Abstract
Purpose of review
Robust and dynamic innate and adaptive responses characterize the acute central nervous system (CNS) response to HIV and other viral infections. In a state of chronic infection or viral latency, persistent immune activation associates with pathology in the CNS. Understanding this process is critical, since immune-mediated pathology in non-renewable CNS cells may result in long-term neurologic sequelae for HIV infected individuals.
Recent findings
In humans, immune activation is reduced by suppressive combination antiretroviral therapy (cART), but persists at abnormally elevated levels on treatment. CNS immune activation is initiated in acute infection and progressively increases until cART is started. Newly identified characteristics of the CNS immune surveillance network include features of homeostasis and function of brain microglial cells, lymphatic drainage from CNS to cervical lymph nodes, and cells in cerebrospinal fluid associated with neurocognitive impairment.
Summary
More research is required to determine whether early intervention to reduce infection limits the immunopathology established by sustained immune responses that ultimately fail to resolve infection, and to unravel mechanisms of persistent immune activation during treated HIV so that strategies can be developed to therapeutically protect the brain.
Keywords: HIV-1, central nervous system, cerebrospinal fluid, immune activation, neuroinflammation
Introduction
We go all wrong by too strenuous a resolution to go all right.
~ Nathaniel Hawthorne, The Marble Faun, 1860
Activation of the immune system is a pathologically appropriate response to invading pathogens including viral infection. In most acute infections, immune activation provides a beneficial role in control and eventual clearance of invading pathogens and resolution of infectious processes. In chronic infections, the role of immune activation becomes more complex. Surveillance and activity of the immune system contributes to control and suppression of pathogen replication and spread. However, stimulation of processes associated with immune surveillance and activity can have long-term deleterious effects. The negative impact of chronic systemic immune activation in untreated as well as treatment suppressed HIV infection has been well documented, and is detailed in other articles in this issue. These considerations are unique in the central nervous system (CNS), where there is a dynamic relationship between the systemic immune environment and that in the CNS compartment, a unique set of factors involved in immune surveillance, and a differential impact of combination antiretroviral therapy (cART). Finally, within the brain the immune process is the primary determinant of neuropathology and the essential functional cells – neurons -- are nonrenewable, leading to a potential long-term impact of even transient immune activation in this compartment. The following review will examine new knowledge about the persistence of immune activation in the setting of suppressive cART, early CNS immune activation in HIV, and the function of cells and tissues involved in the CNS immune response to viral infection.
Considerations for immune activation in the CNS compartment
The CNS is actually a number of tissues (including brain parenchyma, meninges, perivascular spaces, choroid plexus, and cerebrospinal fluid (CSF)) comprising a distinctive immunologic environment with unique immune sentries equipped to respond to and contain viral infections (for review, see:[1]). This CNS immune response is distinct from that in the systemic circulation, but also impacted by the systemic compartment and likely also interactive with it. Prior work has clearly established that immune activation in the CNS in untreated late stages of SIV or HIV infection associates strongly with neuronal injury, pathologic signs of HIV and SIV encephalitis, and HIV-associated dementia (HAD) (for review see: [2, 3]). Both in vivo and in vitro studies have suggested that damage to neurons in HIV is mediated by inflammatory processes and perhaps toxic effects of certain viral proteins, rather than by direct viral infection of neurons. Several clinical studies have confirmed that HIV associated neurocognitive impairment (HAND) in treatment naïve individuals associates with elevated inflammatory cytokines and chemokines in the CSF, but not elevated HIV RNA levels [4].
CNS immune activation in treated HIV infection: persistent immune activation, CSF escape and CD8 encephalitis
Most of our understanding of the role of neuroinflammation in HIV pathogenesis derives from studies in untreated patients with advanced disease or from simian immunodeficiency virus (SIV) models enhanced for neuropathogenesis[5–7]. However, multiple methods of assessment of the structure and function of the brain indicate that neurological injury is detected in humans with HIV despite systemically virologically suppressive treatment. This neurologic injury may be subclinical but detected by sensitive measures used for investigation into pathogenesis and treatment[8–12], or may be clinically evident in rare cases of ‘CSF escape’ or CD8 encephalitis where HIV replication or an immune response to HIV antigen underlies and active progressive neurologic decline[13–15].
A host of innate and adaptive immune responses are involved in the response of the CNS to acute and chronic HIV infection, and the activities of many of these are difficult to discern in the living human. Table 1 lists measures of CNS immune activation that have been used clinically and in research efforts to examine the aspects of CNS immune responses in SIV and HIV. CSF biomarkers have been a primary source of insight into CNS immune activation, given the feasibility of sampling this CNS tissue in living volunteers. A small number of soluble markers have been most commonly measured to assess processes of immune activation in the CNS. CSF measurement of neopterin, a stable pteridine biomarker, reflects activation of macrophages and microglial cells within the CNS[16, 17]. CSF neopterin has been noted to be predicted of HAD in untreated HIV/AIDS, and to decline with the initiation of cART, commensurate with decreased risk of HAD[18, 19]. Prior studies have documented elevations in CSF neopterin above the upper normal reference value after several years of suppressive cART in a majority of individuals compared to a minority with elevated levels in blood [11]. However the source of ongoing macrophage activation in this setting is unclear. Recently, a large study of asymptomatic individuals with cART-suppressed HIV infection to < 50 copies HIV RNA in plasma for up to 10 years demonstrated that elevated levels of CSF neopterin associate with the presence of detectable CSF HIV RNA by a single copy assay. [20]. Individuals with asymptomatic CSF escape, or detectable CSF HIV RNA with concomitant undetectable plasma HIV RNA, also manifest elevation of CSF neopterin[21]. Though there are likely to be several drivers of CNS macrophage activation in individuals on suppressive treatment, these findings demonstrate that HIV release or even ongoing low-level viral replication in the CNS tissue compartment is a cause of, or alternately provoked by, local immune activation.
Table 1.
Research measures used to assess CNS immune activation in HIV infection.
| Cerebrospinal fluid: | Neuroimaging: |
|---|---|
Soluble biomarkers:
|
Magnetic resonance spectroscopy (MRS):
|
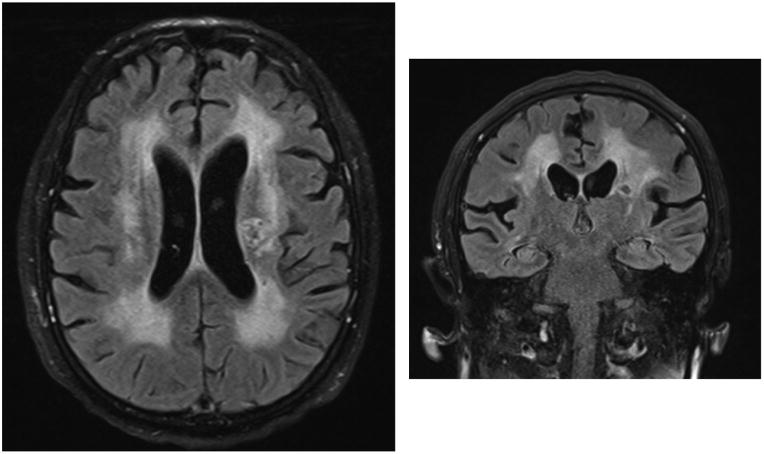
Symptomatic CSF escape and CD8 encephalitis, rare clinically overt neurologic syndromes in patients on suppressive cART, may reflect an extreme consequence of immune activation response to low-level viral antigen. CSF escape is characterized by progressive of neurologic signs and symptoms in patients on cART with discordance between CSF and blood HIV RNA [13, 14, 22, 23]. HIV may be undetectable in blood while detected in CSF; in others, CSF is one log10 higher than a fairly well controlled level in the blood. However, in almost all described cases, the progressive neurologic disorder is dramatic and severe, while the levels of HIV RNA detected in CSF can be as low as 100 copies/ml. In our experience, when available, CSF inflammatory markers and imaging measures have been markedly elevated and may improve with treatment [14]. Figure 1 shows the brain MRI images of an individual with symptomatic CSF escape, demonstrating inflammatory changes throughout the brain despite less than 100 copies/ml CSF HIV. Though CSF escape has been posited to relate to an inability of cART to adequately suppressive local CNS HIV replication, the extent of neuroinflammation involved despite low levels of HIV suggests that this disorder is enhanced by robust local immune responses to antigen in a setting of immune preservation. In this sense symptomatic CSF escape may share pathologic features with CNS immune reconstitution inflammatory syndrome (IRIS) in a more stable stage of immune recovery. The fact that low CD4 nadir is a risk factor for symptomatic CSF escape supports this hypothesis. CSF escape in the setting of concurrent inflammatory CNS opportunistic infection has been well documented, further suggesting that immune perturbation may trigger HIV replication[24].
Figure 1.
Brain MRI images demonstrating abnormalities suggestive of widespread CNS inflammation in symptomatic CNS escape. Axial FLAIR (left) and coronal FLAIR (right) sequences of brain show diffuse periventricular T2 hyperintensities predominantly in the white matter of the brain in a patient presenting with 4 weeks of progressive neurological decline with new paucity of speech, disorientation, slowed movements, and inability to walk. Plasma HIV RNA level was <20 copies/ml, CSF HIV RNA was only 99 copies/ml, and CSF white blood cell count was 18 cells/ul (normal < 5 cells/ul). After adjustment of the patient’s antiretroviral regimen to enhance potency in the CNS, signs and symptoms resolved and CSF HIV RNA was undetectable and pleocytosis resolved.
CD8 T cell encephalitis also presents typically in patients on stably suppressive cART, and is likely on a similar spectrum with CSF escape. In this pathologically proven immune mediated condition, patients present with severe neurologic compromise in the setting of suppressed HIV, and are found to have widespread cerebral inflammation with perivascular CD8+ T lymphocyte infiltration on brain biopsy[15, 25]. In most of these patients, CSF HIV RNA is undetectable, and HIV RNA has not consistently been detected in the brain. This condition can respond dramatically to early initiation of immune suppression with corticosteroids, suggesting a primary aberrant immune response etiology. Though rare disorders, these clinical entities provide an important proof-of-concept that immune mediated pathology can develop in the CNS despite systemically suppressive therapy, and raise the possibility that a form of HIV-associated IRIS may impact the CNS at a subclinical level more broadly in treated HIV infection.
Whether persistent low-grade CNS immune activation in well-controlled HIV is sufficient for progression of clinically manifest HAND is unclear. A recent study suggests that immune activation associates with more subtle progressive neurological impairment in patients on suppressive cART. CSF neopterin, CSF neurofilament light chain (NFL), a measure of active neuronal injury, and neuropsychological test performance were longitudinally measured in 100 cART-treated individuals with consistent plasma viral suppression to < 50 copies/ml HIV RNA [26]. Though CSF NFL did not distinguish individuals with neurocognitive impairment in the study, CSF neopterin was higher at baseline in those with mild forms of HAND than those without HAND. Importantly, elevated CSF neopterin but not NFL at baseline also distinguished those with neurocognitive performance decline over time. Since CSF neopterin reflects CNS macrophage activation, these data suggest that even low-grade immune activation in the CNS in treated patients may underlie mild but progressive neurocognitive impairment in this setting.
Given evidence that immune activation associates with CNS injury in HIV, further studies are needed to determine whether reversal of persistent low-grade CNS immune activation in cART treated patients may be a safe and effective means of preventing or halting the progression of HAND. To date, medications with mild anti-inflammatory properties have been studied as adjunctive therapies in treatment of HAND, without clear evidence of effect. The anti-inflammatory antibiotic minocycline has been suggested as a potential therapy for HAND and neuroprotective in animal models [27, 28], but randomized studies have not indicated an impact on immune or clinical endpoints in humans [29–31]. Anti-inflammatory properties of statins may have beneficial end-organ effects in HIV infected persons; studies of statin use in HAND are in development. Treatment intensification as a means of reducing immune activation through enhanced suppression of viral replication has not demonstrated a benefit in levels of CSF immune activation when instituted in chronic or acute infection[32–34], but larger studies are in progress.
Immune activation in the CNS during early infection and the impact of early cART
Most acute systemic viral infections are accompanied by systemic immune activation that can contribute to neuroimmune activation as result of increased trafficking of activated immune cells to the CNS. In HIV, however, immune activation is triggered in the CNS not only from the impact of systemic immune activation, but by the neurotropic nature of the virus, which passes directly into the CNS compartment during acute infection. Though the systemic immunology of acute HIV in terms of the specific cells and signals involved has been extensively examined[35, 36], much less is known about the immunopathogenic processes involved in the initial CNS response to HIV. Independent studies have documented a mild lymphocytic pleocytosis in the CSF during primary infection, and elevations in soluble cytokines associated with influx of lymphocytes (CXCL10) and monocytes (CCL-2) into the CNS compartment[37–39]. These findings suggest that a cascade of neuroinflammation is established early in infection, enhancing the trafficking of lymphocytes and monocytes into the CNS. During acute infection we observe little divergence between the degree of immune activation in the periphery and in the CNS. In acute infection, this may reflect the fact that a local infection of the CNS with an autonomous source of immune perturbation has not yet been established. HIV replication in the CNS can be established within the first year of infection, however, associated with heightened immune activation in this compartment [40]. CSF neopterin is elevated in acute and primary infection, suggesting that immune activation, while incited by processes in the periphery, may stimulate activation of resident CNS cells.
The Primary Infection Stage CNS Events Study (PISCES) examined the natural history of CNS immune responses to recent HIV infection through assessment of CSF immune responses and inflammation measured by brain magnetic resonance spectroscopy (MRS). Individuals in PISCES were enrolled at a median four months post infection and longitudinally followed prior to and after electively starting cART. Neuroimaging revealed progressive mild increases in levels of inflammatory metabolites in multiple brain regions over the course of the first year of HIV infection prior to cART [41]. CSF measures indicated increases in CSF neopterin over this period in the majority of individuals, and parallel increases in CSF CD4+ and CD8+ T lymphocyte activation (as measured by percent CD38+HLADR+ cells) over time without treatment[42]. These data suggest that CNS immune activation is not a transient process present only during the profound inflammatory setting of acute infection. In fact, it is triggered during this period and, in the absence of treatment, worsens over time even during the early stages of infection.
In a short period of follow up of PISCES participants after cART initiated at a median of six months post infection, inflammatory metabolites on MRS did not normalize in the CNS, consistent with findings of persistent immune activation observed in most studies of chronic HIV. However in a complementary study of individuals identified in Thailand during acute HIV and started on immediate cART within the first month of infection, initially elevated MRS markers of inflammation improved to levels in HIV uninfected controls even after a short time on treatment[43]. The same Thai study examined CSF YKL-40, a biomarker of inflammation derived from activated microglial cells and predictive of SIV encephalitis in a macaque model. CSF YKL-40 levels were lower in cART naïve acute HIV than in chronic HIV, and were similar between acute HIV and HIV-uninfected Thais[44]. After 6 months of cART started during acute infection, CSF YKL-40 remained normal, and was reduced compared to the group treated in chronic infection. This study suggests that microglial activation may be prevented by early cART, and is consistent with other findings from this cohort that treatment during very acute HIV may be neuroprotective[45]. More evidence from this cohort and similar studies of the impact of early cART on the CNS may provide supportive rationale for improved screening for recent HIV infection and prompt referral to treatment.
Immune cells and tissues involved in pathogenesis
Microglial cell activation is a hallmark of the pathology of HIV encephalitis associated with advanced disease[46, 47], and as noted above is a feature of early HIV infection and may associate with subtle forms of HAND. Although microglial activation is known to associate with numerous inflammatory and degenerative neurologic disorders, there has previously been little understanding of the maintenance and stability of these key CNS cells. Microglia are long-lived cells recognized as a possible reservoir for latent HIV infection as well as a persistent source of immune perturbation once activated. Although previously believed to be at least in part derived from trafficking activated peripheral monocytes, recent studies have indicated that brain microglia are of embryonic origin and even under inflammatory conditions do not derive from the migration of circulating myeloid precursor cells [48, 49]. Importantly, intravital imaging of microglial cells in a rodent model has recently demonstrated that the microglial response to CNS viral infection is instantaneous and dynamic [50, 51]. The concept that microglia are static and quiescent has been replaced by an understanding that they are involved in immediate broad immune activation signaling upon exposure to viral invasion, and are mobile cells that ‘scan’ the brain for invading pathogens. Additionally microglial cell induction of inflammosomes (innate immune response protein complexes that respond to pathogen associated molecular patterns) is precipitated during acute lentiviral infection, and inflammosome activation associates with brain pathology in a feline immunodeficiency model of HIV encephalitis [52]. Finally, HIV-Tat protein may be a direct trigger or in a pathway associated with processes leading to microglial activation, though the extent to which this is an important cause of ongoing activation in latent cART treated infection is unclear [53, 54]. Overall, this new understanding of the homeostasis and behavior of microglia supports their major role in contributing to the problem of persistent CNS immune activation in HIV: microglia respond to acute HIV in an immediate and dynamic fashion, and once perturbed they are resident cells of the CNS that may harbor integrated HIV long-term, associated with constant cellular immune activation.
Though the CNS is an immune privileged site wherein immune responses to infection are tightly constrained, new evidence indicates that it is not an isolated system in terms of segregation of immune responses from the systemic circulation. Lymphatic vessels adjacent to the dura that drain from the brain to the deep cervical lymph nodes have been identified in the mouse and in human tissue[55, 56]. These vessels serve as physical conduits from CNS out to the periphery, draining both CSF and brain interstitial fluid. Cells and macromolecules can be transported out of the brain via this newly recognized system, stimulating peripheral immune responses. This finding has particular import to HIV in that it suggests that persistent immune activation in the CNS could provoke immune responses in the systemic circulation. Furthermore, it suggests that HIV infected cells in the CNS, if mobile, could theoretically travel out of this compartment and ‘re-seed’ the periphery, raising the issue that HIV infection in a CNS ‘reservoir’ could present a barrier to systemic HIV eradication.
Finally, despite a longstanding consideration that macrophages and microglia are the main mediators of HIV-related CNS injury, potentially important roles are emerging for CNS CD4+ and CD8+ T lymphocytes in HAND. Certain brain disorders in treated HIV are associated with copious CD8+ T lymphocyte infiltration rather than microglial nodules or activated macrophages[25]. Although brain pathology of HIV has not classically shown numerous parenchymal T lymphocytes, is possible that pathogenically important lymphocytes may cluster in CNS tissues such as the meninges, where lymphoid cell collections have been noted in patients with multiple sclerosis [57, 58]. Compartmentalized CSF HIV in the early stages of infection appears to be produced by lymphocytes within the CNS, rather than by the macrophages that are a key source of CNS HIV in advanced infection[40]. Cellular phenotypes of CD4+ and CD8+ T lymphocytes and monocytes in CSF and blood have been defined by multiparameter flow cytometry studies HIV-infected subjects and controls, indicating that CSF is primarily composed of CD8+ T lymphocytes both pre-and post-cART, with CD8+ T cell % higher in CSF than blood [59, 60]. Recent work demonstrates that percentage of CD8+ T lymphocytes in CSF, and in particular those that express interferon gamma, positively associate with the presence of HAND even in individuals on virologically successful cART [61]. In an independent study of participants mostly on suppressive cART, increased markers of CSF CD4+ CD8+ T lymphocyte activation and exhaustion correlated with poorer performance on neuropsychological testing and periventricular brain imaging abnormalities suggestive of inflammation [62]. These studies suggest a pathologic role of CNS lymphocyte activation that persists despite cART. Finally, adoptive immunotherapy using virus-specific CD8+ T lymphocytes has recently been shown to clear persistent lymphocytic choriomeningitis virus (LCMV) from mouse neurons and microglia without inciting significant cytopathic changes, suggesting that HIV-specific T lymphocyte responses themselves may benefit the CNS if the accompanying pathologic aspects of more generalized CNS immune activation can be limited[63].
Conclusions
Treatment with cART typically prevents the most severe form of HIV associated neurocognitive disorder (HAND), HIV-associated dementia (HAD), and is associated with a marked improvement in active CNS replication, immune activation, and neuronal injury [18, 60, 64, 65]. However, the CNS is persistently abnormal in some individuals with apparently successful systemic viral suppression on cART, and the underlying mechanisms of persistent CNS perturbation in humans on cART are currently poorly understood. Recent studies have confirmed that immune activation is a ubiquitous feature of untreated HIV infection, beginning during the earliest stages of infection. Its features – tissues, cells, and pathways involved – may differ between individuals and perhaps change over the course of infection and treatment. CNS immune activation in the earlier stages of HIV derives from lymphocytes and monocytes, while in chronic or advanced disease is a product of microglia and tissue macrophages. Multiple measures of the CNS reveal that though systemically suppressive cART treatment substantially blunts immune activation, there are persistent levels of CNS immune abnormality in some individuals on cART. In extreme cases, CNS immune activation is a hallmark of clinically relevant disorders of CSF escape and CD8 encephalitis in patients on cART, posited to be the result of balance between pathogen persistence and the response of a robust immune system. It is possible that in some stages of disease, immune activation in the CNS is beneficial, or even critical in targeting infected cells and checking levels of viral replication. On the other hand, activation of the immune system allows for enhanced trafficking of cells into the CNS, presumably amplifying the amount of HIV brought into this compartment, stimulating a cascade of cytokine release and further influx of cells into the CNS, and allowing for activation of resident cells of the CNS including tissue macrophages and microglial cells. Once these processes have been established, it may be that even in the face of complete suppression of viral replication, abnormal chronic microglial activation contributes to ongoing neuronal dysfunction, and clinical HAND. Future investigations are needed to explore the characteristics and mechanisms of persistent CNS immune activation and the potential value of earlier cART or adjunctive immune-modifying therapies in the prevention and treatment of CNS-related inflammation and injury.
Key points.
HIV-associated neurocognitive disorder (HAND) in individuals off of combination antiretroviral therapy (cART) is primarily caused by immune activation within the CNS.
Elevated central nervous system markers of immune activation despite suppressive cART associate with low levels of persistent detectable HIV and may contribute to ongoing HAND.
Immune activation is initiated in the CNS within days of initial HIV infection and progressively increases over time prior to the initiation of cART.
Newly recognized characteristics of the robust and complex system relevant to CNS immune activation include dynamic nature of microglial cells, presence of a brain lymphatic system draining to the cervical lymph nodes, and pathogenic functions of T lymphocytes in the CNS.
Acknowledgments
The author thanks her colleagues especially Dr. Richard W. Price for their discussions surrounding the content of this manuscript, and Ms. Leah T. Le and Zaina Zayyad for their support.
Financial support and sponsorship
This author was supported by NIH awards R01MH095613, R01NS084911, and R21 MH099979 and the Department of Neurology, Yale University, New Haven, USA.
Footnotes
Conflicts of interest
None.
References
- 1.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spudich S, Gonzalez-Scarano F. HIV-1-Related Central Nervous System Disease: Current Issues in Pathogenesis, Diagnosis, and Treatment. Cold Spring Harb Perspect Med. 2012;2:a007120. doi: 10.1101/cshperspect.a007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol. 2009;22:315–320. doi: 10.1097/WCO.0b013e328329cf3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Airoldi M. Neurocognitive impairment in HIV-infected naïve patients with advanced disease: the role of virus and intrathecal immune activation. Clinical & developmental immunology. 2012;2012:467154. doi: 10.1155/2012/467154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams K, Burdo TH. Monocyte mobilization, activation markers, and unique macrophage populations in the brain: observations from SIV infected monkeys are informative with regard to pathogenic mechanisms of HIV infection in humans. J Neuroimmune Pharmacol. 2012;7:363–371. doi: 10.1007/s11481-011-9330-3. [DOI] [PubMed] [Google Scholar]
- 8.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 9.Becker JT, Sanders J, Madsen SK, Ragin A, Kingsley L, Maruca V, et al. Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav. 2011;5:77–85. doi: 10.1007/s11682-011-9113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardenas VA, Meyerhoff DJ, Studholme C, Kornak J, Rothlind J, Lampiris H, et al. Evidence for ongoing brain injury in human immunodeficiency virus-positive patients treated with antiretroviral therapy. J Neurovirol. 2009;15:324–333. doi: 10.1080/13550280902973960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50:773–778. doi: 10.1086/650538. [DOI] [PubMed] [Google Scholar]
- 14.Peluso MJ, Ferretti F, Peterson J, Lee E, Fuchs D, Boschini A, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26:1765–1774. doi: 10.1097/QAD.0b013e328355e6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lescure FX, Moulignier A, Savatovsky J, Amiel C, Carcelain G, Molina JM, et al. CD8 Encephalitis in HIV-Infected Patients Receiving cART: A Treatable Entity. Clin Infect Dis. 2013 doi: 10.1093/cid/cit175. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs D, Chiodi F, Albert J, Asjo B, Hagberg L, Hausen A, et al. Neopterin concentrations in cerebrospinal fluid and serum of individuals infected with HIV-1. AIDS. 1989;3:285–288. doi: 10.1097/00002030-198905000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Kuehne LK, Reiber H, Bechter K, Hagberg L, Fuchs D. Cerebrospinal fluid neopterin is brain-derived and not associated with blood-CSF barrier dysfunction in non-inflammatory affective and schizophrenic spectrum disorders. J Psychiatr Res. 2013;47:1417–1422. doi: 10.1016/j.jpsychires.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz A, Yiannoutsos CT, Fuchs D, Price RW, Crozier K, Hagberg L, et al. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J Neuroinflammation. 2013;10:62. doi: 10.1186/1742-2094-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brew BJ, Dunbar N, Pemberton L, Kaldor J. Predictive markers of AIDS dementia complex: CD4 cell count and cerebrospinal fluid concentrations of beta 2-microglobulin and neopterin. J Infect Dis. 1996;174:294–298. doi: 10.1093/infdis/174.2.294. [DOI] [PubMed] [Google Scholar]
- **20.Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS. 2014;28:2251–2258. doi: 10.1097/QAD.0000000000000400. Study revealing perisistent macrophage activation in cART-treated HIV in individuals with detectable levels of HIV RNA in cerebrospinal fluid, suggesting that immune activation in treated patients relates to CNS viral persistence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202:1819–1825. doi: 10.1086/657342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogoch II, Davis BT, Venna N. Reversible dementia in a patient with central nervous system escape of human immunodeficiency virus. J Infect. 2011;63:236–239. doi: 10.1016/j.jinf.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Bingham R, Ahmed N, Rangi P, Johnson M, Tyrer M, Green J. HIV encephalitis despite suppressed viraemia: a case of compartmentalized viral escape. Int J STD AIDS. 2011;22:608–609. doi: 10.1258/ijsa.2011.010507. [DOI] [PubMed] [Google Scholar]
- 24.Falcone EL, Adegbulugbe AA, Sheikh V, Imamichi H, Dewar RL, Hammoud DA, et al. Cerebrospinal fluid HIV-1 compartmentalization in a patient with AIDS and acute varicella-zoster virus meningomyeloradiculitis. Clin Infect Dis. 2013;57:e135–142. doi: 10.1093/cid/cit356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray F, Lescure FX, Adle-Biassette H, Polivka M, Gallien S, Pialoux G, et al. Encephalitis with Infiltration by CD8+ Lymphocytes in HIV Patients Receiving Combination Antiretroviral Treatment. Brain Pathol. 2013 doi: 10.1111/bpa.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Eden A, Franklin D, Zetterberg H, Fuchs D, Heaton R, Letendre S, et al. CNS immunoactivation and neuronal damage in patients wtih progressive neurocognitive impairment (CROI Abstract 474). Special Issue: Abstracts From the 2015 Conference on Retroviruses and Opportunistic Infections, Topics in Antiviral Medicine; Seattle, WA. 2015; p. 197. Preliminary report of an association between persistent abnormal CNS immune activation in virologically-suppressed HIV and presence and progression of HAND. [Google Scholar]
- 27.Campbell JH, Burdo TH, Autissier P, Bombardier JP, Westmoreland SV, Soulas C, et al. Minocycline inhibition of monocyte activation correlates with neuronal protection in SIV neuroAIDS. PLoS One. 2011;6:e18688. doi: 10.1371/journal.pone.0018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratai EM, Bombardier JP, Joo CG, Annamalai L, Burdo TH, Campbell J, et al. Proton magnetic resonance spectroscopy reveals neuroprotection by oral minocycline in a nonhuman primate model of accelerated NeuroAIDS. PLoS One. 2010;5:e10523. doi: 10.1371/journal.pone.0010523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho EL, Spudich SS, Lee E, Fuchs D, Sinclair E, Price RW. Minocycline fails to modulate cerebrospinal fluid HIV infection or immune activation in chronic untreated HIV-1 infection: results of a pilot study. AIDS Res Ther. 2011;8:17. doi: 10.1186/1742-6405-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakasujja N, Miyahara S, Evans S, Lee A, Musisi S, Katabira E, et al. Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology. 2013;80:196–202. doi: 10.1212/WNL.0b013e31827b9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacktor N, Miyahara S, Evans S, Schifitto G, Cohen B, Haughey N, et al. Impact of minocycline on cerebrospinal fluid markers of oxidative stress, neuronal injury, and inflammation in HIV-seropositive individuals with cognitive impairment. J Neurovirol. 2014;20:620–626. doi: 10.1007/s13365-014-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yilmaz A, Verhofstede C, D’Avolio A, Watson V, Hagberg L, Fuchs D, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55:590–596. doi: 10.1097/QAI.0b013e3181f5b3d1. [DOI] [PubMed] [Google Scholar]
- 33.Dahl V, Lee E, Peterson J, Spudich SS, Leppla I, Sinclair E, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis. 2011;204:1936–1945. doi: 10.1093/infdis/jir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valcour VG, Spudich SS, Sailasuta N, Phanuphak N, Lerdlum S, Fletcher JL, et al. Neurological Response to cART vs. cART plus Integrase Inhibitor and CCR5 Antagonist Initiated during Acute HIV. PLoS One. 2015;10:e0142600. doi: 10.1371/journal.pone.0142600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Picker LJ. Immunopathogenesis of acute AIDS virus infection. Curr Opin Immunol. 2006;18:399–405. doi: 10.1016/j.coi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Chang JJ, Altfeld M. Innate immune activation in primary HIV-1 infection. J Infect Dis. 2010;202(Suppl 2):S297–301. doi: 10.1086/655657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enting RH, Prins JM, Jurriaans S, Brinkman K, Portegies P, Lange JM. Concentrations of human immunodeficiency virus type 1 (HIV-1) RNA in cerebrospinal fluid after antiretroviral treatment initiated during primary HIV-1 infection. Clin Infect Dis. 2001;32:1095–1099. doi: 10.1086/319602. [DOI] [PubMed] [Google Scholar]
- 38.Spudich S. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. The Journal of infectious diseases. 2011;204:753–760. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *40.Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog. 2015;11:e1004720. doi: 10.1371/journal.ppat.1004720. In the first two years of HIV infection, cerebrospinal fluid pleocytosis associates with amplified HIV replication and compartmentalized HIV is adapted to replicated within T lymphocytes rather than macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young AC, Yiannoutsos CT, Hegde M, Lee E, Peterson J, Walter R, et al. Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology. 2014 doi: 10.1212/WNL.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **42.Suh J, Sinclair E, Peterson J, Lee E, Kyriakides TC, Li FY, et al. Progressive increase in central nervous system immune activation in untreated primary HIV-1 infection. J Neuroinflammation. 2014;11:199. doi: 10.1186/s12974-014-0199-y. CNS immune activation as measured by cerebrospinal fluid neopterin and percentage of CD38+HLADR+ CD4+ and CD8+ lymphocytes progressively increases after primary infection until the initiation of cART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sailasuta N, Ross W, Ananworanich J, Chalermchai T, DeGruttola V, Lerdlum S, et al. Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PLoS One. 2012;7:e49272. doi: 10.1371/journal.pone.0049272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peluso M, Valcour V, Ananworanich J, Fletcher JL, Tipsuk S, Slike B, et al. Astrocyte and microglial activation in acute and chronic HIV pre- and post-cART (CROI Abstract 473). Special Issue: Abstracts From the 2015 Conference on Retroviruses and Opportunistic Infections, Topics in Antiviral Medicine; Seattle, WA, USA. 2015; p. 195. [Google Scholar]
- 45.Peluso MJ, Valcour V, Ananworanich J, Sithinamsuwan P, Chalermchai T, Fletcher JL, et al. Absence of Cerebrospinal Fluid Signs of Neuronal Injury Before and After Immediate Antiretroviral Therapy in Acute HIV Infection. J Infect Dis. 2015;212:1759–1767. doi: 10.1093/infdis/jiv296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghorpade A, Persidsky Y, Swindells S, Borgmann K, Persidsky R, Holter S, et al. Neuroinflammatory responses from microglia recovered from HIV-1-infected and seronegative subjects. J Neuroimmunol. 2005;163:145–156. doi: 10.1016/j.jneuroim.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4:430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greter M, Merad M. Regulation of microglia development and homeostasis. Glia. 2013;61:121–127. doi: 10.1002/glia.22408. [DOI] [PubMed] [Google Scholar]
- 49.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *50.Rua R, McGavern DB. Elucidation of monocyte/macrophage dynamics and function by intravital imaging. J Leukoc Biol. 2015;98:319–332. doi: 10.1189/jlb.4RI0115-006RR. Review article detailing the use of novel live imaging techniques to visualize myeloid cell dynamics in tissues, including trafficking monocytes and resident microglial cells in brain tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nayak D, Zinselmeyer BH, Corps KN, McGavern DB. In vivo dynamics of innate immune sentinels in the CNS. Intravital. 2012;1:95–106. doi: 10.4161/intv.22823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *52.Walsh JG, Reinke SN, Mamik MK, McKenzie BA, Maingat F, Branton WG, et al. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology. 2014;11:35. doi: 10.1186/1742-4690-11-35. Activation of microglial cells inflammasomes occurs immediately after lentiviral infection in a feline immunodeficiency model and associates with neuropathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan M, Yao H, Cai Y, Liao K, Seth P, Buch S. HIV-1 Tat disrupts CX3CL1-CX3CR1 axis in microglia via the NF-kappaBYY1 pathway. Curr HIV Res. 2014;12:189–200. doi: 10.2174/1570162x12666140526123119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paris JJ, Singh HD, Carey AN, McLaughlin JP. Exposure to HIV-1 Tat in brain impairs sensorimotor gating and activates microglia in limbic and extralimbic brain regions of male mice. Behav Brain Res. 2015;291:209–218. doi: 10.1016/j.bbr.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **55.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. Description of lymphatic system in mouse brain that drains CSF and brain interstial fluid components to cervical lymph nodes, directly connecting what was previously thought to be isolated immune environment to the periphery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **56.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. Complementary publication to the above. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 59.Ho EL, Ronquillo R, Altmeppen H, Spudich SS, Price RW, Sinclair E. Cellular Composition of Cerebrospinal Fluid in HIV-1 Infected and Uninfected Subjects. PLoS One. 2013;8:e66188. doi: 10.1371/journal.pone.0066188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinclair E, Ronquillo R, Lollo N, Deeks SG, Hunt P, Yiannoutsos CT, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47:544–552. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *61.Schrier RD, Hong S, Crescini M, Ellis R, Perez-Santiago J, Spina C, et al. Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND) PLoS One. 2015;10:e0116526. doi: 10.1371/journal.pone.0116526. One of the first reports to associate percentage and function of CSF CD8+ T lymphocytes with HAND in treated HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *62.Grauer OM, Reichelt D, Gruneberg U, Lohmann H, Schneider-Hohendorf T, Schulte-Mecklenbeck A, et al. Neurocognitive decline in HIV patients is associated with ongoing T-cell activation in the cerebrospinal fluid. Ann Clin Transl Neurol. 2015;2:906–919. doi: 10.1002/acn3.227. Another study that associates T lymphocyte phenotype and function with the presence of HAND in mostly cART treated and suppressed subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herz J, Johnson KR, McGavern DB. Therapeutic antiviral T cells noncytopathically clear persistently infected microglia after conversion into antigen-presenting cells. J Exp Med. 2015;212:1153–1169. doi: 10.1084/jem.20142047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194:1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 65.Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology. 2007;69:1536–1541. doi: 10.1212/01.wnl.0000277635.05973.55. [DOI] [PubMed] [Google Scholar]