Abstract
Postinfluenza bacterial pneumonia is associated with significant mortality and morbidity. MicroRNAs (miRNAs) are small, noncoding RNAs that regulate gene expression posttranscriptionally. miR-155 has recently emerged as a crucial regulator of innate immunity and inflammatory responses and is induced in macrophages during infection. We hypothesized upregulation of miR-155 inhibits IL-17 and increases susceptibility to secondary bacterial pneumonia. Mice were challenged with 100 plaque-forming units H1N1 intranasally and were infected with 107 colony-forming units of MRSA intratracheally at day 5 postviral challenge. Lungs were harvested 24 h later, and expression of miR-155, IL-17, and IL-23 was measured by real-time RT-PCR. Induction of miR-155 was 3.6-fold higher in dual-infected lungs compared with single infection. miR-155−/− mice were protected with significantly lower (4-fold) bacterial burden and no differences in viral load, associated with robust induction of IL-23 and IL-17 (2.2- and 4.8-fold, respectively) postsequential challenge with virus and bacteria, compared with WT mice. Treatment with miR-155 antagomir improved lung bacterial clearance by 4.2-fold compared with control antagomir postsequential infection with virus and bacteria. Moreover, lung macrophages collected from patients with postviral bacterial pneumonia also had upregulation of miR-155 expression compared with healthy controls, consistent with observations in our murine model. This is the first demonstration that cellular miRNAs regulate postinfluenza immune response to subsequent bacterial challenge by suppressing the IL-17 pathway in the lung. Our findings suggest that antagonizing certain microRNA might serve as a potential therapeutic strategy against secondary bacterial infection.
Keywords: miR-155, IL-17, postviral bacterial pneumonia
the influenza a virus is one of the most prevalent pathogens, causing respiratory illness every winter (61). Nevertheless, the influenza virus still accounts for 250,000 to 500,000 deaths each year and this number increased due to emergence of the 2009 H1N1 pandemic influenza strain (42, 46, 61).
Although the influenza virus itself can lead to severe pneumonia, mortality is most often caused by secondary complications of the infection. A recent report on the 2009 H1N1 influenza strain indicates that nearly 30% of fatal H1N1 cases between May 2009 and August 2009 in the United States were associated with a secondary bacterial infection (6, 31, 48, 66). In the United States, many of these infections have been due to the community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) strain USA300 (16, 20, 24, 30). This association of necrotizing bacterial pneumonia with antecedent influenza virus infection is well recognized. Studies have noted the importance of bacterial infections due to Streptococcus pneumoniae, Haemophilus influenzae, and S. aureus in the fatal cases associated with the 1918 influenza pandemic as well (5, 5a, 39). The increased influenza-associated mortality from coinfections with S. aureus among the pediatric age group resulted in a 2008 health advisory from the Centers for Disease Control and Prevention (CDCHAN-00268-2008-01-30-ADV-N) (14).
Th17 cells have been described as producing high levels of the proinflammatory cytokines IL-17 and IL-22 (2, 13, 29, 68). IL-23 has been implicated in Th17 pathway regulation, proliferation, and cytokine production. STAT3 activation, driven by IL-6 and IL-23, is required for terminal Th17 differentiation (34, 68). In addition to CD4+ T cells, many innate immune cells respond to IL-23 and are also important in both resistance to infection and in mediating autoimmune pathology. Patients with hyper-IgE syndrome (Job's syndrome) were shown to have STAT3 mutations (38). Consequently, these patients fail to develop Th17 cells or produce IL-17A, resulting in S. aureus infection of the skin and lung (36). These patients appear to have enhanced susceptibility to S. aureus due to a requirement for IL-17 signaling in the epithelium (37), suggesting a specific role for Th17 immunity in host defense against this pathogen. Moreover, it has recently been shown that mice challenged with influenza A PR/8/34 H1N1 and subsequently with S. aureus had substantially decreased IL-17 and IL-23 production after S. aureus infection. Overexpression of IL-23 in influenza A, S. aureus-coinfected mice rescued the induction of IL-17 and markedly improved bacterial clearance. These data confirm a role for IL-17 and IL-23 in the clearance of infection in the lung (27).
MicroRNAs are small RNAs that posttranscriptionally regulate eukaryotic gene expression. In addition to their involvement in a wide range of physiological and pathological processes, including viral infections, microRNAs are increasingly implicated in the eukaryotic response to bacterial pathogens. Recent studies have characterized changes in host microRNA expression following infection with exclusively extracellular (Helicobacter pylori) or intracellular (Salmonella enterica) Gram-negative bacteria, as well as in the response to Gram-positive (Listeria monocytogenes) and other pathogens (Mycobacterium and Francisella species). A role for miRNAs in the innate immune response was demonstrated when microRNAs such as miR-146a, miR-155, and miR-21 were shown to be induced in response to Toll-like receptor 4 (TLR4) signaling in monocytes (43, 45, 51, 56). miR-155 is induced by LPS, as well as other TLR ligands and proinflammatory cytokines (45). miR-155 has been found to be upregulated in several activated immune cells, including T lymphocytes, B lymphocytes, macrophages, and dendritic cells (DCs). This microRNA also is upregulated by a broad range of inflammatory mediators [such as tumor necrosis factor (TNF)-α, interferons, and polyriboinosinic:polyribocytidylic acid] during innate immune responses (57). The expression of miRNA-155 is upregulated upon lymphocyte activation (19), and it has been shown to control cell proliferation and differentiation (44, 65). For instance, miRNA-155 regulates B-cell proliferation, malignancy, and antibody production, at least in part through inhibition of activation-induced cytidine deaminase and PU.1 expression (47, 60, 67). miR-155 can also negatively regulate the differentiation and function of IL-17-producing helper T cells (26, 32, 43). However, the role of miR-155 in innate immune response to postviral bacterial pneumonia has not been studied.
In this study, we evaluated the mechanisms of impaired lung antibacterial responses postinfluenza infection. Mice sequentially infected with influenza and MRSA have significant upregulation of IFNγ, which leads to increased expression of miR-155 in whole lung, and this is associated with blunted IL-23 and IL-17 expression. miR-155 −/− mice demonstrate improved bacterial clearance secondary to a more robust IL-23 and IL-17 expression in the lung. Treatment with IL-17-neutralizing antibody largely abolishes the protected phenotype seen in miR-155−/− mice. Macrophages isolated from intubated patients in the intensive care unit (ICU) with postinfluenza bacterial pneumonia also had significantly higher expression of miR-155 compared with healthy controls.
METHODS
Mice.
Six- to eight-week-old wild-type (WT) C57BL/6 mice were purchased from Taconic. miR-155−/− and C57BL/6 control mice were purchased from The Jackson Laboratory. Mice were maintained under pathogen-free conditions. All of the studies were performed on age- and sex-matched mice. All of the animal studies were conducted with approval from the University of Michigan Committee on Use and Care of Animals.
Influenza A PR/8/34 H1N1 infection.
A mouse-adapted influenza A virus strain (strain A/PR8/34: H1N1 isotype; American Type Culture Collection) was inoculated into mice as described. Mice were infected with 100 plaque-forming units (PFU) of influenza A PR/8/34 H1N1 (in 40 μl sterile PBS) from a frozen stock or were mock infected with PBS intranasally.
S. aureus infection.
S. aureus (American Type Culture Collection 49775) producing γ-hemolysin and Panton-Valentine leukocidin was purchased from the American Type Culture Collection. S. aureus was cultured as detailed by American Type Culture Collection instructions in casein hydrolysate yeast extract containing modified medium, overnight for 18 h to stationary growth phase. Five days post-H1N1 challenge mice received 107 colony-forming units (CFU) of S. aureus (in 50 μl sterile PBS) or control PBS by intratracheal instillation. After an additional 24 h, lungs were harvested. Viral burden was determined by quantitative real-time RT-PCR on lung RNA for viral matrix protein as described previously (7).
mRNA extraction and real-time (TaqMan) quantitative PCR.
Total RNA from cells was isolated per the manufacturer's protocol for the RNAeasy Mini Kit (Qiagen, Valencia, CA). RNA amounts were determined by spectrometric analysis at 260 nm. All primers were designed using Primer Express software (Applied Biosystems, Foster City, CA). Levels of mRNA were determined by real-time quantitative RT-PCR analysis using an ABI PRISM 7000 Sequence Detection System (ABI/Perkin Elmer, Foster City, CA).
Necropsy.
Mice were euthanized at various intervals after H1N1 and/or MRSA challenge by inhalation of carbon dioxide and exsanguinated and the lungs were removed.
Lung macrophage isolation.
Lung macrophages (consisting of both alveolar and interstitial macrophages) were isolated from dispersed lung digest cells by adherence purification as previously described (10). This population comprises both resident and exudate/recruited macrophages, and cells are autoflourescent, CD11c+, F4/80+ (alveolar macrophages), and CD11b+ CD11clow (exudate macrophages) on flow cytometry.
Antibody neutralization.
IFNγ- and IL-17-neutralizing antibodies were kindly provided by Dr. Steven Kunkel, and the protocol has been described previously. (21). We treated the mice with 100 μg of anti-IFNγ antibody and 200 μg of IL-17 antibody intraperitoneally for neutralization of IFNγ and IL-17, respectively. The control group received anti-mouse IgG antibody.
IL-23 reconstitution.
Murine recombinant IL-23 was purchased from R&D Systems (Minneapolis, MN). Mice were anesthetized, and 2 μg rmIL-23 were administered intratracheally 4 h before challenge with MRSA.
Whole lung homogenization for CFU determination.
At designated time points, the mice were euthanized by CO2 inhalation. Before lung removal, the pulmonary vasculature was perfused by infusing 1 ml of PBS containing 5 mM EDTA into the right ventricle. Whole lungs were removed, with care taken to dissect away lymph nodes. The lungs were then homogenized in 1 ml of PBS with protease inhibitor (Boehringer Mannheim, Indianapolis, IN). Homogenates were then serially diluted 1:5 in PBS and plated on blood agar to determine lung CFU.
Bronchoalveolar lavage.
Bronchoalveolar lavage (BAL) was performed for collection of BAL fluid (BALF) as previously described (10). Briefly, the trachea was exposed and intubated using a 1.7-mm-outer-diameter polyethylene catheter. BAL was performed by instilling PBS containing 5 mM EDTA in 1-ml aliquots. A total of 3 ml PBS was instilled per mouse, with 90% of lavage fluid retrieved.
Human macrophage isolation.
BALF was collected from patients admitted to the ICU for respiratory failure/aacute respiratory distress syndrome (ARDS) on mechanical ventilation and who had undergone BAL for clinical purposes; Institutional guidelines were followed and patient samples were de-identified. Since the project involved only biological specimens that could not be linked to a specific individual directly or indirectly, in accordance with Office of Human Research Protection guidance on this subject, the University of Michigan Institutional Review Board (IRB) deemed it IRB exempt. We also had IRB approval from the University of Michigan to collect samples from healthy volunteers. Patient characteristics are described in Table 1. BALF was filtered and centrifuged to form cell pellets. Patients included in our analysis were enrolled between October 2013 and February of 2014, were ≥18 yr of age, met ARDS criteria as defined by the Berlin definition (1), required endotracheal intubation and mechanical ventilation, and underwent BAL. Healthy volunteers without lung disease who underwent BAL via bronchoscopy served as control subjects. BAL samples were filtered through sterile gauze to remove noncellular particulate material and mucus. Samples were then centrifuged (1,000 g for 5 min) after collection to separate the cells and supernatant. The supernatant was aliquoted into small volumes (<10 ml). All BAL supernatant samples were stored at −80°C. Cells were resuspended in RPMI and macrophages were isolated by adherence purification.
Table 1.
Demographic characteristics of patients
| Staphylococcus aureus | Streptococcus pneumoniae | |
|---|---|---|
| Men | 77% | 67% |
| Age | 55 ± 12 | 49 ± 11 |
| PaO2/FiO2 ratio | 118 ± 11 | 109 ± 28 |
| Bacterial isolates no. of patients | 9 (75%) | 3 (25%) |
Statistical analysis.
All of the data are presented as the means ± SE. Significance was tested by unpaired t-test (for two means) or one-way ANOVA (for multiple data groups) followed by Tukey post hoc test. Mouse survival data were analyzed by log-rank test using the Graph Pad Prism software package.
RESULTS
Mice have higher bacterial burden and poor survival after influenza infection.
In patients, the peak incidence of secondary bacterial pneumonia occurs between 4–10 days after the initial influenza infection (49). In animal models, influenza infections lead to impaired ability to clear secondary S. pneumoniae administered 4 days to 6 wk following initial influenza challenge (11, 33, 55). Mice were challenged with 100 PFU influenza (PR8 strain) administered intranasally, a dose sufficient to elicit an inflammatory response in the lungs with characteristic histology of human influenza infections (data not shown), followed 5 days later by intratracheal S. aureus (107 CFU). As seen in Fig. 1A mice challenged with either influenza or MRSA alone had 50% mortality. In comparison, mice that were challenged with influenza before S. aureus infection displayed 100% mortality. To assess if the difference in mortality was secondary to lung bacterial burden, mice were challenged with either MRSA alone or were infected with H1N1 as described above and challenged with MRSA. Lungs were harvested 24 h postbacterial challenge and CFU were quantitated. WT mice when challenged with bacteria alone were efficient in clearing the infection. In contrast, mice that were challenged with MRSA after H1N1 had significantly higher bacterial burden (2 log) in the lung (Fig. 1B) compared with mice infected with bacteria alone. We did not find any systemic dissemination of bacteria in blood or in spleen (data not shown).
Fig. 1.
Mice with viral challenge before bacterial infection have higher bacterial burden in the lung. A: wild-type (WT) mice were challenged with 107 colony-forming units (CFU) methicillin-resistant Staphylococcus aureus (MRSA) either alone or 5 days after infection with 100 PFU H1N1, lungs were harvested at 24 h, and CFU were evaluated; n = 6 mice in each group and experiments were repeated 3 times. *P < 0.05, compared with MRSA infection alone. B: mice were challenged intranasally with 100 PFU H1N1 alone or intratracheally with 107 CFU MRSA alone or at day 5 post-H1N1 infection mice were challenged with 107 CFU MRSA. Survival was assessed for each condition; n = 8 mice in each group and experiments were repeated 3 times. *P < 0.01, compared with the double infection group as measured by log rank test.
Mice with dual infection have increased IFNγ expression in lungs, compared with single infection.
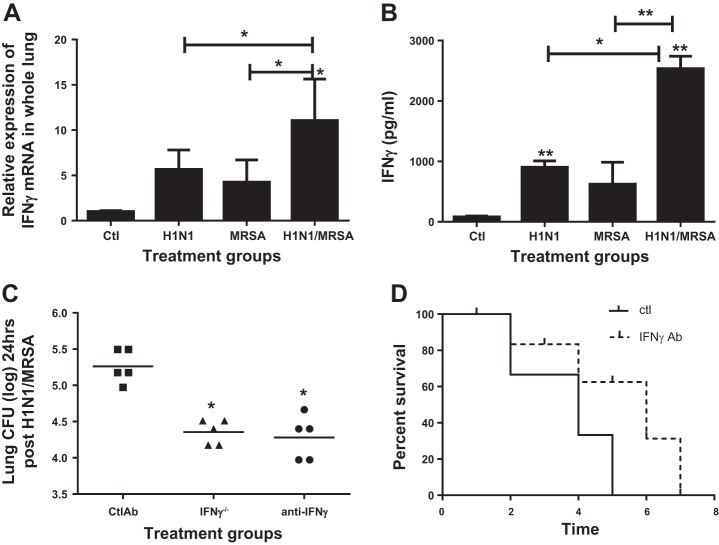
Sun and Metzger (55) have shown that the effect of elevated IFNγ on antibacterial immune response to S. pneumoniae post-H1N1 is to impair phagocytosis by macrophages. To assess IFNγ expression in our model, mice were infected with H1N1 intranasally and on day 5 challenged with 107 MRSA intratracheally. Lungs were harvested at 24 h, and IFNγ was expression measured by real-time RT-PCR. Mice either postviral or postbacterial challenge alone had a fivefold increase in expression of IFNγ mRNA as compared with uninfected controls (Fig. 2A). Interestingly, challenge with virus and bacteria sequentially resulted in a significantly greater increase in expression of IFNγ (10-fold). We also observed a significant increase in IFNγ protein levels in whole lung (Fig. 2B), most prominent in the lungs of dual-infected animals.
Fig. 2.
Dual-infected animals have higher IFNγ in lung compared with either challenge alone. IFNγ−/− mice and mice treated with anti-IFNγ antibody have lower lung bacterial burden compared with WT mice postsequential infection. Mice were infected with 100 PFU H1N1 or 107 CFU MRSA alone or sequentially, and IFN γ levels in lung digests were measured by real-time RT-PCR (A) ELISA (B); n = 5 mice in each group, experiments repeated twice. *P < 0.05, **P < 0.01, compared with untreated controls. C: WT mice and IFNγ−/− were infected with 100 PFU H1N1 intranasally and on day 5 challenged with 107 CFU MRSA, lungs were harvested, and bacterial burden was quantitated. WT mice were also treated with 80 μl anti-IFN γ Ab intraperitoneally or control antibody intraperitoneally 6 h before challenge with intratracheally 107 CFU MRSA and on day 5 post-H1N1 intranasally infection, lungs were harvested 24 h postbacterial challenge, and CFU was quantitated; n = 5 mice in each group and experiments were repeated 2 times. **P < 0.01, as compared mice infected WT mice. D: WT mice were also treated with 80 μl anti-IFNγ Ab intraperitoneally or control antibody intraperitoneally 6 h before challenge with intratracheal 107 CFU MRSA and on day 5 post-H1N1 intranasal infection and survival was assessed.
To determine if this increase in IFNγ was causally linked to impaired antibacterial immunity in the lungs of influenza-infected mice, WT and IFNγ−/− mice were infected with H1N1 and on day 5 challenged with MRSA and the lungs were harvested at 24 h postbacterial challenge for CFU determination. As seen in Fig. 2C, IFNγ−/− mice had significantly lower bacterial burden in the lung (9.6-fold) compared with WT mice postsequential infection. To assess if this protection was specifically mediated by blunted IFNγ−/− responses post-H1N1 infection, WT mice were treated with control antibody or a neutralizing Ab against murine IFNγ intraperitoneally (Fig. 2C). Mice treated with control antibody succumbed to bacterial pneumonia by day 4 (Fig. 2D), which was associated with high bacterial burden in the lung, whereas mice treated with IFNγ antibody had a more prolonged survival (Fig. 2D) and had significantly lower bacterial burden when compared infected mice treated with control Ab (Fig. 2C), indicating that IFNγ plays an important role in postinfluenza suppression of MRSA immunity.
Sequential challenge with influenza and MRSA results in upregulated miR-155 levels in lung and lung macrophages.
MicroRNAs have been recently described to play a role in innate immune responses to viral and bacterial challenge (18, 47). To further investigate if microRNA were contributing to increased host susceptibility to secondary bacterial challenge, we measured the expression of miR-155 in whole lung (Fig. 3A) and lung macrophages (Fig. 3B) harvested from control and infected mice. miR-155 has been shown to upregulate IFNγ and has been implicated in innate responses mediated by NF-κB (64). Infection with either H1N1 or MRSA alone resulted in an approximate fivefold increase in miR-155 expression, compared with untreated controls in lung macrophages. By comparison, mice challenged with both H1N1 and MRSA had significantly higher (9.5-fold higher) expression than untreated controls in lung macrophages. miR-146 has also been described to be activated via the NF-κB pathway and has been shown to be important for host defense (56). To determine whether the elevation of miR-155 was a selective response or if other microRNA were also involved, we measured miR-146 levels in whole lung and found no difference in expression of miR-146 in mice challenged with MRSA alone or after dual infection (data not shown). This suggests that the induction of miR-155 was specific in our model.
Fig. 3.
Macrophages harvested from mice postdual infection have higher expression of miR-155 RNA compared with viral or bacterial infection alone, which is attenuated by anti-IFNγ Ab treatment. A: WT mice were challenged with 107 CFU MRSA either alone or 5 days after infection with 100 PFU H1N1, lungs were harvested at 24 h, and expression of miR-155 was measured by RT-PCR. ***P < 0.001. B: lung macrophages were harvested 24 h postbacterial challenge by collagenase digest and adherence purification and miR-155 was expression measured by real-time RT-PCR. C: WT mice were infected intranasally with 100 PFU H1N1 and on day 5 mice were inoculated with either control antibody or 80 μl anti-IFNγ antibody intraperitoneally and 6 h later mice were challenged intratracheally with 107 CFU MRSA. Lung macrophages were harvested and expression of miR-155 measured by real-time PCR. *P < 0.05, **P < 0.01; n = 4 in each group, and experiment was repeated 3 times.
To assess the role of IFNγ in the upregulation of miR-155, lungs were harvested from mice coinfected with H1N1 and MRSA in the presence or absence of anti-IFNγ antibody intraperitoneally. Similar to the results in Fig. 3A, there was an approximate sixfold increase in expression of miR-155 in lungs harvested from mice post-H1N1 and post-MRSA dual infection treated with control Ab. This induction was significantly reduced in mice that received anti-IFNγ antibody, indicating a direct in vivo role of IFNγ in upregulation of miR-155 (Fig. 3C).
Mice postdual infection have reduced levels of IL-17 and IL-23.
To further understand the mechanism by which influenza suppressed the antibacterial host response, and how this process might be regulated by miR-155, we measured cytokines that are important for lung antibacterial host defense. While there was no difference in protein levels of IL-12 or TNF-α in dual-infected mice (data not shown), we found that sequential infection resulted in significantly blunted IL-23 (Fig. 4A) and IL-17 (Fig. 4B) expression as measured by real-time RT-PCR, and compared with mice infected with H1N1 or MRSA alone, suggesting that preceding H1N1 infection attenuates induction of IL-17 and an IL-17-inducing cytokine, IL-23.
Fig. 4.
Decreased expression of IL-23 and IL-17 in lungs postsequential infection. WT mice were challenged with 107 CFU MRSA either alone or 5 days after infection with 100 PFU H1N1, lungs were harvested at 24 h, and IL-17 (A) and IL-23 (B) gene expression was measured by RT-PCR. *P < 0.05; n = 4 in each group, and experiments were repeated 3 times.
miR-155 mediates increased mortality and impaired bacterial clearance in postinfluenza pneumonia.
Given that we observed a significant upregulation of miR-155 in coinfected mice with impaired antibacterial immunity, WT and miR-155−/− mice were infected with 100 PFU H1N1 and then challenged with 107 CFU MRSA on day 5 as described previously. WT mice had 100% mortality by day 4. By comparison, dually infected miR-155−/− mice were partially protected, with 40% mice alive at day 10 postbacterial challenge (Fig. 5A). No mice died after 10 days. To understand if miR-155 was altering the host response to viral challenge or if the absence of miR-155 had an effect on viral clearance, we measured viral replication in whole lung by RT-PCR. As seen in Fig. 5B, there was no significant difference in viral gene expression between WT and miR 155−/− mice, suggesting that the survival benefit seen in the mutant mice was not secondary to different levels of viral burden between the two groups.
Fig. 5.
MiR-155−/− mice have decreased bacterial burden in the lung and increased expression of IL-23 and IL-17 compared with WT mice. A: WT and miR-155−/− mice were challenged intranasally with 100 PFU H1N1 and on day 5 post-H1N1 infection challenged with 107 CFU MRSA and survival was assessed. WT and miR-155−/− mice were challenged intranasally with 100 PFU H1N1 and on day 5 post-H1N1 infection challenged with 107 CFU MRSA. Lungs were harvested at 24 h and viral gene expression was analyzed by RT-PCR (B) and lung bacterial CFU were quantified (C). Lungs were harvested 24 h postbacterial challenge and expression of IL- 23 (D) and IL- 17 (E) levels and measured by real-time PCR. Lung macrophages 24 h postbacterial challenge from H1N1/MRSA-infected mice or uninfected control mice were harvested by collagenase digest and adherence purification and IL-23p19 and expression was measured by real-time PCR (F). *P < 0.05, **P < 0.01; n = 4 in each group, and experiments were repeated 3 times.
To further understand the impact of miR-155 on antibacterial responses, WT and miR-155−/− mice were infected with either MRSA alone or sequentially infected and bacterial CFU were determined 24 h post-MRSA challenge. There was no difference in lung bacterial burden between WT and miR-155−/− mice post-MRSA challenge alone (data not shown). Interestingly, miR-155−/− mice displayed significantly lower lung bacterial burden, compared with WT mice, after infection with H1N1 and MRSA (Fig. 5C), indicating that miR-155 was mediating increased mortality and impaired bacterial clearance postinfluenza pneumonia.
miR-155 negatively regulates IL-17 and IL-23 expression in postinfluenza pneumonia.
We next performed experiments to determine if improved bacterial clearance observed in dual-infected miR-155−/− mice was attributable to more robust production of IL-17 cytokines. WT and miR-155−/− mice were dually infected as described above, and lungs were harvested 24 h postbacterial challenge. IL-23 and IL-17 mRNA expression was measured by real-time RT-PCR. As previously observed, WT mice displayed reduced expression of IL-23 and IL-17. In contrast, dually infected miR-155−/− mice displayed two- and fourfold higher expression of IL-23 and IL-17, respectively, compared with WT animals (Fig. 5, D and E, respectively), suggesting that the improved clearance in miR-155−/− mice was partially attributable to greater IL-17 responses in the lung postinfection.
To further identify the cellular source of IL-23, macrophages were harvested from infected WT and miR-155−/− mice (as described in methods) 24 h postintracheal challenge with MRSA in H1N1-infected mice and expression was measured by real-time PCR. As shown in Fig. 5F, macrophages harvested from WT mice postdual challenge did not have any significant upregulation in IL-23 expression compared with uninfected macrophages. In contrast, macrophages purified from miR-155−/− mice postdual infection had a robust twofold induction in IL-23 expression compared with macrophages harvested from infected WT mice.
Treatment with IL-17 antibody partially abolishes the protective phenotype observed in miR-155−/− mice.
To establish that the improved antibacterial host response observed in miR-155−/− mice was secondary to enhanced IL-17 expression, WT and miR-155−/− mice were infected with H1N1 intranasally and treated with 100 μg anti-IL-17 antibody or control antibody intraperitonelly 4 h before challenge with MRSA intratracheally and then assessed for lung bacterial clearance 24 h later. WT mice treated with the control antibody had high bacterial burden. As observed previously, miR-155−/− mice treated with control Ab were significantly more efficient in clearing bacteria. However, as seen in Fig. 6A administration of anti-IL-17 Ab 4 h before bacterial challenge partially mitigated the improved bacterial clearance observed in miR-155−/− mice.
Fig. 6.
miR-155−/− mice treated with anti-IL-17 antibody before bacterial challenge have higher bacterial burden in the lung. A: WT and miR-155−/− mice were challenged intranasally with 100 PFU H1N1 and on day 5 post-H1N1 infection challenged with 107 CFU MRSA. Four hours before bacterial challenge miR-155−/− mice were treated with either a control antibody or 100 μg of anti-IL-17 antibody intraperitoneally. Lungs were harvested at 24 h and CFU were quantified. B: WT mice were infected with H1N1 and 4 h before challenge with MRSA given vehicle control or rmIL-23 intratracheally Lungs were harvested at 24 h and CFU quantified. *P < 0.05; n = 5 in each group, and experiments were repeated 2 times.
To provide further evidence that IL-23, which is upstream of IL-17 and is produced more abundantly by macrophages postdual infection in miR-155−/− mice was playing a mechanistic role in our model, WT mice were infected with H1N1 and 4 h before MRSA intratracheally reconstituted with either vehicle control or rmIL-23. Lungs were harvested 24 h after bacterial challenge and CFU were quantitated. As seen in Fig. 6B, mice treated with rmIL-23 had significantly lower bacterial burden in the lung compared with vehicle-treated WT mice postsequential infection.
Treatment with miR-155 antagomir improves bacterial clearance and IL-17 expression in dual-infected animals.
To evaluate a therapeutic potential for miR-155 antagonism, WT mice were infected with H1N1 intranasally, and then 4 h before challenge with intratracheal MRSA, they were treated with either sham or miR-155 antagomir intraperitoneally. Lungs were harvested 24 h after bacterial challenge, and bacterial load was quantitated. Dually infected mice treated with sham antagomir had high lung MRSA CFU whereas treatment with miR-155 antagomir reduced lung MRSA CFU (Fig. 7A) without having any effect on viral titres in the lung (data not shown). Moreover, treatment of H1N1/MRSA-infected mice with miR-155 antagomir resulted in significantly increased expression of both IL-23 and IL-17 (Fig. 7, B and C, respectively). Collectively, these data indicate that antagonizing miR-155 offers protection in postinfluenza bacterial pneumonia.
Fig. 7.
Treatment with miR-155 antagomir decreased bacterial burden in the lung and upregulates expression of IL-23 and IL-17. WT mice were challenged intranasally with 100 PFU H1N1 and on day 5 post-H1N1 infection challenged with 107 CFU MRSA and treated with sham antagomir or miR-155 antagomir intranasally. Lungs were harvested at 24 h and CFU were quantified (A). Cytokine expression of IL-23 and IL-17 were measured in lungs by real-time RT-PCR (B and C). *P < 0.05, **P < 0.01; n = 5 in each group, and experiments were repeated 2 times.
Alveolar macrophages from dual-infected patients have higher miR-155 levels.
To determine whether observations made in the murine model were clinically relevant in patients with influenza pneumonia, we examined expression of miR-155 levels in patients admitted to our ICU with ARDS from bacterial pneumonia postinfluenza A infection. Patient characteristics at study entry are shown in Table 1. Intubated patients that tested positive for influenza A (H1N1) and had tracheal aspirate or sputum positive for Gram-positive bacteria were selected. Healthy volunteers without lung disease who underwent BAL via bronchoscopy served as control subjects. BAL from 12 patients with secondary bacterial pneumonia was harvested, and macrophages were purified by adherence purification with a purity of 96% as determined by flow cytometry. miR-155 expression was measured by real-time RT-PCR. As shown in Fig. 8, macrophages harvested from patients with secondary bacterial pneumonia due to either MRSA or S. pneumoniae demonstrated significantly higher miR-155 expression (4.5- and 3.1-fold, respectively) compared with control macrophages harvested from healthy subjects.
Fig. 8.

Human alveolar macrophages purified from patients with H1N1 and bacterial coinfection had increased expression of miR-155. Alveolar macrophages were purified by adherence purification from bronchoalveolar lavage (BAL) obtained from healthy subjects who underwent bronchoscopy for research purposes or patients admitted to our intensive care unit with respiratory failure from secondary bacterial pneumonia post H1N1, and expression of miR-155 was measured by real-time PCR. *P < 0.05; n = 12 patients and n = 3 controls.
DISCUSSION
Our findings demonstrate a novel molecular mechanism by which influenza A impairs host defense against secondary MRSA infection. The majority of severe and fatal influenza infections are related to secondary bacterial pneumonia (9) highlighting the importance of understanding compromised immune defense in this context. Furthermore, recent findings during the recent H1N1 pandemic have shown a predilection for secondary CA-MRSA infections in fatal influenza A (25). We found that miR-155 is induced by influenza and this microRNA inhibits protective IL-23 and IL-17 responses. Antagonizing miR-155 postinfluenza A infection increased IL-23 and IL-17 expression and improved the immune response to secondary S. aureus challenge. These observations may have implications for the attenuation of host defense against a number of pathogens, as the IL-17 pathway has been implicated in host immunity against bacterial and fungal pathogens.
The observation that influenza A increases susceptibility to S. aureus was reported many years ago (23); however, the molecular mechanisms for this effect have remained elusive. Furthermore, pathologic synergism between influenza A and S. aureus products has been identified (58, 59, 70). Recently, influenza A was shown to exacerbate secondary S. aureus infection in mice as measured by decreased bacterial clearance and increased mortality (54). In the aforementioned study, influenza was shown to inhibit NK cell production of TNF-α, which resulted in impaired antimicrobial function of macrophages. In our S. aureus model, WT and miR-155−/− mice displayed no difference in TNF-α production in the lung induced by S. aureus at 24 h postchallenge, which was significantly upregulated compared with that in control mice despite worsened clearance of S. aureus in WT mice (data not shown). miR-155 is known to affect TNF-α stabilization and increases its translation (63); however, we did not see changes in TNF-α in our current studies.
Sun and Metzger (55) have shown influenza induces IFNγ expression, which increases susceptibility to streptococcal pneumonia. Similarly, we observed increased production of IFNγ in mice with dual infection. Our study indicates that IFNγ drives enhanced miR-155 expression, which mediates impaired innate immune response to bacterial challenge. Sun and Metzger proposed the mechanism of impaired bacterial clearance to be secondary to impaired phagocytosis of bacteria by macrophages, due to reduced expression of the MARCO scavenger receptor. Scavenger receptors may be relevant in our model as well since macrophages transfected with antagomir to miR-155 demonstrate increased ability to phagocytize S. aureus due to upregulation of scavenger receptor SRA (12). We have not directly measured the phagocytic ability of alveolar macrophages from dual-infected mice to engulf MRSA, but it is possible that it would be enhanced given our previous results; thus the defect in vivo likely relates to impaired clearance (12). Type 1 interferons also have been implicated in increased susceptibility to secondary bacterial pneumonia (41, 50) although we did not find any difference in IFNα/β levels in our model (data not shown).
IL-17 has been implicated in immunity against bacterial pathogens including S. aureus (27). In fact, IL-17−/− mice had increased bacterial burden in the lung in mice challenged with H1N1 and subsequently with S. aureus. These mice were shown to have increased inflammation and decreased clearance of bacteria associated with substantially decreased IL-17, IL-22, and IL-23 production after S. aureus infection (27). In addition, they demonstrated that the majority of IL-17A-producing cells were CD4+ or γδ T cells, which were significantly decreased when bacterial infection was preceded by viral challenge. Furthermore, overexpression of IL-23 in influenza A, S. aureus-coinfected WT mice rescued the induction of IL-17 and IL-22 and markedly improved bacterial clearance. This is consistent with what we observed in our model, and our work now links these changes in IL-17 and IL-23 to upregulation of miR-155.
Many studies have demonstrated the critical role of macrophages in antibacterial host defense and specifically in postviral secondary bacterial pneumonia (4, 17, 55). IL-23 has been described to be involved in the clearance of infectious pathogens, immune responses against malignancies, and development of autoimmune diseases (8, 28, 35). CD11c+ cells have been shown to be primary producers of IL-23 in the lungs postinfection or injury, including CD11c+ macrophages/monocytes (3). IL-23 has been implicated in Th17 cell regulation, proliferation, and cytokine production (34, 68). In addition to CD4+ T cells, many innate immune cells respond to IL-23 and are important in resistance to infection. We have shown sequentially infected mice have significantly low expression of IL-23 in lungs, and reconstitution of IL-23 intratracheally improves the ability of WT mice challenged with H1N1 and MRSA to clear bacteria. We postulate that rmIL-23 has a dual role in offering protection: 1) IL-23 potentiates production of IL-17 as well as IL-22, and both these cytokines have been shown to offer protection against secondary bacterial pneumonia postinfluenza infection (22, 27); and 2) IL-23 has also been shown to suppress IL-12-mediated potentiation of IFN-γ production, thus offering additional protection against secondary bacterial pneumonia (53).
miR-155 has been shown to be upregulated by various TLR ligands in macrophages through either the MyD88 or TRIF signaling pathway (45). In mouse models of autoimmune diseases like experimental allergic encephalomyelitis and arthritis, it has been shown that miR-155-deficient animals have defects in skewing toward the Th17 lineage both in vivo and in vitro (40, 43, 69). Contrary to the autoimmune models, in our study we see upregulation of miR-155 in lung, specifically by macrophages postviral and bacterial challenge associated with significantly blunted IL-23 response, which likely results in decreased IL-17 seen in lungs postdual infection.
In our study, we noted increased IFN-γ levels postdual challenge with virus and bacteria and IFN-γ has been shown previously to regulate miR-155 expression (2). Moreover, IFN-γ has also been shown to negatively regulate IL-23 in murine macrophages by histone modification in a model of murine experimental colitis (52). We speculate that the mechanism of decreased IL-23 secretion by lung macrophage is mediated via IFN-γ signaling upregulating miR −155 in macrophages and potentially resulting in histone acetylation. miR-155−/− mice are protected with improved bacterial clearance associated with increased IL-23 production by macrophages and increased IL-17 in whole lung, implicating miR-155 in the impaired immune response seen in postviral bacterial pneumonia. We focused our study on understanding the role of miR-155 expression in macrophages and susceptibility to postviral bacterial pneumonia. We speculate that the IL-17 producing cells are likely CD4+ T cells, although other cells like innate lymphoid cells and γδ T cells could also be playing a role in the model and this is an area of further investigation. IL-23 and IL-17 have previously been implicated in impaired antibacterial response to staphylococcus with improvement in bacteria clearance postreconstitution of IL-23 (27). Our study provides a novel mechanism for the blunted IL-23/IL-17 expression seen in mice postdual infection.
miR-155 is upregulated in malignancy (62) and is increasingly being implicated in immune response to infectious disease. In a recent study, Gracias et al. (18) implicated miR-155 in controlling CD8+ T-cell responses to influenza by regulating interferon signaling. Our study is the first observation of its role in innate immune responses against dual viral and bacterial infection, specifically its role in increasing susceptibility to secondary bacterial infections. The mechanism of increased susceptibility is likely blunting the IL-23 immune response hence decreasing IL-17 production, which has been shown before to be critical for host response against bacterial pneumonia. Considering there are miR-155 antagomirs available commercially, this offers a novel treatment option to protect against mortality and morbidity seen in patients with secondary bacterial pneumonia.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-119682 and HL-123515 and National Institute of Allergy and Infectious Diseases Grant AI-117229.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.P., R.E., P.K.P., and U.B. performed experiments; T.J.S., B.B.M., and U.B. interpreted results of experiments; T.J.S., B.B.M., and U.B. edited and revised manuscript; M.N.B., S.L.K., and U.B. conception and design of research; U.B. analyzed data; U.B. prepared figures; U.B. drafted manuscript; U.B. approved final version of manuscript.
REFERENCES
- 1.ARDS Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307: 2526–2533, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol 19: 377–382, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol 13: 666–678, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosmann M, Grailer JJ, Russkamp NF, Ruemmler R, Zetoune FS, Sarma JV, Ward PA. CD11c+ alveolar macrophages are a source of IL-23 during lipopolysaccharide-induced acute lung injury. Shock 39: 447–452, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet 6: 303–312, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918-19 influenza pandemic. Emerg Infect Dis 14: 1193–1199, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)–United States, May-August 2009. MMWR Morb Mortal Wkly Rep 58: 1071–1074, 2009. [PubMed] [Google Scholar]
- 7.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol 183: 5301–5310, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421: 744–748, 2003. [DOI] [PubMed] [Google Scholar]
- 9.DeLeo FR, Musser JM. Axis of coinfection evil. J Infect Dis 201: 488–490, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest 116: 2532–2542, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med 205: 323–329, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domingo-Gonzalez R, Katz S, Serezani CH, Moore TA, Levine AM, Moore BB. Prostaglandin E2-induced changes in alveolar macrophage scavenger receptor profiles differentially alter phagocytosis of Pseudomonas aeruginosa and Staphylococcus aureus post-bone marrow transplant. J Immunol 190: 5809–5817, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol 8: 337–348, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L, Fry A, Hageman J, Gorwitz R, Bresee J, Uyeki T. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics 122: 805–811, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, Carroll K, Thomas DL, Nuermberger E, Bartlett JG. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 40: 100–107, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Gordon SB, Irving GR, Lawson RA, Lee ME, Read RC. Intracellular trafficking and killing of Streptococcus pneumoniae by human alveolar macrophages are influenced by opsonins. Infect Immun 68: 2286–2293, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gracias DT, Stelekati E, Hope JL, Boesteanu AC, Doering TA, Norton J, Mueller YM, Fraietta JA, Wherry EJ, Turner M, Katsikis PD. The microRNA miR-155 controls CD8(+) T cell responses by regulating interferon signaling. Nat Immunol 14: 593–602, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haasch D, Chen YW, Reilly RM, Chiou XG, Koterski S, Smith ML, Kroeger P, McWeeny K, Halbert DN, Mollison KW, Djuric SW, Trevillyan JM. T cell activation induces a noncoding RNA transcript sensitive to inhibition by immunosuppressant drugs and encoded by the proto-oncogene, BIC. Cell Immunol 217: 78–86, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Hageman JC, Uyeki TM, Francis JS, Jernigan DB, Wheeler JG, Bridges CB, Barenkamp SJ, Sievert DM, Srinivasan A, Doherty MC, McDougal LK, Killgore GE, Lopatin UA, Coffman R, MacDonald JK, McAllister SK, Fosheim GE, Patel JB, McDonald LC. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis 12: 894–899, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito T, Schaller M, Hogaboam CM, Standiford TJ, Sandor M, Lukacs NW, Chensue SW, Kunkel SL. TLR9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through DC-derived Notch ligand delta-like 4. J Clin Invest 119: 33–46, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, Blanc F, De Trez C, Van Maele L, Dumoutier L, Huerre MR, Eberl G, Si-Tahar M, Gosset P, Renauld JC, Sirard JC, Faveeuw C, Trottein F. Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection. J Virol 87: 6911–6924, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakab GJ, Warr GA, Knight ME. Pulmonary and systemic defenses against challenge with Staphylococcus aureus in mice with pneumonia due to influenza A virus. J Infect Dis 140: 105–108, 1979. [DOI] [PubMed] [Google Scholar]
- 24.Kallen AJ, Hageman J, Gorwitz R, Beekmann SE, Polgreen PM. Characteristics of Staphylococcus aureus community-acquired pneumonia during the 2006–2007 influenza season. Clin Infect Dis 45: 1655, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Kallen AJ, Reed C, Patton M, Arnold KE, Finelli L, Hageman J. Staphylococcus aureus community-onset pneumonia in patients admitted to children's hospitals during autumn and winter of 2006–2007. Epidemiol Infect 138: 666–672, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J Immunol 182: 2578–2582, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 186: 1666–1674, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature 442: 461–465, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203: 2271–2279, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 29: 1128–1132, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, Vugia D, Harriman K, Matyas B, Glaser CA, Samuel MC, Rosenberg J, Talarico J, Hatch D; California Pandemic Working Group. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA 302: 1896–1902, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Lu L, Cao HD, Zeng HQ, Wang PL, Wang LJ, Liu SN, Xiang TX. Recombinant Mycobacterium smegmatis mc(2)155 vaccine expressing outer membrane protein 26 kDa antigen affords therapeutic protection against Helicobacter pylori infection. Vaccine 27: 972–978, 2009. [DOI] [PubMed] [Google Scholar]
- 33.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis 186: 341–350, 2002. [DOI] [PubMed] [Google Scholar]
- 34.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity 28: 445–453, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol 183: 8026–8034, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452: 773–776, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, Tsuge I, Karasuyama H. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 206: 1291–1301, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, Metin A, Karasuyama H. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448: 1058–1062, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198: 962–970, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murugaiyan G, Beynon V, Mittal A, Joller N, Weiner HL. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J Immunol 187: 2213–2221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest 121: 3657–3665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team , Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360: 2605–2615, 2009. [DOI] [PubMed] [Google Scholar]
- 43.O′Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 33: 607–619, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med 205: 585–594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA 104: 1604–1609, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol 160: 492–502, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science 316: 608–611, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 38: e91–97, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med 121: 258–264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest 119: 1910–1920, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O'Leary JJ, Ruan Q, Johnson DS, Chen Y, O'Neill LA. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol 11: 141–147, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Sheikh SZ, Matsuoka K, Kobayashi T, Li F, Rubinas T, Plevy SE. Cutting edge: IFN-gamma is a negative regulator of IL-23 in murine macrophages and experimental colitis. J Immunol 184: 4069–4073, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sieve AN, Meeks KD, Lee S, Berg RE. A novel immunoregulatory function for IL-23: inhibition of IL-12-dependent IFN-gamma production. Eur J Immunol 40: 2236–2247, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Small CL, Shaler CR, McCormick S, Jeyanathan M, Damjanovic D, Brown EG, Arck P, Jordana M, Kaushic C, Ashkar AA, Xing Z. Influenza infection leads to increased susceptibility to subsequent bacterial superinfection by impairing NK cell responses in the lung. J Immunol 184: 2048–2056, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 14: 558–564, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103: 12481–12486, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang B, Xiao B, Liu Z, Li N, Zhu ED, Li BS, Xie QH, Zhuang Y, Zou QM, Mao XH. Identification of MyD88 as a novel target of miR-155, involved in negative regulation of Helicobacter pylori-induced inflammation. FEBS Lett 584: 1481–1486, 2010. [DOI] [PubMed] [Google Scholar]
- 58.Tashiro M, Ciborowski P, Klenk HD, Pulverer G, Rott R. Role of Staphylococcus protease in the development of influenza pneumonia. Nature 325: 536–537, 1987. [DOI] [PubMed] [Google Scholar]
- 59.Tashiro M, Ciborowski P, Reinacher M, Pulverer G, Klenk HD, Rott R. Synergistic role of staphylococcal proteases in the induction of influenza virus pathogenicity. Virology 157: 421–430, 1987. [DOI] [PubMed] [Google Scholar]
- 60.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science 316: 604–608, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289: 179–186, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Tili E, Croce CM, Michaille JJ. miR-155: on the crosstalk between inflammation and cancer. Int Rev Immunol 28: 264–284, 2009. [DOI] [PubMed] [Google Scholar]
- 63.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 179: 5082–5089, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. miR-155 regulates IFN-gamma production in natural killer cells. Blood 119: 3478–3485, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turner M, Vigorito E. Regulation of B- and T-cell differentiation by a single microRNA. Biochem Soc Trans 36: 531–533, 2008. [DOI] [PubMed] [Google Scholar]
- 66.van der Sluijs KF, van der Poll T, Lutter R, Juffermans NP, Schultz MJ. Bench-to-bedside review: bacterial pneumonia with influenza - pathogenesis and clinical implications. Crit Care 14: 219, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, Smith KG, Rada C, Enright AJ, Toellner KM, Maclennan IC, Turner M. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity 27: 847–859, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 282: 9358–9363, 2007. [DOI] [PubMed] [Google Scholar]
- 69.Yao R, Ma YL, Liang W, Li HH, Ma ZJ, Yu X, Liao YH. MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One 7: e46082, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang WJ, Sarawar S, Nguyen P, Daly K, Rehg JE, Doherty PC, Woodland DL, Blackman MA. Lethal synergism between influenza infection and staphylococcal enterotoxin B in mice. J Immunol 157: 5049–5060, 1996. [PubMed] [Google Scholar]