To The Editor
Acute pancreatitis (AP) and diabetes have a bidirectional relationship. In a recent systematic review1, the pooled prevalence of pre-diabetes and diabetes after the first-attack of AP was 16% (95% confidence interval [CI] 9–24) and 23% (95% CI 16–31) respectively. The risk of new-onset diabetes at the end of one year was 15% which increased by 2.7 fold at 5 years. Interestingly, age, sex, etiology or the severity of AP had little or no effect on subsequent development of diabetes.
We hypothesized that the risk of diabetes is dependent on the severity of AP. Among patients enrolled in the Severe Acute Pancreatitis Study (SAPS), a well-characterized cohort at the University of Pittsburgh Medical Center2, 3, we evaluated the probability of and factors influencing the development of diabetes after AP.
From 162 patients with first-attack of AP enrolled between 2003–2010, after excluding those who died during the index admission (n=10) or had no follow-up information available (n=25), 127 patients formed the final study cohort4. Medical records were reviewed retrospectively. Diabetes was defined using the World Health Organization criteria5, 6. Transient hyperglycemia during the index hospitalization was not considered to represent diabetes. Treatment with oral pancreatic enzyme replacement therapy (PERT) for clinical suspicion of exocrine insufficiency, pancreatitis-related readmissions and imaging changes of chronic pancreatitis during follow-up were recorded.
Mean age of patients was 53.4±19.1 years, 49% were male, 83% were Caucasians, 49% were transfers from other institutions and 47% had biliary pancreatitis. Consistent with our tertiary care cohort, a high fraction of patients had intensive care unit (ICU) admission (33%), persistent organ failure (OF) (27%), and pancreatic necrosis with/without peripancreatic fluid collections (28%). Median length of stay during index admission was 9 (IQR 5,19) days, and the median follow up was 53 (IQR 19,86) months.
Of the 127 patients, 26 (20.4%) had pre-existing diabetes, 73 (57.4%) did not develop diabetes and 28 (22%) developed new-onset diabetes (19 [67.8%] during index admission, 9 [32.1%] during follow up, median time to diagnosis was 34.5 months [IQR 16.5, 63]). The risk of new-onset diabetes in non-transfered patients without pre-existing diabetes was 17% (8/47). Diabetes in these patients occurred more frequently during follow up (5/8, 62.5%) than during index admission (3/8, 32,5%).
When compared with patients who did not develop diabetes, those who developed new-onset diabetes during index hospitalization were more likely (p<0.05) to be male (63 vs. 49%) and have severe disease as reflected by a high prevalence of transfers (84 vs. 49%), ICU admission (79 vs. 17%), persistent OF (84 vs. 11%) and pancreatic necrosis (79 vs. 10%). All 19 patients with new-onset diabetes during index admission had either pancreatic necrosis or OF (vs. 15% who did not develop diabetes). ICU admission (33%), necrosis (44%) and persistent OF (22%) in patients developing diabetes during the follow-up also tended to be higher when compared with patients who did not develop diabetes (p>0.05, likely due to Type-II error). The risk of new-onset diabetes increased in the presence of OF and with amount of necrosis (Figure 1).
Figure 1.
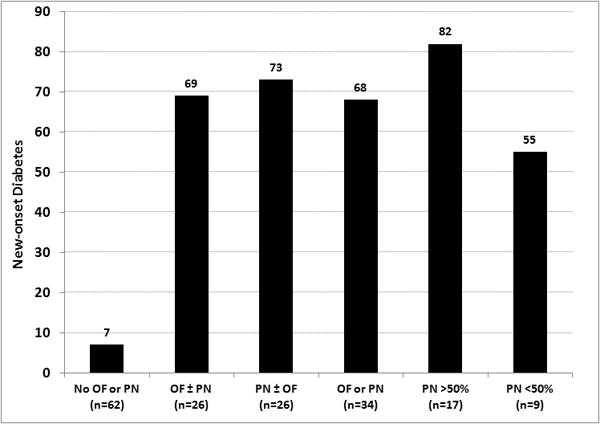
Risk of new-onset diabetes based on persistent organ failure and pancreatic necrosis OF - persistent organ failure, PN - pancreatic necrosis
The risk of readmissions was significantly higher in patients with new-onset diabetes (32.1 vs. 13.7%, p=0.047). Pancreatic calcifications were noted in 6/127 (4.7%) patients (1 with preexisting diabetes, 3 who did not and 2 who developed new-onset diabetes).
Oral PERT was given to 11/127 (8.7%) patients (6 clinical steatorrhea, 5 pancreatic surgery/debridement). Of these, 9 also developed new-onset diabetes (6 index admission, 3 follow-up). Pancreatic necrosis was noted in 7/11 (63.6%) and pancreatic calcifications during follow-up in 4/11 (36.4%) patients.
Primary finding of our analysis is that the risk of new-onset diabetes after AP is determined by disease severity. This observation is driven by the presence and amount of necrosis, which can result in islet cell destruction in the affected part of the pancreas. It is important however to recognize that a subset of patients without necrosis also develop diabetes by mechanisms that need further study. Due to a small sample size, we could not evaluate the effect of demographic factors, family history and etiology on diabetes risk.
Extrapolating our results to a community population will translate into a risk of new-onset diabetes after mild AP of 10–15% and after severe AP/necrosis of 30–50% over a 3–5 year period. This risk appears to be higher than what would be expected during a similar period in an age- and sex-matched population without diabetes7 - although formal studies should determine the risk attributable to AP.
Internists and gastroenterologists should be aware of the high risk of diabetes after AP, particularly in patients with severe disease and those with recurrent disease. Until formal recommendations are available, screening for diabetes at appropriate intervals (once a year) by formal testing (fasting blood glucose and/or hemoglobin A1C levels) should therefore be considered. Studies should also quantify the benefits of early diagnosis on diabetes-related outcomes.
Acknowledgments
This study was presented as an oral presentation at the Annual Meeting of the American College of Gastroenterology in October 2014 and published as an abstract in Am J Gastroenterol 2014;109:S237.
Grant Support: None
Footnotes
Conflict of Interest: The authors report no conflict of interest relevant to this manuscript.
Authorship criteria and contributions:
Kishore Vipperla: Study design, data acquisition, analysis and interpretation, drafting and revising the article, final approval of the version to be published.
Georgios I. Papachristou: Study supervision, data acquisition and interpretation, critical review of the manuscript for important intellectual content, final approval of the version to be published.
Adam Slivka, David C Whitcomb: Data interpretation, critical review of the manuscript for important intellectual content, final approval of the version to be published.
Dhiraj Yadav: Study design, study supervision, data analysis and interpretation, drafting and revising the article, final approval of the version to be published.
References
- 1.Das SL, Singh PP, Phillips AR, et al. Newly diagnosed diabetes mellitus after acute pancreatitis: a systematic review and meta-analysis. Gut. 2014;63:818–831. doi: 10.1136/gutjnl-2013-305062. [DOI] [PubMed] [Google Scholar]
- 2.Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476–1482. doi: 10.1053/j.gastro.2012.03.005. quiz e15–16. [DOI] [PubMed] [Google Scholar]
- 3.Nawaz H, Mounzer R, Yadav D, et al. Revised Atlanta and determinant-based classification: application in a prospective cohort of acute pancreatitis patients. Am J Gastroenterol. 2013;108:1911–1917. doi: 10.1038/ajg.2013.348. [DOI] [PubMed] [Google Scholar]
- 4.Vipperla K, Papachristou GI, Easler J, et al. Risk of and factors associated with readmission after a sentinel attack of acute pancreatitis. Clin Gastroenterol Hepatol. 2014;12:1911–1919. doi: 10.1016/j.cgh.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Definitions and diagnosis of diabetes mellitus and intermediate hyperglycaemia. Available at: http://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ Accessed 12.11.14.
- 6.Use of Glycated hemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Available at: http://www.who.int/diabetes/publications/report-hba1c_2011.pdf. Accessed 12.11.14.
- 7.Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/diabetes/statistics/incidence_national.htm. Accessed 12.20.14.


