Abstract
Alveolar epithelial and endothelial cell injury is a major feature of the acute respiratory distress syndrome, in particular when in conjunction with ventilation therapies. Previously we showed [Kim SC, Kellett T, Wang S, Nishi M, Nagre N, Zhou B, Flodby P, Shilo K, Ghadiali SN, Takeshima H, Hubmayr RD, Zhao X. Am J Physiol Lung Cell Mol Physiol 307: L449–L459, 2014.] that tripartite motif protein 72 (TRIM72) is essential for amending alveolar epithelial cell injury. Here, we posit that TRIM72 improves cellular integrity through its interaction with caveolin 1 (Cav1). Our data show that, in primary type I alveolar epithelial cells, lack of TRIM72 led to significant reduction of Cav1 at the plasma membrane, accompanied by marked attenuation of caveolar endocytosis. Meanwhile, lentivirus-mediated overexpression of TRIM72 selectively increases caveolar endocytosis in rat lung epithelial cells, suggesting a functional association between these two. Further coimmunoprecipitation assays show that deletion of either functional domain of TRIM72, i.e., RING, B-box, coiled-coil, or PRY-SPRY, abolishes the physical interaction between TRIM72 and Cav1, suggesting that all theoretical domains of TRIM72 are required to forge a strong interaction between these two molecules. Moreover, in vivo studies showed that injurious ventilation-induced lung cell death was significantly increased in knockout (KO) TRIM72KO and Cav1KO lungs compared with wild-type controls and was particularly pronounced in double KO mutants. Apoptosis was accompanied by accentuation of gross lung injury manifestations in the TRIM72KO and Cav1KO mice. Our data show that TRIM72 directly and indirectly modulates caveolar endocytosis, an essential process involved in repair of lung epithelial cells through removal of plasma membrane wounds. Given TRIM72's role in endomembrane trafficking and cell repair, we consider this molecule an attractive therapeutic target for patients with injured lungs.
Keywords: lung injury, alveolar epithelial cells, apoptosis, caveolar endocytosis, tripartite motif protein 72, acute respiratory distress syndrome
cells and tissues of the human body are subjected to mechanical stress on a daily basis, the consequence of which depends on the variable intrinsic ability of different cells to repair plasma membrane wounds and the relative regenerative ability of the remaining cell population. Lung tissue is exposed to a mechanical strain as total lung volume fluctuates within the vital capacity range during breathing. The associated deformation of the lung matrix and overlaying alveolar resident cells is thought to promote cell growth and proliferation (10, 72). However, under pathological conditions, such as acute respiratory distress syndrome (ARDS), heterogeneous lung unit recruitment and fluid accumulation in alveolar spaces result in high tensile and interfacial stress (45), which are amplified during mechanical ventilation (46, 63). As a result, alveolus resident epithelial and endothelial cells are often wounded, and as a result may die through necrosis and apoptosis (13, 39), which is, in turn, associated with loss of cellular function and dysregulated inflammation (2, 26, 41, 60). In addition, fibrin and collagen deposition begin at the denuded basement membrane (69) and may initiate signaling cascades responsible for pulmonary fibrosis. However, the molecular mechanisms of lung cell wounding and repair remain poorly understood.
Repair of plasma membrane wounds requires protein-protein interactions among various repair molecules in close proximity to the plasma membrane to regulate the coordinated interplay of membrane trafficking and cytoskeletal remodeling (20, 42, 43). Specifically, there is a critical contribution of deformation-induced lipid trafficking from endomembrane to the plasma membrane (i.e., exocytic process) that is involved in protecting lung cells against stress failure and/or aiding repair of plasma membrane wounds (64, 65). However, the specific source of recruited endomembrane to the plasma membrane wound sites is under debate (8, 62). Furthermore, prior studies suggest that endocytosis is indispensable for successful repair of plasma membrane wounding (11), which is linked to, and possibly triggered by, endomembrane exocytosis following plasma membrane wounding (28, 62). Other study in primary type I alveolar epithelial (ATI) cells have indicated an important role for caveolar endocytosis but not clathrin and fluid-phase endocytosis in repairing plasma membrane wounding (67).
Our laboratory has reported that tripartite motif protein 72 (TRIM72) (also known as mitsugumin 53), a lipid vesicle-associated protein (4, 32), is required for effective repair of alveolar epithelial cell wounding following mechanical stress (34). Experimental data support the role of TRIM72 in mediating endomembrane exocytosis to form a repair patch at the plasma membrane (5, 71) and that the TRIM72-vesicle complex localizes in close proximity to or on the plasma membrane (4, 5), possibly for priming of membrane breakage repair. Furthermore, we have identified a physical and physiological interaction between TRIM72 and caveolin 1 (Cav1) (34), the major type of caveolar protein expressed in ATI cells and endothelial cells, inferring that specific mechanisms of endomembrane trafficking and endocytosis cross talk during repair of plasma membrane wounding.
In the present study, we aimed to identify whether TRIM72 specifically modulates caveolar endocytosis in the context of alveolar epithelial cell repair. We applied immunobiochemical and pharmacological tools to assess the machinery and dynamics of the caveolar endocytic pathway in primary ATI cells isolated from genetically modified mouse models. Our data indicate that Cav1 targeting to the plasma membrane is significantly compromised in primary ATI cells lacking TRIM72, and that TRIM72 facilitates endocytic activity through the caveolar pathway. Further analysis shows that deletion of either functional domain of TRIM72, i.e., RING, B-box, coiled-coil (CC), or PRY-SPRY, abolishes the physical interaction between TRIM72 and Cav1. The physiological significance of the TRIM72/Cav1 interaction is supported by our in vivo data showing that mice deficient in both TRIM72 and Cav1 [double knockout (DKO)] exhibit significantly more apoptotic cell death in the lung compared with mice with single-gene knockout (KO) of TRIM72 or Cav1 when subjected to injurious lung ventilation. Moreover, our data show that TRIM72KO and Cav1KO mice have a marked increase in gross lung injury symptoms compared with the wild-type (WT) controls following mechanical ventilation. Altogether, our data indicate that modulation of Cav1-mediated endocytosis is one of the mechanisms through which TRIM72 regulates lipid trafficking to facilitate membrane repair, and that TRIM72 protects lung cells in an integrative fashion through modulation of both inward and outward lipid trafficking events during membrane repair. This finding highlights the central role of TRIM72 in lung cell repair and ARDS.
MATERIALS AND METHODS
Plasmid construction and lentiviral production.
Four deletion mutation constructs of TRIM72 ΔRING [49–189 nucleotides (nt)], ΔB-box (250–375 nt), ΔCC (412–516 nt), and ΔPRY-SPRY (820–1434 nt) were generated by PCR-based method from full-length TRIM72 (1–1434 nt) in enhanced green fluorescent protein pEGFP-C1 vector. Full-length TRIM72 was also subcloned into the L309C vector (generous gift from Dr. Zhiping Pang, Rutgers University), a third-generation lentiviral expression vector (50, 74). Successful subcloning of all constructs was confirmed by sequencing analysis (Mutagenex, Suwanee, GA).
To produce lentivirus, human embryonic kidney (HEK)-293 cells were cultured until 80–90% confluence and transfected with L309-TRIM72, vesicular somatitis virus G glycoprotein, Rev, and Rev response element at 2:1:1:1 using Xfect reagent (Clontech, Mountain View, CA). Viral soup was harvested 48 h after transfection by centrifugation at 2,000 rpm, yielding average viral titer of 4 × 105 viral particles/ml. One to approximately two × 105 viral particles per milliliter of viral particles were used to infect rat lung epithelial cells (RLE) to achieve infection efficiency of >90%.
Animals.
Generation and genotyping methods of the TRIM72KO mice have been described (4, 34). Cav1KO mice were obtained from the Jackson Laboratory (stock no. 004585, Bar Harbor, ME) and were genotyped following instruction. DKO mice were generated by crossing TRIM72KO and Cav1KO mice and identifying positive pups using PCR-based genotyping method (primers oIMR1972: 5′-GTGTATGACGCGCACACCAAG-3′; oIMR1973: 5′-CTAGTGAGACGTGCTACTTCC-3′; and oIMR1974: 5′-CTTGAGTTCTGTTAGCCCAG; Cav1KO: 410 bp, WT: 690 bp, heterozygote: both bands). Mice were housed in a sterile ventilated facility of the University Laboratory Animal Resources at The Ohio State University and the Comparative Medicine Animal Facility at Eastern Virginia Medical School under standard husbandry. Mice of mixed sex and 2∼6 mo of age were used for experiments. Control WT mice were littermates of the TRIM72KO mice, which have mixed 129/Sv and C57BL/6J background, similar to that of the Cav1KO and DKO mice. All experiments were approved by the Institutional Animal Care and Use Committee of the Ohio State University and Eastern Virginia Medical School.
Cell culture and isolation of primary cells from mouse lung.
RLE were cultured in F-12K culture medium containing 10% FBS and 1% Pen/Strep (P/S). Cells were infected with L309-TRIM72 for 4 days and used for in vitro pulse-chase endocytic experiments. HEK-293-T cells were cultured in DMEM containing 10% FBS and 1% P/S until ∼60% confluence and transfected with full-length or truncated green fluorescent protein (GFP)-TRIM72 constructs plus full-length GFP-Cav1 in pEGFP-N1 vector using Xfect reagent (Clontech). Cells were maintained in complete DMEM for 2 days after transfection before lysis for coimmunoprecipitation experiments.
Our laboratory previously reported a protocol to isolate primary alveolar epithelial cells from mouse lung tissue (34). Here we modified this protocol to harvest other types of primary cells as well. Briefly, murine lungs were lavaged using phosphate-buffered saline buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4), and alveolar macrophages in bronchoalveolar lavage fluid (BALF) were harvested by centrifugation. Lungs were digested in 1.8 U/ml dispase (Life Technologies, Carlsbad, CA). Cell suspensions were filtered through 100-μm mesh and panned on IgG-coated dish to remove leukocytes. Unattached cells were incubated first with rat anti-mouse CD90 antibody (Thermo Scientific, Waltham, MA) to remove fibroblasts (35) and then with rat anti-mouse CD31 antibody (Biolegend, San Diego, CA) conjugated to anti-rat IgG Dynabeads (Life Technologies, Carlsbad, CA) to capture endothelial cells (34). Cells that were not bound to CD31 were harvested as mixed type I and type II alveolar epithelial (ATII) cells. ATI cells were identified by positive staining for T1α. Cells were either lysed directly for Western blot or cultured in F-12K culture medium containing 10% FBS and 1% P/S for 5∼6 days for in vitro pulse-chase experiments.
Immunocytochemistry and quantification of Cav1 membrane distribution.
Primary alveolar epithelial cells at day 5 of culture were fixed with 4% paraformaldehyde for 30 min. Cells were either directly stained with an antibody that preferably recognizes a plasma membrane Cav-1 pool (53) (catalog no. 610494, BD Biosciences, Franklin Lakes, NJ), or stained with an antibody for total cellular Cav1 (catalog no. 610060, BD Biosciences) after permeabilization with 0.1% saponin (67). Cells were either costained with hamster anti-mouse T1α (catalog no. 8.1.1, DSHB, Iowa City, IA) and rabbit anti-pro-surfactant protein C (SPC) (Santa Cruz Biotechnology, Dallas, TX) to identify ATI and ATII cells in the mixed culture, or T1α plus the above Cav1 antibodies to evaluate Cav1 cellular distribution in ATI cells. Cells were then incubated with fluorophore-conjugated secondary antibodies (goat anti-hamster Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 594) and counterstained with 4,6-diamidino-2-phenylindole (DAPI) before imaged under an Olympus AX70 microscope. A total of 54 images from WT and 49 images from TRIM72KO primary cells were analyzed. Blind scoring was performed by two investigators. Membrane-bound, paranuclear-bound, or dual staining of Cav1 in T1α+ ATI cells was assigned a score of 3, 2, or 1, respectively, and percentages of each Cav1 staining pattern per group were compared and plotted. Experiments were repeated in three pairs of animals.
Western blot and RT-PCR.
Total denatured protein samples from primary cells and lung tissue were separated on SDS polyacrylamide gel and transferred onto polyvinylidene fluoride membranes. The following primary antibodies were used: rabbit anti-TRIM72, rabbit anti-caveolin-1 (Cell Signaling, Danvers, MA), and mouse anti-β-actin antibodies (Sigma-Aldrich, St. Louis, MO).
Total RNA was isolated from primary cells using Trizol reagent (Life Technologies, Carlsbad, CA). RNA (1 μg) was reverse transcribed into cDNA with a High Capacity cDNA Synthesis kit (Life Technologies, Carlsbad, CA) using random primers. PCR was performed with 1 μl cDNA using Platinum Taq DNA polymerase High Fidelity PCR Kit (Life Technologies, Carlsbad, CA). Primers used were as follows: TRIM72 sense, 5′-CTGGAGCATCAGCTGGTGGAG-3′; antisense, 5′-CAGGCAGAATTTCATGAGGA-3′; product size of 741 bp; and GAPDH sense, 5′-TATGTCGTGGAGTCTACTGG-3′; antisense, 5′-CATTGCTGACAATCTTGAGT-3′; product size of 169 bp.
Endocytosis assay.
Endocytosis experiments were carried out using BODIPY-lactosyl ceramide (LacCer, catalog no. B34402, Molecular Probes-Thermo Scientific), Alexa Fluor 488 transferrin (Tfn, catalog no. T13342, Molecular Probes), and dextran fluorescein (Dex, catalog no. D1821, Molecular Probes) as pathway-specific cargos for the caveolar, clathrin-mediated, and fluid-chase endocytosis, as previously described (9, 58, 67). Pharmacological inhibitors for the above three pathways were nystatin (Sigma, catalog no. N4014), chlorpromazine (CPZ) (Sigma, catalog no. C8138), and clostridium difficile toxin B (C. toxin B) (Calbiochem, catalog no. 616377), respectively. In brief, RLE cells were infected with L309C-TRIM72 for 4 days, washed with HEPES minimum essential medium (HMEM; Sigma-Aldrich, St. Louis, MO), and incubated with either BODIPY-LacCer (2.5 μM) or Alexa Fluor 488-Tfn (5 μg/ml) at 4∼10°C for 30 min to prelabel the plasma membrane. For experiments involving Tfn, cells were first serum-starved for 2 h at 37°C to upregulate the Tfn receptor. To assess the internalization of the endocytic markers, cells were transferred to a 37°C water bath for 5 min. Excess plasma membrane-bound BODIPY-LacCer was removed by back exchange at 4∼10°C with 5% fatty acid-free BSA-HMEM (10 min each for a total of 6 washes). Excess plasma membrane-bound Tfn was removed by acid stripping on ice for 30 s. Experiments involving fluid-phase marker Dex were devoid of preincubation. Cells were treated at 37°C for 20 min with 100 μg/ml prewarmed Dex and briefly washed. Cargo internalization was observed and quantified using image processing programs on an Olympus AX70 or a Nikon Ti-s/L 100 epifluorescence microscope.
Pharmacological inhibitors of endocytosis were used to test the pathway specificity. RLE cells were pretreated with nystatin (25 μg/ml), CPZ (8 μg/ml), and C. toxin B (100 μM) at 37°C for 30 min before prelabeling the plasma membrane. RLE cells without TRIM72 overexpression were used as controls.
Coimmunoprecipitation assay.
Two days after transfection with full-length or truncated TRIM72 constructs (ΔRING, ΔB-box, ΔCC, or ΔPRY-SPRY) in pEGFP-C1 vector, plus full-length Cav1 in pEGFP-N1 vector, HEK-293-T cells transfected were gently rinsed with cold PBS and lysed on ice with immunoprecipitation buffer (20 mM HEPES, 2 mM EDTA, 140 mM NaCl, 10% glycerol, 1% NP-40, and 1% Triton X-100) supplemented with Protease Inhibitor Cocktail Set III (Calbiochem, Billerica, MA). Lysates were centrifuged to clear undissolved debris and incubated with rabbit anti-Cav1 antibody (Cell Signaling Technology, Danvers, MA) or rabbit IgG antibody for 2 h at 4°C. Protein G Plus/protein A agarose beads were then added and incubated with lysates for 1 h at 4°C. Immunoprecipitation beads were collected by centrifugation and washed with immunoprecipitation buffer and resuspended in 2× SDS sample buffer for Western blot analysis using mouse anti-TRIM72 and rabbit anti-Cav1 antibodies.
High tidal volume ventilation-induced lung injury.
The procedure to introduce ventilation-induced lung injury was modified from previous reports (34, 52). Briefly, WT, TRIM72KO, Cav1KO, and DKO mice were anesthetized with ketamine-xylazine, paralyzed with 0.3 mg/kg pancuronium, and mechanically ventilated with 100% O2 and end-expiratory pressure of 3 cmH2O on a FlexiVent ventilator (SCIREQ, Montreal, QC, Canada) continuously for 3 h. A tidal volume of 30 ml/kg body weight (BW) at a rate of 100/min was used for overventilation (OV) and 6 ml/kg BW at a rate of 150/min was used for normal ventilation (NV). Mice in the OV group were first acclimated with NV regimen for 15 min before being switched to the OV regimen. Baseline lung mechanics were measured at the initial NV regimen using programmed perturbation (snapshot) in FlexiWare software (SCIREQ). After ventilation, mice were killed by exsanguination, and the left lung lobe was lavaged, fixed with 4% paraformaldehyde at a transpulmonary pressure of 20 cmH2O for 30 min at 4°C, and embedded in paraffin. Hematoxylin and eosin staining of lung sections was performed by the Histology Core Facility at Eastern Virginia Medical School.
Two upper right lung lobes were used to measure wet-to-dry ratios. Lung lobes were immediately weighed after excision (wet weight) and then dried at 65°C for 72 h until no further decrease in weight was observed (dry weight). Wet-to-dry weight ratio was calculated. Total protein in BALF was measured using Bradford assay, and total lactate dehydrogenase (LDH) level in BALF was measured using a LDH cytotoxicity detection kit (Roche, Indianapolis, IN).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling assay.
Unstained paraffin sections (5 μm) from ventilated lungs were assayed for apoptosis using the DeadEnd fluorometric terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) system (Promega, Madison, WI), according to the manufacturer's instructions. Lung sections were deparaffinized, hydrated, and subjected to antigen retrieval. TUNEL-positive cells were counted from five randomly chosen fields/section in a blinded fashion. Experiments were repeated in five animals from each group. In a separate experiment, coimmunostaining of an ATI cell marker was performed using rat anti-T1α (Biolegend, San Diego, CA) to identify localization of TUNEL-positive cells. The secondary antibody for anti-T1α antibody used Alexa Fluor 594 chicken anti-rat IgG (Life Technologies, Carlsbad, CA). DAPI was used for nuclear staining. Images were captured under Nikon Ti-s/L 100 epifluorescence microscope.
Statistical analyses.
All data were analyzed using Origin 6.0 (OriginLab, Northampton, MA). Data are presented as means ± SD. Normality of continuous data was determined by Anderson-Darling Normality Test Calculator. Comparison of means between two continuous datasets was performed using Student's t-test and among multiple groups was conducted using one-way ANOVA with post hoc analysis. The χ2 test was used for comparison of Cav1 membrane staining between the WT and TRIM72KO groups in Fig. 1 (category data). Paired Student's t-test was used for comparison of caveolar endocytosis in paired WT and TRIM72KO samples assayed at the same time in Fig. 2. Statistical significance was assumed at a P < 0.05.
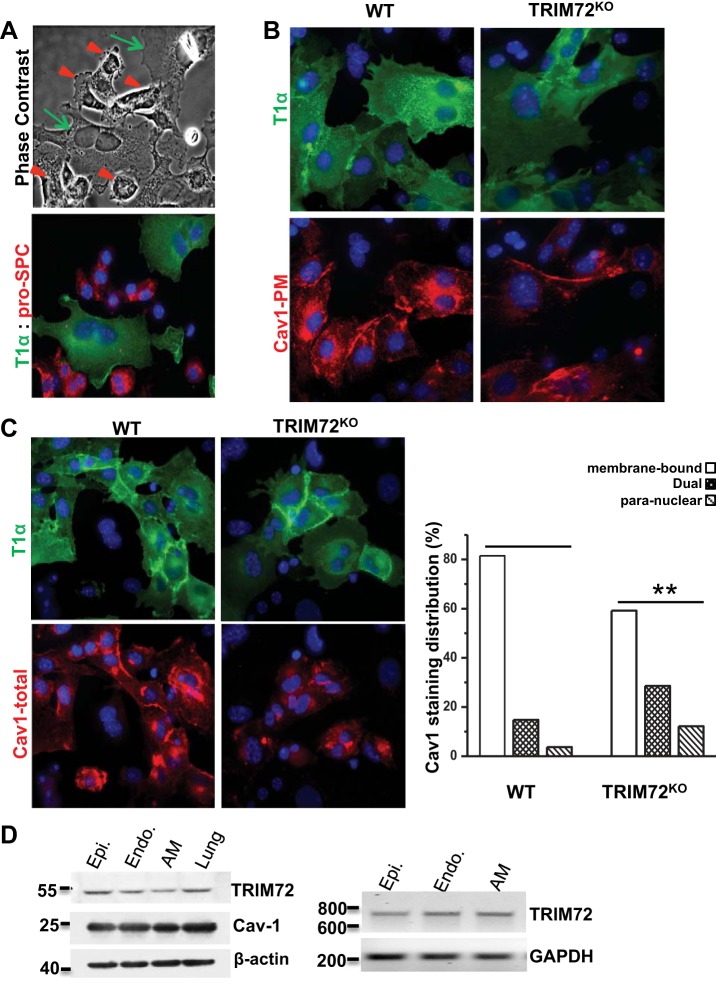
Fig. 1.
Decreased caveolin 1 (Cav1) membrane distribution in type I alveolar epithelial (ATI) cells with loss of tripartite motif protein 72 (TRIM72). A: representative images of primary alveolar epithelial cells isolated from mouse. T1α immunostaining was used to identify ATI cells, and pro-surfactant protein C (SPC) was used to identify type II alveolar epithelial (ATII) cells; note the difference in cell appearance of T1α+ ATI cells (green arrows) vs. pro-SPC+ ATII cells (red arrowheads) under phase contrast. B: immunostaining images of plasma membrane-bound Cav1 (Cav1-PM) in T1α+ cells (ATI) isolated from the wild-type (WT) and knockout (KO) TRIM72KO mice. C: representative images (left) and quantification (right) of total Cav1 immunostaining (Cav1-total) in T1α+ cells (ATI) isolated from the WT and TRIM72KO mice. Right: percentage of cells with each of the staining patterns was calculated and plotted. Intergroup difference was detected by χ2 test (χ2 = 11.91, n = 49∼54, **P < 0.01). Experiments were repeated in >3 pairs of mice. D: expression of TRIM72 in primary alveolar epithelial cells (Epi), endothelial cells (Endo), and alveolar macrophage (AM) by Western blot (left) and RT-PCR (right). Whole lung homogenate was included as a positive control for Western blot. RT-PCR detects specific amplification of a 712-bp fragment on trim72 mRNA. β-Actin and GAPDH were used as internal control for Western blot and RT-PCR, respectively. Experiments were repeated 3 times.
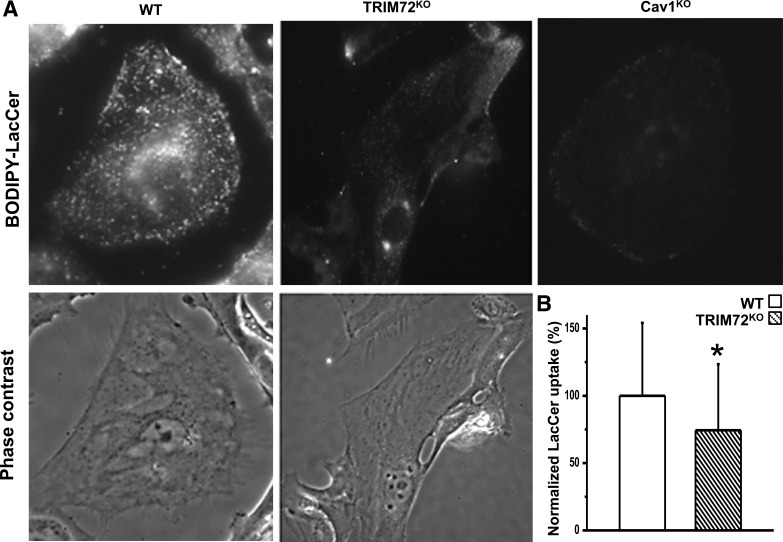
Fig. 2.
Compromised caveolar endocytosis in ATI cells with absence of TRIM72. A: representative phase contrast and fluorescent images of BODIPY-LacCer (lactosyl ceramide) uptake in primary alveolar epithelial cells isolated from the WT, TRIM72KO, and Cav1KO mice. Cells were prelabeled with BODIPY-LacCer at 4∼10°C for 30 min and incubated at 37°C for 5 min to initiate endocytosis. Imaging was performed following sufficient back-exchange with 5% BSA-HEPES minimum essential medium to remove excess BODIPY-LacCer on the plasma membrane. B: statistical analysis of internalized BODIPY-LacCer in the WT and TRIM72KO. Values are means ± SD; n = 5 for each experimental group. *P < 0.05 compared with WT by paired Student's t-tests. BODIPY-LacCer uptake in the WT group was set as 100% for normalization.
RESULTS
Decreased Cav1 membrane distribution in ATI cells with loss of TRIM72.
Cav1's role as a critical regulator of lung injury has been recognized (29). Cav1 is synthesized at the rough endoplasmic reticulum (ER) and preassembled in exocytic carrier vesicles in ER and Golgi before being transported to the plasma membrane to form a stable caveolar structure (23). Thus it can be detected throughout its biosynthetic pathway in different intracellular pools (23, 59). Our published data show that lack of TRIM72 in the lung was associated with reduced Cav1 expression (33). However, in these TRIM72 KOs, the specific pool of Cav1 affected was not determined. Here we quantified the subcellular localization of Cav1 in primary alveolar epithelial cells isolated from the WT and TRIM72KO lung.
As shown in Fig. 1A, ATI and ATII cells are the major cell types detected in isolated primary alveolar epithelial cells and can be differentiated by the relatively large cell size and smooth texture of ATI cells (arrows) vs. the smaller and rough ATII cells (arrowheads). In addition, ATI cells are selectively stained by T1α antibody (green), while ATII cells are positive for pro-SPC antibody (red), cell-specific markers for ATI and ATII cells, respectively (66). We first assessed Cav1 distribution under nonpermeabilized conditions using a Cav1 antibody that preferably binds to the plasma membrane pool (53). As shown in Fig. 1B, plasma membrane-bound Cav1 was reduced in primary ATI cells isolated from the TRIM72KO mice compared with the WT. We next performed Cav1 immunostaining under membrane permeabilization conditions to allow for identification of both the intracellular and plasma membrane Cav1 (Fig. 1C) (67). Subcellular distribution of Cav1 in T1α-positive ATI cells was analyzed. As shown in Fig. 1C, WT ATI cells had prominent membrane-bound Cav1 staining in 82% of examined cells (staining score of 3), 15% with dual staining of membrane and paranuclear (staining score of 2), and 4% with paranuclear Cav1 staining (staining score of 1), indicating that the majority of Cav1 exists at stable caveolar assemblies at the plasma membrane location. Interestingly, the Cav1 staining pattern was very different in TRIM72KO ATI cells, with only 59% of cells showed primarily membrane bound-Cav1 staining, 29% for dual staining and 12% for paranuclear staining. Averaging the staining scores for all Cav1 at all sites showed an overall ∼22.3% reduction in membrane distribution in TRIM72KO compared with WT (χ2 test, χ2 = 11.91, P < 0.01). Taken together with our laboratory's previous data showing enhanced Cav1 membrane staining in cells cotransfected with Cav1 and TRIM72 (33), we hypothesize that the interaction between TRIM72 and Cav1 is critical for proper targeting of Cav1 carrier vesicles to their plasma membrane destination.
Since both epithelial and endothelial cell injury are prominent in ARDS (12), and it is known that alveolar macrophages are important for the resolution of ARDS (24), we next utilized our refined primary cell culture technique to extend our analyses in epithelial cells to examine TRIM72 expression in lung endothelial cells and lung macrophages. As shown in Fig. 1D, we detected TRIM72 expression in both cell types by Western blot and RT-PCR. Cav1 expression was detected at roughly equal amount in all of these primary cell types, indicating the likelihood that TRIM72 and Cav1 interaction exist in different types of lung cells.
Compromised caveolar endocytosis in ATI cells with absence of TRIM72.
A previous report showed that caveolae internalization plays a direct role in removing membrane wounds by pore-forming toxins (11). In primary ATI cells, it was also shown that pharmacological inhibition of the caveolar endocytosis abolished the protective effect of hypertonic saline on repair of plasma membrane wounding (67). Here we examined whether the altered Cav1 membrane distribution associated with lack of TRIM72 affects caveolar endocytic activities (Fig. 2). BODIPY-LacCer complexed with BSA, a fluorophore-labeled glycosphingolipid that specifically stimulates the caveolar uptake pathway (58), was used to assess the caveolar endocytosis quantitatively and dynamically. As shown in Fig. 2, A and B, WT alveolar epithelial cells showed robust uptake of BODIPY-LacCer when incubated at 37°C for 5 min. In contrast, cells isolated from TRIM72KO lung exhibited a statistically significant reduction in BODIPY-LacCer internalization compared with WT cells (P < 0.05), suggesting that TRIM72 is required for effective caveolar endocytosis. BODIPY-LacCer internalization in primary alveolar epithelial cells isolated from Cav1KO mice serves as a negative control (Fig. 2A) where minimal level of caveolar endocytosis was seen. Normalization to WT (100%) showed that the level of reduction in caveolar endocytosis in the TRIM72KO cells is ∼26% of that of the WT controls.
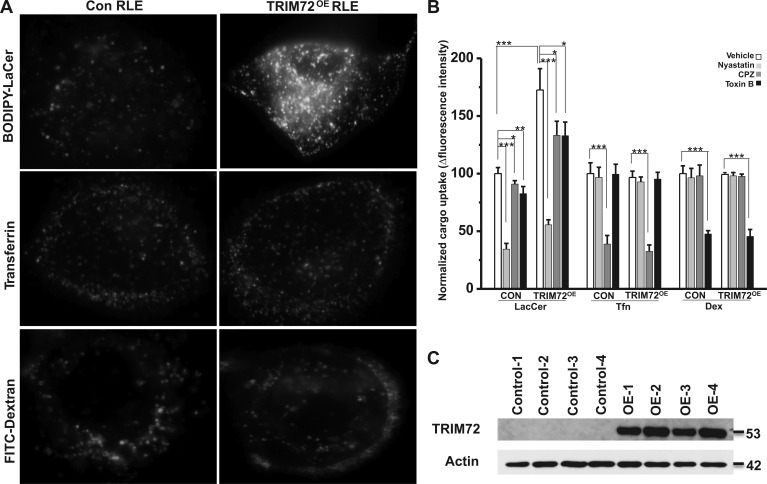
TRIM72 overexpression boosts caveolar endocytosis in RLE cells.
To determine whether TRIM72 directly modulates caveolar endocytosis, we performed lentivirus-mediated full-length TRIM72 overexpression in RLE cells and probed the effect on caveolar endocytosis (Fig. 3). Our laboratory's previous report has characterized pathway selectivity of pharmacological inhibitors for the three main endocytic pathways, i.e., caveolar endocytosis and clathrin-mediated and fluid-phase endocytosis (67) in primary ATI cells. Here we chose nystatin as a relatively specific inhibitor of the caveolar pathway, CPZ as inhibitor of the clathrin-mediated endocytosis, and C. toxin B as inhibitor of the fluid-phase endocytosis. As shown in Fig. 3, A and B, TRIM72 overexpression selectively upregulated internalization of BODIPY-LaCer, but not Alexa Fluor 488-Tfn nor FITC-dextran, primary cargo of the caveolar, clathrin-mediated, and fluid-chase pathways, respectively (67). Pharmacological inhibitor profiling in Fig. 3B confirmed the specificity of each inhibitor for the specific endocytic pathway, i.e., although internalization of BODIPY-LacCer can be slightly inhibited by CPZ and C. toxin B, nystatin treatment led to reduction of BODIPY-LacCer by >50%. On the other hand, Tfn and Dex internalization were relatively more specifically inhibited by CPZ or C. toxin B, respectively, consistent with previous results (67). Our data show that TRIM72 overexpression-induced upregulation in caveolar endocytosis can be significantly inhibited by application of nystatin in the cell culture medium (Fig. 3B). Cells were lysed at the end of the endocytosis assay to confirm ectopic expression efficiency of TRIM72, and good expression levels were shown by our Western blot results (Fig. 3C).
Fig. 3.
Enhanced caveolar endocytosis by TRIM72 overexpression (OE) in rat lung epithelial cells (RLE) cells. A: representative images of BODIPY-LacCer, Alexa Fluor 488-transferrin (Tfn), and FITC-dextran (Dex) uptake in RLE without (Con) and with TRIM72 OE (TRIM72OE). B: statistical analysis of internalized BODIPY-LacCer, Alexa Fluor 488-Tfn, and FITC-Dex in Con and TRIM72OE RLE cells, and inhibition of each cargo by pharmaceutical inhibitors, i.e., nystatin, chlorpromazine (CPZ), and Clostridium difficile toxin B (Toxin B). Values are means ± SD; n = 4 for each experimental group. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the vehicle control by Student's t-tests. C: Western blot shows ectopic TRIM72 expression in RLE cells. β-Actin was used as loading control.
Conformational interaction between TRIM72 and Cav1.
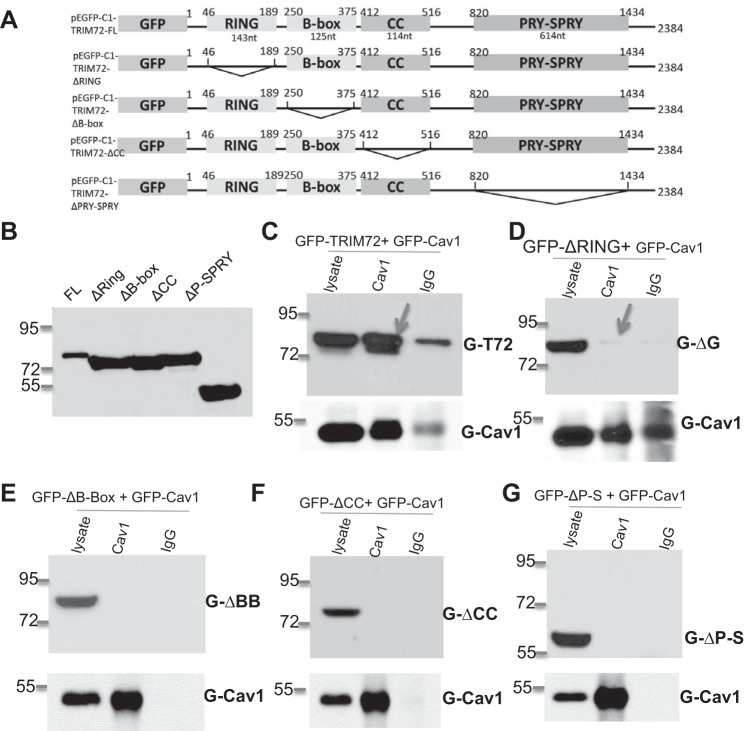
Our laboratory's previous publication identified a direct physical interaction between Cav1 and TRIM72 (34). Heretofore, the molecular mechanisms of this interaction had not been defined. To resolve this, we first determined the nucleotide sequences corresponding to the theoretical RING, B-box, CC, and PRY-SPRY domains on TRIM72 (4) and constructed deletion mutation for each of the domain (the 143 nt RING domain, the 125 nt B-box, the 114 nt CC domain, and the 614 nt PRY-SPRY domain, Fig. 4A). Successful construction of the truncated constructs were confirmed by sequencing analysis. To detect TRIM72 mutants, we screened available TRIM72 antibodies and selected a mouse anti-human TRIM72 antibody clone 257, since it is able to recognize all mutant forms of TRIM72 as well as the full length TRIM72 (Fig. 4B). Western blot showed that the GFP-fused TRIM72 mutants ΔRING, ΔB-box, ΔCC, and ΔPRY-SPRY migrated at the predicted molecular size of ∼75.2, 75.4, 75.8, and 57.1 kDa, along with the 80-kDa full-length GFP-TRIM72 (Fig. 4B). Subsequent coimmunoprecipitation assays using Cav1 antibody confirmed that full-length TRIM72 could form a strong interaction with GFP-tagged full-length Cav1 (34) (Fig. 4C), as great amount of TRIM72 was pulled down by anti-Cav1 antibody. However, to our surprise, none of the TRIM72 truncation mutants were able to physically interact with Cav1 (Fig. 4, D–G), indicating that all four domains are required to form a conformational interaction between TRIM72 and Cav1.
Fig. 4.
Conformational interaction between Cav1 and TRIM72. A: schematic design of TRIM72 deletion mutants based on pEGFP-C1-TRIM72-FL (full length), i.e., deletion of the 143-nucleotide (nt) RING domain in pEGFP-C1-TRIM72-ΔRING, the 125-nt B-box in pEGFP-C1-TRIM72-ΔB-box, the 114-nt coiled-coil (CC) domain in pEGFP-C1-TRIM72-ΔCC, and the 614-nt PRY-SPRY domain in pEGFP-C1-TRIM72-ΔP-S. B: Western blot shows that mouse anti-human TRIM72 clone 257 recognizes all mutant forms of TRIM72. C: coimmunoprecipitation of FL green fluorescent protein (GFP)-TRIM72 using rabbit anti-human Cav1 antibody in human embryonic kidney-293 cells cotransfected with GFP-TRIM72 and GFP-Cav1. Lysate shows sufficient GFP-TRIM72 and GFP-Cav1 input, and rabbit IgG antibody serves as a negative control for the coimmunoprecipitation process. D–G: Cav1 antibody failed to coimmunoprecipitate GFP-ΔRING, GFP-ΔB-Box, GFP-ΔCC, and GFP-ΔP-S, respectively.
Increased apoptotic cell death following OV in lungs lacking TRIM72 and/or Cav1.
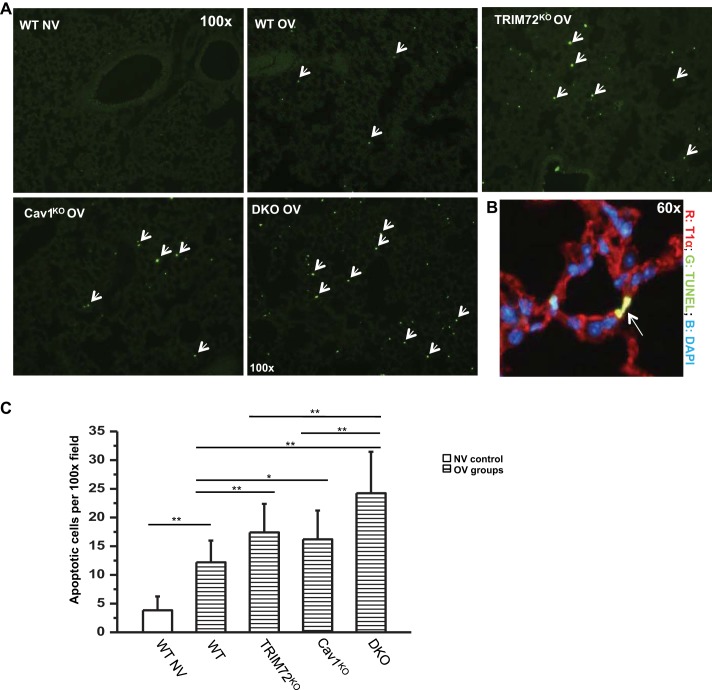
As both TRIM72 (34) and caveolar endocytosis (11) have been shown to be critically involved in repair of alveolar epithelial cells, and we identified an intrinsic link between these two here, next we examined the integrated effect of TRIM72/Cav1 for cell repair at the whole organ level using OV-induced lung injury model. Lung cell injury was introduced by high tidal volume mechanical ventilation, an experimental procedure that induces cell death through apoptosis and necrosis (39) as well as macroscopic lung injury, such as edema and microvascular leakage (30, 36). WT, TRIM72KO, Cav1KO, and double TRIM72KO/Cav1KO (DKO) mice were subjected to injurious ventilation for 3 h with 100% O2, a fraction of inspired O2 sometimes used in mechanically ventilated patients with severely low arterial Po2. The extent of apoptotic cell death following OV was examined using a TUNEL assay to detect nicked DNA in apoptotic cells (Fig. 5A, arrows). Representative coimmunostaining of TUNEL-positive cells with T1α in OV DKO lungs showed that these apoptotic cells are alveolar resident cells (Fig. 5B). Further quantitative analysis (Fig. 5C) revealed that WT lungs subjected to normal tidal volume ventilation (NV, 6 ml/kg BW) contain very few apoptotic cells, while injurious ventilation (OV, 30 ml/kg BW) for 3 h caused a statistically significant increase in apoptotic cells in the WT lungs (Fig. 5, 3.8 ± 2.4 in WT NV vs. 12.2 ± 3.8 TUNEL-positive cells per ×100 image in WT OV, **P < 0.01). Lungs lacking repair molecules, either TRIM72 or Cav1, had a statistically significant increase in lung cell death under stressed conditions (OV) compared with the WT (17.4 ± 5.0 TUNEL+ cells/field for TRIM72KO OV and 16.4 ± 5.0 TUNEL+ cells/field for Cav1KO OV respectively, **P < 0.01 and *P < 0.05 compared with WT OV, respectively). Interestingly, lungs of the TRIM72/Cav1 DKO mice experienced a further increase in apoptotic cell death following OV compared with both TRIM72KO and Cav1KO lungs (24.2 ± 7.2 TUNEL+ cells/field, **P < 0.01 compared with TRIM72KO or Cav1KO). Taken together, results from Figs. 1–5 support the idea that TRIM72 and Cav1 work in coordination, yet autonomously, in preventing lung cell death.
Fig. 5.
Increased apoptotic cell death following overventilation (OV) in lungs lacking TRIM72 and/or Cav1. A: representative terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining on lung tissue at ×100; WT, TRIM72KO, Cav1KO, and double knockout (DKO) mice were subjected to mechanical ventilation at 6 ml/kg body weight (BW) [normal ventilation (NV)] or 30 ml/kg BW (OV) for 3 h before TUNEL assay. Bright green dots represent nicked DNA in apoptotic cells. B: representative TUNEL staining on lung slides at ×60. Sections are counterstained with T1α (ATI cell marker, red) and 4,6-diamidino-2-phenylindole (DAPI) (nuclear staining, blue). C: statistical analysis of apoptotic cells per ×100 field in WT NV, WT OV, TRIM72KO OV, Cav1KO OV, and DKO OV experimental groups. Results shown are means ± SD from 5 animals in each of the 5 experimental groups, examining 5 fields per lung section in each animal. ANOVA analysis identified significant differences among the 5 experimental groups (P < 0.001). Post hoc Tukey honestly significant difference test identified intergroup differences as labeled, *P < 0.05 and **P < 0.01.
Aggravated lung injury with loss of TRIM72 and Cav1 following injurious ventilation.
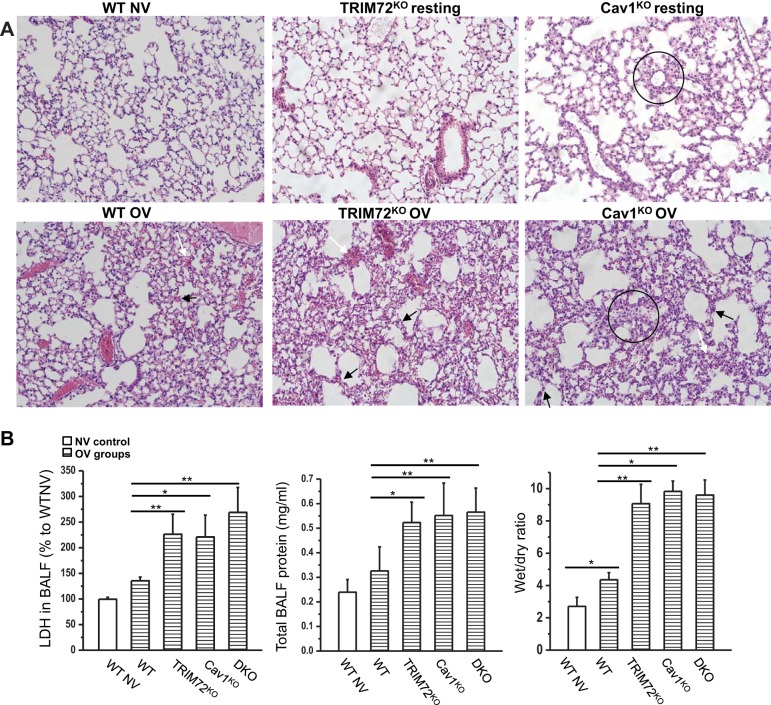
In addition to apoptotic cell death, macroscopic lung injury in WT, TRIM72KO, Cav1KO, and DKO mice subjected to injurious ventilation was also analyzed and compared (Fig. 6). As shown in Fig. 6A, there were no significant signs of lung injury in WT lung following 6 ml/kg BW ventilation (NV). Injurious ventilation (30 ml/kg BW, OV) led to septal thickening (black arrows) and minor hemorrhage (white arrows) in the WT lungs. In contrast, more severe lung pathology was observed in the OV TRIM72KO and Cav1KO lungs, including increased amount of hemorrhage in the alveolar space and septal thickening (arrows, Fig. 6A), consistent with previous reports (31, 54). It has been described that the structure of Cav1KO mice is altered due to extracellular fibrillar deposits, irregular alveolar space, and hypercellularity (16, 54), and we observed some hypercellularization of alveolar wall in the Cav1KO lungs (circles, Fig. 6A). To test if the resting lung compliance of WT, TRIM72KO, Cav1KO, and DKO mice is altered, we compared the baseline mechanical properties of the lungs from WT, TRIM72KO, Cav1KO, and DKO mice. As shown in Table 1, genetic alteration of TRIM72 and Cav1 did not change the compliance and resistance of the lung significantly at baseline level. Thus we conclude that the altered ventilator-induced lung injury profiles observed in mice lacking TRIM72 and/or Cav1 were not the result of different ventilation pressures on different mouse strains, but are rather largely due to the lack of these two repair molecules in the lung.
Fig. 6.
Aggravated lung injury with loss of TRIM72 and Cav1 following injurious ventilation. A: representative hematoxylin and eosin images from WT NV, WT OV, TRIM72KO resting, TRIM72KO OV, Cav1KO resting, and Cav1KO OV experimental groups. WT OV lungs show minor hemorrhage in the alveolar space (white arrow) and septal thickening (black arrows). These signs of lung injury were more severe in TRIM72KO OV and Cav1KO OV groups (arrows). TRIM72KO and Cav1KO show no signs of lung injury at resting stage, and WT NV shows basically normal structure. Hypercellularization was seen in Cav1KO lungs (circles). B: statistical analysis of bronchoalveolar lavage fluid (BALF) lactate dehydrogenase (LDH), BALF total protein, and wet-to-dry ratio in the above experimental groups. Values are means ± SD; n = 6 for WT NV and TRIM72KO groups and n = 5 for WT OV, Cav1KO OV, and DKO group. ANOVA analysis identified significant differences in BALF LDH, total protein, and wet-to-dry ratio among the 5 experimental groups (P < 0.001 for all 3 comparisons). Post hoc Tukey honestly significant difference test identified group-group differences as labeled, *P < 0.05 and **P < 0.01.
Table 1.
Lung mechanics of WT, TRIM72KO, Cav1KO, and DKO mice at baseline
| WT | TRIM72KO | Cav1KO | DKO | |
|---|---|---|---|---|
| Rrs, cmH2O/ml | 1.039 ± 0.368 | 0.961 ± 0.546 | 0.893 ± 0.158 | 0.948 ± 0.251 |
| Crs, ml/cmH2O | 0.023 ± 0.011 | 0.027 ± 0.012 | 0.023 ± 0.005 | 0.021 ± 0.006 |
Values are means ± SD; n = 12 for wild type (WT), n = 7 for tripartite motif protein 72 knockout (TRIM72KO), and n = 5 for caveolin 1 knockout (Cav1KO) and double knockout (DKO) groups. Rrs, resistance of the respiratory system; Crs, compliance of the respiratory system. P > 0.05 based on one-way ANOVA analysis.
Furthermore, BALF LDH, a marker for cell injury (14), total BALF protein, and wet-to-dry ratios were assessed as surrogates of vascular permeability and pulmonary edema, respectively (Fig. 6B). OV WT lungs demonstrated greater wet-to-dry ratios compared with WT lungs exposed to NV (*P < 0.05). In addition, OV in TRIM72KO and Cav1KO resulted in significantly greater BALF LDH, total protein, and wet-to-dry ratios compared with OV in WT lungs (**P < 0.01 and *P < 0.05 as indicated in Fig. 6B). We did not observe an additional increase in these measures of the TRIM72/Cav1 DKO lungs compared with the TRIM72KO and Cav1KO lungs, suggesting that a saturating effect on pathology of lung injury has been reached in the single mutants.
DISCUSSION
In this report, we evaluated the role and possible mechanism(s) for TRIM72-mediated regulation of caveolar endocytosis in lung cells, and the deleterious consequences of TRIM72 and Cav1 KOs in ventilator-associated lung injury (VALI). Our results show that TRIM72 protects lung cells at least partially through altering the plasma membrane distribution of Cav1 (Fig. 1) and critically modulates caveolar endocytic activity (Figs. 2 and 3). A physical interaction between TRIM72 and Cav1 that is dependent on an intact TRIM72 was also identified (Fig. 4). Nevertheless, TRIM72 and Cav1 likely govern two separate yet linked critical steps of the membrane repair process, since a greater effect on apoptotic cell death was observed in DKO lungs following injurious ventilation (Fig. 5). In addition, KO of either Cav1 or TRIM72 resulted in more severe lung injury compared with WT under stressed conditions (Fig. 6), supporting a significant physiological role for both TRIM72 and Cav1 in VALI and ARDS.
Injury of lung epithelial and endothelial cells has been recognized as a contributing factor for the pathogenesis of ARDS (12), in particular when in conjunction with the mechanical ventilation therapies. In addition, abnormal repair of microinjury to lung cells is viewed as an attractive theory for the initiation of pulmonary fibrosis (3, 57). Thus elucidating the molecular mechanism(s) of lung cell repair is of great interest to basic and clinical researchers. Previous studies show that lung cells use similar strategies compared with other general cell types for engaging plasma membrane repair. For example, dynamic lipid trafficking both to and from the plasma membrane has been recognized as an intrinsic component of successful plasma membrane repair in immortalized lung cell and primary ATI cells (20, 64, 67). While the exact source of endomembrane that contributes to patching of wounded plasma membrane is controversial (8, 62), previous studies show that TRIM72, a protein of limited tissue expression, exists in a protein-vesicle complex near and/or on the plasma membrane (4) and is essential for successful repair of injured alveolar epithelial cells (33). This may represent a ready-to-go repair assembly in a few injury-prone tissue types, including striated muscle and the lung.
The association of TRIM72 with lipid vesicles results from its demonstrated ability to bind specific lipid component(s), which is an important feature contributing to its membrane repair capacity. For example, prior results suggest that recombinant TRIM72 protein (mitsugumin 53) binds to phosphatidylserine, but not to other membrane lipids, such as sphingosine-1-P, phosphatidic acid, phosphatidylcholine, phosphatidylethanolamine, and various phosphoinositol metabolites on a phosphatidylinositol 4,5-bisphosphate strip (4). A later study confirmed TRIM72's higher affinity binding to phosphatidylserine and lower affinity binding to a variety of other membrane lipid components, including palmitate, stearate, and sphingosine (32). Furthermore, although not directly associated with cholesterol, the member repair capacity of TRIM72 is significantly impacted by cholesterol content in the plasma membrane. Specifically, depletion of membrane cholesterol abolished, and recovery of cholesterol restored, injury-induced membrane translocation of TRIM72 during ischemia-reperfusion-induced cardiomyocyte injury (68). Interestingly, published reports indicate that TRIM72 interacts with a caveolar component polymerase I and transcript release factor (also known as cavin) and, therefore, is brought into close contact with plasma membrane cholesterol to facilitate its membrane repair function (75). We previously identified a direct physical interaction between TRIM72 and Cav1 (33), the major protein component of plasma membrane lipid raft caveolae (23), and ectopic coexpression of TRIM72 and Cav1 led to enhanced plasma membrane distribution of Cav1 (33). This motivated our further investigation on the specific motif involved in TRIM72/Cav1 interaction.
TRIM72 belongs to the TRIM family proteins, which contains a conserved RING, B-box, and a CC motif at the NH2-terminus, and a variable SPRY domain at the COOH-terminus to distinguish TRIMs as specific isoforms (49), indicating the functional importance of these domains. Each domain dictates a distinct theoretical function. For example, RING domain and B-box are thought to have E3 ubiquitin ligase activity; CC domain may be involved in protein-protein interaction; and PRY-SPRY has been shown to modulate certain immune responses. Our coimmunoprecipitation results here showed that all TRIM72 domains are important for its interaction with Cav1 (Fig. 4). Given the relatively small fragment that was deleted from TRIM72, an intriguing possibility is TRIM72 and Cav1 forms a conformational complex during their interaction, a common transitional configuration for many E3 ubiquitin ligases (70). The significance of this interaction may be above and beyond membrane repair, as described here, as TRIM72 may play an indispensable role in the broad biological functions of caveolae in many other cell types.
In the lung tissue, Cav1 is mainly expressed in ATI and endothelial cells (17, 67), and it has been shown that ATII cells do not express Cav1 and have no caveolar structure (7). In this study, we quantified the subcellular distribution of Cav1 in primary ATI cells (Fig. 1), the major cell type comprising the alveolar wall and one of the major targeted cells for lung injury. Our data showed that the plasma membrane localization of Cav1 in TRIM72KO ATI cells was reduced by 22% compared with that of the WT cells, suggesting that TRIM72 is critically involved in the membrane targeting of Cav1 and, presumably, the biogenesis of the stable caveolae structure at the plasma membrane.
Caveolar biogenesis is linked to the biosynthetic trafficking of Cav1 and assembly with other caveolar components (23). Specifically, newly synthesized Cav1 first forms homo-oligomers in the ER membrane, which are then transported to the Golgi complex to form larger complexes to incorporate glycosylphosphatidylinositol-anchored protein in a cholesterol-enriched fraction. Small exocytic carrier vesicles then deliver the relatively stable Cav1 assemblies to the plasma membrane, where incorporation of cavin-1 further stabilizes the cholesterol-enriched lipid rafts. Taking into consideration the previous results showing retention of TRIM72 in the Golgi network in C2C12 myogenic cell line by overexpressing Cav3 mutant (6), one appealing hypothesis is that TRIM72-mediated exocytosis facilitates trafficking of the Cav1-cholesterol-glycosylphosphatidylinositol assembly from the Golgi apparatus to plasma membrane domains. However, dynamic caveolar biogenesis needs to be tracked in the presence and absence of TRIM72 to confirm this hypothesis.
One major physiological function of caveolae is to mediate clathrin-independent endocytosis (22), which has been shown to be critically involved in the repair process of ATI and other cell types (11, 67). Here we investigated the activity of caveolar endocytosis in primary alveolar epithelial cells through pulse-chase experiments. Specific cargo of the caveolar pathway BODIPY-LacCer was applied to stimulate caveolar endocytosis (58). Our results demonstrate a significant reduction in BODIPY-LacCer uptake in TRIM72KO cells compared with the WT cells and a 72.6% increase in BODIPY-LacCer uptake in cells overexpressing TRIM72 (Figs. 2 and 3), suggesting a direct link between TRIM72 and dynamic caveolar endocytosis. However, we presume that the compromised caveolar endocytosis is at least partially a result of reduced Cav1 at the plasma membrane in cells lacking TRIM72. We speculate that this reduced caveolar endocytosis contributes to the repair-defect phenotype of the TRIM72KO lung cells (34).
A variety of cells and tissues in the human body possess cell repair machinery/mechanism(s) to maintain function under stressed conditions (44). Our previous studies showed that >50% injured WT alveolar epithelial cells repair membrane breakage following mechanical insults, as evidenced by trapping of membrane-impermeable large molecules inside their cytosol (34). Consistently, only a handful of lung resident cells undergo cell death following injurious ventilation in vivo, as indicated by propidium iodide labeling of the nucleus in permanently damaged cells (34), despite initial insults. Although it is conceivable that wounds at the plasma membrane would lead to a necrotic rather than to an apoptotic cell death, experimental evidences show that both necrosis or apoptosis cell death mechanisms are identified in ARDS (38). The precise cause and consequences of lung cell apoptosis in ARDS are not clear, but the association between apoptotic cell death and ARDS is well established (17). As there is no specific marker to detect cellular necrosis pertinent to our experimental setting, we use apoptosis to probe lung cell injury, but considering that there may be a population of dead cell missed by this assay. Nevertheless, a more complex overlap may exist between the two cell death mechanisms, as apoptosis can lead to secondary necrosis (59) and a cross talk between lung cell apoptosis and cell repair pathways has been indicated (1, 19, 55). Our laboratory's previous results showed that trim72 ablation led to increased lung cell death, while trim72 overexpression had a protective effect, following transient injurious ventilation (34). However, reports on the in vivo impact of Cav1 in determining lung cell fate largely depend on the nature of the injurious models, i.e., mechanical stress or oxygen tension. For example, it was shown that carbon monoxide reduces pathology of VALI through upregulating Cav1 (25). However, data from the same group also showed that deletion of Cav1 protects hyperoxia-induced lung cell injury (73). A study by Maniatis et al. (40) emphasized that the presence of Cav1 is required for an increase in endothelial permeability in an OV model. In the present study, we employed a 3-h OV model to examine cell death and lung injury in transgenic mice lacking trim72 and/or cav1. Our data revealed a significant role for TRIM72 and for Cav1 in protecting lung cells from apoptotic death (Fig. 5). Since DKO of TRIM72 and Cav1 exhibited even greater apoptosis than TRIM72KO or Cav1KO lungs, we conclude that both TRIM72 and Cav1 protect lung cells from stress injury through partially overlapping mechanisms. The increase in apoptotic cell death in repair-defective lungs raises the intriguing possibility that lung cells lacking TRIM72 or Cav1 for proper plasma membrane repair may be prone to programmed cell death.
The protective role of TRIM72 and Cav1 was further confirmed by increased lung pathology in TRIM72KO, Cav1KO, and DKO mice following 3-h OV (Fig. 6). The equivalent level of gross lung injury in TRIM72KO and Cav1KO mice vs. the DKO mice is probably due to complex factors causing these pathologies or to a saturating effect that has been reached with ablation of either trim72 or cav1. It is worth noting that we did observe a significant increase in lung water (wet-to-dry ratio) in the Cav1KO and DKO following injurious ventilation, similar to that observed in the TRIM72KO mice. This result is in contrast to the report by Maniatis et al. (40), where the microvascular permeability following OV measured by BALF 125I-albumin level in Cav1KO mice was lower than that of the WT mice. The discrepancy between our results and theirs may be due to the larger tidal volume we used in our study for OV (30 ml/kg BW instead of 21 ml/kg BW) that could result in edema above and beyond any potential Cav1 requirement for eliciting endothelial cell osmotic control. Nevertheless, as both epithelial cells and endothelial cells are major targets of injurious insults in ARDS (12, 26), and we have confirmed the expression of TRIM72 in endothelial cells and alveolar macrophages in addition to its expression in alveolar epithelial cells (Fig. 1C), the physiological role of TRIM72 in these cell types demands more detailed investigations.
The limitations of the present study are that we assume that the effects of tracheotomy surgery did not differentially alter the lung injury parameters measured in our study among different transgenic lines (e.g., cell apoptosis, histology, LDH, and total protein in BALF and wet-to-dry ratio) and thus only performed baseline ventilation in the WT control group. However, since tracheotomy only affects the upper airway, this is not likely to change the conclusion of our study. In addition, our 3-h OV model introduces a number of insults to the lung, including mechanical stress, edema, hyperoxia (15), hypoxemia, and reduced blood flow due to shock. These factors should be considered when interpreting the mechanisms through which TRIM72/Cav1 protects the lung cells from gross injury and apoptotic death.
In summary, we have shown that TRIM72 is required for maintaining membrane localization of Cav1 and directly modulates caveolar endocytosis in alveolar epithelial cells. This interplay is important for protecting lung cells from apoptotic death and preventing lung pathology following injurious ventilation in an animal model of ARDS. The significance of this study is that it provides systematic, mechanistic evidence for a cellular association of two important effectors of lung repair and their role in regulating cell survival and overall lung pathology. We speculate that an attractive therapeutic option for ARDS and VALI is to upregulate TRIM72 expression in the lung to provide cytoprotection through enhancement of endosomal and plasma membrane lipid trafficking.
GRANTS
This work is supported by Grant R01-HL-116826 from the National Heart, Lung, and Blood Institute and Eastern Virginia Medical School institutional funds to X. Zhao, and core-to-core program from Japan Society for Promotion of Science to H. Takeshima.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.N.N., S.W., T.K., R.K., J.D., M.N., H.T., and R.D.H. performed experiments; N.N.N., S.W., T.K., R.K., J.D., M.N., K.S., R.A.O., H.T., R.D.H., and X.Z. analyzed data; N.N.N., S.W., R.K., K.S., and X.Z. prepared figures; N.N.N., S.W., T.K., R.K., J.D., M.N., K.S., R.A.O., J.C.Y., H.T., J.W.C., R.D.H., and X.Z. approved final version of manuscript; J.D., M.N., K.S., R.A.O., J.C.Y., H.T., J.W.C., R.D.H., and X.Z. edited and revised manuscript; K.S., R.A.O., J.C.Y., H.T., J.W.C., R.D.H., and X.Z. interpreted results of experiments; R.D.H. and X.Z. conception and design of research; X.Z. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Lane Wallace (Division of Pharmacology, College of Pharmacy, The Ohio State University) for critical review of the manuscript, and Dr. Tina Duong Cunningham (The Center for Health Analytics and Discovery, Eastern Virginia Medical School) for helpful discussion on statistical methods.
REFERENCES
- 1.Akram KM, Lomas NJ, Spiteri MA, Forsyth NR. Club cells inhibit alveolar epithelial wound repair via TRAIL-dependent apoptosis. Eur Respir J 41: 683–694, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Andrews NW. Membrane repair and immunological danger. EMBO Rep 6: 826–830, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackwell TS, Tager AM, Borok Z, Moore BB, Schwartz DA, Anstrom KJ, Bar-Joseph Z, Bitterman P, Blackburn MR, Bradford W, Brown KK, Chapman HA, Collard HR, Cosgrove GP, Deterding R, Doyle R, Flaherty KR, Garcia CK, Hagood JS, Henke CA, Herzog E, Hogaboam CM, Horowitz JC, King TE Jr, Loyd JE, Lawson WE, Marsh CB, Noble PW, Noth I, Sheppard D, Olsson J, Ortiz LA, O'Riordan TG, Oury TD, Raghu G, Roman J, Sime PJ, Sisson TH, Tschumperlin D, Violette SM, Weaver TE, Wells RG, White ES, Kaminski N, Martinez FJ, Wynn TA, Thannickal VJ, Eu JP. Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am J Respir Crit Care Med 189: 214–222, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol 11: 56–64, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai C, Masumiya H, Weisleder N, Pan Z, Nishi M, Komazaki S, Takeshima H, Ma J. MG53 regulates membrane budding and exocytosis in muscle cells. J Biol Chem 284: 3314–3322, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai C, Weisleder N, Ko JK, Komazaki S, Sunada Y, Nishi M, Takeshima H, Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J Biol Chem 284: 15894–15902, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell L, Hollins AJ, Al-Eid A, Newman GR, von Ruhland C, Gumbleton M. Caveolin-1 expression and caveolae biogenesis during cell transdifferentiation in lung alveolar epithelial primary cultures. Biochem Biophys Res Commun 262: 744–751, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Cerny J, Feng Y, Yu A, Miyake K, Borgonovo B, Klumperman J, Meldolesi J, McNeil PL, Kirchhausen T. The small chemical vacuolin-1 inhibits Ca(2+)-dependent lysosomal exocytosis but not cell resealing. EMBO Rep 5: 883–888, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng ZJ, Singh RD, Sharma DK, Holicky EL, Hanada K, Marks DL, Pagano RE. Distinct mechanisms of clathrin-independent endocytosis have unique sphingolipid requirements. Mol Biol Cell 17: 3197–3210, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correa-Meyer E, Pesce L, Guerrero C, Sznajder JI. Cyclic stretch activates ERK1/2 via G proteins and EGFR in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 282: L883–L891, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Corrotte M, Almeida PE, Tam C, Castro-Gomes T, Fernandes MC, Millis BA, Cortez M, Miller H, Song W, Maugel TK, Andrews NW. Caveolae internalization repairs wounded cells and muscle fibers. Elife 2: e00926, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello ML, Mathieu-Costello O, West JB. Stress failure of alveolar epithelial cells studied by scanning electron microscopy. Am Rev Respir Dis 145: 1446–1455, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Crimi E, Zhang H, Han RN, Del Sorbo L, Ranieri VM, Slutsky AS. Ischemia and reperfusion increases susceptibility to ventilator-induced lung injury in rats. Am J Respir Crit Care Med 174: 178–186, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Danpure CJ. Lactate dehydrogenase and cell injury. Cell Biochem Funct 2: 144–148, 1984. [DOI] [PubMed] [Google Scholar]
- 15.De Paepe ME, Mao Q, Chao Y, Powell JL, Rubin LP, Sharma S. Hyperoxia-induced apoptosis and Fas/FasL expression in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 289: L647–L659, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293: 2449–2452, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol 23: 1161–1168, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Galani V, Tatsaki E, Bai M, Kitsoulis P, Lekka M, Nakos G, Kanavaros P. The role of apoptosis in the pathophysiology of Acute Respiratory Distress Syndrome (ARDS): an up-to-date cell-specific review. Pathol Res Pract 206: 145–150, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Geiser T, Ishigaki M, van Leer C, Matthay MA, Broaddus VC. H2O2 inhibits alveolar epithelial wound repair in vitro by induction of apoptosis. Am J Physiol Lung Cell Mol Physiol 287: L448–L453, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Godin LM, Vergen J, Prakash YS, Pagano RE, Hubmayr RD. Spatiotemporal dynamics of actin remodeling and endomembrane trafficking in alveolar epithelial type I cell wound healing. Am J Physiol Lung Cell Mol Physiol 300: L615–L623, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gvaramia D, Blaauboer ME, Hanemaaijer R, Everts V. Role of caveolin-1 in fibrotic diseases. Matrix Biol 32: 307–315, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Hansen CG, Nichols BJ. Molecular mechanisms of clathrin-independent endocytosis. J Cell Sci 122: 1713–1721, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayer A, Stoeber M, Bissig C, Helenius A. Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic 11: 361–382, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2: 65, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoetzel A, Schmidt R, Vallbracht S, Goebel U, Dolinay T, Kim HP, Ifedigbo E, Ryter SW, Choi AM. Carbon monoxide prevents ventilator-induced lung injury via caveolin-1. Crit Care Med 37: 1708–1715, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussein O, Walters B, Stroetz R, Valencia P, McCall D, Hubmayr RD. Biophysical determinants of alveolar epithelial plasma membrane wounding associated with mechanical ventilation. Am J Physiol Lung Cell Mol Physiol 305: L478–L484, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 14: 81–93, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Idone V, Tam C, Andrews NW. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol 18: 552–559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin Y, Lee SJ, Minshall RD, Choi AM. Caveolin-1: a critical regulator of lung injury. Am J Physiol Lung Cell Mol Physiol 300: L151–L160, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 23: 243–252, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasper M, Reimann T, Hempel U, Wenzel KW, Bierhaus A, Schuh D, Dimmer V, Haroske G, Muller M. Loss of caveolin expression in type I pneumocytes as an indicator of subcellular alterations during lung fibrogenesis. Histochem Cell Biol 109: 41–48, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Seo J, Ko YG, Huh YD, Park H. Lipid-binding properties of TRIM72. BMB Rep 45: 26–31, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Kim SC, Kellett T, Wang S, Nishi M, Nagre N, Zhou B, Flodby P, Shilo K, Ghadiali SN, Takeshima H, Hubmayr RD, Zhao X. TRIM72 is required for effective repair of alveolar epithelial cell wounding. Am J Physiol Lung Cell Mol Physiol 307: L449–L459, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SC, Kellett T, Wang S, Nishi M, Nagre N, Zhou B, Flodby P, Shilo K, Ghadiali SN, Takeshima H, Hubmayr RD, Zhao X. TRIM72 is required for effective repair of alveolar epithelial cell wounding. Am J Physiol Lung Cell Mol Physiol 307: L449–L459, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kisselbach L, Merges M, Bossie A, Boyd A. CD90 Expression on human primary cells and elimination of contaminating fibroblasts from cell cultures. Cytotechnology 59: 31–44, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kor DJ, Warner DO, Carter RE, Meade LA, Wilson GA, Li M, Hamersma MJ, Hubmayr RD, Mauermann WJ, Gajic O. Extravascular lung water and pulmonary vascular permeability index as markers predictive of postoperative acute respiratory distress syndrome: a prospective cohort investigation. Crit Care Med 43: 665–673, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Le Saux O, Teeters K, Miyasato S, Choi J, Nakamatsu G, Richardson JA, Starcher B, Davis EC, Tam EK, Jourdan-Le Saux C. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am J Physiol Lung Cell Mol Physiol 295: L1007–L1017, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YG, Jeong JJ, Nyenhuis S, Berdyshev E, Chung S, Ranjan R, Karpurapu M, Deng J, Qian F, Kelly EA, Jarjour NN, Ackerman SJ, Natarajan V, Christman JW, Park GY. Recruited alveolar macrophages, in response to airway epithelial-derived MCP-1/CCL2, regulate airway inflammation and remodeling in allergic asthma. Am J Respir Cell Mol Biol 52: 772–784, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lionetti V, Recchia FA, Ranieri VM. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care 11: 82–86, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Maniatis NA, Kardara M, Hecimovich D, Letsiou E, Castellon M, Roussos C, Shinin V, Votta-Vellis EG, Schwartz DE, Minshall RD. Role of caveolin-1 expression in the pathogenesis of pulmonary edema in ventilator-induced lung injury. Pulm Circ 2: 452–460, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manicone AM. Role of the pulmonary epithelium and inflammatory signals in acute lung injury. Expert Rev Clin Immunol 5: 63–75, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol 6: 499–505, 2005. [DOI] [PubMed] [Google Scholar]
- 43.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol 19: 697–731, 2003. [DOI] [PubMed] [Google Scholar]
- 44.McNeil PL, Terasaki M. Coping with the inevitable: how cells repair a torn surface membrane. Nat Cell Biol 3: E124–E129, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Mendez JL, Hubmayr RD. New insights into the pathology of acute respiratory failure. Curr Opin Crit Care 11: 29–36, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149: 1327–1334, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol 204: 594–604, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Opalek JM, Ali NA, Lobb JM, Hunter MG, Marsh CB. Alveolar macrophages lack CCR2 expression and do not migrate to CCL2. J Inflamm 4: 19, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ozato K, Shin DM, Chang TH, Morse HC 3rd. TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol 8: 849–860, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pang ZP, Cao P, Xu W, Sudhof TC. Calmodulin controls synaptic strength via presynaptic activation of calmodulin kinase II. J Neurosci 30: 4132–4142, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plataki M, Lee YD, Rasmussen DL, Hubmayr RD. Poloxamer 188 facilitates the repair of alveolus resident cells in ventilator-injured lungs. Am J Respir Crit Care Med 184: 939–947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plataki M, Lee YD, Rasmussen DL, Hubmayr RD. Poloxamer 188 facilitates the repair of alveolus resident cells in ventilator injured lungs. Am J Respir Crit Care Med 184: 939–947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, Enrich C, Parton RG. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol Biol Cell 16: 2091–2105, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem 277: 8635–8647, 2002. [DOI] [PubMed] [Google Scholar]
- 55.Roberts JR, Perkins GD, Fujisawa T, Pettigrew KA, Gao F, Ahmed A, Thickett DR. Vascular endothelial growth factor promotes physical wound repair and is anti-apoptotic in primary distal lung epithelial and A549 cells. Crit Care Med 35: 2164–2170, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Ryter SW, Choi AM, Kim HP. Profibrogenic phenotype in caveolin-1 deficiency via differential regulation of STAT-1/3 proteins. Biochem Cell Biol 92: 370–378, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Selman M, Pardo A. Idiopathic pulmonary fibrosis: misunderstandings between epithelial cells and fibroblasts? Sarcoidosis Vasc Diffuse Lung Dis 21: 165–172, 2004. [PubMed] [Google Scholar]
- 58.Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, Marks DL, Simari R, Parton RG, Pagano RE. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell 15: 3114–3122, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma DK, Choudhury A, Singh RD, Wheatley CL, Marks DL, Pagano RE. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J Biol Chem 278: 7564–7572, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Slutsky AS. Ventilator-induced lung injury: from barotrauma to biotrauma. Respir Care 50: 646–659, 2005. [PubMed] [Google Scholar]
- 61.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 369: 2126–2136, 2013. [DOI] [PubMed] [Google Scholar]
- 62.Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol 189: 1027–1038, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med 171: 1328–1342, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Deformation-induced lipid trafficking in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 280: L938–L946, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Role of deformation-induced lipid trafficking in the prevention of plasma membrane stress failure. Am J Respir Crit Care Med 166: 1282–1289, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Wang S, Hubmayr RD. Type I alveolar epithelial phenotype in primary culture. Am J Respir Cell Mol Biol 44: 692–699, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang S, Singh RD, Godin L, Pagano RE, Hubmayr RD. Endocytic response of type I alveolar epithelial cells to hypertonic stress. Am J Physiol Lung Cell Mol Physiol 300: L560–L568, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Xie W, Zhang Y, Lin P, Han L, Han P, Wang Y, Chen Z, Ji G, Zheng M, Weisleder N, Xiao RP, Takeshima H, Ma J, Cheng H. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res 107: 76–83, 2010. [DOI] [PubMed] [Google Scholar]
- 69.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Weathington NM, Mallampalli RK. New insights on the function of SCF ubiquitin E3 ligases in the lung. Cell Signal 25: 1792–1798, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weisleder N, Takeshima H, Ma J. Mitsugumin 53 (MG53) facilitates vesicle trafficking in striated muscle to contribute to cell membrane repair. Commun Integr Biol 2: 225–226, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto H, Teramoto H, Uetani K, Igawa K, Shimizu E. Stretch induces a growth factor in alveolar cells via protein kinase. Respir Physiol 127: 105–111, 2001. [DOI] [PubMed] [Google Scholar]
- 73.Zhang M, Lin L, Lee SJ, Mo L, Cao J, Ifedigbo E, Jin Y. Deletion of caveolin-1 protects hyperoxia-induced apoptosis via survivin-mediated pathways. Am J Physiol Lung Cell Mol Physiol 297: L945–L953, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, Sudhof TC. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78: 785–798, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu H, Lin P, De G, Choi KH, Takeshima H, Weisleder N, Ma J. Polymerase transcriptase release factor (PTRF) anchors MG53 protein to cell injury site for initiation of membrane repair. J Biol Chem 286: 12820–12824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]