Abstract
Influenza causes an acute infection characterized by virus replication in respiratory epithelial cells. The severity of influenza and other respiratory diseases changes over the life course and during pregnancy in women, suggesting that sex steroid hormones, such as estrogens, may be involved. Using primary, differentiated human nasal epithelial cell (hNEC) cultures from adult male and female donors, we exposed cultures to the endogenous 17β-estradiol (E2) or select estrogen receptor modulators (SERMs) and then infected cultures with a seasonal influenza A virus (IAV) to determine whether estrogenic signaling could affect the outcome of IAV infection and whether these effects were sex dependent. Estradiol, raloxifene, and bisphenol A decreased IAV titers in hNECs from female, but not male, donors. The estrogenic decrease in viral titer was dependent on the genomic estrogen receptor-2 (ESR2) as neither genomic ESR1 nor nongenomic GPR30 was expressed in hNEC cultures and addition of the genomic ER antagonist ICI 182,780 reversed the antiviral effects of E2. Treatment of hNECs with E2 had no effect on interferon or chemokine secretion but significantly downregulated cell metabolic processes, including genes that encode for zinc finger proteins, many of which contain estrogen response elements in their promoters. These data provide novel insights into the cellular and molecular mechanisms of how natural and synthetic estrogens impact IAV infection in respiratory epithelial cells derived from humans.
Keywords: antiviral defenses, estradiol, respiratory disease, SERMs, zinc finger proteins
biological differences exist between males and females, yet these differences are often overlooked in experimental studies in immunology and infectious diseases (2). The recent policy announcement by the National Institutes of Health (NIH) that applicants consider balancing the sexes in preclinical, cell culture, and animal studies speaks to the importance of sex as a biological variable that can help predict the outcome of diseases (4, 19). Clinical observations reveal that respiratory diseases, such as asthma, chronic obstructive pulmonary disorder, and influenza are often more severe in women than men, being highly dependent on both age and hormonal status (3, 18). Influenza, in particular, is a major recurring cause of morbidity and mortality worldwide, affecting 10% of the population annually through epidemics, pandemics, and seasonal outbreaks. When age and sex are included in analyses of influenza pathogenesis, young adult women experience more severe outcomes compared with age-matched men (42). Young adult women were reportedly two to six times more likely to die from H5N1 or H7N9 avian influenza infection and during the initial wave of the 2009 H1N1 influenza pandemic (14, 51, 64). Influenza is considered an immune-mediated disease, in which the disease severity is largely determined by the strength of the host inflammatory response to infection (56). Murine models have been instrumental in characterizing sex differences in the outcome of influenza A virus (IAV) infection (26, 46, 47). These studies collectively illustrate that young adult female mice suffer a worse outcome from infection with IAV than their male counterparts. Sex differences in the outcome of IAV in mice are not caused by differences in virus replication in the lower respiratory tract (i.e., lungs) but rather are associated with an exacerbated proinflammatory cytokine and chemokine response in the lungs (26, 47). Regulating the balance between immune responses causing protection or pathology is integral for repair of pulmonary tissue and recovery from infection.
Murine models further reveal that sex differences in the outcome of respiratory diseases, including IAV, are mitigated in the absence of sex steroid hormones (46, 47), suggesting that the hormonal milieu plays a fundamental role in determining sex-specific differences in the outcome of infection. Endogenous sex steroid hormones, such as 17β-estradiol (E2), are immunomodulatory and regulate cellular immune responses to infection (54, 62). In mouse models of asthma, allergy, and IAV, females suffer a worse outcome from pulmonary diseases compared with males (30, 47). Among female rodents, endogenous changes in circulating E2 concentrations alter lung function and exogenous E2 treatment reduces pulmonary inflammation and disease symptoms (22, 30, 45, 65). Continuous E2 exposure, but not cyclical or ablated E2, in adult female mice prolongs survival following IAV infection (47). In women, sustained concentrations of E2 are associated with improved outcome of respiratory diseases, such as asthma. For example, administration of oral contraceptives containing E2 reduces allergic inflammation in the respiratory tract and asthma exacerbations associated with premenstrual asthma (22, 59).
Estrogens, primarily E2, regulate cellular function in diverse cell types, via binding to intracellular, genomic estrogen receptors (ERs), such ERα (ESR1) and ERβ (ESR2), or to membrane-associated, nongenomic ERs, such as the G protein-coupled estrogen receptor (GPER). These receptors are cell type specific and can activate gene expression directly by serving as ligand-dependent transcriptional factors (ESR1 and ESR2) or indirectly by altering signaling pathways, including ERK/MAPK, NF-κB, and STAT activity (10, 54). Both immune cells and nonimmune cells (e.g., respiratory epithelial cells) have intracellular ERs that regulate cellular functions, including inflammatory responses (10). Targeting ER activity might be therapeutic for diseases that are caused by excessive inflammation and that disproportionately affect females. Selective estrogen receptor modulators (SERMs) bind to ERs to cause conformational changes that alter engagement with coactivator and corepressor proteins to initiate either induction or suppression of target gene transcription (21, 38). A recent screen revealed antiviral properties of two SERMs against Ebola virus infection: clomiphene, which is used to treat infertility, and raloxifene, approved for treatment of osteoporosis and to decrease the risk of breast cancer in postmenopausal women (17). Environmental estrogens, including xenoestrogens, such as bisphenol A (BPA), also can interact with ERs to alter transcriptional activity (57), but the effects on cellular responses to viruses have not been evaluated. In this study, we used primary differentiated human nasal epithelial cell (hNEC) cultures isolated from healthy tissue of male and female donors (8, 20, 44) to study the effects of estrogenic compounds on the human cellular response to IAV infection. Nasal epithelial cells are the primary cell type infected with IAV, and these cultures allowed us to investigate IAV infection and pathogenesis based on the sex and hormonal milieu of the donor cultures.
MATERIALS AND METHODS
Madin-Darby canine kidney cells.
Madin-Darby canine kidney (MDCK) cells were propagated in Dulbecco's modified Eagle's low-phenol, high-glucose medium (DMEM; Invitrogen) containing 10% fetal bovine serum (FBS; Life Technologies), 100 U/ml penicillin (Life Technologies), 100 μg/ml streptomycin (Life Technologies), and 2 mM l-glutamine (Life Technologies). The cells were maintained at 37°C in a humidified environment with 5% CO2.
Virus.
The recombinant influenza A/Udorn/307/72 H3N2 virus (IAV) used in this study was described previously (41). The hemagglutinin (HA) protein contains amino acid changes (L226Q/S228G in the H3 numbering system) that allows for preferential binding to α2,3-linked sialic acid. Viral working stocks of were generated by infecting MDCK cells at a multiplicity of infection (MOI) of ∼0.01 infectious units per cell in low-phenol, high-glucose DMEM containing 0.25% bovine serum albumin (BSA; Life Technologies), acetylated trypsin from bovine pancreas (5 μg/ml; N-acetyl trypsin; Sigma), 100 U/ml penicillin, 100 μg/ml streptomycin, 6 mM l-glutamine, and 1 mM sodium pyruvate (Life Technologies), which we refer to as infection media. The infected cells were incubated at 37°C for 72 h, and infected cell supernatant was harvested, clarified by centrifugation, aliquoted, and stored at −80°C. Infectious virus titers were determined by 50% tissue culture infectious dose (TCID50) as described previously (33).
Chemicals.
Stock solutions of 17β-estradiol (E2; 1 mM; Sigma) were made in ethanol, while raloxifene (100 mM; Sigma), ospemifene (100 mM; Sigma), clomiphene citrate (100 mM; Sigma), ICI 182,780 (ICI; 2 mM; Tocris-Cookson), and BPA (44 mM; Sigma; provided by Dr. Jodi A. Flaws, University of Illinois at Urbana-Champaign) were made in dimethyl sulfoxide (DMSO; Sigma). All stock solutions were stored at −20°C.
hNEC cultures.
Primary hNECs were collected from nondiseased nasal mucosa of patients undergoing endoscopic sinonasal surgery for nonsinusitis indications, including dacryocystorhinostomy, approach to anterior skull base pathology, or removal of benign nasal masses. The cells were collected from male (n = 10) and female (n = 42) donors (age range 18–45 yr). The research protocol was approved through the Johns Hopkins Institutional Review Board, and all subjects gave signed informed consent. The cells were differentiated at an air-liquid interface in 24-well Falcon filter inserts (0.4-μM pore; 0.33 cm2; Becton Dickinson) coated with human type IV placental collagen (Sigma-Aldrich) as described previously (8, 20, 44).
Infection and treatment of hNEC cultures.
Before infection, the apical surface of hNEC cultures was washed with Dulbecco's PBS with calcium and magnesium (DPBS; Life Technologies). The cultures were infected via the apical chamber with a MOI of 0.1 TCID50/cell or mock infected at 32°C in 200 μl of infection media. After 2 h, the inoculums were aspirated, the apical surfaces washed twice with DPBS, and cells were incubated at 32°C. At the indicated hours postinfection (hpi), 200 μl of infection media were added to the apical surface, incubated at 32°C for five min, and then collected. All samples were stored at −80°C. At the indicated times pre- or postinfection, the basolateral media were replaced by media containing doses of vehicle control (EtOH or DMSO as appropriate), E2 (0.1, 1.0, or 10 nM), BPA (44 μM), raloxifene (50 μM), ospemifene (50 μM), clomiphene citrate (50 μM), or ICI (100 nM). Basolateral media were collected and replaced with media containing fresh vehicle or compound at 48 and 96 hpi.
TCID50 assay.
MDCK cells were plated in a 96-well plate and cultured for 72 h in growth DMEM until 90–100% confluent. The cells were washed twice with DPBS and then covered with 180 μl of infection media. Serial dilutions (10−1 to 10−8) of hNEC apical samples at each time point were made in separate, 96-well plates by adding 20 μl of each apical sample to 180 μl of infection DMEM and then serially diluted 10-fold. Twenty microliters of each dilution were then added to the MDCK plates in replicates of six, resulting in final dilutions of each sample ranging from 10−2 to 10−9. The infection proceeded for 7 days at 32°C, and then the cells were fixed with 4% formaldehyde and stained with napthol blue-black solution. The cytopathic effect was scored visually and the Reed and Muench calculation was used to determine the titer of infectious virus at each time point.
Multiplex chemokine assay analysis.
The Meso Scale Discovery (MSD) multiplex assay system was used to measure chemokines collected from apical samples of hNECs after infection. In each well, the Chemokine Panel 1 kit quantitatively determined the concentration of eight C-C ligand motif (CCL2, CCL3, CCL4, CCL11, CCL13, CCL17, CCL22, and CCL26) and two C-X-C ligand motif (CXCL8 and CXCL10) chemokines, according to the manufacturer's protocol. All samples were run in duplicate. MSD plates were read on the MSD SECTOR Imager 2400 at the Becton Dickinson Core Facility at the Johns Hopkins Bloomberg School of Public Health and analyzed on the accompanying software (MSD Discovery Workbench version 4).
Enzyme-linked immunosorbent assay.
The PBL Assay Science DIY Human IFN Lambda 1/2/3 (IL-29/28A/28B) enzyme-linked immunosorbent assay (ELISA) was used to measure levels of interferon-λ (IFNλ) collected from apical samples of hNECs after infection. All samples were run in duplicate and read on the FilterMax F3 Multi-Mode Microplate Reader (Molecular Devices). The data were analyzed with the accompanying software (SoftMax Pro Data Acquisition and Analysis Software).
Real-time RT-PCR.
At the indicated times postinfection, hNEC cultures were treated with TRIzol Reagent (Life Technologies) and total RNA was extracted using the PureLink RNA Mini Kit (Ambion by Life Technologies) according to the manufacturer's protocol. Reverse transcriptase generation of complementary DNA (cDNA) was performed with 0.5 μg of total RNA, primed with a blend of random hexamer and oligo (dT)s against the RNA library, using an iScript RT Kit (Bio-Rad Laboratories). Real-time PCR (qPCR) using Ssofast qPCR Supermix with EvaGreen (Bio-Rad Laboratories) was conducted using the StepOnePlus Real-Time PCR System (Life Technologies) and accompanying software (StepOne Software) according to the manufacturer's instructions. A dilution curve was generated from a compilation of the samples and qPCR analysis was performed using forward and reverse primers for estrogen receptor-α (ESR1), estrogen receptor-β (ESR2), G protein-coupled estrogen receptor 1 (GPER), and the zinc finger protein genes ZNF91, ZMYM6, and ZFAND4. An initial incubation of 95°C for 10 min was followed by 94°C for 10 s, annealing at 60°C for 10 s, and extension at 72°C for 10 s, for 40 cycles, followed by a final extension at 72°C for 10 min. A melting curve was generated at 55–90°C to monitor the generation of a single product. 18S RNA (RNA18S) was used as a reference gene for each sample. Final values of relative gene expression were calculated and expressed as the ratio normalized to RNA18S. All analyses were performed in duplicate or triplicate.
Western blot analysis.
At the indicated times postinfection cells were lysed with 1% sodium dodecyl sulfate (SDS; Fisher Scientific) in PBS and total protein was measured using the Pierce BCA Protein Assay Kit (Life Technologies) according to the manufacturer's instructions. BSA dilutions (Pierce BCA Protein Assay Kit) were used as the standards. For Western blot analysis, 7–30 μg of total protein from each sample were separated on 4–15% Mini-PROTEAN TGX Precast Gels (Bio-Rad) and transferred to a polyvinylidene fluoride Immunobilon-FL membrane (PVDF; Millipore). The membranes were blocked with PBS containing 5% dry milk powder and 0.05% Tween-20 (Sigma). Wash buffer contained PBS with 0.05% Tween-20. Membranes were blocked for 30 min at room temperature (RT), incubated for 1.5 h at RT with primary antibody, washed three times each for 5 min, incubated for 1 h at RT with secondary antibody, and then washed three times each for 5 min. Primary and secondary antibodies were diluted in blocking buffer. The primary antibodies used were mouse anti-β-actin (AC-15 MAb; 1:5000 dilution; Abcam-ab6276), rabbit anti-ESR2 (PAb; 1:100 dilution; Thermo- PA1-310B), and rabbit anti-ESR1 (1:500 dilution; Genetex-GTX62423). The Alexa Fluor 647-conjugated secondary antibodies used were a goat anti-mouse immunoglobulin G (IgG) and a donkey anti-rabbit IgG (both at a 1:1,000 dilution; Invitrogen). For visualization, membranes were imaged using a FluorChemQ phosphorimager (ProteinSimple) and signal intensities quantified by ImageJ analyses (NIH).
Identification of putative estrogen response elements.
The Dragon ERE Finder version 3.0 (http://datam.i2r.a-star.edu.sg/ereV3/) was used to identify putative estrogen response elements (EREs) in the promoters of select genes (1). The NCBI BLAST program was used search for the annotated start sites of genes of interest against ∼11,000 human promoters. These sequences were then used to pinpoint the location of the putative ERE pattern by expanding them to cover an extended region of [−5,000, +3,000] relative to genes of interest start site. We used the recommended search sensitivity of 83%.
Microarray analysis.
Trizol reagent and the PureLink RNA Mini kit (Ambion/Life Technologies) were used for extraction and purification of RNA. Cells were processed according to the manufacturer's protocol. Following elution of purified RNA from the PureLink columns with Nuclease-free water, quantitation was performed using a NanoDrop spectrophotometer and quality assessment was determined by RNA Nano LabChip analysis on an Agilent BioAnalyzer 2100 or RNA Screen Tape on an Agilent TapeStation 2200. One hundred nanograms of total RNA were processed for hybridization to Affymetrix Human Gene ST 2.0 microarrays using the Affymetrix GeneChip WT PLUS Reagent Kit according to the manufacturer's recommended protocol. The signal amplification protocol for washing and staining of eukaryotic targets was performed in an automated fluidics station (Affymetrix FS450) using Affymetrix protocol FS450_0002. The arrays were scanned in the GCS3000 laser scanner with autoloader and 3G upgrade (Affymetrix). Quality assessment of hybridizations and scans was performed with Expression Console software (Affymetrix).
Statistical analyses.
For analysis of virus titers, cytokine/chemokine levels, and real-time PCR data, comparisons among treatment groups were performed using multivariate analysis of variance (MANOVA; for repeat measures) followed by planned comparisons or one-way ANOVA with appropriate post hoc tests using SigmaPlot 12 (Systat Software). For microarray analyses, Partek Genomics Suite version 6.6 was used, with the robust multichip average (RMA) algorithm used for background correction, normalization, and summarization of probes. ANOVAs with linear contrasts were performed to generate relative fold change, P values, and gene lists. Statistical significance was assigned at P ≤ 0.05.
RESULTS
Pretreatment with E2 reduces viral titers in hNECs from female, but not male, donors.
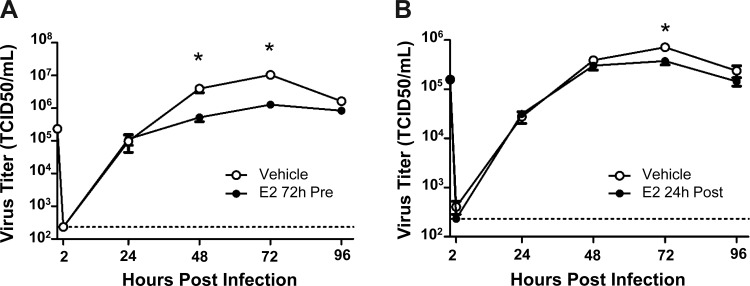
To test the hypothesis that E2 has antiviral effects on IAV infection, hNEC cultures from female and male donors were pretreated in the basolateral media with E2 (0.1, 1, or 10 nM) or vehicle for 24 h before infection with IAV. During peak virus replication (i.e., 72–96 hpi), E2 pretreatment of hNEC cultures from female (Fig. 1A) but not male (Fig. 1B) donors significantly reduced virus titers compared with vehicle-treated hNEC cultures (P < 0.05). We interpret these data to suggest that E2 has a sex-specific antiviral activity in hNEC cultures.
Fig. 1.
17β-estradiol (E2) inhibits infectious virus production in human nasal epithelial cell (hNEC) from female, but not male, donors. hNEC cultures from female (A) or male (B) donors were pretreated with E2 (0.1, 1, and 10 nM) or vehicle for 24 h in the basolateral media and then infected with influenza A virus [IAV; multiplicity of infection (MOI) = 0.1 50% tissue culture infectious dose (TCID50)/cell] via the apical membrane. Supernatants were collected every 24 h postinfection (hpi) and virus titers were analyzed by TCID50 assay. The limit of detection is indicated by a dotted line. The data represent means ± SE. *P < 0.05, E2-treated cultures were significantly different from sex-matched vehicle controls based on a multivariate analysis of variance (MANOVA) followed by planned comparisons, with n = 15 hNEC wells per treatment group.
To determine whether the duration of E2 exposure could shift the timing of the antiviral effects of E2, hNEC cultures from female donors were exposed to E2 72 h before or 24 h after infection with IAV. Pretreatment of hNEC cultures with E2 for 72 h resulted in a significant reduction in virus titers 48 and 72 hpi compared with vehicle-treated cultures (P < 0.05; Fig. 2A), while treatment of hNEC cultures 24 hpi significantly reduced virus titers at 72 hpi (P < 0.05; Fig. 2B). Taken together, these data suggest that the antiviral activity of E2 in hNEC cultures from female donors can be induced with treatments over an extended timeframe.
Fig. 2.
Extended E2 pretreatment and E2 treatment postinfection inhibits infectious virus production in hNECs from female donors. hNEC cultures from females were pretreated with E2 (10 nM) for 72 h before IAV infection (A) or treated with E2 (10 nM) 24 h after infection with IAV (B) (MOI = 0.1 TCID50/cell). Supernatants were collected every 24 hpi and virus titers were analyzed by TCID50 assay. The limit of detection is indicated by a dotted line. The data represent means ± SE. *P < 0.05, E2-treated cultures were significantly different from vehicle controls based on a MANOVA followed by planned comparisons, with n = 12 hNEC wells per treatment group.
E2 does not affect production of cytokines in response to IAV infection in female donors.
Respiratory epithelial cells are significant producers of cytokines, chemokines, and type III interferons in response to IAV infection (36, 49). To test the hypothesis that E2 reduces IAV titers by altering host immune responses to infection, hNEC cultures from female donors were pretreated with E2 (0.1, 1, and 10 nM) or vehicle for 24 h before infection, and the apical supernatants were analyzed at 72 hpi (i.e., during peak virus replication) for production of a panel of chemokines and type III interferons. Concentrations of IFNλ and all chemokines measured, with the exception of CCL13 and CCL26, were significantly increased in IAV-infected hNEC cultures compared with mock infected cultures derived from females (P < 0.05 in each case; Table 1). In contrast to the immunomodulatory effects of E2 reported in reproductive epithelial cells (11, 50, 52), E2 did not significantly alter secretion of any chemokine or IFNλ in either the mock- or IAV-infected hNECs compared with the vehicle-treated hNECs. Therefore, the antiviral effects of E2 on IAV infection do not involve altered apical production of IFNλ or chemokines.
Table 1.
Effects of E2 on chemokine and interferon production in hNECs from females
| Mock |
IAV |
|||||||
|---|---|---|---|---|---|---|---|---|
| E2, nM |
E2, nM |
|||||||
| Protein, pg/ml | Vehicle | 0.1 | 1 | 10 | Vehicle | 0.1 | 1 | 10 |
| CCL11 | 13.1 ± 2.64 | 19.7 ± 1.83 | 15.9 ± 2.58 | 15.6 ± 1.39 | 103 ± 10.5* | 116 ± 17.5* | 129 ± 16.9* | 102 ± 9.10* |
| CCL26 | 5.71 ± 0.51 | 6.56 ± 0.92 | 6.31 ± 0.68 | 5.82 ± 0.72 | 2.27 ± 0.51 | 1.87 ± 0.62 | 3.51 ± 0.85 | 2.43 ± 0.84 |
| CXCL8 | 1340 ± 302 | 715 ± 78.0 | 1,100 ± 326 | 824 ± 105 | 2,920 ± 386* | 2,940 ± 414* | 2,840 ± 479* | 3,070 ± 466* |
| CXCL10 | 2.50 ± 0.56 | 1.88 ± 0.26 | 9.46 ± 3.65 | 2.50 ± 0.42 | 14,100 ± 2,600* | 14,900 ± 3,080* | 18,400 ± 3,870* | 15,100 ± 3,010* |
| CCL2 | 0.32 ± 0.09 | 0.38 ± 0.08 | 0.17 ± 0.05 | 0.32 ± 0.12 | 4.82 ± 0.54* | 5.16 ± 0.63* | 4.22 ± 0.53* | 5.32 ± 0.61* |
| CCL13 | 14.7 ± 3.38 | 12.4 ± 1.89 | 13.3 ± 1.39 | 11.7 ± 2.78 | 18.5 ± 3.42 | 13.2 ± 1.47 | 14.3 ± 2.88 | 16.0 ± 1.82 |
| CCL22 | 17.5 ± 2.59 | 15.7 ± 4.79 | 17.8 ± 2.14 | 16.9 ± 2.76 | 115 ± 18.2* | 164 ± 23.8* | 164 ± 26.7* | 110 ± 16.5* |
| CCL3 | 21.1 ± 3.09 | 28.4 ± 2.56 | 27.8 ± 2.27 | 21.3 ± 2.07 | 76.4 ± 6.09* | 137 ± 51.3* | 86.4 ± 7.66* | 83.5 ± 6.80* |
| CCL4 | 6.73 ± 0.70 | 6.07 ± 0.81 | 7.04 ± 0.78 | 5.16 ± 0.57 | 42.4 ± 4.69* | 48.2 ± 6.36* | 44.2 ± 5.06* | 45.2 ± 4.52* |
| CCL17 | 4.31 ± 0.30 | 3.93 ± 0.32 | 4.00 ± 0.25 | 4.36 ± 0.25 | 23.1 ± 2.41* | 32.1 ± 5.61* | 25.1 ± 3.69* | 23.1 ± 2.10* |
| IFNλ | ND | ND | ND | ND | 20,100 ± 7540* | 14,200 ± 4,060* | 14,800 ± 4,960* | 16,800 ± 6,850* |
All data are presented as means ± SE.
E2; 17β-estradiol; hNECs, human nasal epithelial cells; ND, not determined.
P ≤ 0.001, influenza A virus (IAV) > mock within treatment group based on two-way ANOVA.
The antiviral effects of E2 require intracellular ESR2 signaling in hNECs from female donors.
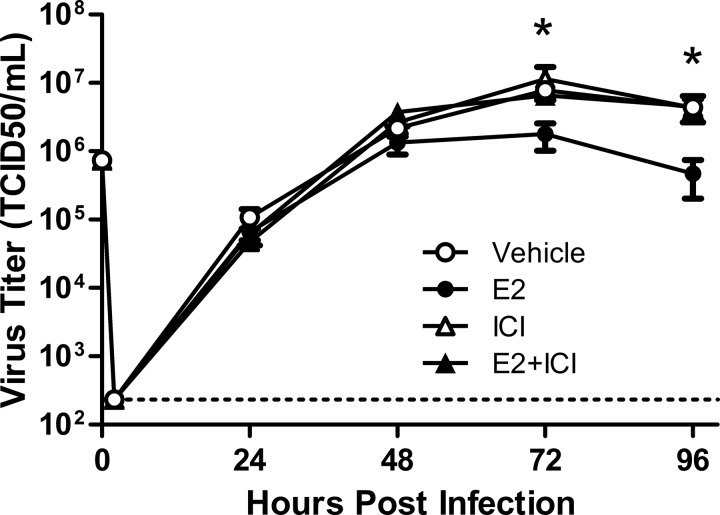
The ER family is comprised of many ER and ER-like proteins, including the intracellular genomic E2 receptors ESR1 and ESR2 and the nongenomic G protein-coupled receptors (e.g., GPER). To test the hypothesis that the antiviral effects of E2 were mediated by signaling through genomic rather than nongenomic receptors, we pretreated hNEC cultures from females with E2 (10 nM), the genomic ER blocker ICI 182,780 (100 nM), E2 + ICI, or vehicle for 24 h before inoculation with IAV. Pretreatment of hNEC cultures with E2 significantly reduced virus titers at 72 and 96 hpi compared with vehicle-treated cells (P < 0.05; Fig. 3), but the addition of ICI with E2 reversed the antiviral effects of E2 resulting in virus titers that were indistinguishable from vehicle-treated cells (Fig. 3). These data suggest that the antiviral effects of E2 are mediated by genomic signaling through intracellular ERs.
Fig. 3.
The estrogen receptor antagonist ICI 182,780 reverses the effects of E2 on virus titers in hNECs from females. hNEC cultures from female donors were pretreated with E2 (10 nM), ICI (100 nM), E2+ ICI, or vehicle for 24 h in the basolateral media and then infected with IAV (MOI = 0.1 TCID50/cell) via the apical membrane. Supernatants were collected every 24 hpi and virus titers were analyzed by TCID50 assay. The limit of detection is indicated by a dotted line. The data represent means ± SE. *P < 0.05, E2-treated cultures were significantly different from vehicle controls based on a MANOVA followed by planned comparisons, with n = 12 hNEC wells per treatment group.
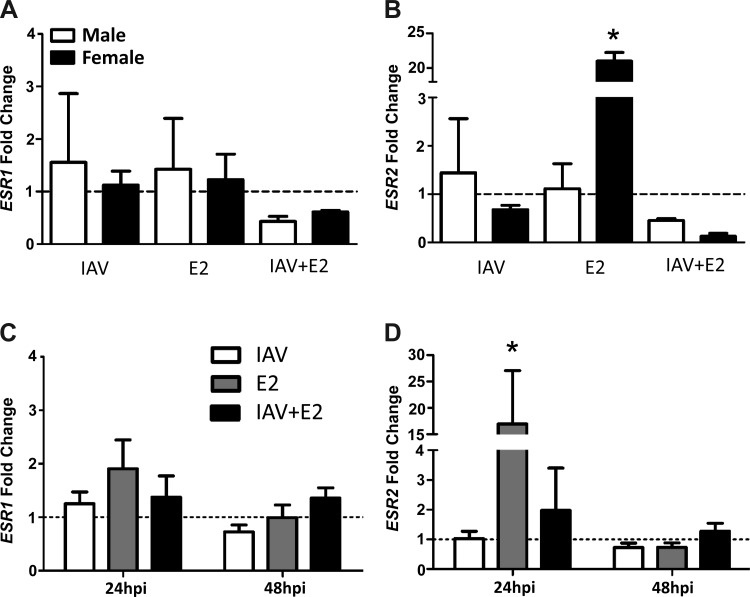
To determine whether hNEC cultures expressed genomic and nongenomic ERs, we measured transcripts of ESR1, ESR2, and GPER in hNECs derived from male or female donors treated with either vehicle or E2 before mock or IAV infection. At 24 hpi, transcripts of GPER were not detected in hNEC cultures from male or female donors (data not shown). Although ESR1 was expressed in hNECs derived from both male and female donors, the expression level was low and was not altered in the presence of E2 (Fig. 4A). In contrast, ESR2 was expressed to higher levels in hNECs derived from female than male donors following exposure to E2 (P < 0.05; Fig. 4B). To evaluate the kinetics of genomic ER expression, transcripts of ESR1 and ESR2 were measured 24 and 48 hpi in hNEC cultures from female donors. Detection of ESR1 mRNA remained low at both 24 and 48 hpi and was not altered by treatment with E2 (Fig. 4C). In contrast, treatment of hNECs from females with E2 significantly increased the expression of ESR2 at 24 hpi but not 48 hpi (P < 0.05; Fig. 4D).
Fig. 4.
E2 stimulates the expression of ESR2, but not ESR1, mRNA in hNECs from female donors only. hNEC cultures from female and male donors were pretreated with E2 (10 nM) or vehicle for 24 h in the basolateral media and infected with IAV (MOI = 0.1 TCID50/cell) via the apical membrane, and after 24 h, the cells were collected for analysis of ESR1 (A) and ESR2 (B) expression. In hNECs from females only, the kinetics of mRNA expression of ESR1 (C) and ESR2 (D) was analyzed in cultures collected at 24 and 48 hpi. The fold change of ESR1 and ESR2 mRNA was determined by the ΔΔCt method using 18sRNA as the reference gene. The data represent means ± SE with the stippled line indicating the normalized expression level of genes in vehicle-treated, mock-infected cultures. *P < 0.05, significant difference from the mock vehicle control by one-way ANOVA and Tukey post hoc tests, with n = 3–5 hNEC wells per treatment group per sex.
To confirm that hNEC cultures derived from female donors expressed genomic ER proteins, cell lysates at 24 hpi were subjected to Western Blot analysis. At 24 hpi, ESR2, but not ESR1, was detected in hNECs derived from females (Fig. 5, A and B). Both proteins were detected in MCF-7 cells, indicating the lack of ESR1 expression in hNEC cultures was not due to an inability of the antibodies to detect the protein. Taken together, these data suggest that the activation of ESR2 by E2 in female, but not male, hNECs mediate the sex-specific antiviral effects of E2.
Fig. 5.
hNEC cultures derived from female donors express ESR2, but not ESR1, protein. hNEC cultures from female donors were pretreated with E2 (10 nM) or vehicle for 24 h in the basolateral media and then infected with IAV (MOI = 0.1 TCID50/cell) via the apical membrane. After 24 h, the cells were lysed and subjected to Western blot analysis for ESR1, ESR2, and ACTB measurements (A). Protein isolated from MCF-7 cells was used as a positive control for estrogen receptor expression. Quantification of ESR2 relative to ACTB was quantified by ImageJ analyses (B).
E2 reduces metabolic processes in hNEC cultures from females following IAV infection.
To uncover potential pathways affected by E2-ESR2 signaling in hNEC cultures derived from females, we conducted microarray analyses on RNA isolated at 24 and 48 hpi. To determine the selective effects of E2 on gene expression during IAV infection, samples from IAV-infected cultures were normalized to mock-infected cultures from the same hormone treatment condition. Overall, significantly more genes were altered by E2 treatment at 24 compared with 48 hpi. Using a fold change of 1.3 and P < 0.05, we determined that 666 transcripts were differentially expressed in hNECs treated with E2 compared with vehicle at 24 hpi and only 234 transcripts were differentially expressed at 48 hpi (Fig. 6A). There were only seven overlapping genes that were differentially expressed in hNECs treated with E2 compared with vehicle at both 24 and 48 hpi (Fig. 6A). Gene Ontology Enrichment revealed that the biological functions with the greatest number of differentially expressed genes in hNECs treated with E2 compared with vehicle at 24 hpi included organelle organization and cellular metabolic processes, both of which received significant enrichment scores (i.e., > a score of 3; Fig. 6B). Further analyses revealed that a family of zinc finger protein (ZFP) genes was the largest cluster of genes differentially expressed at 24 hpi in response to E2 treatment of hNEC cultures (Fig. 6C).
Fig. 6.
E2 treatment reduces metabolic processes in IAV-infected hNECs from females. hNEC cultures from female donors were pretreated with E2 (10 nM) or vehicle for 24 h in the basolateral media and then infected with IAV (MOI = 0.1 TCID50/cell) via the apical membrane. At 24 and 48 hpi, cells were harvested, and RNA was isolated and spotted on Affymetrix Human GeneST2.0 arrays. Data from infected hNECs were normalized to uninfected hNECs within the same treatment group and ANOVAs in Partek Genomics Suite version 6.0 were used to determine significant differences between E2- and vehicle-treated, IAV-infected hNECs. The Venn diagram (A) illustrates the number of differentially expressed genes at 24 and 48 hpi. Gene ontology (B) was used to group genes differentially expressed between E2- and vehicle-treated, IAV-infected hNECs at 24 hpi into functional hierarchies based on enrichment score, with scores >3 indicating significant differences. C: heat map was created using unbiased hierarchical clustering to represent the largest family of differentially expressed genes [i.e., genes that encode zinc finger proteins (ZFP)] between E2- and vehicle-treated, IAV-infected hNEC cultures at 24 hpi.
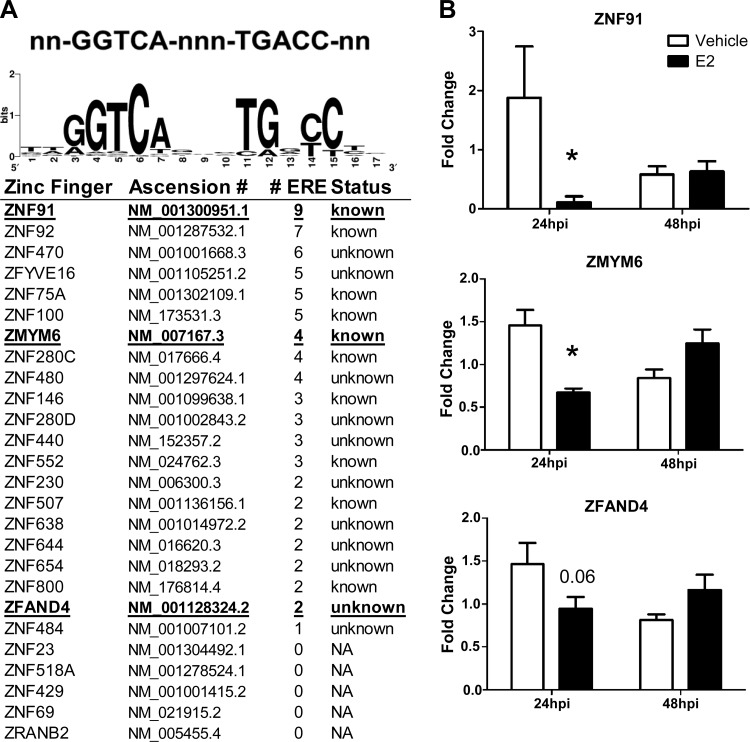
To determine whether E2 could transcriptionally regulate the activity of these zinc finger genes, we used Dragon ERE Finder version 3.0 (http://datam.i2r.a-star.edu.sg/ereV3/) to identify putative EREs in the promoter regions of ZFP genes. Of the 38 ZFP genes that were downregulated during IAV infection by E2, 21 had at least one putative or known EREs present in their promoters (Fig. 7A). Of the 21 ZFP genes that had putative EREs in their promoters, 20 of these genes had two or more putative EREs, 11 of which have been experimentally confirmed to bind to ERs (1). ZFPs play diverse roles in regulating cellular function and responses to damage; their role in IAV infection, however, is largely unknown. Of the 38 ZFP genes that were downregulated in the presence of E2 during IAV infection, ZNF91, ZMYM6, and ZFAND4 were of particular interest because of the large number of EREs present in their promoters (i.e., ZNF91 and ZMYM6) or because they belong to a family of ZFPs that can have antiviral activity (i.e., ZFAND4) (28). The effects of E2 on the expression of ZNF91, ZMYM6, and ZFAND4 transcripts were validated using real time RT-PCR, which confirmed that E2 significantly downregulated ZNF91 and ZMYM6 at 24 hpi but not 48 hpi (P < 0.05; Fig. 7B). While ZFAND4 expression was reduced at 24 hpi, this did not achieve statistical significance (P = 0.057; Fig. 7B). Taken together, these data suggest that E2 has the potential to directly regulate transcription of ZFP genes, which may provide a novel mechanism for reducing late stage virus replication.
Fig. 7.
Identification of putative estrogen response elements (EREs) in ZFP gene promoters. A: Dragon ERE Finder version 3.0 (http://datam.i2r.a-star.edu.sg/ereV3/) was used to identify putative EREs in the promoters of ZFP genes. The WebLogo sequence generator was used to develop a graphical representation of the ERE nucleic acid sequence conservation (GGTCA-nnn-TGACC) among the identified ZFP genes (6). B: cells were collected for analysis by real time RT-PCR to validate the ZNF91, ZMYM6, and ZFAND4 mRNA expression levels. Fold change of the target genes was determined by the ΔΔCt method using 18sRNA as the reference gene. The data represent means ± SE. *P < 0.05, significant differences compared with vehicle based on Student's t-tests at each time point, with n = 4–5 hNEC wells per treatment group.
Estrogenic chemicals that selectively bind to ESR2 reduce IAV titers in hNECs from females.
Selective estrogen receptor modulators are a class of FDA-approved therapeutic estrogenic chemicals, while BPA is a xenoestrogen forming the backbone of various plastic and consumer products. These estrogenic chemicals have differential affinity for ERs in a variety of tissues (21). To determine if compounds that bind genomic ERs could decrease IAV titers, similar to E2, hNEC cultures from females were pretreated with raloxifene, clomiphene citrate, ospemifene, BPA, or vehicle 24 h before infection with IAV. Raloxifene, but not clomiphene citrate or ospemifene, significantly reduced viral titers at 72 and 96 hpi compared with vehicle-treated cultures (P < 0.05; Fig. 8A). Treatment of hNECs with BPA also significantly reduced viral titer at 48 and 72 hpi compared with vehicle-treated hNECs (P < 0.05; Fig. 8B). Both raloxifene and BPA have stronger binding affinities for ESR2 than ESR1 (24), suggesting that chemicals that selectively bind ESR2 have antiviral activity against IAV infection of respiratory epithelial cells from females.
Fig. 8.
Select estrogenic compounds with affinity for ESR2 reduce virus titers in hNECs from female donors. hNEC cultures from female donors were pretreated with vehicle and either selective estrogen receptor modulators (A), including clomiphene citrate, ospemifene, and raloxifene or bisphenol (BPA) (B) for 24 h in the basolateral media and infected with IAV (MOI = 0.1 TCID50/cell) via the apical membrane. Supernatants were collected every 24 hpi and virus titers were analyzed by TCID50. The limit of detection is indicated by a dotted line. The data represent means ± SE. *P < 0.05, significant difference between vehicle-treated hNECs and raloxifene-treated (A) or BPA-treated (B) based on one-way ANOVAs and Tukey post hoc tests, with n = 9 hNEC wells per treatment group.
DISCUSSION
In this study, E2 and select estrogenic chemicals had antiviral effects against IAV infection. These estrogenic compounds reduced peak viral titer, which did not involve changes in the secretion of chemokines or IFNλ, but rather was associated with reduced metabolic processes. The effects of estrogens were specific for hNEC cultures from female, but not male, donors. The antiviral effects were most likely mediated by signaling through ESR2 as ESR2, but not ESR1 or GPER, was expressed in hNEC cultures and the protective effects of E2 were eliminated in the presence of the genomic ER antagonist ICI 182,780.
Antiviral effects of E2 and estrogenic chemicals have been reported previously against viruses including HIV, hepatitis C virus (HCV), Ebola virus, and human cytomegalovirus (HCMV). Estradiol can prevent HIV transcription by blocking HIV promoter activity in peripheral blood mononuclear cells and preventing HIV entry in CD4+ T cell and macrophages (48, 55). Estradiol also prevents the production of infectious HCV particles (12). Certain FDA-approved SERMs inhibit Ebola and other Marburg viruses in vivo and in vitro (17). Furthermore, SERMs, including clomiphene and raloxifene, inhibit HCV infection at multiple stages of the virus life cycle and, raloxifene, in particular, acts as an adjuvant to improve antiviral HCV therapies in postmenopausal women (9, 37). While E2 has antiviral activities against a wide range of viruses, it is still not clear whether the molecular mechanisms involved are conserved across virus families or result from changes in different cellular pathways resulting from the broad effects of E2 on cellular gene expression. Implications of these findings are far reaching as a significantly greater proportion of the world population is exposed to IAV and suffer from influenza annually than Ebola, HCV, HCMV, or HIV (63).
The inhibition of IAV infectious virus production in E2 or SERMs treated hNECs from female donors only occurred at late times after low MOI infection, with the magnitude of the antiviral effects being greater when cells were treated before rather than after the initiation of infection. Because the inhibition of virus titer occurs at a time when the infection in the hNECs is no longer synchronized among the cells in the cultures, the E2-induced reduction in titer could be resulting from E2 effects at multiple stages of the virus life cycle (i.e., entry, replication, reinfection). Therefore, we hypothesized that there may be some combination of host response and virus infection occurring synergistically to impair virus replication at later time points of infection.
Our microarray data indicate that E2 significantly alters the expression of genes involved in metabolic processes. Within the differentially regulated genes comprising metabolic processes, the largest family of E2-downregulated genes in IAV-infected hNECs from females was the one that encodes for ZFPs. Very little is known about the function of many of these ZFPs, let alone their potential roles in the IAV life cycle. In general, ZFPs are transcriptional regulators that can induce or repress host gene transcription (25). Because these ZFPs are all similarly downregulated in response to E2 treatment and IAV infection and expressed EREs in their promoters, this may point to a common transcription factor or activation pathway responsible for the antiviral effects of E2. There is a growing appreciation of the importance of various metabolic, cell cycle, and lipid metabolism pathways for productive IAV infection (60), and downregulation of these ZFPs may be exerting an antiviral activity by altering those pathways.
Both ESR1 and ESR2 are present in the upper and lower respiratory tract and are required for the development and function of the lung but exhibit distinct cell-type-specific expression patterns (5, 15, 29). ESR2 has been found in bronchial epithelial cells and alveoli, whereas ESR1 is mostly limited to alveolar macrophages and endothelial blood vessel cells, with modest expression in bronchial epithelial cells (15, 29, 40, 58). In primary human bronchial epithelial cells (HBECs) and HBEC cell lines from males and females, the amount of ESR2 is almost doubled compared with ESR1 (15). In the nasal mucosa, ESR2 is also the dominant ER present and, in some studies, the only ER detectable (34, 43, 53), which is consistent with our data. Similar to our results, in lung adenocarcinoma cells, only the ERs in cells from females respond to E2 and other ER ligands (7). Although no studies have investigated the ESR1:ESR2 relationship in the respiratory tract, in mammary, uterine, and prostate tissue, where both subtypes of ER are expressed, ESR2 is antagonistic to ESR1, inhibiting ESR1-mediated gene expression (13, 31). The expression of ESR2 has also been shown to alter the recruitment of ESR1 transcriptional cofactors and increase the degradation of ESR1, suggesting that ESR2 can mediate or dominate ESR1 signaling in the same cell (31, 32), which may explain why low levels of ESR1 mRNA, but not ESR1 protein, is detectable in hNECs.
ESR1 and ESR2 have similar DNA binding homology but only share 57% homology in their ligand binding domains, contributing to differences in transcriptional activities and tissue-specific cellular responses triggered through ligand-dependent ER activation (27). For example, E2, through ESR1, the dominant receptor in the female reproductive tract, decreases the production of human β-defensin 2 (HBD2) and elafin in the vaginal epithelium but increases HBD2 and elafin in the uterine epithelium (61). ESR2 is required for overall lung function, whereas ESR1 is required to control inflammation in the lung (35, 58). In mice, signaling through ESR1, but not ESR2, reduces pulmonary concentrations of proinflammatory cytokines and chemokines in the lungs and improves the outcome of influenza in female mice (47). In addition to the tissue type, the biological effects of E2 are contingent on the concentration of E2. Physiological levels of E2, signaling through ESR1, promote proinflammatory type 1 IFN production via Toll-like receptor stimulation and induction type 1 IFN genes (23). Conversely, high E2 levels consistent with those found before ovulation and during pregnancy are protective against excessive inflammatory responses (54). In our study, physiologically relevant levels of E2 had no effect on the induction of chemokines or IFNλ in cells from the upper respiratory tract. This may reflect a fundamental difference between signaling through ESR2 in upper respiratory tract epithelial cells and signaling through ESR1 in immune cells in the lower respiratory tract and reproductive tract. Future studies will need to compare ER signaling in mouse and human epithelial and immune cells in the upper and lower respiratory tract to definitively resolve the differences in how E2 protects against IAV in both hNECs and mice. Collectively our data illustrate that cell type-specific differences in ER expression result in differential modes of protection.
In the current study, of the SERMs tested, only raloxifene and BPA significantly decreased IAV titer in a manner similar to E2. Raloxifene can bind to both ESR1 and ESR2 but has a higher affinity for ESR2 (16). Furthermore, raloxifene binding to ESR2 results in an increase in ESR2 reporter gene expression but minimal expression after binding to ESR1 (39). BPA can have varying affinity for ERs depending on the cell or tissue in which it is measured (24). Ospemifene can bind both ERs with similar affinity, and, while the affinities of clomiphene citrate for ESR1 and ESR2 are unknown, clomiphene citrate is a well-documented ESR2 antagonist, inhibiting ESR2-mediated cellular events. We hypothesize that ospemifene and clomiphene citrate did not inhibit virus replication because they do not signal primarily through ESR2 (21). The data from the present study implicate signaling through ESR2 as being critical for the antiviral effects of E2 in hNEC cultures from female donors.
After the Women's Health Initiative raised concerns about estrogen replacement therapy, the use of SERMs as safer alternatives has greatly increased in the United States (21). Repurposing SERMs as antivirals could be a beneficial and a novel therapeutic for the many women currently prescribed SERMs for other diseases. The expression of ERs in the respiratory tract implies that E2 and SERMs can affect the function of tissues and organs outside of the reproductive tract. Understanding these effects will help shed light on additional novel functions of E2 in pulmonary cellular physiology.
GRANTS
This work was funded by the Center for Alternatives to Animal Testing Grant 2013-09 (to S. L. Klein) and National Institutes of Health Grants R01-AI-097417 and HHSN272201400007C (to A. Pekosz), R01-AI-72502 (to A. P. Lane), and T32-AI-007417 (to J. Peretz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.P., A.P., and S.L.K. conception and design of research; J.P. performed experiments; J.P. analyzed data; J.P., A.P., A.P.L., and S.L.K. interpreted results of experiments; J.P. prepared figures; J.P. and S.L.K. drafted manuscript; J.P., A.P., A.P.L., and S.L.K. edited and revised manuscript; J.P., A.P., A.P.L., and S.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Klein and Pekosz laboratory members for insightful discussions about these data. We also thank Anne Jedlicka and Amanda Dziedzic of the Johns Hopkins Bloomberg School of Public Health Genomic Analysis and Sequencing Core for assistance with microarray analyses.
REFERENCES
- 1.Bajic VB, Tan SL, Chong A, Tang S, Strom A, Gustafsson JA, Lin CY, Liu ET. Dragon ERE Finder version 2: a tool for accurate detection and analysis of estrogen response elements in vertebrate genomes. Nucleic Acids Res 31: 3605–3607, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35: 565–572, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casimir GJ, Lefevre N, Corazza F, Duchateau J. Sex and inflammation in respiratory diseases: a clinical viewpoint. Biol Sex Differ 4: 16, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 509: 282–283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology 138: 4613–4621, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res 14: 1188–1190, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dougherty SM, Mazhawidza W, Bohn AR, Robinson KA, Mattingly KA, Blankenship KA, Huff MO, McGregor WG, Klinge CM. Gender difference in the activity but not expression of estrogen receptors alpha and beta in human lung adenocarcinoma cells. Endocr Relat Cancer 13: 113–134, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer WA, King LS 2nd, Lane AP, Pekosz A. Restricted replication of the live attenuated influenza A virus vaccine during infection of primary differentiated human nasal epithelial cells. Vaccine 33: 4495–4504, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furusyo N, Ogawa E, Sudoh M, Murata M, Ihara T, Hayashi T, Ikezaki H, Hiramine S, Mukae H, Toyoda K, Taniai H, Okada K, Kainuma M, Kajiwara E, Hayashi J. Raloxifene hydrochloride is an adjuvant antiviral treatment of postmenopausal women with chronic hepatitis C: a randomized trial. J Hepatol 57: 1186–1192, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Gilliver SC. Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol 120: 105–115, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Haddad SN, Wira CR. Estradiol regulation of constitutive and keratinocyte growth factor-induced CCL20 and CXCL1 secretion by mouse uterine epithelial cells. Am J Reprod Immunol 72: 34–44, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashida K, Shoji I, Deng L, Jiang DP, Ide YH, Hotta H. 17beta-estradiol inhibits the production of infectious particles of hepatitis C virus. Microbiol Immunol 54: 684–690, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87: 905–931, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann J, Otte A, Thiele S, Lotter H, Shu Y, Gabriel G. Sex differences in H7N9 influenza A virus pathogenesis. Vaccine 33: 6949–6954, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Ivanova MM, Mazhawidza W, Dougherty SM, Minna JD, Klinge CM. Activity and intracellular location of estrogen receptors alpha and beta in human bronchial epithelial cells. Mol Cell Endocrinol 305: 12–21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jisa E, Dornstauder E, Ogawa S, Inoue S, Muramatsu M, Jungbauer A. Transcriptional activities of estrogen receptor alpha and beta in yeast properties of raloxifene. Biochem Pharmacol 62: 953–961, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Johansen LM, Brannan JM, Delos SE, Shoemaker CJ, Stossel A, Lear C, Hoffstrom BG, Dewald LE, Schornberg KL, Scully C, Lehar J, Hensley LE, White JM, Olinger GG. FDA-approved selective estrogen receptor modulators inhibit Ebola virus infection. Sci Transl Med 5: 190ra179, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein SL, Hodgson A, Robinson DP. Mechanisms of sex disparities in influenza pathogenesis. J Leukoc Biol 92: 67–73, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein SL, Schiebinger L, Stefanick ML, Cahill L, Danska J, de Vries GJ, Kibbe MR, McCarthy MM, Mogil JS, Woodruff TK, Zucker I. Opinion: sex inclusion in basic research drives discovery. Proc Natl Acad Sci USA 112: 5257–5258, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohanski MA, Lane AP. Sinonasal epithelial cell response to Staphylococcus aureus burden in chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg 141: 341–349, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol 143C: 207–222, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Kos-Kudla B, Ostrowska Z, Marek B, Ciesielska-Kopacz N, Sieminska L, Kajdaniuk D, Nowak M, Kudla M. Hormone replacement therapy in postmenopausal asthmatic women. J Clin Pharm Ther 25: 461–466, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294: 63–69, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurosawa T, Hiroi H, Tsutsumi O, Ishikawa T, Osuga Y, Fujiwara T, Inoue S, Muramatsu M, Momoeda M, Taketani Y. The activity of bisphenol A depends on both the estrogen receptor subtype and the cell type. Endocr J 49: 465–471, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Laity JH, Lee BM, Wright PE. Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol 11: 39–46, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Larcombe AN, Foong RE, Bozanich EM, Berry LJ, Garratt LW, Gualano RC, Jones JE, Dousha LF, Zosky GR, Sly PD. Sexual dimorphism in lung function responses to acute influenza A infection. Influenza Other Respir Viruses 5: 334–342, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HR, Kim TH, Choi KC. Functions and physiological roles of two types of estrogen receptors, ERalpha and ERbeta, identified by estrogen receptor knockout mouse. Lab Anim Res 28: 71–76, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin W, Zhu C, Hong J, Zhao L, Jilg N, Fusco DN, Schaefer EA, Brisac C, Liu X, Peng LF, Xu Q, Chung RT. The spliceosome factor SART1 exerts its anti-HCV action through mRNA splicing. J Hepatol 62: 1024–1032, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massaro D, Massaro GD. Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice. Am J Physiol Lung Cell Mol Physiol 287: L1154–L1159, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, Balhorn A, Miyahara N, Takeda K, Gelfand EW. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol 38: 501–508, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv 3: 281–292, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Matthews J, Wihlen B, Tujague M, Wan J, Strom A, Gustafsson JA. Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol 20: 534–543, 2006. [DOI] [PubMed] [Google Scholar]
- 33.McCown MF, Pekosz A. The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging. J Virol 79: 3595–3605, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millas I, Liquidato BM, de Sousa Buck H, Barros MD, Paes RA, Dolci JE. Evaluation of estrogenic receptors in the nasal mucosa of women taking oral contraceptives. Contraception 83: 571–577, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, Gustafsson JA. Lung dysfunction causes systemic hypoxia in estrogen receptor beta knockout (ERbeta-/-) mice. Proc Natl Acad Sci USA 103: 7165–7169, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller L, Jaspers I. Epithelial cells, the “switchboard” of respiratory immune defense responses: effects of air pollutants. Swiss Med Wkly 142: w13653, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami Y, Fukasawa M, Kaneko Y, Suzuki T, Wakita T, Fukazawa H. Selective estrogen receptor modulators inhibit hepatitis C virus infection at multiple steps of the virus life cycle. Microbes Infect 15: 45–55, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Osborne CK, Zhao H, Fuqua SA. Selective estrogen receptor modulators: structure, function, and clinical use. J Clin Oncol 18: 3172–3186, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277: 1508–1510, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, Ciana P, Maggi A, Warner M, Gustafsson JA, Nord M. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol 23: 8542–8552, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pekosz A, Newby C, Bose PS, Lutz A. Sialic acid recognition is a key determinant of influenza A virus tropism in murine trachea epithelial cell cultures. Virology 386: 61–67, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peretz J, Hall OJ, Klein SL. Sex Differences in Influenza Virus infection, Vaccination, and Therapies. In: Sex and Gender Differences in Infection and Treatments for Infectious Diseases, edited by Klein SL, Roberts CW. Cham, Switzerland: Springer International, 2015, p. 183–210. [Google Scholar]
- 43.Philpott CM, Wild DC, Wolstensholme CR, Murty GE. The presence of ovarian hormone receptors in the nasal mucosa and their relationship to nasal symptoms. Rhinology 46: 221–225, 2008. [PubMed] [Google Scholar]
- 44.Ramanathan M Jr, Lane AP. A comparison of experimental methods in molecular chronic rhinosinusitis research. Am J Rhinol 21: 373–377, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol 88: 4711–4720, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, Arnold AP, Klein SL. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ 2: 8, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17beta-estradiol protects females from influenza a virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7: e1002149, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez-Garcia M, Biswas N, Patel MV, Barr FD, Crist SG, Ochsenbauer C, Fahey JV, Wira CR. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PLoS One 8: e62069, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders CJ, Doherty PC, Thomas PG. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res 343: 13–21, 2011. [DOI] [PubMed] [Google Scholar]
- 50.Schaefer TM, Wright JA, Pioli PA, Wira CR. IL-1beta-mediated proinflammatory responses are inhibited by estradiol via down-regulation of IL-1 receptor type I in uterine epithelial cells. J Immunol 175: 6509–6516, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Sedyaningsih ER, Isfandari S, Setiawaty V, Rifati L, Harun S, Purba W, Imari S, Giriputra S, Blair PJ, Putnam SD, Uyeki TM, Soendoro T. Epidemiology of cases of H5N1 virus infection in Indonesia, July 2005-June 2006. J Infect Dis 196: 522–527, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Sentman CL, Meadows SK, Wira CR, Eriksson M. Recruitment of uterine NK cells: induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone. J Immunol 173: 6760–6766, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Shirasaki H, Watanabe K, Kanaizumi E, Konno N, Sato J, Narita SI, Himi T. Expression and localization of steroid receptors in human nasal mucosa. Acta Otolaryngol 124: 958–963, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 28: 521–574, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Szotek EL, Narasipura SD, Al-Harthi L. 17β-estradiol inhibits HIV-1 by inducing a complex formation between β-catenin and estrogen receptor on the HIV promoter to suppress HIV transcription. Virology 443: 375–383, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc Natl Acad Sci USA 111: 3799–3804, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33: 378–455, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vegeto E, Cuzzocrea S, Crisafulli C, Mazzon E, Sala A, Krust A, Maggi A. Estrogen receptor-α as a drug target candidate for preventing lung inflammation. Endocrinology 151: 174–184, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velez-Ortega AC, Temprano J, Reneer MC, Ellis GI, McCool A, Gardner T, Khosravi M, Marti F. Enhanced generation of suppressor T cells in patients with asthma taking oral contraceptives. J Asthma 50: 223–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe T, Watanabe S, Kawaoka Y. Cellular networks involved in the influenza virus life cycle. Cell Host Microbe 7: 427–439, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wira CR, Fahey JV, Rodriguez-Garcia M, Shen Z, Patel MV. Regulation of mucosal immunity in the female reproductive tract: the role of sex hormones in immune protection against sexually transmitted pathogens. Am J Reprod Immunol 72: 236–258, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol 15: 217–230, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organization. Disease and Injury Regional Mortality Estimates, 2000–2012; Global Summary Estimates (Online). http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html [10 Nov. 2015].
- 64.Zarychanski R, Stuart TL, Kumar A, Doucette S, Elliott L, Kettner J, Plummer F. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ 182: 257–264, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmerman JL, Woodruff PG, Clark S, Camargo CA. Relation between phase of menstrual cycle and emergency department visits for acute asthma. Am J Respir Crit Care Med 162: 512–515, 2000. [DOI] [PubMed] [Google Scholar]