Summary
In a patient who had metastatic anaplastic lymphoma kinase (ALK)-rearranged lung cancer, resistance to crizotinib developed because of a mutation in the ALK kinase domain. This mutation is predicted to result in a substitution of cysteine by tyrosine at amino acid residue 1156 (C1156Y). Her tumor did not respond to a second-generation ALK inhibitor, but it did respond to lorlatinib (PF-06463922), a third-generation inhibitor. When her tumor relapsed, sequencing of the resistant tumor revealed an ALK L1198F mutation in addition to the C1156Y mutation. The L1198F substitution confers resistance to lorlatinib through steric interference with drug binding. However, L1198F paradoxically enhances binding to crizotinib, negating the effect of C1156Y and resensitizing resistant cancers to crizotinib. The patient received crizotinib again, and her cancer-related symptoms and liver failure resolved.
Small-molecule tyrosine kinase inhibitors are standard therapies for several types of cancer, including chronic myeloid leukemia,1 epidermal growth factor receptor (EGFR)–mutated non–small-cell lung cancer (NSCLC),2,3 and ALK-rearranged NSCLC.4 Although these therapies can be highly effective, resistance often develops. In patients whose disease relapses while they are receiving first-generation inhibitors, treatment with more potent and selective next-generation inhibitors can frequently reinduce durable responses.5-9 The success of next-generation inhibitors in overcoming resistance has led to the common clinical practice of sequential treatment with increasingly potent and selective targeted therapies.
In NSCLC, ALK rearrangement identifies a subgroup of patients who have sensitivity to crizotinib, the first ALK inhibitor tested in the clinic.10 Resistance to crizotinib typically develops within the first year or two after treatment is initiated, and it is mediated by a variety of different mechanisms, including secondary mutations within the ALK tyrosine kinase domain and activation of alternative signaling pathways.11 Despite the diversity of resistance mechanisms, most crizotinib-resistant tumors remain ALK-dependent and are sensitive to more potent, structurally distinct, second-generation ALK inhibitors such as ceritinib, alectinib, and brigatinib.8,9,12 As with crizotinib, however, resistance invariably develops.13,14
Lorlatinib (PF-06463922, Pfizer) is a new, reversible, ATP-competitive small-molecule inhibitor of ALK and the related tyrosine kinase ROS1.15 In cell lines, this third-generation inhibitor has subnanomolar to low nanomolar potency against ALK and retains potency against all known resistant mutants.16 Lorlatinib is also highly selective for ALK.15 The selectivity of lorlatinib was enhanced by the targeting of a specific residue in the ALK tyrosine kinase domain — leucine at position 1198 (L1198) — which is detected in only approximately 25% of kinases.15 At this position, most kinases have a larger tyrosine or phenylalanine that sterically interferes with lorlatinib binding. Lorlatinib is in early-phase clinical testing.
Here we describe a woman with metastatic ALK-rearranged NSCLC who had received multiple prior therapies, including crizotinib and ceritinib, and who subsequently had a response to lorlatinib (Fig. 1A). The disease eventually relapsed while she was receiving lorlatinib, but she again had a response to crizotinib. We describe the molecular basis for the resistance to lorlatinib and resensitization to crizotinib in this patient.
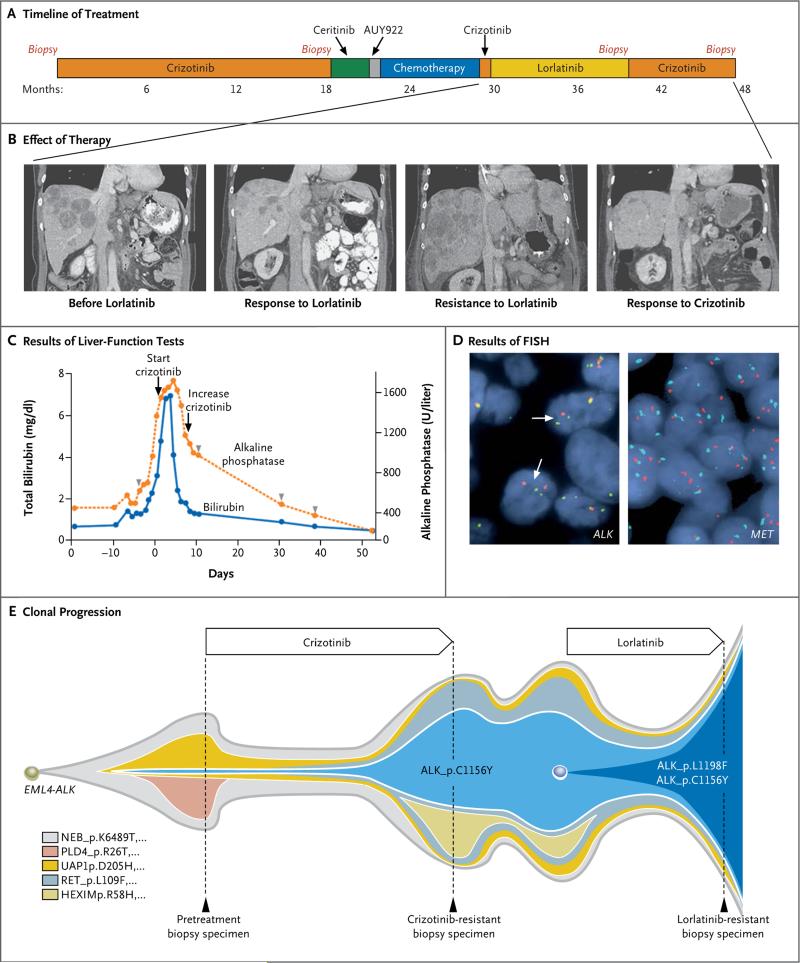
Figure 1. Acquired Resistance to Lorlatinib and Resensitization to Crizotinib.
Panel A shows the various treatments the patient received for metastatic anaplastic lymphoma kinase (ALK)–rearranged non–small-cell lung cancer as well as the duration of each treatment. Panel B shows computed tomographic (CT) images of the patient's metastatic liver disease before she received lorlatinib, during the time she had a response to lorlatinib, when the disease subsequently relapsed at 9 months, and during the patient's second response to crizotinib therapy. A radiologic second response to crizotinib was noted on the first restaging CT obtained after 8 weeks of treatment. Shown is the patient's second response after she had received crizotinib for 12 weeks. Panel C shows serial monitoring of total bilirubin levels and alkaline phosphatase levels before and after retreatment with crizotinib. To convert values for bilirubin to micromoles per liter, multiply by 17.1. The starting dose of crizotinib was 250 mg once daily, which was increased to 250 mg twice daily once the patient's liver-function tests showed improvement. Crizotinib treatment was initiated on day 1. Arrowheads indicate intermittent administration of low-dose vinorelbine, which was eventually discontinued because of neutropenia. The relative dose intensity (the proportion of administered doses relative to planned doses) of vinorelbine was 0.54. Panel D shows the results of fluorescence in situ hybridization (FISH) assays of tumor cells for ALK (left) and MET (right). The standard break-apart ALK FISH assay was used to screen the lorlatinib-resistant sample for ALK rearrangement and gene amplification. Split signals (white arrows) were observed in 30% of the cells, a finding consistent with ALK rearrangement; no amplification of ALK was detected. A dual-color FISH assay with a C-MET probe (Repeat-Free Poseidon C-MET [7q31] probe, Kreatech) and a copy-number control probe (centromere 7 [CEP7], Abbott–Vysis) were used to screen for MET amplification. The MET-to-CEP7 ratio (i.e., the ratio of red signals to cyan signals) was 1.0, indicating no amplification. Amplification of C-MET would appear as more red signals than cyan signals. Panel E shows clonal evolution of resistance to ALK inhibitors in the patient. This model is based on an analysis of whole-exome sequencing of pretreatment and resistant biopsy samples. A founder ALK C1156Y subclone was detectable at low frequency in the pretreatment tumor specimen. With crizotinib therapy, this subclone expanded to 50% of the tumorcell population and led to the patient's relapse. Lorlatinib was effective against the crizotinib-resistant tumor, but the C1156Y subclone acquired a second ALK mutation, L1198F. The double-mutant subclone (C1156Y–L1198F) was insensitive to lorlatinib and became the dominant subclone in the relapsed tumor. Selected mutations identifying each subclone are shown. Biopsies were performed where indicated. No biopsy was performed when the patient received intervening therapies between crizotinib and lorlatinib, so the proportion of subclones shown during this time period are extrapolated from the post-crizotinib sample.
Case Report
A 52-year-old woman with metastatic ALK-rearranged NSCLC received first-line crizotinib and had a clinically significant response that lasted for 18 months. Computed tomography (CT) at that time revealed new abdominal lymphadenopathy. She underwent biopsy of a lymph node that was enlarging while she was receiving crizotinib; the biopsy specimen showed a crizotinib-resistant ALK C1156Y mutation.17 Crizotinib was discontinued, and she began to receive ceritinib. First restaging CT scans at 5 weeks showed progressive disease with numerous new metastatic liver lesions. She then received a heat shock protein 90 (HSP90) inhibitor (AUY922) and had rapidly worsening disease. Chemotherapy (carboplatin–pemetrexed) was then administered, and she had a response that lasted for 6 months. After the cancer relapsed while the patient was receiving chemotherapy, she received crizotinib again and had no response.
The patient then enrolled in a phase 1 trial of lorlatinib. The first restaging CT after 5 weeks of treatment showed a 41% reduction in tumor burden (Fig. 1B). She did well until 8 months later, when CT showed worsening liver metastases. She underwent biopsy of a resistant liver lesion and continued to receive lorlatinib. Two weeks later, nausea and indigestion developed, and her total bilirubin level was 0.8 mg per deci-liter (14 μmol per liter) (baseline, 0.3 mg per deciliter [5.1 μmol per liter]). Lorlatinib was discontinued. Three days later, she was admitted to the hospital with progressive nausea, abdominal pain, and fatigue. Laboratory studies were notable for worsening hepatic dysfunction, with an elevated total bilirubin level of 1.4 mg per deciliter (24 μmol per liter). CT showed markedly worsening disease with nearly confluent metastatic lesions in the liver, splenomegaly, and ascites (Fig. 1B). She received a single dose of low-dose vinorelbine (22.5 mg per square meter of body weight). Over the next 5 days, she had further clinical deterioration and impending liver failure, with a total bilirubin level increasing to 4.8 mg per deciliter (82 μmol per liter) (Fig. 1C).
At this point, molecular testing revealed two ALK resistance mutations (detailed below). Examination of the structure of the ALK kinase domain suggested that crizotinib could have activity against this compound mutant. Treatment with crizotinib was then restarted. The patient had a rapid and dramatic clinical improvement, with resolution of her liver failure (Fig. 1C). She was discharged from the hospital and continued to receive therapy with full-dose crizotinib. She also received intermittent low-dose vinorelbine, but chemotherapy was frequently interrupted, the dose was further reduced, and eventually it was discontinued because of neutropenia. Serial restaging CT showed a clinically significant radiologic response that lasted almost 6 months (Fig. 1B).
Methods
Patient
The patient provided written informed consent to participate in the clinical trial. All biopsies and molecular testing were performed in accordance with protocols approved by the institutional review board at Massachusetts General Hospital.
Genetic Studies
Screening for ALK rearrangement and amplification of the MET proto-oncogene (MET) were performed with the use of fluorescence in situ hybridization (FISH), as described previously.10,18 ALK resistance mutations were identified with the use of a targeted next-generation sequencing (NGS) assay19 and Sanger sequencing of complementary DNA. Whole-exome sequencing was performed as described in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Ba/F3 Cell-Line Studies
Ba/F3 cells were engineered to express echinoderm microtubule-associated protein-like 4 (EML4)–ALK harboring different ALK resistance mutations. Cell-survival assays were performed as described previously.13
Biochemical and Structural Studies
Details of the methods for determination of Ki inhibition constants, kinetic characteristics, and co-crystal structures are provided in the Supplementary Appendix. The co-crystal structures have been deposited in the Worldwide Protein Data Bank (identifiers 5A9U, 5AA8, 5AA9, 5AAA, 5AAB, and 5AAC).
Results
Genetic Analysis of Resistant Tumor Specimens
To identify the mechanism of resistance in this patient, we obtained a biopsy specimen of a liver lesion that was enlarging while the patient was receiving lorlatinib so that we could perform molecular testing. FISH showed ALK rearrangement and no evidence of ALK amplification (Fig. 1D). Since MET is inhibited by crizotinib and has been identified as a potential resistance mechanism to more selective second-generation ALK inhibitors,20 we performed FISH to determine whether MET was amplified (i.e., there were multiple copies of MET in the DNA of the tumor). No MET amplification was observed (Fig. 1D).
Using a targeted NGS-based polymerase-chain-reaction (PCR) assay (referred to as Snapshot NGS), we identified two mutations in the ALK kinase domain: C1156Y, which was previously detected in this patient's post-crizotinib biopsy, and L1198F. No other mutations in 38 cancer-associated genes (e.g., EGFR and KRAS) were detected by means of Snapshot NGS. ALK C1156Y is a known crizotinib-resistance mutation,17 whereas ALK L1198F has been reported as a gain-of-function mutation in anaplastic thyroid cancer21 and as a brigatinib-resistance mutation in combination with F1174V in a nucleophosmin-ALK–rearranged cell line.22 C1156Y and L1198F mutations were present at similar frequencies (29% and 35%, respectively) in the woman's tumor, suggesting that the two mutations were on the same allele of the ALK fusion gene. To confirm this, we amplified EML4-ALK from complementary DNA derived from a frozen tumor specimen, subcloned the PCR products into the pCR4-TOPO vector, and sequenced individual bacterial colonies (Fig. S1 in the Supplementary Appendix). The two mutations were on the same allele.
We next performed whole-exome sequencing of samples of tumor tissue obtained from the patient before treatment, after the disease relapsed when the patient was receiving crizotinib, and after the disease relapsed when the patient was receiving lorlatinib. We detected the double mutation (ALK C1156Y–L1198F) in the lorlatinib-resistant sample and the single ALK C1156Y mutation in the crizotinib-resistant sample. ALK C1156Y was also detected at low frequency in the pretreatment tumor. The fraction of tumor cells harboring ALK C1156Y was less than 7% in the pretreatment tumor, approximately 50% in the crizotinib-resistant tumor, and approximately 100% in the lorlatinib-resistant tumor. The ALK L1198F mutation was detected only in the lorlatinib-resistant specimen.
Clonal analysis suggested that a minor subclone harboring C1156Y existed before crizotinib treatment. This subclone was enriched during treatment with crizotinib and subsequently evolved to acquire the L1198F mutation under the selective pressure of lorlatinib (Fig. 1E, and Fig. S2 in the Supplementary Appendix). After the patient had a response to crizotinib a second time, relapse occurred and another biopsy specimen was obtained. Snapshot NGS testing of the crizotinib-resistant specimen showed that the ALK L1198F mutation was no longer detectable. These findings are consistent with effective suppression of the C1156Y–L1198F subclone by crizotinib.
Cellular and Biochemical Characterization of Drug-Resistant ALK Mutants
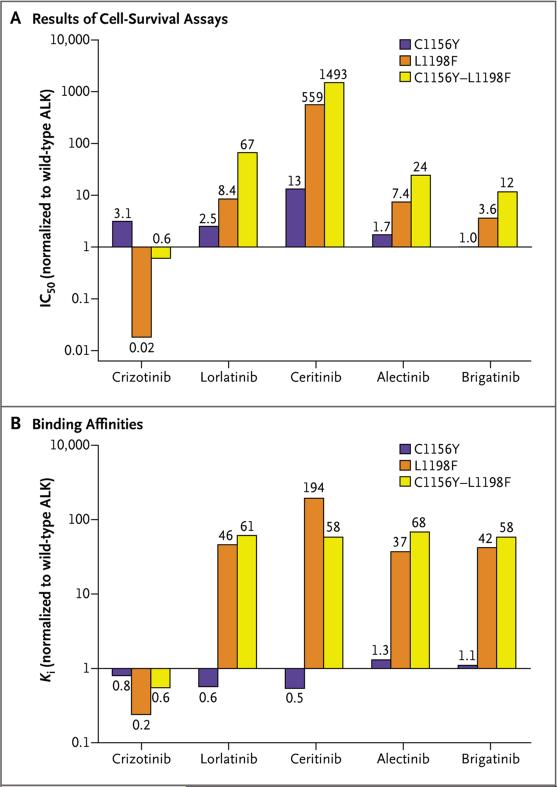
To confirm that ALK C1156Y–L1198F mediates resistance to lorlatinib, we generated Ba/F3 cell lines expressing ALK C1156Y, L1198F, or the double mutant. According to the results of cell-survival assays, ALK C1156Y–L1198F was resistant to lorlatinib as well as to second-generation ALK inhibitors (Fig. 2A, and Table S1 in the Supplementary Appendix). In contrast, crizotinib inhibited ALK C1156Y–L1198F and wild-type (i.e., nonmutant) ALK with similar potencies (half-maximal inhibitory concentration [IC50], 12nM and 20 nM, respectively). The single ALK L1198F mutant was particularly sensitive to crizotinib, with an IC50 of 0.4 nM, but it was associated with variable degrees of resistance to next-generation ALK inhibitors, including lorlatinib (Fig. 2A, and Table S1 in the Supplementary Appendix). Consistent with these results, phosphorylation of ALK C1156Y–L1198F was effectively suppressed by crizotinib, but not by lorlatinib (Fig. S3 in the Supplementary Appendix). These findings corroborate the clinical observation that C1156Y–L1198F causes resistance to lorlatinib but sensitization to crizotinib.
Figure 2. Cellular and Biochemical Characterization of ALK C1156Y–L1198F.
As shown in Panel A, Ba/F3 cells harboring wild-type echinoderm microtubule-associated protein-like 4 (EML4)–ALK or different mutant versions of EML4-ALK were treated with various ALK inhibitors for 48 hours. Cell survival was determined with the use of a CellTiter-Glo assay. Half-maximal inhibitory concentration (IC50) values for the EML4-ALK mutants were determined and were normalized to the IC50 for wild-type EML4-ALK. Ratios equal to 1 correspond to similar cellular potency between mutant and wild-type ALK proteins, whereas ratios less than 1 correspond to greater potency of the mutant as compared with wild-type ALK. As shown in Panel B, inhibition constants (Ki's) for binding of wild-type and mutant ALK kinases with various ALK inhibitors were determined. Shown are the binding affinities of ALK mutants relative to wild-type ALK. Ratios close to 1 correspond to similar binding affinities between mutant and wild-type ALK proteins, whereas ratios less than 1 correspond to greater affinity of the mutant relative to the wild-type kinase.
To determine whether the L1198F mutation can resensitize other crizotinib-resistant mutants, we generated additional Ba/F3 cell lines expressing single and double ALK mutants. In almost all cases, the addition of L1198F increased sensitivity to crizotinib but promoted resistance to the other ALK inhibitors (Fig. S4 and Table S2 in the Supplementary Appendix). In particular, for the highly refractory ALK G1202R mutation (IC50, 382 nM), the double mutant (G1202R–L1198F) was nearly as sensitive as wild-type ALK to crizotinib (IC50, 31 nM vs. 20 nM).
We next determined the binding affinities (Ki) of wild-type and mutant ALK kinase domains for various ALK inhibitors. Consistent with the cell-line results, L1198F and C1156Y–L1198F mutants had decreased binding affinity for lorlatinib, as well as other next-generation ALK inhibitors, relative to wild-type ALK but increased affinity for crizotinib (Fig. 2B, and Table S3 in the Supplementary Appendix). In contrast, the single C1156Y mutant showed affinity that was equivalent to that of wild-type ALK for both crizotinib and lorlatinib. However, the enzymatic activity of the C1156Y mutant (Kcat/KM,substrate) was 5.6 times as high as that of wild-type ALK (Table S4 in the Supplementary Appendix). The activity of the C1156Y–L1198F double mutant was mildly higher than that of the wild-type (1.7 times as high), whereas the activity of the ALK L1198F mutant was actually 2.5 times as low. These biochemical data suggest that alterations in drug binding, more so than changes in kinase activity, are probably the primary mechanism underlying resistance of C1156Y–L1198F to lorlatinib and sensitivity to crizotinib.
Structural Studies of Drug-Resistant ALK Mutants
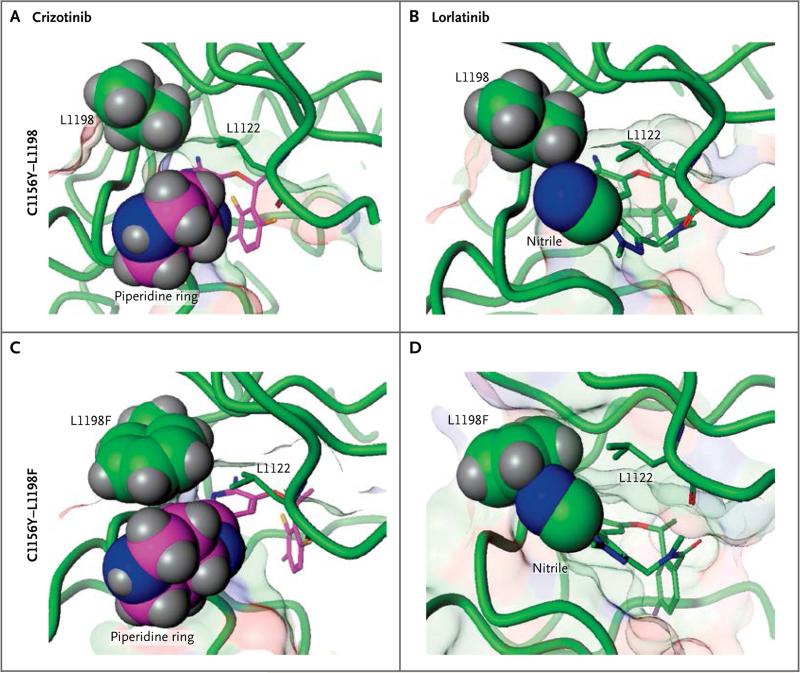
We determined the co-crystal structures of wild-type and mutant ALK kinase domains bound to crizotinib or lorlatinib. The cysteine at position 1156 (C1156) is located far (13 Å) from the inhibitor binding site and is not predicted to directly affect drug binding (Fig. S5 in the Supplementary Appendix). The C1156Y substitution does affect the ordering and position of the tip of the glycine-rich loop, increasing kinase activity.
In contrast, L1198 resides near the ATP-binding site, and substitution of leucine with the larger phenylalanine leads to a steric clash with the nitrile of lorlatinib (Fig. 3). Co-crystal structures reveal that binding of lorlatinib to the L1198F mutant requires that the rigid macrocyclic inhibitor rotate away from the phenylalanine, worsening the hinge-binding interaction and potentially introducing strain into the kinase (Fig. S6 in the Supplementary Appendix). This is unfavorable for binding, with a decrease of 1.8 kcal of binding energy as compared with wild-type ALK in both computational and experimental measurements of compound binding (Tables S4 and S5 in the Supplementary Appendix). As shown in Figure 3, the phenylalanine does not clash with crizotinib and in fact moves slightly closer to the inhibitor. This proximity does not alter hinge-binding and may be structurally beneficial, since crizotinib binding to L1198F is more favorable by 0.8 kcal relative to wild-type ALK (Tables S4 and S5 in the Supplementary Appendix). In cancer cells with the double mutation, the enhanced binding due to L1198F probably offsets the increased kinase activity due to C1156Y, leading to crizotinib sensitization.
Figure 3. Structural Basis for Resistance to Lorlatinib and Sensitivity to Crizotinib Mediated by ALK C1156Y–L1198F.
Nonphosphorylated ALK wild-type and mutant (C1156Y, L1198F, C1156Y–L1198F) kinase domains were co-crystallized with crizotinib or lorlatinib. Shown are the co-crystal structures of crizotinib bound to the single ALK C1156Y mutant (Panel A), lorlatinib bound to the single ALK C1156Y mutant (Panel B), and crizotinib bound to the ALK C1156Y–L1198F double mutant (Panel C). Panel D shows modeling of lorlatinib bound to the double ALK C1156Y–L1198F mutant, highlighting the steric clash between the phenylalanine residue and lorlatinib. L1122 is a g-loop leucine residue that creates a binding pocket with L1198F just above the piperidine and nitrile groups. The co-crystal structures of lorlatinib and crizotinib bound to ALK C1156Y and ALK C1156Y–L1198F are shown in Figure S6 in the Supplementary Appendix.
Discussion
This patient with metastatic ALK-rearranged lung cancer received multiple ALK inhibitors during her treatment course, including first-, second-, and third-generation inhibitors. Resistance to ALK inhibition was a dynamic and clonal process, originating with a founder ALK C1156Y clone in the pretreatment tumor and culminating in a double-mutant subclone (ALK C1156Y–L1198F) in the post-lorlatinib tumor that led to the relapse. This acquired L1198F mutation conferred resistance to lorlatinib, but unexpectedly restored sensitivity to crizotinib, a less potent and less selective first-generation inhibitor.
Although we cannot rule out the potential role of low-dose vinorelbine, we think that chemotherapy was not the cause of this patient's dramatic clinical recovery. This assessment is based on phase 3 trial data showing a response rate with vinorelbine of 0.8% among patients with previously treated NSCLC23 and on our cellular, biochemical, and structural data that provide the mechanistic basis for resensitization to crizotinib.
These results highlight the clinical usefulness of developing multiple, structurally distinct inhibitors that target the same oncogenic kinase. When resistance develops to one inhibitor, repeat biopsy can provide critical information as to whether sequential therapy with a different inhibitor may be effective. Over the course of disease, serial repeat biopsies, as performed in this patient, can not only provide clinically relevant insights but can also offer an invaluable glimpse into the longitudinal evolution of cancer.
Our results also highlight ALK L1198F as a novel resistance mechanism in ALK-rearranged NSCLC. Remarkably, this substitution changes the exact residue used to enhance selectivity of lorlatinib for ALK over other kinases.15 Targeting the extra space created by the smaller leucine residue formed the basis for the medicinal chemistry approach taken to gain selectivity. This selectivity strategy was not used in the development of crizotinib, and in fact crizotinib binds and inhibits ALK L1198F even more potently than it binds and inhibits wild-type ALK. Overall, these results suggest that detailed knowledge of structure and functional features of drug targets can be exploited in strategies for drug design and lead to clinically relevant predictions of drug activity.
Supplementary Material
Acknowledgments
Supported by Pfizer, grants from the National Cancer Institute (5R01CA164273, to Drs. Shaw and Engelman) and the National Foundation for Cancer Research (to Dr. Shaw), Be a Piece of the Solution, and Lungstrong.
We thank Steve Moskowitz of Advanced Medical Graphics and Emily Chin for assistance with earlier versions of the figures.
Funded by Pfizer and others; ClinicalTrials.gov number, NCT01970865.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 4.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 5.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome–positive leukemias. N Engl J Med. 2012;367:2075–88. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor–resistant non– small-cell lung cancer. N Engl J Med. 2015;372:1689–99. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 7.Sequist LV, Soria JC, Goldman JW, et al. Rociletinib in EGFR-mutated non–small-cell lung cancer. N Engl J Med. 2015;372:1700–9. doi: 10.1056/NEJMoa1413654. [DOI] [PubMed] [Google Scholar]
- 8.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non–small-cell lung cancer. N Engl J Med. 2014;370:1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–28. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 10.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015;21:2227–35. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camidge DR, Bazhenova L, Salgia R, et al. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non-small cell lung cancer (NSCLC). J Clin Oncol. 2015;33:8062. abstract. [Google Scholar]
- 13.Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4:662–73. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katayama R, Yanagitani N, Koike S, et al. Resistance mechanisms to ALK inhibitors. American Association for Cancer Research; Philadelphia: 2015. [Google Scholar]
- 15.Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro -2,10,16-trimethyl-15-oxo-10,15,16,17 -tetrahydro-2H-8,4-(metheno)pyrazolo [4,3-h][2,5,11]-benzoxadiazacyclotetra decine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57:4720–44. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
- 16.Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015;28:70–81. doi: 10.1016/j.ccell.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–9. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 18.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non–small-cell lung cancer. N Engl J Med. 2014;371:1963–71. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–84. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 20.Gouji T, Takashi S, Mitsuhiro T, Yukito I. Crizotinib can overcome acquired resistance to CH5424802: is amplification of the MET gene a key factor? J Thorac Oncol. 2014;9(3):e27–8. doi: 10.1097/JTO.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 21.Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 2011;71:4403–11. doi: 10.1158/0008-5472.CAN-10-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceccon M, Mologni L, Giudici G, et al. Treatment efficacy and resistance mechanisms using the second-generation ALK Inhibitor AP26113 in human NPM-ALK-positive anaplastic large cell lymphoma. Mol Cancer Res. 2015;13:775–83. doi: 10.1158/1541-7786.MCR-14-0157. [DOI] [PubMed] [Google Scholar]
- 23.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.