals who were identified in the Synthetic Derivative Database with DNA samples in the BioVU DNA repository at Vanderbilt University (Nashville, TN). A χ2-test and multivariable logistic regression analysis with adjustments for age, sex, baseline serum creatinine, and underlying comorbid disease covariates showed a significant association between rs2276415 and the prevalence of any event of acute kidney injury and chronic kidney disease (CKD) in diabetic patients but not in patients without diabetes. This result was replicated in the second independent study. Diabetic CKD patients over 55 yrs old with the minor AQP11 allele had a significantly faster progression of estimated glomerular filtration rate decline than patients with the wild-type genotype. Three-dimensional structural analysis suggested a functional impairment of AQP11 with rs2276415, which could place diabetic patients at a higher risk for kidney disease. These studies identified rs2276415 as a candidate genetic factor predisposing patients with type 2 diabetes to CKD.
Keywords: diabetes mellitus, acute kidney injury, chronic kidney injury, proximal tubules
chronic kidney disease (CKD) is characterized by progressive structural damage to the kidney and/or by a decrease of kidney function for >3 mo. Hyperglycemia is clearly a prerequisite for the development of CKD and affects 25–40% of diabetic patients (17, 21, 22). However, the genetic factors predisposing diabetic patients at risk for the development of CKD remain uncertain. Identification of genes underlying the genetic susceptibility to CKD in diabetic patients could impact disease prevention strategies and reduce the overall burden of end-stage renal disease.
Recent evidence strongly indicates a link of acute kidney injury (AKI) episodes to the subsequent development of CKD in diabetic patients (39). A significant proportion of AKI cases involve tubular injury, and proximal tubules (PTs) are specifically susceptible to tubular damage and dysfunction (3, 35). There is increasing awareness that in addition to the glomerulus, PT may be a target in diabetic kidney disease, and PT injury might be an important contributor to the development of diabetic CKD (23).
We recently identified aquaporin (AQP)11 haploinsufficiency as a factor predisposing to hyperglycemia-induced nephropathy (2, 36). AQPs, primarily water transport proteins, are characterized by the presence of two highly conserved Asn-Pro-Ala (NPA) motifs that, along with the surrounding sequences, are important for pore formation and its functioning (27, 28). Sequences around NPA boxes generally correspond to AQP protein selectivity. Thus, arginine or aspartic acid situated immediately after COOH-terminal NPA boxes in two different groups of AQP superfamily are determinants of the pore size for water or glycerol passage, respectively (14). AQP11, with its unusual NH2-terminal Asn-Pro-Cys (NPC) motif, which is important for its function, is a member of the novel third group of the AQP superfamily (16, 26). AQP11 resides in the endoplasmic reticulum (ER) (26), and previously published data have indicated that AQP11 may maintain ER homeostasis and cytosolic and/or vesicle osmoregulation in the PT (2, 20, 29, 45).
In the kidney, AQP11 is exclusively expressed in all PT segments and is regulated by glucose (2, 26, 37). Our and other studies have demonstrated that in mice, loss of AQP11 function in PTs leads to AKI (26, 37) and that AQP11 insufficiency predisposes to hyperglycemia-induced renal dysfunction (2).
Our preliminary analysis indicated that the rs2276415 AQP11 variant was significantly associated with the prevalence of CKD in patients with diabetes (4). Frequency data analysis revealed that the AQP11 rs2276415 variant is the only nonsynonymous variant in the human AQP11 gene, with a mean allele frequency (MAF) of >5% (1, 7, 19). This variant corresponds to the substitution of a neutral glycine at position 102, which is highly conserved among vertebrate species, by a polar serine situated immediately after the NPC motif in the functional, pore-forming region of AQP11. Mutation analysis demonstrated that mutations in this region resulted in a downregulation of AQP11 water transport function (16).
Therefore, we hypothesized that AQP11 gene polymorphism rs2276415, which causes a Gly102Ser substitution in the functional region of AQP11, may be associated with an increased risk for the development of kidney disease in type 2 diabetic patients.
The purpose of the present studies was to determine whether this specific AQP11 genetic variant confers a susceptibility to AKI or CKD in type 2 diabetes and to evaluate the association of the AQP11 gene polymorphism with the prevalence of kidney disease in diabetic patients. To test whether this association was specific to diabetes, we performed a similar analysis in nondiabetic patients. Our findings were replicated in a second independent retrospective study with diabetic and nondiabetic patients that were not included in the original study.
METHODS
Case and Control Definitions
Studies were restricted to Caucasian participants of 18 yr of age and older. Exclusion criteria included at least one of the following: 1) estimated glomerular filtration rate (eGFR) of <15 ml·min−1·1.73 m−2, 2) diagnosis of CKD stage 5 or end-stage renal disease at baseline, 3) current or prior kidney malignancy, 4) kidney transplant, or 5) pregnancy. As the primary outcome, the development of AKI was defined by 0.3–0.5 mg/dl or a 25–50% increase of serum creatinine from a baseline level. The development of CKD, regardless of its etiology, was defined by at least two eGFR values of <50 ml·min−1·1.73 m−2 as calculated by the Modification of Diet in Renal Disease formula validated in type 2 diabetic patients and nondiabetic individuals (22). Only patients with data between 5 and 10 yr of observation and at least 5 total outpatient eGFR values in that timeframe were included for the CKD study.
Case definition.
Cases were defined as those patients who experienced AKI or CKD. In each group, patients had documentation of acute renal failure or CKD by ICD-9 codes 584.x, 585.x, or 586.x.
Control definition.
Patients matching the case by sex and age within 5 yr were included if they 1) did not meet any case definitions for AKI or CKD, with average eGFR values as calculated from creatinine data of >70 ml·min−1·1.73 m−2 between 5 and 10 yr of observation and no episodes of eGFR values of <60 ml·min−1·1.73 m−2 during this time; 2) had no outpatient or inpatient ICD-9 codes for an acute or chronic kidney condition; and 3) had no serum or plasma creatinine values of >1 mg/dl. The incidence of type 2 diabetes was determined by ICD-9 codes for diabetes mellitus type 2 and confirmed by diagnostic tests and treatment.
Covariates of interest.
We selected an a priori panel of covariates including age, sex, baseline serum creatinine, and the following underlying comorbid diseases: hypertension as defined by the use of antihypertensive medication, myocardial infarction as identified by ICD-9 codes 410.9 or 412, and ischemia as defined by the use of vasopressor or inotropic medication.
Targeted AQP11 Single-Nucleotide Polymorphism Genotyping
During AQP11 single-nucleotide polymorphism (SNP) selection using publicly available data, we excluded from our study coding AQP11 variants with a MAF of <5% and identified only one coding c.304G>A variant, rs2276415, with a MAF of 14% (1, 7, 19). This variant affects AQP11 translation through a Gly102Ser substitution within the highly conserved region of AQP11.
We used the Synthetic Derivative Database to extract cases and controls, as defined above. BioVU provided corresponding DNA samples obtained from discarded blood samples collected during routine patient care. The amplification of the DNA sequence flanking the polymorphic region surrounding the c.304G>A variant was conducted by the Vanderbilt DNA Resources Core using the ABI Prism 7900HT Sequence Detection System and TaqMan SNP genotyping assay (call rate: >96%), designed and validated by Applied Biosystems, following the manufacturer's protocol.
Annual eGFR Decline Calculation and Analysis
The annual eGFR decline was calculated using the following formula: (last eGFR − basal eGFR)/(years of observation) in the corresponding AQP11 genotype and age groups. Annual eGFR decline differences between CKD and control subjects with corresponding AQP11 genotype and age (changes in eGFR) were calculated by the following formula: CKD annual eGFR decline value − control annual eGFR decline value.
Computerized Analysis of the Three-Dimensional Structure of Human AQP11
Despite the low sequence identity with other AQPs, we built a three-dimensional (3-D) homology model, using as a template the 1.9-Å resolution crystal structure of AQP0 (Protein Database identifier: 2B6O) (11), using the alignment of human AQP11 and other AQPs, as previously described (45).
Statistical Analysis
An unpaired Student's t-test was used to compare quantitative data between case and control subjects. Allele frequencies were estimated by allele counting. To determine the departure from the Hardy-Weinberg equilibrium, Pearson's χ2-test was used. Logistic regression analysis was used to test whether patients with AQP11 SNP rs2276415 are at a higher risk for the development of kidney disease (AKI or CKD) than patients with the common allele only. Logistic regression, in which a dichotomous outcome (kidney disease − or kidney disease +) was regressed on independent variables (see Covariates of interest), generated the coefficients of a prediction formula (SEs of estimate and significance levels as P values), and odds ratios (with 95% confidence intervals). Estimates of power were performed using a one-sided test at an α level of 0.05 in each sample subset. The dominant genetic model was used to assess genotype. To address multiple testing issues in the CKD component cohort in study I, the Bonferroni correction method was applied for two tests. The F-test was used to estimate the difference in regression lines for the eGFR reduction in subjects with GG or AG/AA genotypes under the null hypothesis that the two lines were equal.
Study Approval
The study protocol complied with the Declaration of Helsinki; the protocol and data set access for research purposes were approved by the Vanderbilt Institutional Review Board (IRB protocol no. 110555) and BioVU Review Committee.
RESULTS
Our working hypothesis was that patients with the AQP11 polymorphism are more susceptible to renal tubular dysfunction and that hyperglycemia in patients with diabetes might aggravate renal tubular injury, thus placing these diabetic patients at greater risk for the development of kidney disease than diabetic patients carrying the wild-type allele.
Target Gene and SNP Selection
Our previous studies in an animal model linked AQP11 to AKI, hyperglycemia-induced renal dysfunction, and a higher risk for kidney disease in diabetes (2, 36, 37). Following our animal model data, we selected this candidate gene to test common AQP11 polymorphisms in the coding elements of the gene in AKI or CKD patients with or without type 2 diabetes.
After the exclusion of SNPs with a MAF of <5% frequency from our study, publicly available data revealed only one nonsynonymous sequence variant, rs2276415 (c.304G>A), in the human AQP11 gene with a MAF of 14% (1, 7, 19). We have previously reported an association of the AQP11 rs2276415 (HGVM1698529) marker with an increased risk for contrast-induced nephropathy in diabetic patients (4). While this AQP11 marker was found to be associated with susceptibility to asthma, Hodgkin lymphoma, flucloxacillin-induced liver injury (http://www.gwascentral.org), and poor graft survival after kidney transplantation (4, 31), it has not been previously tested in Caucasian CKD patients with type 2 diabetes. This SNP was not included in the Affymetrix array in the previous genome-wide association study on diabetic nephropathy (32) (communication with Affymetrix). SNP rs2276415 results in a Gly102Ser substitution immediately next to the important NPC motif in the pore-forming region that is responsible for AQP11 functioning (16). Database analysis showed that Gly102 in AQP11 is highly conserved across mammalian species. Remarkably, for Tupaia belangeri, one of the few unique mammals carrying Ser102 in AQP11, stress-induced kidney failure is the most common cause of death (43). These mammals develop accelerated diabetic nephropathy after the induction of diabetes with moderate-dose streptozotocin injections (30). Taken together, these data present strong evidence that SNP rs2276415 might be a genetic marker of AQP11 impaired function predisposing the host to kidney disease.
Study Population
Available medical and demographic data were obtained from the Synthetic Derivative Database, a deidentified electronic medical record system that is linked to BioVU, a DNA biobank repository at Vanderbilt University (Nashville, TN) (34). We queried the Synthetic Derivative Database to identify a group of 2,694 subjects with DNA samples in BioVU; 1,075 diabetic and 1,619 nondiabetic subjects, who underwent between 5 and 10 yr of examinations within 1997–2007, were selected for 2 independent studies at Vanderbilt University Medical Center. Study I consisted of 826 case and 629 age- and sex-matched control subjects; study II had 579 case and 660 control subjects. Comparisons were made and analyzed in diabetic and nondiabetic subjects.
Initially, we performed an association study for the AQP11 SNP rs2276415 variant and AKI in subjects who visited the Vanderbilt outpatient clinic or were admitted to the Vanderbilt hospital (study I). For further analysis, in the same study, a phenotypically more homogenous CKD component cohort was dissected from the parent AKI cohort (Fig. 1).
Fig. 1.
Flow diagram describing the acute kidney injury (AKI) parent and chronic kidney disease (CKD) component cohorts in study I. DM, diabetes mellitus.
We validated our CKD study findings in the independent replica CKD study (study II) in case and control subjects with or without type 2 diabetes who visited the outpatient clinic at Vanderbilt University and were not included in study I.
Study I. Relation of the AQP11 rs2276415 polymorphism to the prevalence of AKI or CKD in diabetic and nondiabetic subjects.
In study I, we initially studied subjects with an AKI phenotype as defined in methods. The characteristics of the patients in the case and control groups are shown in Table 1. Since patients with diabetes have a higher burden of CKD, it was also important to determine whether the AQP11 polymorphism conferred a susceptibility to CKD independently of AKI. To address this question, we identified a phenotypically homogenous CKD component cohort that was dissected from diabetic and nondiabetic AKI in study I (Fig. 1; see the definition of CKD in methods).
Table 1.
Characteristics of study I patients in parent AKI and control cohorts
| Case | Control | P Value | |
|---|---|---|---|
| n | 826 | 629 | |
| Mean age, yr | 65 ± 6 | 65 ± 7 | >0.05 |
| Men, n | 331 | 324 | <0.001 |
| Women, n | 495 | 305 | <0.001 |
| Ischemic stroke, n | 438 | 202 | <0.001 |
| Myocardial infarction, n | 102 | 65 | >0.20 |
| Diabetes mellitus, n | 248 | 135 | <0.001 |
| Hypertension, n | 538 | 358 | <0.001 |
| Nonrenal transplant, n | 183 | 45 | <0.001 |
| Sepsis, n | 192 | 15 | <0.001 |
| CKD, n | 349 | 0 | N/A |
| Diabetes duration, yr | 11 ± 8 | 8 ± 9 | >0.05 |
| Baseline serum creatinine, mg/dl | 1.08 ± 0.29 | 0.89 ± 0.18 | <0.001 |
| Baseline eGFR, ml·min−1·1.73 m−2 | 78.71 ± 26.29 | 88.07 ± 19.41 | <0.001 |
| Serum creatinine at CKD, mg/dl | 1.60 ± 0.42 | 0.79 ± 0.003 | <0.005 |
| eGFR at CKD, ml·min−1·1.73 m−2 | 47.90 ± 13.09 | 87.13 ± 0.25 | <0.001 |
Data are means ± SD; n, number of patients.
AKI, acute kidney injury; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; N/A, not applicable.
Allele frequencies and genotype distribution analysis (Table 2) revealed that the AQP11 rs2276415 polymorphism was associated with the prevalence of AKI and with the prevalence of CKD in patients with type 2 diabetes (P = 0.036, sample size: 383, and P = 0.004, sample size: 295, respectively) but not in patients without diabetes (sample size for AKI: 1,072 and sample size for CKD: 683). Pearson's χ2-test revealed no departure from the Hardy-Weinberg equilibrium in the genotype distribution in AKI and CKD subgroups (P > 0.05).
Table 2.
Genotype distribution of the AQP11 c.304G>A variant in case and control subjects in AKI and component CKD cohorts with and without diabetes in study I
| Parent AKI Cohorts |
Component CKD Cohorts |
|||||
|---|---|---|---|---|---|---|
| Case | Control | P Value (Allele Frequency Difference) | Case | Control | P Value (Allele Frequency Difference) | |
| Diabetes | 0.036 | 0.004 | ||||
| n (%) | 248 (100) | 135 (100) | 160 (100) | 135 (100) | ||
| GG, n (%) | 189 (76.2) | 115 (85.2) | 115 (71.9) | 115 (85.2) | ||
| AG, n (%) | 57 (23.0) | 20 (14.8) | 42 (26.2) | 20 (14.8) | ||
| AA, n (%) | 2 (0.8) | 0 (0) | 3 (1.9) | 0 (0) | ||
| Hardy-Weinberg P value* | 0.30 | 0.35 | 0.71 | 0.35 | ||
| No diabetes | 0.096 | 0.080 | ||||
| n (%) | 578 (100) | 494 (100) | 189 (100) | 494 (100) | ||
| GG, n (%) | 492 (85.1) | 402 (81.4) | 163 (86.2) | 402 (81.4) | ||
| AG, n (%) | 81 (14.0) | 86 (17.4) | 26 (13.8) | 86 (17.4) | ||
| AA, n (%) | 5 (0.9) | 6 (1.2) | 0 (0) | 6 (1.2) | ||
| Hardy-Weinberg P value | 0.42 | 0.57 | 0.31 | 0.57 | ||
n, Number of subjects. AQP11, aquaporin 11. An unpaired Student's t-test with no covariate adjustment was used for the comparisons.
P = Pearson's goodness-of-fit χ2 (degree of freedom = 1).
Multivariable logistic regression analysis showed that diabetic patients with the AQP11 rs2276415 polymorphism were at 1.795 times (P = 0.038) and 2.250 times (P = 0.006) at higher risk of the development of AKI and CKD, respectively, compared with diabetic patients with the wild-type allele. No association of the AQP11 polymorphysm with an increased risk for AKI or CKD was detected in nondiabetic subjects (Table 3). Bonferroni correction for two comparisons was applied in the CKD component cohort. The association of the AQP11 SNP with CKD in diabetic patients, as a component, was still strongly statistically significant (adjusted P = 0.018). Our results clearly indicate that the AQP11 minor allele is likely to be a factor placing diabetic patients at a higher risk of development of AKI or CKD.
Table 3.
Risk of AKI or CKD associated with the minor allele in study I subjects
| Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Parent AKI cohort | |||
| Diabetes | 1.795* | 1.028–3.135 | 0.038 |
| No diabetes | 0.764 | 0.553–1.054 | 0.100 |
| Component CKD cohort | |||
| Diabetes | 2.250† | 1.251–4.046 | 0.006 |
| No diabetes | 0.697 | 0.435–1.117 | 0.132 |
Multivariable logistic regression analysis was performed on independent variables including age, sex, baseline serum creatinine, hypertension, myocardial infarction, and ischemia.
Estimated power of 0.69.
Estimated power of 0.87.
Study II. Relation of the AQP11 rs2276415 polymorphism to the prevalence of CKD in diabetic and nondiabetic subjects.
To validate our findings in study I, we performed independent replication study II of the AQP11 rs2276415 polymorphism association with CKD in diabetic patients using a modified protocol. The Synthetic Derivative Database at Vanderbilt University was queried, and we found a total of 1,239 subjects with DNA samples in BioVU who visited the outpatient clinic, were not admitted to the hospital, and were not identified in study I (Table 4).
Table 4.
Characteristics of patients in study II (CKD cohort)
| Case | Control | P Value | |
|---|---|---|---|
| n | 579 | 660 | |
| Mean age, yr | 63 ± 8 | 57 ± 10 | >0.05 |
| Men, n | 276 | 339 | >0.10 |
| Women, n | 303 | 321 | >0.10 |
| Ischemic stroke, n | 303 | 194 | <0.001 |
| Myocardial infarction, n | 123 | 61 | <0.001 |
| Diabetes mellitus, n | 334 | 358 | >0.20 |
| Hypertension, n | 549 | 491 | <0.001 |
| Nonrenal transplant, n | 106 | 30 | <0.001 |
| Sepsis, n | 74 | 18 | <0.001 |
| AKI, n | 285 | 0 | N/A |
| Diabetes duration, yr | 7 ± 3 | 7 ± 2 | >0.50 |
| Baseline serum creatinine, mg/dl | 1.06 ± 0.23 | 0.78 ± 0.12 | <0.001 |
| Baseline eGFR, ml·min−1·1.73 m−2 | 72.34 ± 16.15 | 87.13 ± 0.25 | <0.001 |
| Serum creatinine at CKD, mg/dl | 1.64 ± 0.59 | 0.88 ± 0.13 | <0.001 |
| eGFR at CKD, ml·min−1·1.73 m−2 | 48.25 ± 12.88 | 86.20 ± 12.60 | <0.001 |
| Urinary protein, g/24 h | 2.07 ± 2.39 | 0.18 ± 0.08 | <0.001 |
| Urine albumin-to-creatinine ratio, mg/dl | 82.81 ± 95.44 | 14.19 ± 8.76 | <0.05 |
Data are means ± SD; n, number of patients.
The relation of AQP11 SNP rs2276415 to the prevalence of CKD in diabetic but not in nondiabetic subjects (sample size: 692 and 547, respectively) was replicated in study II (Table 5). The comparison of distribution confirmed an association of SNP rs2276415 with the prevalence of CKD only in diabetic patients (P = 0.01). Pearson's χ2-test revealed that the genotype distribution of AQP11 SNP rs2276415 was in Hardy-Weinberg equilibrium in both diabetic and nondiabetic cohorts in study II, thus validating our prior findings (Table 5). Diabetic patients carrying SNP rs2276415 were at a 1.6 higher risk of the development of CKD (P = 0.01), whereas this coding SNP was not associated with the prevalence of CKD in nondiabetic subjects (P = 0.73; Table 6).
Table 5.
Genotype distribution of the AQP11 c.304G>A variant in CKD subjects in study II and in combined CKD studies I and I
| Parent AKI Cohorts |
Component CKD Cohorts |
|||||
|---|---|---|---|---|---|---|
| Case | Control | P Value (Allele Frequency Difference) | Case | Control | P Value (Allele Frequency Difference) | |
| Diabetes | 0.01 | <0.001 | ||||
| n (%) | 334 (100) | 358 (100) | 494 (100) | 493 (100) | ||
| GG, n (%) | 255 (76.35) | 301 (84.08) | 370 (74.90) | 416 (84.38) | ||
| AG, n (%) | 75 (22.46) | 55 (15.36) | 117 (23.68) | 75 (15.21) | ||
| AA, n (%) | 4 (1.19) | 2 (0.55) | 7 (1.42) | 2 (0.41) | ||
| Hardy-Weinberg P value* | 0.56 | 0.76 | 0.51 | 0.48 | ||
| No diabetes | 0.54 | 0.095 | ||||
| n (%) | 245 (100) | 302 (100) | 434 (100) | 796 (100) | ||
| GG, n (%) | 204 (83.27) | 248 (82.12) | 367 (84.56) | 650 (81.66) | ||
| AG, n (%) | 41 (16.73) | 51 (16.89) | 67 (15.44) | 137 (17.21) | ||
| AA, n (%) | 0 (0) | 3 (0.99) | 0 (0) | 9 (1.13) | ||
| Hardy-Weinberg P value | 0.15 | 0.83 | 0.08 | 0.56 | ||
n, Number of subjects. An unpaired Student's t-test with no covariate adjustment was used for the comparisons.
P = Pearson's goodness-of-fit χ2 (degree of freedom = 1).
Table 6.
Risk of CKD associated with the minor allele in subjects in study II and in combined CKD studies I and II
| Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|
| Study II | |||
| Diabetes | 1.636* | 1.120–2.391 | 0.011 |
| No diabetes | 0.923 | 0.591–1.442 | 0.73 |
| Combined studies I and II | |||
| Diabetes | 1.811† | 1.319–2.486 | 0.001 |
| No diabetes | 0.813 | 0.592–1.115 | 0.198 |
Multivariable logistic regression analysis was performed on independent variables including age, sex, baseline serum creatinine, hypertension, myocardial infarction, and ischemia.
Estimated power of 0.83.
Estimated power of 0.98.
Combined data analysis from both studies (Tables 5 and 6) confirmed the increased risk of CKD in diabetic patients (odds ratio: 1.811, P < 0.001) but not in patients without diabetes (P = 0.198).
Age-Related eGFR Reduction in Diabetic CKD Patients
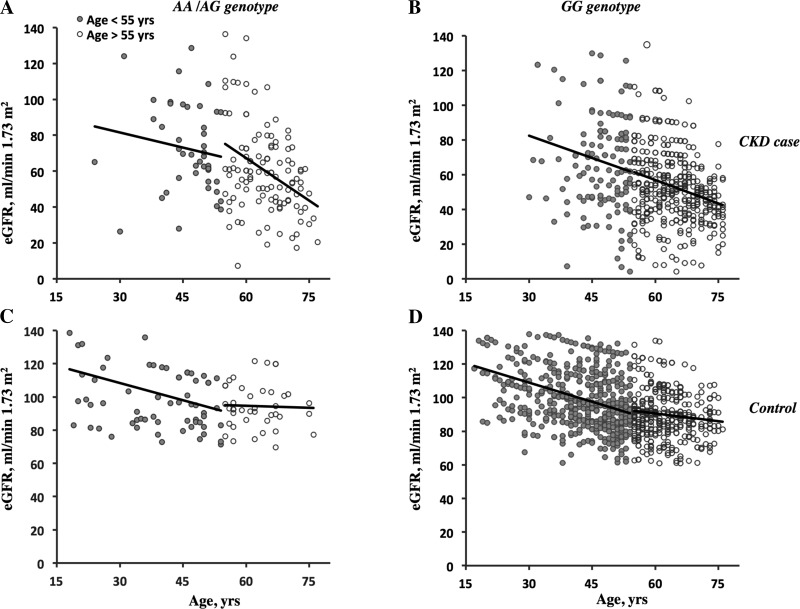
We found that the presence of the minor allele affected the progression of the eGFR reduction in diabetic CKD patients (Fig. 2, A and B). To demonstrate the significance of this eGFR decline difference, we performed further F-tests for linear regression. Importantly, in patients who carried the minor allele, eGFR declined sharply after 55 yr of age compared with homozygous wild-type allele carriers [slopes of regression lines: −1.509 ± 0.308 vs. −0.739 ± 0.174; Table 7; regression lines were significantly different with P = 0.023, F = 5.19758, degrees of freedom (df)n = 1, dfd = 485]. For diabetic CKD patients with the minor allele who were younger than 55 yr old, the regression line was not significantly different from that of wild-type allele carriers (slopes of regression lines: −0.771 ± 0.557 vs. −0.858 ± 0.410, P = 0.91, F = 0.0131484, dfn = 1, dfd = 173). No differences in eGFR decline related to genotype and age were detected in diabetic control cohorts (Fig. 2, C and D) or nondiabetic CKD and control cohorts (not shown).
Fig. 2.
Age- and genotype-related estimated glomerular filtration rate (eGFR) reductions in diabetic CKD patients. The eGFR reduction for diabetic CKD (top) and control (bottom) subjects with different aquaporin (AQP)11 genotypes before (open circles) and after (solid circles) 55 yr of age are shown. The eGFR value at each time point was quantified using the Modification of Diet in Renal Disease formula. A and B: CKD case cohort. A: patients with the minor AQP11 allele (AA/AG genotypes). B: patients with the wild-type AQP11 allele (GG genotype). C and D: control cohort. C: subjects with the minor AQP11 allele (AA/AG genotypes). D: subjects with the wild-type AQP11 allele (GG genotype). Each eGFR value was plotted versus the age of the patient. See Table 7 for the statistical analysis.
Table 7.
Linear regression analysis of the eGFR reduction in diabetic CKD patients at ages of <55 or >55 yr old
| AA/AG Genotype |
GG Genotype |
|||
|---|---|---|---|---|
| Age < 55 yr old | Age > 55 yr old | Age < 55 yr old | Age > 55 yr old | |
| Slope | −0.771 ± 0.557 | −1.509 ± 0.308 | −0.858 ± 0.410 | −0.739 ± 0.174 |
| y-Intercept | 98.12 ± 25.94 | 155.1 ± 20.04 | 108.2 ± 19.48 | 100.5 ± 11.24 |
| 95% Confidence interval | −1.897 to 0.354 | −2.118 to −0.899 | −1.661 to −0.054 | −1.079 to −0.398 |
| R2 | 0.046 | 0.168 | 0.032 | 0.047 |
| P value (nonzero slope) | 0.174 | < 0.001 | 0.038 | < 0.001 |
| Number of values | 42 | 121 | 135 | 368 |
Data are means ± SD.
Homology 3-D Model of Human AQP11
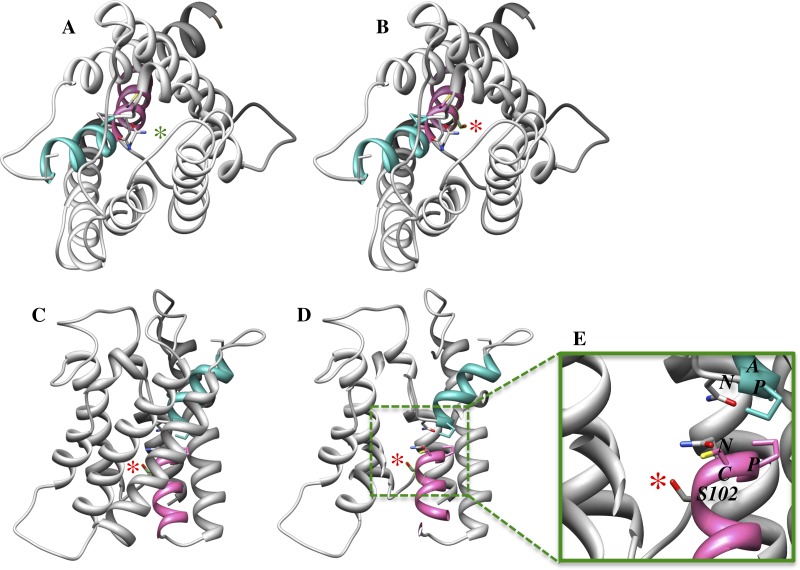
Using a 3-D homology model of AQP11, we investigated how the Gly102Ser substitution affected the functionality of the AQP11 protein (Fig. 3). AQP11 modeling revealed a conserved helical topology similar to that found in other AQPs with a bundle of transmembrane helixes connected by nonhelical extramembrane loops. These helixes are arranged around a central pore with an hourglass-like structure. The narrowest part of the pore is formed by two short nonmembrane-crossing helixes (pink and green in Fig. 3) oriented 180° from each other crossing near the center of the molecule. These short helixes contain the NPC and NPA motifs, which are essential for the transport functionality of AQP11 (44). AQP11 c.304G>A results in a Gly102Ser substitution (red star in Fig. 3) immediately next to Cys101 in the NH-terminal NPC motif. Mutations in this motif downregulate AQP11 function, indicating that the NPC motif, unlike the NH2-terminal NPA motif in conventional AQPs, is indispensable for its function (16). Our model of AQP11 clearly indicates the presence of the side chain of Ser102 in the AQP11 channel in which the extra hydroxyl group protrudes into through the pore. Alteration of the steric and electrostatic features may uncouple the normal interactions that drive the passage of water molecules. Therefore, this critical location of the Gly102Ser substitution might modify the water pore functionality and thus affect water transport in PTs.
Fig. 3.
Computerized three-dimensional structure of the pore-forming region in the AQP11 monomer. A and B: top views from the proximal side. A: computerized position of Gly102 (green star). B: computerized position of Ser102 (highlighted by the green line) with the charged chain (red star). C–E: lateral views of AQP11 with the Gly102Ser substitution. C: AQP11 monomer. D: sliced AQP11 monomer with the selected area (in the box). The red star indicates the position of Ser102. E: magnification of the selected area in the box. The altered pore-forming region includes the Asn-Pro-Cys (NPC) motif following Ser102 with the charged chain (red star). The analysis revealed that the polar side chain of Ser102 protrudes into the pore of AQP11 and could potentially interfere with the transport capacity of the AQP11 channel due to the steric and electrostatic changes in the pore. NPA, Asn-Pro-Ala motif.
DISCUSSION
In the present study, we examined whether the common c.304G>A variant of AQP11 (rs2276415), resulting in a substitution of the highly conserved glycine residue at position 102 to the serine residue in the functionally important pore-forming, NPC-containing region of the channel, could predispose to the development or progression of kidney injury. The association of AQP11 SNP rs2276415 with the development of AKI or CKD was tested in study I and replicated for CKD patients in study II in an independently identified cohort with subjects that were not involved in study I.
Previously published data have suggested the importance of AQP11 in ER homeostasis and in the maintenance of cytosolic and/or vesicle osmolality of PTs (2, 20, 26, 29, 45). In the kidney, AQP11 is exclusively expressed in all segments of the PT, a major site of renal water and glucose flux (2). While the exact mechanism of AQP11 functioning is yet to be elucidated, it is known that AQP11 functions as a water channel with slow water conduction (45). We recently demonstrated that the loss of AQP11 protein functionality caused AKI and the induction of ER stress, the unfolded protein response, and apoptosis as molecular mechanisms of PT-specific injury in the kidney (2, 37). The AQP11 haploinsufficiency in our animal model was a factor predisposing to high glucose-induced dysfunction of PT cells, hyperglycemia-induced oxidative stress, fibrosis in the kidney, and diabetic kidney disease (2, 36).
We hypothesized that exonic AQP11 SNP rs2276415, resulting in the Gly102Ser substitution and not previously tested, might be a risk factor predisposing patients with hyperglycemia (type 2 diabetes mellitus) to the development of kidney disease. Indeed, in study I, we showed that only in the diabetic population was the AQP11 SNP associated with a significantly increased risk for AKI or CKD, but not in patients without diabetes. In study II, designed only for CKD, our prior data were replicated and confirmed the significant association of AQP11 SNP rs2276415 with the prevalence of CKD in type 2 diabetic patients.
Glomerulopathy and podocyte loss are important factors in the development of diabetic kidney disease (42, 47). However, functional and structural changes in PTs are apparent after a few days of hyperglycemia (12, 13, 25, 41, 46). Chronically activated kidney injury molecule-1 in PT promoted a proinflammatory response and kidney fibrosis in an animal model of CKD (15). Thus, accumulating evidence suggests that injured PTs might be a triggering site of tubulointerstitial fibrosis and the progression of diabetic CKD (23). These findings have been strongly supported by a recent study (40) that showed an association of lower levels of tubular injury with the regression of microalbuminuria in patients with type 1 diabetes.
PTs mediate insulin-independent glucose reabsorption, and PT cells are unable to prevent tubular glucose overload and tubulopathy at the early stages of diabetes (33). At later stages of diabetes, CKD strongly associates with glomerular injury and renal function decline in CKD patients (9, 10, 18, 24). Our analysis of the progression of the eGFR reduction in diabetic CKD patients clearly indicates that patients with the AQP11 minor allele older than 55 yr of age have a significantly higher rate of eGFR decline compared with patients with wild-type AQP11.
How hyperglycemia relates to the function of AQP11 is still unclear. Our recently published in vivo and in vitro data indicated that high glucose overload requires a compensatory increase of AQP11 expression. However, haploinsufficiency of AQP11 cannot provide the AQP11 functional compensation in a hyperglycemic animal model (2). Here, we suggest that in diabetic patients with Gly102Ser, the insufficiency of the AQP11 compensation is likely to be a factor placing these patients at a higher risk for the development of CKD. How AQP11 protects the kidney against prolonged hyperglycemia-induced tubulopathy in concordance with glucose overload in PT cells is yet to be determined.
Our 3-D homology modeling revealed the possibility of structural alterations in the AQP11 pore-forming region that might be linked to AQP11 dysfunction and a higher risk of development of CKD in diabetic patients with the Gly102Ser substitution next to the NPC motif. Our cross-species database analysis of the AQP11 sequence showed that glycine in the NH2-terminal NPXG sequence is well conserved in plant AQPs (SIP1.1 and SIP1.2) and across the species of vertebrates, including one of the oldest species on the earth, Latimeria chalumnae, according to its recently discovered genome (1a). Evolutionary cross-species conservation of NPXG and, particularly, NPCG (Gly102) sequences in AQP11 in higher vertebrates, birds, and mammals implies an important role of this structure for protein functioning.
Renal failure due to psychosocial stress is a main cause of death in tree shrews (T. belangeri). This is an “outgroup” species with a recently reported genome shares common ancestry with primates but carries Ser102, identical with that we tested in human AQP11 here (5, 6, 43). In a recently established streptozotocin-induced model of diabetic nephropathy, T. belangeri demonstrated an accelerated development of kidney disease compared with what has been previously described for rodent models (30, 38). Thus, database analysis and previously reported data strongly suggest that the substitution of highly conserved Gly102 to Ser102 in the functional region in AQP11 is an essential contributor to stress-induced kidney injury. A critical location of the resulting Gly102Ser substitution suggested modification of the AQP11 functionality and water transport modulation in PTs (Fig. 3). In future studies, it would be clinically important to evaluate the functionality of Gly102Ser AQP11 and the molecular mechanism behind its relation to diabetic CKD.
Given the importance of AQP11 in ER homeostasis in PTs and the link of ER stress with kidney fibrosis (2, 20, 26, 29, 45), we propose that AQP11 channel dysfunction might predispose the diabetic host to CKD via PT-specific hyperglycemia-induced ER stress and unfolded protein response activation followed by profibrotic pathways and fibrotic remodeling in the kidney (Fig. 4).
Fig. 4.

Hypothetical two-hit scheme for the increased risk of CKD in patients with diabetes that carry the AQP11 minor allele. SNP, single-nucleotide polymorphism; ER, endoplasmic reticulum; ECM, extracellular matrix.
The current studies had some limitations, including the exclusion of patients with type 1 diabetes, the exclusion of patients from control cohorts who developed renal disease by the end of the study, the lack of histological confirmation of the etiology of AKI or CKD in every patient, and the lack of ethnic diversity. To fully cover the genetic variability within the AQP11 gene and neighboring genes, including functional regions such as the promoter, splice sites, and transcription factor-binding motifs, association analyses using haplotype tagging SNPs would be required. In addition, the molecular mechanism of AQP11 Gly102Ser on the development of kidney disease was only modeled and the crystal structure of AQP11 has not yet been determined. Nevertheless, our investigations confirmed the important role of AQP11 in kidney homeostasis. While we identified the specific SNP in AQP11 as a potential genetic marker of increased risk for the development of CKD in patients with type 2 diabetes, to predict CKD progression in diabetic patients with AQP11 polymorphism, a longitudinal protocol would be required.
In summary, our prior analysis identified AQP11 SNP rs2276415 as a risk factor candidate predisposing patients with type 2 diabetes to CKD. These data were replicated and confirmed that diabetic patients with at least one minor allele at this position in AQP11 were at a higher risk for the development of CKD and had a higher rate of eGFR decline than patients with the wild-type genotype.
GRANTS
The data sets used for the analyses described here were obtained from Vanderbilt University Medical Center's BioVU, which is supported by institutional funding and by Vanderbilt Clinical and Translational Science Award Grant 1-UL1-RR-024975-01 from the National Institutes of Health (NIH). The research reported in this publication was supported by NIH Grant UL1-TR-000445 (VR685 and VR2107 to E. E. Tchekneva), by a Development Grant from the Department of Medicine (to E. E. Tchekneva), Vanderbilt University School of Medicine, by NIH Grants DK-51265, DK-95785, and DK-62794, and funds from a Veterans Affairs Merit Review Grant (to R. C. Harris).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.P. Choma, M.M.D., and E.E.T. conception and design of research; D.P. Choma, R.V., H.N., I.A.Z., Andrei Pavlichenko, Artyom Pavlichenko, L.F., M.M.D., and E.E.T. analyzed data; D.P. Choma, R.V., M.M.D., and E.E.T. drafted manuscript; D.P. Choma, R.V., H.N., D.P. Carbone, R.C.H., M.M.D., and E.E.T. edited and revised manuscript; D.P. Choma, R.V., H.N., I.A.Z., Andrei Pavlichenko, Artyom Pavlichenko, L.F., D.P. Carbone, R.C.H., M.M.D., and E.E.T. approved final version of manuscript; R.V., D.P. Carbone, R.C.H., M.M.D., and E.E.T. interpreted results of experiments; R.V. and E.E.T. prepared figures; E.E.T. performed experiments.
ACKNOWLEDGMENTS
The authors thank Dr. Eric G. Neilson, Dr. Alp Ikizler, Dr. William E. Lawson, Dr. Kelly Birdwell, Dr. Edward Siew, and Dr. Pinelopi P. Kapitsinou for the invaluable expert assistance and manuscript editing as well as Anneleise Antonnucci and Elena M. Dikova for the manuscript editing.
REFERENCES
- 1.1000 Genomes Project Consortium, Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, Hurles ME, McVean GA. A map of human genome variation from population-scale sequencing. Nature 467: 1061–1073, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Amemiya CT, Alfoldi J, Lee AP, Fan S, Philippe H, Maccallum I, Braasch I, Manousaki T, Schneider I, Rohner N, Organ C, Chalopin D, Smith JJ, Robinson M, Dorrington RA, Gerdol M, Aken B, Biscotti MA, Barucca M, Baurain D, Berlin AM, Blatch GL, Buonocore F, Burmester T, Campbell MS, Canapa A, Cannon JP, Christoffels A, De Moro G, Edkins AL, Fan L, Fausto AM, Feiner N, Forconi M, Gamieldien J, Gnerre S, Gnirke A, Goldstone JV, Haerty W, Hahn ME, Hesse U, Hoffmann S, Johnson J, Karchner SI, Kuraku S, Lara M, Levin JZ, Litman GW, Mauceli E, Miyake T, Mueller MG, Nelson DR, Nitsche A, Olmo E, Ota T, Pallavicini A, Panji S, Picone B, Ponting CP, Prohaska SJ, Przybylski D, Saha NR, Ravi V, Ribeiro FJ, Sauka-Spengler T, Scapigliati G, Searle SM, Sharpe T, Simakov O, Stadler PF, Stegeman JJ, Sumiyama K, Tabbaa D, Tafer H, Turner-Maier J, van Heusden P, White S, Williams L, Yandell M, Brinkmann H, Volff JN, Tabin CJ, Shubin N, Schartl M, Jaffe DB, Postlethwait JH, Venkatesh B, Di Palma F, Lander ES, Meyer A, Lindblad-Toh K. The African coelacanth genome provides insights into tetrapod evolution. Nature 496: 311–316, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atochina-Vasserman E, Biktasova A, Abramova E, Cheng DS, Polosukhin VV, Tanjore H, Takahashi S, Sonoda H, Foye L, Venkov C, Ryzhov SV, Novitskiy S, Shlonimskaya N, Ikeda M, Blackwell TS, Lawson WE, Gow AJ, Harris RC, Dikov MM, Tchekneva EE. Aquaporin 11 insufficiency modulates kidney susceptibility to oxidative stress. Am J Physiol Renal Physiol 304: F1295–F1307, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choma DP, Harris RC, Tchekneva EE. A Single Nucleotide Polymorphism in the AQP11 Gene Is Associated With an Increased Risk for Developing Chronic Kidney Disease in Diabetic Patients. Philadelphia, PA: American Society of Nephrology, 2011. [Google Scholar]
- 5.Fan Y, Huang ZY, Cao CC, Chen CS, Chen YX, Fan DD, He J, Hou HL, Hu L, Hu XT, Jiang XT, Lai R, Lang YS, Liang B, Liao SG, Mu D, Ma YY, Niu YY, Sun XQ, Xia JQ, Xiao J, Xiong ZQ, Xu L, Yang L, Zhang Y, Zhao W, Zhao XD, Zheng YT, Zhou JM, Zhu YB, Zhang GJ, Wang J, Yao YG. Genome of the Chinese tree shrew. Nat Commun 4: 1426, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Fan Y, Yu D, Yao YG. Tree shrew database (TreeshrewDB): a genomic knowledge base for the Chinese tree shrew. Sci Rep 4: 7145, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu W, O'Connor TD, Jun G, Kang HM, Abecasis G, Leal SM, Gabriel S, Rieder MJ, Altshuler D, Shendure J, Nickerson DA, Bamshad MJ; NHLBI Exome Sequencing Project, Akey JM. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature 493: 216–220, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giunti S, Barit D, Cooper ME. Diabetic nephropathy: from mechanisms to rational therapies. Minerva Med 97: 241–262, 2006. [PubMed] [Google Scholar]
- 10.Giunti S, Barit D, Cooper ME. Mechanisms of diabetic nephropathy: role of hypertension. Hypertension 48: 519–526, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature 438: 633–638, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannedouche TP, Delgado AG, Gnionsahe DA, Boitard C, Lacour B, Grunfeld JP. Renal hemodynamics and segmental tubular reabsorption in early type 1 diabetes. Kidney Int 37: 1126–1133, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Huang HC, Preisig PA. G1 kinases and transforming growth factor-beta signaling are associated with a growth pattern switch in diabetes-induced renal growth. Kidney Int 58: 162–172, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Hub JS, de Groot BL. Mechanism of selectivity in aquaporins and aquaglyceroporins. Proc Natl Acad Sci USA 105: 1198–1203, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphreys BD, Xu F, Sabbisetti V, Grgic I, Naini SM, Wang N, Chen G, Xiao S, Patel D, Henderson JM, Ichimura T, Mou S, Soeung S, McMahon AP, Kuchroo VK, Bonventre JV. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest 123: 4023–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda M, Andoo A, Shimono M, Takamatsu N, Taki A, Muta K, Matsushita W, Uechi T, Matsuzaki T, Kenmochi N, Takata K, Sasaki S, Ito K, Ishibashi K. The NPC motif of aquaporin-11, unlike the NPA motif of known aquaporins, is essential for full expression of molecular function. J Biol Chem 286: 3342–3350, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imperatore G, Knowler WC, Nelson RG, Hanson RL. Genetics of diabetic nephropathy in the Pima Indians. Curr Diab Rep 1: 275–281, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Ina K, Kitamura H, Tatsukawa S, Takayama T, Fujikura Y, Shimada T. Transformation of interstitial fibroblasts and tubulointerstitial fibrosis in diabetic nephropathy. Med Electron Microsc 35: 87–95, 2002. [DOI] [PubMed] [Google Scholar]
- 19.International HapMap Consortium, Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, Peltonen L, Dermitzakis E, Bonnen PE, Altshuler DM, Gibbs RA, de Bakker PI, Deloukas P, Gabriel SB, Gwilliam R, Hunt S, Inouye M, Jia X, Palotie A, Parkin M, Whittaker P, Yu F, Chang K, Hawes A, Lewis LR, Ren Y, Wheeler D, Gibbs RA, Muzny DM, Barnes C, Darvishi K, Hurles M, Korn JM, Kristiansson K, Lee C, McCarrol SA, Nemesh J, Dermitzakis E, Keinan A, Montgomery SB, Pollack S, Price AL, Soranzo N, Bonnen PE, Gibbs RA, Gonzaga-Jauregui C, Keinan A, Price AL, Yu F, Anttila V, Brodeur W, Daly MJ, Leslie S, McVean G, Moutsianas L, Nguyen H, Schaffner SF, Zhang Q, Ghori MJ, McGinnis R, McLaren W, Pollack S, Price AL, Schaffner SF, Takeuchi F, Grossman SR, Shlyakhter I, Hostetter EB, Sabeti PC, Adebamowo CA, Foster MW, Gordon DR, Licinio J, Manca MC, Marshall PA, Matsuda I, Ngare D, Wang VO, Reddy D, Rotimi CN, Royal CD, Sharp RR, Zeng C, Brooks LD, McEwen JE. Integrating common and rare genetic variation in diverse human populations. Nature 467: 52–58, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi K, Kobayashi K, Sohara E, Sasaki S, Saihara Y. Aquaporin-11 (AQP11) is important for cytosolic osmoregulation. J Am Soc Nephrol 17: 539A, 2006. [Google Scholar]
- 21.Krolewski AS, Canessa M, Warram JH, Laffel LM, Christlieb AR, Knowler WC, Rand LI. Predisposition to hypertension and susceptibility to renal disease in insulin-dependent diabetes mellitus. N Engl J Med 318: 140–145, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Magri CJ, Fava S. The role of tubular injury in diabetic nephropathy. Eur J Intern Med 20: 551–555, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol 14: 1358–1373, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Mbanya JC, Thomas TH, Taylor R, Alberti KG, Wilkinson R. Increased proximal tubular sodium reabsorption in hypertensive patients with type 2 diabetes. Diabet Med 6: 614–620, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Morishita Y, Matsuzaki T, Hara-chikuma M, Andoo A, Shimono M, Matsuki A, Kobayashi K, Ikeda M, Yamamoto T, Verkman A, Kusano E, Ookawara S, Takata K, Sasaki S, Ishibashi K. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule. Mol Cell Biol 25: 7770–7779, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature 407: 599–605, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Nozaki K, Ishii D, Ishibashi K. Intracellular aquaporins: clues for intracellular water transport? Pflügers Arch 456: 701–707, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Pan XH, Yang XY, Yao X, Sun XM, Zhu L, Wang JX, Pang RQ, Cai XM, Dai JJ, Ruan GP. Bone-marrow mesenchymal stem cell transplantation to treat diabetic nephropathy in tree shrews. Cell Biochem Funct 32: 453–463, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Park JI, Yang SH, Lee JP, Yoo SH, Kim YS. Genetic predisposition of donors affects the allograft outcome in kidney transplantation: single-nucleotide polymorphism of aquaporin-11. Kidney Res Clin Pract 34: 42–57, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezzolesi MG, Skupien J, Mychaleckyj JC, Warram JH, Krolewski AS. Insights to the genetics of diabetic nephropathy through a genome-wide association study of the GoKinD collection. Semin Nephrol 30: 126–140, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54: 3427–3434, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, Masys DR. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther 84: 362–369, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Tchekneva EE, Harris RC. Aquaporin 11 Insufficient Mice Are Predisposed to the Development of Diabetic Kidney Disease. San Diego, CA: American Society of Nephrology, 2012. [Google Scholar]
- 37.Tchekneva EE, Khuchua Z, Davis LS, Kadkina V, Dunn SR, Bachman S, Ishibashi K, Rinchik EM, Harris RC, Dikov MM, Breyer MD. Single amino acid substitution in aquaporin 11 causes renal failure. J Am Soc Nephrol 19: 1955–1964, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 12: 261–266, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Thakar CV, Christianson A, Himmelfarb J, Leonard AC. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol 6: 2567–2572, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaidya VS, Ficociello LH, Johnson AC, Collings FB, Warram JH, Krolewski AS, Bonventre JV. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-d-glucosaminidase. Kidney Int 79: 464–470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallon V, Blantz R, Thomson S. The salt paradox and its possible implications in managing hypertensive diabetic patients. Curr Hypertens Rep 7: 141–147, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Vallon VKR. Pathophysiology of the diabetic kidney. Compr Physiol 1: 1–58, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Holst D. Renal failure as the cause of death in Tupaia belangeri exposed to persistent social stress. J Comp Physiol 78: 236, 1972. [Google Scholar]
- 44.Yakata K, Hiroaki Y, Ishibashi K, Sohara E, Sasaki S, Mitsuoka K, Fujiyoshi Y. Aquaporin-11 containing a divergent NPA motif has normal water channel activity. Biochim Biophys Acta 1768: 688–693, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Yakata K, Tani K, Fujiyoshi Y. Water permeability and characterization of aquaporin-11. J Struct Biol 174: 315–320, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Zerbini G, Bonfanti R, Meschi F, Bognetti E, Paesano PL, Gianolli L, Querques M, Maestroni A, Calori G, Del Maschio A, Fazio F, Luzi L, Chiumello G. Persistent renal hypertrophy and faster decline of glomerular filtration rate precede the development of microalbuminuria in type 1 diabetes. Diabetes 55: 2620–2625, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev 4: 39–45, 2008. [DOI] [PubMed] [Google Scholar]