Abstract
Despite numerous attempts to discover genetic variants associated with elite athletic performance, injury predisposition, and elite/world-class athletic status, there has been limited progress to date. Past reliance on candidate gene studies predominantly focusing on genotyping a limited number of single nucleotide polymorphisms or the insertion/deletion variants in small, often heterogeneous cohorts (i.e., made up of athletes of quite different sport specialties) have not generated the kind of results that could offer solid opportunities to bridge the gap between basic research in exercise sciences and deliverables in biomedicine. A retrospective view of genetic association studies with complex disease traits indicates that transition to hypothesis-free genome-wide approaches will be more fruitful. In studies of complex disease, it is well recognized that the magnitude of genetic association is often smaller than initially anticipated, and, as such, large sample sizes are required to identify the gene effects robustly. A symposium was held in Athens and on the Greek island of Santorini from 14–17 May 2015 to review the main findings in exercise genetics and genomics and to explore promising trends and possibilities. The symposium also offered a forum for the development of a position stand (the Santorini Declaration). Among the participants, many were involved in ongoing collaborative studies (e.g., ELITE, GAMES, Gene SMART, GENESIS, and POWERGENE). A consensus emerged among participants that it would be advantageous to bring together all current studies and those recently launched into one new large collaborative initiative, which was subsequently named the Athlome Project Consortium.
Keywords: genetics, performance, sports genomics
at the outset, the Athlome Project aims to collectively study the genotype and phenotype data currently available on elite athletes, in adaptation to exercise training (in both human and animal models) and on exercise-related musculoskeletal injuries from individual studies and from consortia worldwide. To achieve this, several steps are set out:
1) To establish an ethically sound international research consortium (Athlome Project Consortium) and biobank resource systematically across individual centers;
2) To discover genetic variants associated with exercise performance, adaptive response to exercise-training, and skeletal-muscle injuries using the genome-wide association study (GWAS) approach, targeted sequencing or whole genome sequencing, where possible;
3) To validate and replicate the genetic markers from the discovery phase across sex and ethnicity; and
4) To conduct functional investigations following replicated findings [e.g., study the replicated single nucleotide polymorphisms (SNPs) and their linkage disequilibrium regions, in vitro expression studies and knockouts of nearby genes] to better understand the associated biology.
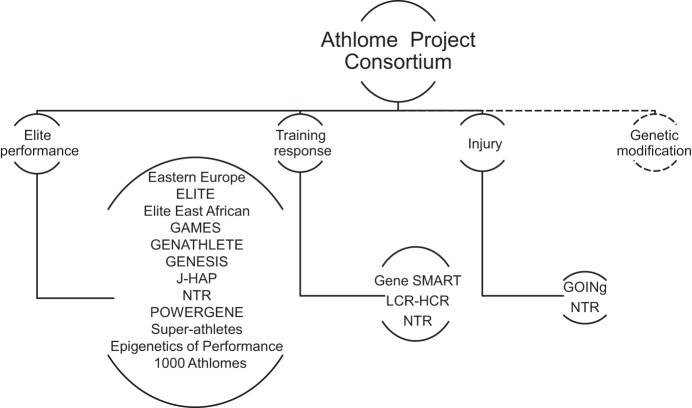
During the development of the initial phase of the Athlome Project, in determining the genetic variations related to elite athletic performance and injury predisposition, epigenomic, transcriptomic, and proteomic analyses need also be carefully planned to strengthen the understanding of gene functions. Linking these findings with metabolic profiling (the end products of the cellular processes) is also a future aspiration of the Athlome Project. Another challenge is to be able to efficiently integrate the multiple “omics” datasets generated from the different approaches. The ultimate goal of the Athlome Project Consortium is to generate the ethically sound environment, interest, and capacity needed to develop the specialist knowledge to inform personalized training and injury prevention, as well as doping detection. The following individual or collaborative study groups have agreed to work together in the global partnership that constitutes the Athlome Project Consortium. The participating cohorts and the focus of each are depicted in Fig. 1.
Fig. 1.
The Athlome Project Consortium. Genomic, epigenomic, transcriptomic, proteomic, and metabolomic studies are being conducted by the participating centers to address questions in the 3 main research areas: elite performance, training response, and injury. Future investigations planned include studies on genetic modification.
Eastern Europe Population Studies (The Russian and Belarusian Cohorts, GELAK, GELAV, and GUAP)
The Russian and Belarusian cohorts, the Genetics and Epigenetics of Lithuanian Athletes from Kaunas (GELAK) and Vilnius (GELAV), and the Genome of Ukrainian Athletes Project (GUAP) have consolidated to identify genetic and epigenetic variations associated with high-level sports performance. The cohort comprises East Europeans (from Belarus, Lithuania, Russia, and Ukraine; in total n = 8,228 athletes and n = 4,121 controls). The athletes are grouped into international (including participants in Olympics and world championships), national, regional, or local/noncompetitive categories. These include biathletes, distance runners, cyclists, triathletes, kayakers, rowers, canoers, modern pentathletes, orienteers, skiers, speed skaters, short-trackers, walkers, weightlifters, bodybuilders, powerlifters, strongmen, sprint runners (≤ 400 m), sprint swimmers (50–100 m), decathletes, heptathletes, combat athletes, field athletes, bobsleigh athletes, rhythmic and artistic gymnasts, figure skaters, fencers, and team ball-sport players. A portion of the participants have been evaluated with a variety of quantitative performance- and health-related assessments, including strength/power-related measurements, agility/speed-related measurements, balance, flexibility and coordination measurements, endurance-related measurement, skeletal muscle biopsy, and health-related measurements.
Principal Investigators: Ildus I. Ahmetov [Volga Region State Academy of Physical Culture, Sport and Tourism, Russia (RUS)], Svitlana B. Drozdovska [National University of Physical Education of Ukraine, Ukraine (UKR)], Colin N. Moran [University of Stirling, United Kingdom (UK)], Valentina Ginevičienė [Vilnius University, Lithuania (LTU)], Andrei A. Gilep [Institute of Bioorganic Chemistry NASB, Belaurs (BLR)].
ELITE http://elite.stanford.edu
The Exercise at the Limit - Inherited Traits of Endurance (ELITE) consortium is a global initiative with the main objective to map the role that genetics plays in athletic ability vs. environmental factors, such as training. Study participant (n > 600) selection is based on a physiological variable relevant for both health and sport performance, i.e., maximum oxygen uptake (V̇o2max). The main inclusion criterion is V̇o2max > 75 ml·kg−1·min−1 for men and > 63 ml·kg−1·min−1 for women, respectively. The consortium is continuously expanding and is recruiting athletes from all over the globe (with main focus on Caucasians, North East Africans, East Asians, and South Americans) who are successful in endurance sports (running, cycling, cross-country skiing, triathlon, and rowing). Analyses currently include enhanced whole exome sequencing and GWAS (1.7 million SNPs). The combination of analytic methods will enable findings and differentiation between common variants with small effects and novel rare variants with larger effects. The aim is also to investigate sex and ethnic differences.
Principal Investigators: Euan A. Ashley, C. Mikael Mattsson, Matthew Wheeler, Daryl Waggott (Stanford University, USA).
Elite East African Athlete Cohort
The consortium also aims to study the East African running success by analyzing data from previously recruited subjects: 1) 76 endurance runners (64 men) and 38 sprint and power event athletes (18 men) from the Ethiopian national athletics teams, 315 controls from the general Ethiopian population (281 men), 93 controls from the Arsi region of Ethiopia (80 men) and 2) 291 elite Kenyan endurance athletes (232 men) and 85 control participants (40 men). Seventy (59 men) Kenyan athletes had competed internationally and achieved outstanding success.
Principal Investigators: Yannis Pitsiladis (University of Brighton, UK), Robert Scott (University of Cambridge, UK).
GAMES
An international consortium (GAMES) was established to compare allele frequencies between elite endurance athletes and ethnicity-matched controls. GWASs were undertaken on two cohorts of elite endurance athletes (GENATHLETE and Japanese endurance runners) and their respective controls, from which a panel of 45 candidate SNPs was identified. These markers were tested for replication in seven additional cohorts of endurance athletes and controls from Australia, Ethiopia, Japan, Kenya, Poland, Russia, and Spain. The study is based on a total of 1,520 endurance athletes (835 of them had competed in world championships or Olympic Games) and 2,760 controls.
Principal Investigators: Claude Bouchard, Tuomo Rankinen (Pennington Biomedical Research Center, Louisiana State University, USA), Noriyuki Fuku [Juntendo University, Japan (JPN)], Yannis Pitsiladis (University of Brighton, UK), Bernd Wolfarth [Humboldt University, Germany (DEU)], Alejandro Lucia [Universidad Europea de Madrid, Spain (ESP)].
GENATHLETE
The study was launched in 1993 with the aim of identifying DNA variants that are present at different frequencies between elite endurance athletes and sedentary controls. Male endurance athletes and controls were recruited from Canada, Finland, Germany, and the USA. The cohort assembled to date includes 315 elite endurance athletes and 320 matched controls. Selection criteria for the all-male endurance athlete sample include that they had to be athletes of national or international caliber with a V̇o2max of at least 75 ml·kg−1·min−1. The mean value for the 315 athletes is currently 79 ml·kg−1·min−1, while the mean for the 320 control subjects reached 40 ml·kg−1·min−1. Multiple candidate genes have been studied using the resources of GENATHLETE. A genome-wide screen for common variants has been performed on GENATHLETE (see GAMES cohort above) and further studies are focusing on nuclear and mitochondrial DNA sequencing.
Principal Investigators: Claude Bouchard, Tuomo Rankinen (Pennington Biomedical Research Center, Louisiana State University System, USA), Bernd Wolfarth (Department of Sports Medicine, Charité Medical School, Berlin, DEU), Louis Perusse (Laval University, Quebec, Canada), Rainer Rauramaa (University of Eastern Finland, Kuopio, Finland).
GENESIS
The GENetics of Elite Status In Sport (GENESIS) consortium aims to identify molecular genetic characteristics associated with successful sports performance. The cohort (current n > 1,200) is mainly composed of UK athletes. Sports include marathon running and other track-and-field athletics, cycling, and team sports (e.g., soccer). The RugbyGene Study is a major subcomponent of GENESIS and focuses on rugby (both union and league codes). Objectives of GENESIS are: 1) to increase current cohort size substantially, 2) to apply hypothesis-free approaches to identify molecular genomic markers, 3) to expand GENESIS from genomics to other omics, and 4) to combine the omics data with athlete health and performance data to maximize practical impact of GENESIS.
Principal Investigators: Alun G. Williams, Stephen H. Day, Georgina K. Stebbings (Manchester Metropolitan University, UK), Robert M. Erskine (Liverpool John Moores University, UK), Hugh E. Montgomery (University College London, UK).
Gene SMART Study http://www.vu.edu.au/speed-gene
The Gene SMART (Skeletal Muscle Adaptive Response to Training) study aims to identify the gene variants that predict the skeletal muscle response to both a single bout and 4 wk of high-intensity interval training in three different training centers. While the lead training and testing center is located in Victoria University, Melbourne, two other centers have been launched at Bond University, Australia, and the University of Sao Paulo, Brazil. A fourth center (University of Brighton, UK) will focus on the omics analyses. The cohort comprises moderately trained, healthy male participants (aged 20–45 yr, body mass index ≤30 kg/m2). Participants are undergoing similar exercise testing and exercise training in three different laboratories. Dietary habits are assessed by questionnaire and nutritionist consultation. Activity history is assessed by questionnaire and current activity level is assessed by activity monitoring. A number of muscle and blood analyses are to be performed, including genotyping, mitochondrial respiration, transcriptomics, proteomics, and enzyme activity before, during and after training, where appropriate. Currently ∼40 participants have finished the study, and the aim is to train a total of 250 participants. The Gene SMART also includes baseline and posttraining testing and sampling for all participants.
Principal Investigators: David Bishop, Nir Eynon [Victoria University, Australia (AUS)].
GOINg
The recently established Genomics Of INjuries (GOINg) consortium aims to identify DNA variants that modify the risk of anterior cruciate ligament (ACL) injuries. It is the only consortium within the Athlome Project to specifically investigate exercise-associated musculoskeletal injuries. The plan is to screen current known loci for ACL injury susceptibility in larger data sets in an attempt to determine if they remain as susceptibility loci across all populations using the hypothesis-driven candidate gene case-control study design. Care will be taken to use the same criteria to accurately phenotype, with respect to ancestry, sporting, and occupational details, injury profile and mechanism(s) of injury, other injury history and family history, as well as other appropriate medical history and medication use. The actual functional significance of the identified variants will also be investigated. This initial phase will be followed by sequencing and the research objectives will be eventually expanded to include other omics. Thus far, ACL rupture consortium has collected DNA samples and clinical, as well as physical and occupational activity information from subjects from South Africa, Poland, Australia, Russia, and Italy.
Principal Investigators: Malcolm Collins, Alison September, Michael Posthumus [University of Cape Town, South Africa (ZAF)], Nir Eynon (Victoria University, AUS), Pawel Cieszczyk [University of Szczecin, Poland (POL)].
J-HAP
The Japanese Human Athlome Project (J-HAP) focuses on the study of genes associated with physical performance and its related phenotypes (e.g., muscle mass, muscle fiber type, V̇o2max). The cohort comprises Japanese athletes (currently > 2,400, mainly international and national levels) and healthy Japanese controls (currently > 1,000). These athletes are mainly track-and-field athletes and swimmers competing in endurance- and sprint/power-oriented events. Multiple omics approaches will be used to determine genes in talent identification in the Japanese population. Among the collected Japanese athletes' and controls' samples, ∼200 muscle biopsies were obtained from both athletes and controls to investigate genetic variants associated with muscle fiber type distribution.
Primary Investigators: Noriyuki Fuku (Juntendo University, JPN), Naoki Kikuchi (Nippon Sport Science University, JPN), Eri Miyamoto-Mikami (The National Institute of Fitness and Sports in Kanoya, JPN).
NTR
The Netherlands Twin Register (NTR) is a population-based cohort recruiting both newborn and adult multiples and their family members with continuous longitudinal data collection. In the past 25+ yr, around 40% of all twins and multiples in the Netherlands have taken part in the NTR research projects. Family members and spouses of twins also took part, leading to a total of over 185,000 participants across multiple research projects. The longitudinal information that has been collected extends from genotype to biomarkers, gene expression to rich behavioral information including biennial reports on (competitive) sports participation and performance level and on injuries related to sports. In its sports research track, NTR aims to understand the interplay between genetic and environmental factors shaping individual differences in sports participation and performance. In the NTR, participants are recruited as newborns and followed into young adulthood, 520 have played competitively at a regional and 189 at a national level. The main sports that Dutch adolescents/young adults engage in are swimming, tennis, bicycling, soccer, and field hockey. The longitudinal data collection of the NTR is ongoing and securely funded for the next 5 yr.
Principal Investigators: Eco de Geus, Meike Bartels [Vrije Universiteit (VU University) and VU Medical Centre, the Netherlands (NLD)].
POWERGENE
The POWERGENE consortium aims to characterize the elite sprint/power athlete genotype. The internationally competitive (Olympic/world championship qualifiers) sprint/power athletes are from: Australia, Belgium, Greece, Italy, Jamaica, Japan, Lithuania, Poland, Spain, the USA, Brazil, and Russia. They will be compared with subelite athletes (national qualifiers), endurance athletes, team athletes, and controls. The current cohort consists of female (n = 264) and male (n = 481) specialist power athletes across three major ethnicities (i.e., European, West African, and East Asian ancestries). Sprint/power athletes include those individuals competing in track (≤ 800 m) and field (jump, throw) events, cycling (track), swimming (≤ 200 m), gymnastics (artistic), weightlifting, judo, speed-skating, and power lifting. Endurance athletes (n = 586) include track and road running specialists (> 800 m), rowers, cyclists, swimmers (> 200 m), triathletes and ironmen. Team sports (n = 862) include football (soccer), cricket, hockey, volleyball, and basketball.
Principal Investigators: Yannis Pitsiladis (University of Brighton, UK), Kathryn North (Murdoch Childrens Research Institute, AUS), Nir Eynon (Victoria University, AUS).
Super-athletes: Genes and Sweat
The study aims to 1) identify genetic variants associated with elite athletic performance, 2) study potential ethnic differences, and 3) study the functional significance of the identified variants. A GWAS will be carried out in 3,000 consented elite athletes, tested negative for doping substances at the Anti-Doping Laboratories, Federazione Medico Sportiva Italiana (FMSI), and Anti-Doping Lab Qatar (ADLQ), using Illumina genotyping technologies. Examining genotype frequency distribution of elite athletes from European countries (where most of FMSI samples will be obtained) against those from South Asian and African countries (where most of ADLQ samples are expected to be obtained) would help to identify potential ethnic differences in the genetic predisposition to athletic performance. Subsequently, urine metabolome in a subset of these athletes (1,000 subjects) will be performed and will be related to the athlete's sporting discipline.
Principal Investigators: Mohamed El-Rayess, Costas Georgakopoulos, Mohammed Alsayrafi [ADLQ, Qatar (QAT)], Francesco Botre [FMSI, Italy (ITA)], Karsten Suhre (Weill Cornell Medical College in Qatar, QAT), Mike Hubank (University College London, UK).
Epigenetics of Elite Athletic Performance
It is clear from animal and human studies that epigenetic marks play a role in the modulation of gene expression in relevant tissues. There also are indications that epigenetic marks can be altered by acute and chronic exercise in skeletal muscle and adipose tissue where they have been studied. Thus individual differences in any exercise-related traits can potentially be explained not only by the impact of DNA sequence variation on biology and behavior but also by the effects of epigenomic signaling on gene expression. We are formulating the hypothesis that elite athletic performance is influenced by epigenomic alterations, facilitating morphological, physiological, metabolic, cognitive, emotional, and behavioral changes that empower the athlete to push performance beyond existing boundaries. We envisage testing this hypothesis by recruiting twin athletes competing at the Olympic or world championship levels.
Principal Investigators: Vassilis Klissouras [University of Athens, Greece (GRC)], Yannis Pitsiladis (University of Brighton, UK).
Rat Models of Exercise and Health (LCR-HCR rat model)
The purpose of the Low Capacity Rats-High Capacity Rats (LCR-HCR) model is to serve as a resource for the in-depth study of rat models to resolve the extremes of exercise and health. By connecting clinical observation with a theoretical base, the working hypothesis is that: variation in capacity for energy transfer is the central mechanistic determinant between disease and health (energy transfer hypothesis). As an unbiased test of this hypothesis, this study showed that two-way artificial selective breeding of rats for low and high intrinsic endurance exercise capacity also produced rats that differed for numerous disease risks, including the metabolic syndrome, premature aging, fatty liver disease, obesity, and Alzheimer's disease. Exercise capacity is a result of intrinsic capacity plus adaptation to all aspects of physical activity. To capture this biology, rats for low and high response to 8 wk of treadmill running exercise were selectively bred. Thus, the study has models that represent the four “corners” of exercise capacity. These contrasting animal model systems may prove to be translationally superior relative to more widely used simplistic models for understanding disease conditions. The rat models may be deeply explored to discover causal mechanisms and develop effective therapeutics. These rats are being studied at over 50 institutions in 11 countries.
Principal Investigators: Steven Britton, Lauren Koch (University of Michigan, USA).
1000 Athlomes Project
The 1000 Athlomes Project aims to sequence 1000 genomes of sprinters and distance runners of West and East African descent. Phase 1 of the project has already commended and involves the sequencing of 12 sprinters and 12 distance runners of the highest level (i.e., world record holders, Olympians, and world champions). Phase 2 (2016–2018) will involve increasing the sample size for sequencing to 100 genomes. The pool of the runners to be sequenced will be expanded to 1,000 by 2020 (phase 3). An important aim of this sequencing project is to document the genotype distribution of elite East and West African athletes. The large amount of genotype data to be generated from the 1000 Athlomes Project will serve as 1) a reference panel for future performance studies and 2) a guide for other extreme phenotype studies in medical science.
Principal Investigators: Masashi Tanaka (Tokyo Metropolitan Institute of Gerontology, JPN), Yannis Pitsiladis (University of Brighton, UK).
Ethical Principles for Athlome Biobanking
The rise of biobanking has brought about a whole range of issues that are not all wholly relevant to the Athlome Project. Nevertheless, certain key principles must be noted here that will inform the governance framework for Athlome: 1) the consortia are global in reach, but there is no universal agreement on the precise nature of ethically justifiable governance for biobanking; 2) given the globality of the consortia, no single regional (e.g., European, American) framework ought to be adopted; 3) a general framework drawing on widely shared principles should be discussed and adopted. Chief among the concerns, but only one among several, is the problem of consent.
Each of the projects that comprise Athlome are existing bioguardians with a duty to protect the rights of participants who have contributed their samples to the individual projects noted above. The collection, storage, access to, and use by researchers of those samples have been approved by relevant regulatory authorities (e.g., institutional review boards, research ethics committees, national health services research ethics services) appropriate to the lead institution of the individual projects/consortia. Existing procedures do not currently extend to the sharing of samples beyond the study, since consent models are prospective (i.e., they guide future actions of researchers) and typically entail a form of specificity and the specific consent obtained varies between project partners. No retrospective consent is feasible, and this is a widely shared problem for biobank development. Since the form of collaboration Athlome envisages was not laid out before participants gave their consent, it might be concluded that the sharing of data beyond the original research group and its stated purposes invalidates that consent. The problem for Athlome is not an uncommon one for biobank collaborations since it seeks retrospective extension of the consent model.
An ethical solution to this problem and related consent problems for new participants is to consider the use of a technique such as “broad consent.” The nomenclature here is important since this notion is variously described as “broad consent,” “blanket consent,” “future consent,” “hypothetical consent,” “passive/tacit/silent consent,” or “waived consent” (4, 5). This would entail asking participants to agree to future unspecified uses of their data that are un(der)determined in the consent process and relevant forms (6). Without sufficient grasp of the uses of the data or with whom it might be shared, this process fails the test of “comprehension”: a user must understand sufficiently what they are agreeing to (3). Another possibility going forward would be “meta-consent,” where consent is sought for broad categories of unspecified future research (7, 8). Others have argued with respect to biobanking that the ethical issues entailed (e.g., privacy, confidentiality, ownership of access to the data) may be sufficiently assuaged by rigorous anonymization (1) and associated practices of data storage, though this is far from universally agreed upon (2).
The Athlome project will develop principles and protocols for safeguarding participants rights to access, confidentiality, privacy of data, and assurances that there is no significant mission drift of the kind of which is permitted under some conceptions of broad consent (or its similes). This would, for example, prohibit commercialization of participants' data. To preserve the integrity of this process and the principles, rigorous anonymization processes will be developed by a partner institution that does not have any direct role in data collection, storage, or analysis. This will assure independence and integrity to the process. This is especially important in this case since some of the research participants are public figures, which increases the likelihood that someone might be interested in reidentifying their data and genomic sequences. The independent institution would also have an oversight of each new proposal for the Athlome Project going forward to ensure compliance with those principles and protocols.
In conclusion, by presenting the main study cohorts and projects that are currently included in the Athlome consortium not only do we intend to show a global view of the main studies and initiatives that will be performed in the foreseeable future in the field of sports genomics (and that are likely to provide new exciting findings), we also wish to motivate potential collaboration initiatives with other research groups worldwide. International collaborations are likely to go well beyond the study of sports performance per se. Indeed, the Athlome consortium presents a unique chance to study the biology of the best elite athletes across most ethnicities, which is profoundly interesting from a medical point of view. World-class athletes represent the actual end-point of the human continuum of fitness-related phenotypes. In this regard, there is growing evidence (coming from both human and rodent study approaches, such as those included in the consortium) that not only physical activity levels but also individual fitness levels (a trait that has a strong genetic component independent of activity levels) are inversely associated with the risk of major cardiometabolic diseases of Western civilization, several cancer types, and Alzheimer's disease. Thus, studying the genes of elite athletes offers a unique chance to gain insight into important medical conditions, including genetic predisposition (or resilience) to chronic disease. Indeed, the “rare-common” strategy, underpinned by ethically sound research governance, is a valuable approach model to examine general mechanisms of disease pathophysiology, with world-class athletes representing the “rare” (“super-fit”) human phenotype. Finally, identifying genetic markers of exercise capacity, adaptation to exercise programs, and the predisposition to injury is certain to provide useful information to prescribe personalized exercise interventions in the context of 21st-century medicine, which should not be based only on identifying new drug targets but also on implementing lifestyle interventions for disease prevention at the individual level.
ATHLOME PROJECT CONSORTIUM
Corresponding author: Yannis P. Pitsiladis
Steering Committee: Yannis P. Pitsiladis (Chair), Nir Eynon, Claude Bouchard, Kathryn N. North, Alun G. Williams, Malcolm Collins, Colin N. Moran, Steven L. Britton, Noriyuki Fuku, Eco de Geus, Vassilis Klissouras, Euan A. Ashley, Alejandro Lucia, Ildus I. Ahmetov, Mohammed Alsayrafi, and Masashi Tanaka.
Consortium Group: University of Brighton, UK: Yannis P. Pitsiladis1 (principal investigator), Nick Webborn,1 Guan Wang1; Victoria University, AUS: Nir Eynon2 (Principal Investigator), David J. Bishop2 (principal investigator), Ioannis Papadimitriou,2 Xu Yan,2 Oren Tirosh,2 Jujiao Kuang2; Pennington Biomedical Center, USA: Claude Bouchard,3 Tuomo Rankinen,3 Mark Sarzinsky3; Stanford University, USA: Euan A. Ashley,4 C. Mikael Mattsson,4 Matthew Wheeler,4 Daryl Waggott4; Bond University, AUS: Nuala M. Byrne5; University of Sao Paulo, BRA: Guilherme G. Artioli6; University of Cape Town, ZAF: Malcolm Collins7 (principal investigator), Alison September,7 Michael Posthumus,7 Willem van der Merwe7; Gdansk University of Physical Education and Sport, POL; University of Szczecin, POL: Pawel Cieszczyk8,9 (principal investigator), Agata Leonska-Duniec,8,9 Krzysztof Ficek,9 Agnieszka Maciejewska-Karlowska,9 Marek Sawczuk,9 Marta Stepien-Slodkowska9; Epworth Healthcare Melbourne, AUS: Julian Feller10; Aspetar, QAT: Paul Dijkstra11; Ural State University of Physical Culture, RUS: Aleksandr M. Chmutov,12 Dmitry A. Dyatlov12, Evgeniy F. Orekhov,12 Yuliya E. Pushkareva,12 Irina A. Shvedkaya12; University of Cagliari, ITA: Myosotis Massidda,13 Carla M. Calò13; Manchester Metropolitan University, UK; University College London, UK; Liverpool John Moores University, UK: Alun G. Williams14,15 (principal investigator), Stephen H. Day,14 Georgina K. Stebbings,14 Robert M. Erskine,15,16 Hugh E. Montgomery15; Murdoch Childrens Research Institute, AUS: Kathryn N. North17 (principal investigator), Fleur C. Garton,17 Peter Houweling17; Ghent University, BEL: Wim Derave,18 Audrey Baguet18; Universidad Europea de Madrid, ESP: Alejandro Lucia,19 Carlos A. Muniesa19; University of Foggia, ITA: Francesco Sessa,20 Annamarie Petito20; Nottingham Trent University, UK: Craig Sale,21 David C. Hughes21, Ian Varley21 (principal investigator); VU University Amsterdam, NLD: Eco de Geus22 (principal investigator), Dorret Boomsma,22 Meike Bartels,22 Gareth E. Davies22; Vilnius University, LTU; Lithuanian Olympic Sports Centre, LTU: Valentina Ginevičienė23,24 (principal investigator), Audronė Jakaitienė,23 Vaidutis Kučinskas,23 Linas Tubelis,24 Algirdas Utkus23; Lithuanian University of Educational Sciences, LTU: Kazys Milašius25 (principal investigator), Linas Tubelis25; University of Stirling, UK: Colin N. Moran26 (principal investigator); Lithuanian Sports University, LTU: Tomas Venckunas,27 Albertas Skurvydas,27 Arvydas Stasiulis27; University of Glasgow, UK: Dalia Malkova,28 Richard Wilson28; University of Michigan, USA: Steven L. Britton29 (principal investigator), Lauren G. Koch29 (principal investigator); Juntendo University, JPN: Noriyuki Fuku30 (principal investigator), Hirofumi Zempo,30 Hisashi Naito,30 Noriko Ichinoseki-Sekine; Nippon Sport Science University, JPN: Naoki Kikuchi31; National Institute of Fitness and Sports in Kanoya, JPN: Eri Miyamoto-Mikami32; National Institute of Health and Nutrition, NIBIOHN, JPN: Haruka Murakami,33 Motohiko Miyachi33; Japan Institute of Sports Sciences, JPN: Hideyuki Takahashi,34 Nao Ohiwa,34 Takashi Kawahara34; Toyo University, JPN: Hiroyasu Tsuchie35; University of Nagasaki, JPN: Takuro Tobina36; The Open University of Japan, JPN: Noriko Ichinoseki-Sekine37; Fukuoka University, JPN: Hiroaki Tanaka38; Waseda University, JPN: Koji Kaneoka39; Nippon Sport Science University, JPN: Koichi Nakazato40; Sport Technology Research Centre Kazan, RUS; Kazan State Medical University, RUS: Ildus I. Ahmetov41,42 (principal investigator), Emiliya S. Egorova,42 Leysan J. Gabdrakhmanova,41,42 Alina A. Arkhipova,42 Alyona V. Borisova,42 Rashid T. Gabbasov,42 Albina A. Stepanova,41 Ravil I. Kashapov41; St. Petersburg Research Institute of Physical Culture, RUS: Victor A. Rogozkin43 (principal investigator), Irina V. Astratenkova,43, Anastasiya M. Druzhevskaya,43 Olga N. Fedotovskaya,43 Natalya D. Golberg,43 Albina M. Hakimullina43; Research Institute for Physical-Chemical Medicine Moscow, RUS: Elena S. Kostryukova,44 Dmitry G. Alexeev,44 Edward V. Generozov,44 Dmitry S. Ischenko,44 Nickolay A. Kulemin,44 Andrey K. Larin,44 Elena A. Ospanova,44 Alexander V. Pavlenko,44 Vadim M. Govorun44; The National Academy of Sciences of Belarus, BLR: Andrei A. Gilep45 (principal investigator), Irina L. Gilep,45 Irina V. Haidukevich,45 Irina L. Rybina45; National University of Physical Education and Sport of Ukraine, UKR: Svitlana B. Drozdovska46 (principal investigator), Victor E. Docenko,46 Vladimir N. Ilyin46; M. Akmullah Bashkir State Pedagogical University, RUS: Eugeniy Lekontsev47; Moscow Department of Physical Culture and Sport, RUS: Egor B. Akimov48; Anti-Doping Lab Qatar, QAT: Mohamed El-Rayess,49 Costas Georgakopoulos,49 Mohammed Alsayrafi49; FMSI, ITA: Francesco Botre50; Weill Cornell Medical College in Qatar, QAT: Karsten Suhre51; University College London, UK: Mike Hubank52; University of Athens, GRC: Vassilis Klissouras53; University of Humboldt and Charité Medical University, DEU: Bernd Wolfarth54,55; Army Recruiting and Training Division, UK: Julie P. Greeves56; Canadian Sport Institute Pacific, CAN: Trent Stellingwerff57; Cardiff Metropolitan University, UK: Craig Ranson58; University of East Anglia, UK: William D. Fraser59,60; Institute of Health and Biomedical Innovation, Queensland University of Technology, AUS: Rebecca Grealy,61 Lyn Griffiths61; University of Cambridge, UK: Robert Scott62; The Swedish School of Sport and Health Sciences, SWE: C. Mikael Mattsson63; Tokyo Metropolitan Institute of Gerontology, JPN: Masashi Tanaka64,65; Physiology Laboratory of the Urals Research Center for Radiation Medicine of the Federal Medical-Biological Agency of Russia, RUS: Vladimir P. Pushkarev66.
Affiliations: 1FIMS Reference Collaborating Centre of Sports Medicine for Anti-Doping Research, University of Brighton, Eastbourne, UK; 2Institute of Sport, Exercise, and Active Living (ISEAL), Victoria University, Melbourne, Australia; 3Human Genomics Laboratory, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, USA; 4Stanford University Medical Center, Stanford, USA; 5Bond Institute of Health and Sport (BIHS), Bond University, Gold Coast, Australia; 6Laboratory of Applied Nutrition and Metabolism, School of Physical Education and Sport, University of Sao Paulo, Sao Paulo, Brazil; 7Division of Exercise Science and Sports Medicine, Department of Human Biology, University of Cape Town, Cape Town, South Africa; 8Department of Tourism and Recreation, Academy of Physical Education and Sport, Gdansk, Poland; 9Department of Physical Culture and Health Promotion, University of Szczecin, Szczecin, Poland; 10Epworth Healthcare, Melbourne, Australia; 11Department of Sport Medicine, Aspetar, Doha, Qatar; 12Genetic Laboratory, Ural State University of Physical Culture, Chelyabinsk, Russia; 13Department of Environmental and Life Science, University of Cagliari, Cagliari, Italy; 14Department of Exercise and Sport Science, Manchester Metropolitan University, Crewe, UK; 15Institute of Sport, Exercise and Health (ISEH), University College London, London, UK; 16Research Institute for Sport and Exercise Sciences (RISES), Liverpool John Moores University, Liverpool, UK; 17Murdoch Childrens Research Institute, Royal Children's Hospital, Melbourne, Australia; 18Department of Movement and Sports Sciences, Ghent University, Ghent, Belgium; 19School of Doctorate Studies & Research, Universidad Europea de Madrid, Madrid, Spain; 20Medical Genetics Unit, Department of Medical Sciences and Department of Clinical and Experimental Medicine, University of Foggia, Foggia, Italy; 21Musculoskeletal Physiology Research Group, Sport, Health and Performance Enhancement Research Centre, Nottingham Trent University, Nottingham, UK; 22VU University and VU Medical Centre, Amsterdam, Netherlands; 23Department of Human and Medical Genetics, Vilnius University, Vilnius, Lithuania; 24Lithuanian Olympic Sports Centre, Vilnius, Lithuania; 25Department of Sport Teaching Methods, Lithuanian University of Educational Sciences, Vilnius, Lithuania; 26Health and Exercise Sciences Research Group, University of Stirling, Stirling, UK; 27Lithuanian Sports University, Kaunas, Lithuania; 28School of Medicine, University of Glasgow, Glasgow, UK; 29Department of Anesthesiology, University of Michigan Medical School, Ann Arbor, USA; 30Graduate School of Health and Sports Science, Juntendo University, Chiba, Japan; 31Sports Training Center, Nippon Sport Science University, Tokyo, Japan; 32Department of Sports and Life Science, National Institute of Fitness and Sports in Kanoya, Kagoshima, Japan; 33Department of Health Promotion and Exercise, National Institute of Health and Nutrition, Tokyo, Japan; 34Department of Sports Science/Medical Centre, Japan Institute of Sports Sciences, Tokyo, Japan; 35Faculty of Law, Toyo University, Tokyo, Japan; 36Faculty of Nursing and Nutrition, University of Nagasaki, Nagasaki, Japan; 37Faculty of Liberal Arts, The Open University of Japan, Chiba, Japan; 38Institute for Physical Activity, Fukuoka University, Fukuoka, Japan; 39Faculty of Sports Sciences, Waseda University, Saitama, Japan; 40Department of Exercise Physiology, Nippon Sport Science University, Tokyo, Japan; 41Sport Technology Research Centre, Volga Region State Academy of Physical Culture, Sport and Tourism, Kazan, Russia; 42Laboratory of Molecular Genetics, Kazan State Medical University, Kazan, Russia; 43Sports Genetics Laboratory, St. Petersburg Research Institute of Physical Culture, St. Petersburg, Russia; 44Research Institute for Physical-Chemical Medicine, Moscow, Russia; 45Institute of Bioorganic Chemistry NASB, Minsk, Belarus; 46National University of Physical Education and Sport of Ukraine, Kiev, Ukraine; 47M. Akmulla Bashkir State Pedagogical University, Ufa, Russia; 48Centre for Sports Innovation Technologies and National Teams of the Moscow Department of Physical Culture and Sport, Moscow, Russia; 49Anti-Doping Lab Qatar (ADLQ), Doha, Qatar; 50Anti-Doping Lab, Federazione Medico Sportiva Italiana (FMSI), Italy; 51Department of Physiology and Biophysics, Weill Cornell Medical College in Qatar, Doha, Qatar; 52Centre for Translational Omics, University College London, London, UK; 53Ergophysiology Research Laboratory, Department of Sport Medicine and Biology of Physical Activity, University of Athens, Athens, Greece; 54Department of Sports Sciences, University of Humboldt, Berlin, Germany; 55Laboratory of Sports Medicine, Charite Medical University, Berlin, Germany; 56Department of Occupational Medicine, Headquarters Army Recruiting and Training Division, UK; 57Canadian Sport Institute Pacific, Pacific Institute for Sport Excellence, Victoria, British Columbia, Canada; 58Sports injury Research Group, Cardiff School of Sport, Cardiff Metropolitan University, Cardiff, UK; 59Norwich Medical School, University of East Anglia, UK; 60Norfolk and Norwich University Hospital, Norwich, UK; 61Genomics Research Centre, Institute of Health and Biomedical Innovation (IHBI), Queensland University of Technology, Brisbane, Australia; 62MRC Epidemiology Unit, University of Cambridge, Cambridge, UK; 63Åstrand Laboratory of Work Physiology, The Swedish School of Sport and Health Sciences, Stockholm, Sweden; 64Department of Longevity and Health, Tokyo Metropolitan Institute of Gerontology, Tokyo, Japan; 65Department of Clinical Laboratory, Tokyo Metropolitan Geriatric Hospital, Tokyo, Japan; 66Physiology Laboratory of the Urals Research Center for Radiation Medicine of the Federal Medical-Biological Agency of Russia, Chelyabinsk, Russia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.P.P., M.T., N.E., C.B., K.N.N., A.G.W., M.C., C.N.M., S.L.B., N.F., E.A.A., V.K., A.L., I.I.A., E.J.d.G., and M.A. drafted manuscript.
ACKNOWLEDGMENTS AND GRANTS
Eastern Europe population studies (The Russian and Belarusian cohorts, GELAK, GELAV, GUAP) were supported by the grants from the Federal Agency for Physical Culture and Sport of the Russian Federation and the Ministry of Education and Science of the Russian Federation (contract number 02.522.11.2004), the Federal Medical-Biological Agency of the Russian Federation (“Sportgen project”), Republic of Belarus (State Program of Development of Physical Culture and Sports for 2011–2015), and Royal Society International joint project grant from the UK (code F-90014). GELAV (Epigenetics of Lithuanian Athletes from Vilnius) project was developed by the Lithuanian National Olympic Committee and Lithuanian Olympic Sports Centre, while actual research was carried out at the Vilnius University, where Lithuanian athletes DNA samples are stored. We thank Prof. Vaidutis Kučinskas, Department of Human and Medical Genetics, Faculty of Medicine, Vilnius University, Lithuania, for providing ideas and support for accessing the control samples.
ELITE is supported by SAP/Stanford Sequencing Initiative and Women's Heart Health at Stanford.
GAMES was partially funded by the Prince Faisal Prize awarded to C. Bouchard, T. Rankinen, M. Sarzynski, and B. Wolfarth. CB is partially funded by the John W. Barton Jr. Chair in Genetics and Nutrition. The study in Russia was supported by a grant from the Federal Medical-Biological Agency (“Sportgen project”), http://fmbaros.ru/en/fmba/infor/. The Spanish group is funded by Cátedra Real-Madrid Universidad Europea and Fondo de Investigaciones Sanitarias and Fondos Feder (Grant PI12/00914). This work in Japan was supported in part by grants from the programs Grants-in-Aid for Challenging Exploratory Research (24650414 to NF) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and by a Grants-in-aid for Scientific Research (KAKENHI) from the Ministry of Health, Labor, and Welfare of Japan (to MM). No specific funding for this work was received for the studies performed in Australia, Poland, Kenya, and Ethiopia.
Gene SMART Study is partly supported by N. Eynon's Australian Research Council Early Career Fellowship DE#140100864 and by the Victoria University Central Research Grant Scheme.
GOINg is supported by funds from the National Research Foundation of South Africa and the Thembakazi Trust.
J-HAP project was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grants 21680050, 11J04771, 24650414, 26882041, 15H03081, and 15K16467) and by MEXT-commissioned projects.
NTR's funding was obtained from the Netherlands Organization for Scientific Research (NWO) and The Netherlands Organisation for Health Research and Development (ZonMW) grants 904-61-090, 985-10-002, 912-10-020, 904-61-193, 480-04-004, 463-06-001, 451-04-034, 400v05-717, Addiction-31160008, Middelgroot-911-09-032, Spinozapremie 56-464-14192, Center for Medical Systems Biology (CSMB, NWO Genomics), NBIC/BioAssist/RK(2008.024), Biobanking and Biomolecular Resources Research Infrastructure (BBMRI-NL, 184.021.007). VU University's Institute for Health and Care Research (EMGO+) and Neuroscience Campus Amsterdam; the European Science Foundation (EU/QLRT-2001-01254), the European Community's Seventh Framework Program (FP7/2007–2013), ENGAGE (HEALTH-F4-2007–201413); the European Research Council (ERC Advanced, 230374, ERC Starting Grant 284 167), Rutgers University Cell and DNA Repository (NIMH U24 MH-068457-06), the Avera Institute, Sioux Falls, SD (USA), and the National Institutes of Health (NIH, R01D0042157-01A, MH-081802; R01 DK-092127-04, Grand Opportunity Grants 1RC2 MH-089951 and 1RC2 MH-089995). Computing was supported by BiG Grid, the Dutch e-Science Grid, which is financially supported by NWO.
Super-athletes: Genes and Sweat is partly funded by Qatar National Research Foundation NPRP Grant (NPRP 7-272-1-041).
POWERGENE GWAS genotyping in Jamaicans, African-Americans (the USA cohort) and Japanese was funded by JSPS KAKENHI (Grants 21680050 and 24650414).
Rat models of exercise and health (LCR-HCR rat model) was funded by the NIH Office of Research Infrastructure Programs/OD Grant R24OD-010950 and by Grant R01DK-099034 (to LGK and SLB). We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp. Contact: LGK lgkoch@umich.edu or SLB brittons@umich.edu for information on the LCR and HCR rats; these rat models are maintained as an international resource with support from the Department of Anesthesiology at the University of Michigan, Ann Arbor, MI.
1000 Athlomes Project is funded by KAKENHI (A)-15200051, (A)-22240072, (A)-25242062 from MEXT and JSPS.
REFERENCES
- 1.Caplan AL. What no one knows cannot hurt you: the limits of informed consent in the emerging world of biobanking. In: The Ethics of Research Biobanking, edited by Solbakk JH, Holm S, Hofmann B. Springer US, 2009, p. 25–32. [Google Scholar]
- 2.Greely HT. The uneasy ethical and legal underpinnings of large-scale genomic biobanks. Annu Rev Genomics Hum Genet 8: 343–364, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Hansson MG. Ethics and biobanks. Br J Cancer 100: 8–12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansson MG, Dillner J, Bartram CR, Carlson JA, Helgesson G. Should donors be allowed to give broad consent to future biobank research? Lancet Oncol 7: 266–269, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann B. Broadening consent-and diluting ethics? J Med Ethics 35: 125–129, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann B, Solbakk JH, Holm S. Consent to biobank research: one size fits all? In: The Ethics of Research Biobanking edited by Solbakk JH, Holm S, Hofmann B. Springer US, 2009, p. 3–23. [Google Scholar]
- 7.Holm S, Ploug T. Patient choice and preventive genomic sequencing-more trouble upstream. Am J Bioeth 15: 24–26, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Ploug T, Holm S. Meta consent: a flexible and autonomous way of obtaining informed consent for secondary research. BMJ 350: h2146, 2015. [DOI] [PubMed] [Google Scholar]