Abstract
Ex vivo-expanded cynomolgus monkey CD4+CD25+CD127− regulatory T cells (Treg) maintained Foxp3 demethylation status at the Treg-Specific Demethylation Region (TSDR), and potently suppressed T cell proliferation through 3 rounds of expansion. When CFSE- or VPD450-labeled autologous (auto) and non-autologous (non-auto) expanded Treg were infused into monkeys, the number of labeled auto-Treg in peripheral blood declined rapidly during the first week, but persisted at low levels in both normal and anti-thymocyte globulin plus rapamycin-treated (immunosuppressed; IS) animals for at least 3 weeks. By contrast, MHC-mismatched non-auto-Treg could not be detected in normal monkey blood or in blood of two out of the three IS monkeys by day 6 post-infusion. They were also more difficult to detect than auto-Treg in peripheral lymphoid tissue. Both auto- and non-auto-Treg maintained Ki67 expression early after infusion. Sequential monitoring revealed that adoptively-transferred auto-Treg maintained similarly high levels of Foxp3 and CD25 and low CD127 compared with endogenous Treg, although Foxp3 staining diminished over time in these non-transplanted recipients. Thus, infused ex vivo-expanded auto-Treg persist longer than MHC-mismatched non-auto-Treg in blood of non-human primates and can be detected in secondary lymphoid tissue. Host lymphodepletion and rapamycin administration did not consistently prolong the persistence of non-auto-Treg in these sites.
Keywords: regulatory T cells, ex vivo expansion, immune suppression, cynomolgus macaques
Introduction
Protracted dependency on non-specific immune suppressants renders transplant recipients susceptible to metabolic disorders, cardiovascular disease, serious infections and malignancy (1). Moreover, immunosuppressive (IS) drugs fail to prevent chronic graft rejection. More effective control of alloimmunity will likely require active immune regulation, as well as suppression. A promising adjunctive therapy to inhibit rejection may be the use of regulatory immune cells, in particular, regulatory T cells (Treg) (2-4), with tolerogenic properties. Although rare, naturally-occurring (n) CD4+CD25hi forkhead box p3 (Foxp3)+ Treg have potent immunomodulatory activity (4, 5) and in mice, their adoptive transfer promotes allograft tolerance. In non-human primates (NHP), endogenous (6) or adoptively-transferred Treg (7, 8) appear important in suppression of renal allograft rejection. In humans, while graft-infiltrating Treg correlate positively with reduced inflammation and better renal transplant outcome (9, 10), patients with operational liver transplant tolerance exhibit higher levels of circulating Treg than non-tolerant patients and healthy controls (11, 12). Moreover, in humanized mouse models (13, 14), adoptively-transferred ex vivo-expanded Treg protect against human arterial or islet allograft rejection.
The regulatory function of human nTreg (15-18) is associated with sustained levels of Foxp3 and low CD127 (IL-7R) expression (19, 20). Importantly, like murine Treg, purified human Treg can be expanded ex vivo by crosslinking CD3 and CD28 in the presence of exogenous IL-2, while their ability to suppress effector T cell responses to cognate or polyclonal stimuli is retained (16, 21). Similarly, nTreg expanded robustly ex vivo from blood of NHP retain the ability to suppress alloreactive T cell responses (22-24). Since NHP are important pre-clinical models for testing promising therapeutic agents (25), including regulatory immune cells with potential to promote transplant tolerance (22, 26, 27), detailed characterization of NHP Treg in vitro and in vivo is likely to prove instructive for therapeutic testing of these cells in clinical organ transplantation.
In addition to use of autologous Treg (auto-Treg), the concept of ‘off-the-shelf’, non-autologous (non-auto) Treg for adoptive cell therapy may offer several advantages. These include advance preparation of large numbers, catering for repeated infusions, quality control, and screening for micro-organisms. Murine studies have demonstrated the potential of non-auto (‘third party’) Treg for control of rejection (28) and tolerance induction (29), whereas recent clinical trials have confirmed the safety and potential efficacy of partially HLA-matched Treg for therapy of graft-versus-host disease after hematopoietic stem cell transplantation (30, 31). In addition to their suppressive function, an important question regarding the comparative efficacy of auto-versus non-auto-Treg is their in vivo persistence following adoptive transfer, including the influence of IS agents used to inhibit allo- and autoimmunity.
Clinical organ transplantation often involves the use of depleting induction agents (e.g. anti-CD52 mAb [Campath] or anti-thymocyte globulin [ATG]). ATG induces prolonged T cell depletion in humans, but (rabbit ATG) can promote Treg expansion in vitro (32, 33) and induce or spare Treg in vivo (34, 35). Also, mechanistic target of rapamycin (mTOR) inhibition promotes the tolerogenic function of adoptively-transferred Treg in rodents after organ transplantation (36), and can enhance the ability of human Treg to suppress transplant vasculopathy (37). In anticipation of testing the efficacy of Treg in organ-transplanted NHP, we have characterized ex-vivo expanded cynomolgus macaque Treg before and following their adoptive transfer into autologous or non-autologous MHC-mismatched recipients given ATG and rapamycin maintenance IS. The data demonstrate that auto-Treg persist longer than non-auto-Treg in the circulation of both control and immunosuppressed hosts, and retain a stable Treg phenotype.
Materials and Methods
Animals
Healthy male cynomolgus macaques (Macaca fascicularis) of Indonesian origin (2.9-5 kg; 5-7 years old), were obtained from specific pathogen-free colonies at Alpha Genesis, Inc, or the NIAID NHP colony (both Yamassee, SC). Immunosuppression comprised 2 consecutive intravenous (i.v.) infusions of rabbit ATG (Genzyme, Boston, MA) 2 or 3 days apart (day-2 or day-3 and day 0) at doses of 10 and 5 mg/kg, respectively. Tacrolimus was given intramuscularly (i.m.) from day −2 to 2 (target whole blood trough levels between 10 and 15 ng/ml) followed by i.m. rapamycin (LC Laboratories, Woburn, MA) monotherapy from day 3 (target trough levels between 5 and 15 ng/ml) through the duration of the experiment. All animal procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and conducted under a University of Pittsburgh Institutional Animal Care and Use Committee-approved protocol. Specific environment enrichment was provided.
MHC typing
Total cellular RNA was isolated from peripheral blood mononuclear cells (PBMC) and converted to cDNA with the Superscript III First-Strand Synthesis System (Invitrogen; Carlsbad, CA). These cDNAs were used to generate primary PCR amplicons with high-fidelity Phusion polymerase (New England BioLabs; lpswich, MA). Gene-specific primers targeting conserved sequences that flank the highly polymorphic peptide-binding domains encoded by exon 2 allowed simultaneous amplification of 195 bp or 283 bp amplicons for all MHC class I or DRB loci, respectively. Primer sequences within exon 2 of class I and DRB loci, as well as protocols are available on the Nonhuman Primate MHC Contract Web Portal (http://go.wisc.edu/173j30). After purification with AMPure XP beads (Agencourt; Beverly, MA), amplicons were pooled at equimolar concentrations for 250 bp paired-end sequencing on a MiSeq instrument (Illumina; San Diego, CA). MHC genotypes were determined using a custom workflow and curated database of Mafa MHC sequences (Mafa_MHC_mRNA-allseq-13.09.01.fasta). Mafa-A, -B and -DRB haplotypes were inferred based on comparisons with previous genotyping results with related cynomolgus macaques in the NIAID-sponsored breeding colony at Alpha Genesis Inc. (38). Table 1 shows the degree of MHC disparity between the Treg donor and recipient pairs. The full genotypes of the monkeys are provided in Supplementary Table 1.
Table 1.
MHC disparity between recipients and Treg donors
| Pairs | Monkey | Mafa-A Haplotype 1 | Mafa-A Haplotype 2 | Mafa-B Haplotype 1 | Mafa-B Haplotype 2 | Mafa-DRB Haplotype 1 | Mafa-DRB Haplotype 2 | |
|---|---|---|---|---|---|---|---|---|
| control | Recipient | CM123 | A018 | A003 | B147a | B028b | DR17 | DR09b |
| Donor | B1117 | A060 | A066 | B013b | B164 | DR19 | DR21 | |
| Recipient | CM116 | A101b | A004 | B004a | B056c | DR15 | DR13 | |
| Donor | CM117 | A007 | A008 | B008b | B184 | DR04 | DR13 | |
| Recipient | CM117 | A007 | A008 | B008b | B184 | DR04 | DR13 | |
| Donor | CM116 | A101b | A004 | B004a | B056c | DR15 | DR13 | |
| IS | Recipient | CM118 | A101b | A091 | B004a | B147b | DR15 | DR09a |
| Donor | B1117 | A060 | A066 | B013b | B164 | DR19 | DR21 | |
| Recipient | CM122 | A004 | A006 | B036b | B002 | DR08 | DR03a | |
| Donor | CM119 | A092 | A008 | B056b | B154 | DR14a | DR03a | |
| Recipient | CM119 | A092 | A008 | B056b | B154 | DR14a | DR03a | |
| Donor | CM122 | A004 | A006 | B036b | B002 | DR08 | DR03a |
IS=immunosuppression
Treg isolation and expansion
PBMC were isolated from freshly-drawn blood and CD4+ T cells negatively enriched using NHP CD4+ T cell isolation kits (Miltenyi Biotech, Auburn, CA). The CD4+ cells were then flow-sorted using a BD FacsAria (BD Biosciences, San Jose, CA) into populations of CD4+CD25+CD127− Treg (20, 24) and CD4+CD25−CD127+ effector T cells (Teff). The purity of both Treg and Teff was consistently >95%. Foxp3 expression by the cynomolgus Treg was significantly higher than by Teff. Artificial antigen-presenting cells (aAPCs) (L-32) (39) expressing CD32, CD80 and CD58 were kindly provided by Dr. M. K. Levings, University of British Columbia, Vancouver, Canada. They were irradiated (80 Gy), loaded with anti-CD3 (BD Bioscience), and cultured with sorted Treg at a T cell/APC ratio of 1:1 for 7-8 days initially in complete RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 10% v/v fetal bovine serum, 2 mM L-glutamine (Mediatech, Inc., Herndon, VA), 100U/ml penicillin-streptomycin (BioWhittaker), 25 mM HEPES (Mediatech) and 55 μM β-2 mercaptoethanol (Invitrogen) in the presence of 300 U/ml recombinant human IL-2 (R&D Systems, Minneapolis, MN) and 100 ng/ml rapamycin (LC Laboratories). Teff were stimulated in parallel and without rapamycin as controls. Thereafter, non-adherent T cells were re-stimulated with aAPC on days 7 and 14 as in the first round, for an additional 2 rounds, except that no rapamycin was added. During each round, half the media was replaced at intervals with fresh media containing 600 U/ml IL-2, with or without 100ng/ml rapamycin, the standard dose for ex vivo expansion of human Treg (40, 41). Cells were harvested on day 21. In some experiments, cells were cryo-preserved at the end of the second round and restimulated for a third round a week before infusion.
Flow cytometry
Single cell suspensions of T cells were stained as described (24) with a fluorochrome-labeled mAb mixture directed against the cell surface markers CD3, CD4, CD25, CD39, CD44, CD45RA, CD62L, CD95, CD127, CCR7 and CXCR3 (BD PharMingen, Franklin Lakes, NJ, or BioLegend, San Diego, CA). Intracellular B-cell lymphoma 2 (Bcl-2), Foxp3, the proliferation marker Ki67, cytotoxic T lymphocyte Ag-4 (CTLA-4) and Helios were stained using an eBioscience Foxp3 Staining kit, according to the manufacturer's instructions. Apoptosis was determined by Annexin V staining (BD PharMingen) and membrane integrity by propidium iodide (PI) staining. Data were acquired on a LSR II or LSR Fortessa (BD Bioscience) and analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
TSDR methylation analysis
Purified Treg were frozen and aliquots submitted to EpigenDX Inc. (Hopkinton, MA) for Treg-specific demethylation region (TSDR) analysis on 10 cytosine-phosphate-guanine (CpG) motifs (42).
In vivo tracking of infused auto- and non-auto-Treg
Auto- and non-auto-Treg were labeled with 2 μM CFSE or VPD450. A mixture of distinctly-labeled auto and non-auto-Treg at 1:1 ratio was injected intravenously into normal untreated or immunosuppressed monkeys. Doses of Treg infused are shown in Table 2. At various times thereafter, PBMC were isolated from 1 ml blood samples and stained for CD3, CD4, CD25, CD62L, CD127, CCR7, CXCR3, and Foxp3. Duplicate samples were processed at each time point. On day 1 post-infusion, inguinal lymph nodes (LN) on one side of the body were biopsied. Single LN cells were stained for CD3, CD4, CD25, CCR7 and CXCR3. Absolute counts of endogenous and infused Treg were determined by CountBright™ absolute counting beads, (Invitrogen) according to the manufacturer's protocol. Data were analyzed with FlowJo software (Tree Star) and graphed with GraphPad Prism (Graph Pad Software, San Diego).
Table 2.
Doses of ex vivo-expanded Treg infused
| Monkey ID | Weight (kg) | IS | auto Treg (cells/kg) | Non-auto Treg (cells/kg) |
|---|---|---|---|---|
| CM123 | 4.5 | - | 3.1×106 CFSE-CM123 | 1.1×107 VPD450-B1117 |
| CM116 | 4.8 | - | 1.87×107 CFSE-CM116 | 1.87×107 VPD450-CM117 |
| CM117 | 2.9 | - | 3.1×107 VPD450-CM117 | 3.1×107 CFSE-CM116 |
| CM118 | 5 | + | 1.2×107 CFSE-CM118 | 1.2×107 VPD450-B1117 |
| CM122 | 5 | + | 2×107 VPD450-CM122 | 2×107 CFSE-CM119 |
| CM119 | 4.6 | + | 2.1×107 CFSE-CM119 | 2.1×107 VPD450-CM122 |
IS=immunosuppression
Enumeration of host immune cell populations
Absolute numbers of mononuclear cell populations in peripheral blood were determined by flow cytometry as described (43), with the addition of anti-NK cell (CD159) antibody (NKG2A; clone Z199, Beckman Coulter).
Statistical analyses
Differences between means were evaluated using Student's paired ‘t’-test or the non-parametric Mann-Whitney U test, as appropriate. Statistical analyses were conducted using the standard formula in Microsoft Excel software.
Results
Ex vivo-expanded cynomolgus Treg maintain expression of Foxp3 and other Treg signature molecules
Previously (24), we expanded cynomolgus Treg ex vivo using anti-CD3/CD28 mAb-coated beads. To minimize any possible bead contamination and to increase the yield of Treg for prospective in vivo monitoring, we employed adherent aAPC (mouse fibroblast L-32 cells) that express the high-affinity Fc receptor CD32 and the costimulatory molecule CD80 (39) to stimulate Treg expansion. This protocol consistently expanded Treg 1000-fold after 3 rounds of expansion (22-24 days) (data not shown).
High levels of CD25, CTLA-4 and Foxp3 expression correlate with suppressive function of Treg (44), whereas Helios expression is associated with nTreg (45). We therefore examined the expression of these markers by the ex vivo-expanded cynomolgus Treg, compared to fresh bulk CD4+ T cells and ex vivo-expanded conventional T cells (Teff). As shown in Figure 1A, both expanded Treg and Teff upregulated the activation markers CD25 and CD44 and downregulated CD127, however the expanded Treg consistently displayed relatively high levels of the ecto-nucleotidase CD39,- a marker of activated Treg (46-48), Foxp3, and Helios. This finding suggested that ex vivo-expanded cynomolgus nTreg were phenotypically distinct from expanded Teff and maintained the expression of Treg functional molecules. Treg harvested after 2 or 3 rounds of expansion potently suppressed anti-CD3/CD28-induced proliferative responses of CD4+ and CD8+ T cells (data not shown).
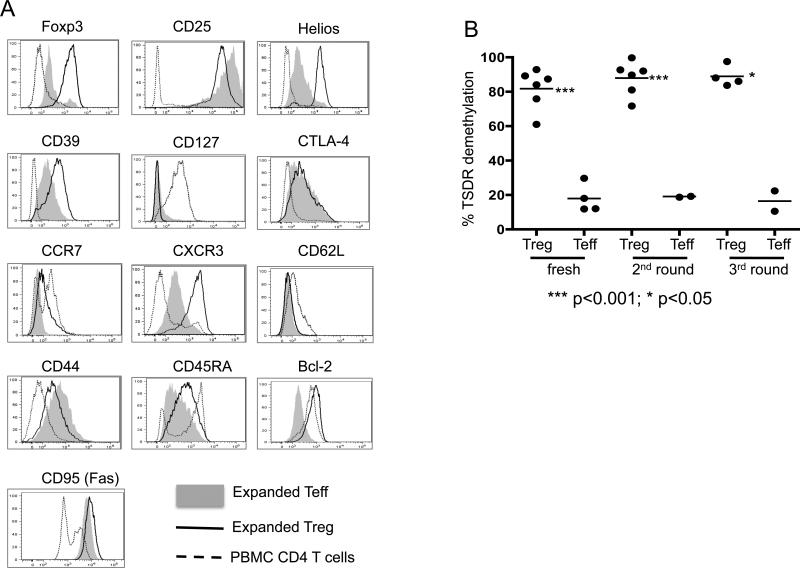
Figure 1. Characteristics of expanded cynomolgus monkey Treg and Teff.
(A) Expanded Treg (black lines) and Teff (grey shading) were stained for Foxp3, CD25, CD39, CD45RA, CD62L, CD95, CD127, Bcl-2, Helios, CTLA-4, CCR7 and CXCR3 at the end of round 3 of expansion. Compared to expanded Teff, expanded Treg showed much higher expression of Foxp3, Helios, CD39 and CXCR3, and similar high expression of CD25. Data are representative of 3 experiments. (B) Demethylation status of Foxp3 TSDR (Treg-specific demethylation region) in fresh and expanded Treg and Teff, assessed at the end of the 2nd and 3rd round of expansion. The % TSDR demethylation in each experiment is shown, as well as the mean % for all experiments in each group (horizontal line). TSDR demethylation was significantly higher (at least 4-fold) in Treg than in Teff under each condition. ***, p<0.001; *, p<0.05.
Expression of activation, tissue homing and survival-associated molecules by expanded cynomolgus Treg
Differential expression of activation markers, homing receptors, and T helper (Th) cell-associated transcription factors delineate subsets of Treg (49) with different migratory and functional properties. In order to suppress normal (naïve) T cell priming, Treg need to migrate to secondary lymphoid tissues, whereas to suppress effector T cell function, they must migrate to inflammatory sites. To more accurately predict the potential behavior of the expanded cynomolgus Treg, we further interrogated their phenotype. As shown in Figure 1A, the expanded cynomolgus Treg displayed an activated phenotype, with upregulated CD44 and intermediate CD45RA expression compared to normal bulk CD4+ T cells and expanded Teff, respectively. They also expressed low levels of CD62L and the lymph node (LN)- and spleen-homing molecule CCR7, but comparatively high levels of the Th1-mediated inflammatory site migration molecule CXCR3 (50, 51), suggesting their potential to migrate preferentially to sites of inflammation (52-54). As indicators of their survival, we also analyzed Treg expression of the anti-apoptotic protein Bcl-2. Unlike expanded Teff, expanded cynomolgus Treg expressed high levels of Bcl-2, suggesting that, after extended expansion, they might have greater survival potential than conventional T cells.
Ex vivo-expanded cynomolgus Treg maintain high levels of demethylated Foxp3 at the TSDR
Stable Foxp3 expression is critical for maintenance of the suppressive capacity of Foxp3+ Treg and there is evidence that demethylation of the TSDR at the Foxp3 locus correlates with stability of Foxp3 expression (42). To determine whether the expansion protocol that we used produced stable Foxp3+ cynomolgus Treg, we tested DNA of freshly-isolated CD4+CD25+CD127− nTreg and CD4+CD25−CD127+ Teff, as well as Treg and Teff expanded for 2 or 3 rounds for their average percent methylation of 10 CpG sites in the TSDR. As shown in Figure 1B, compared to their Teff counterparts, both expanded and fresh Treg displayed a much higher percentage demethylation. These data indicated that our expansion protocol generated cynomolgus Treg with a stable phenotype.
Detection, viability and in vivo persistence of Treg labeled with different fluorochromes
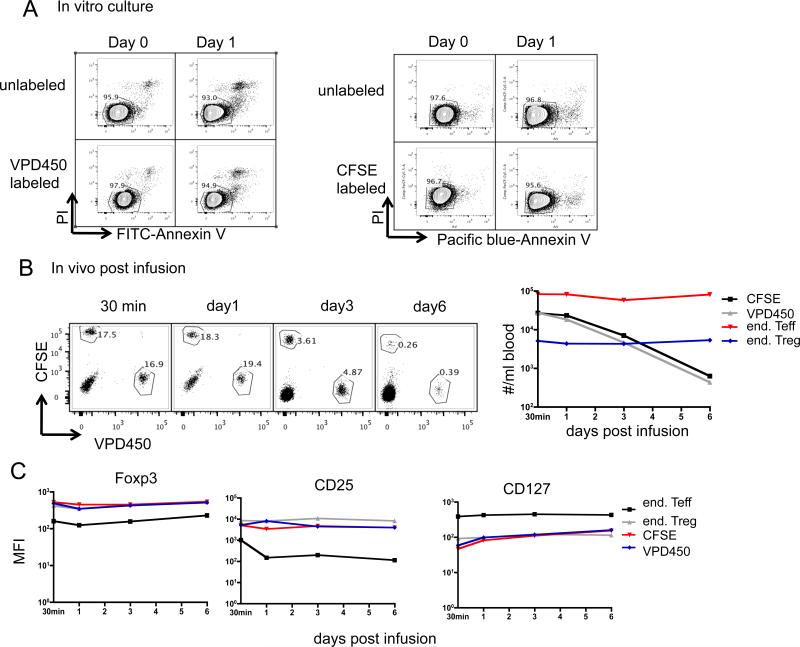
To validate our ability to track infused cells in relatively small numbers and in comparison to endogenous T cells, we tested the detectability and quantification of Treg labeled with CFSE or VPD450 when added in graded numbers to normal whole blood. As shown in Supplementary Figure 2, a linear relationship between added and detected dye-labeled cells was obtained. with as low as 10 added Treg/ml being detected. Dye labeling has been associated with cell death, thus we compared the in vitro viability and in vivo persistence of Treg labeled with CFSE versus VPD450. Immediately (day 0) and day 1 after labeling, CFSE- and VPD450-labeled cells were stained for apoptosis with Annexin V-Pacific blue/PI and Annexin V-FITC/PI, respectively. As shown in Figure 2A, no significant cell death was observed on either day 0 or day 1. When equal numbers of CFSE- and VPD450-labeled auto-Treg were mixed and infused i.v., similar numbers of CFSE+ and VPD450+ cells were detected in the blood from 30 min to 6 days post-infusion (Figure 2B). In addition, the infused Treg labeled with either dye displayed similar levels of Foxp3, CD25 and CD127. Collectively, these data indicate that labeling of Treg with CFSE or VPD450 does not differentially affect their survival.
Figure 2. CFSE / VPD450 labeling does not affect the survival of expanded Treg in vitro or in vivo.
Expanded Treg (round 3) were labeled with CFSE or VPD450. (A) They were then cultured in the presence of 300 U/ml of IL-2 for an additional day, and apoptosis analysed by staining with Annexin V and 7-AAD. Unlabeled cells were used as controls. Data are representative of 3 independent experiments. (B, C) Expanded non-auto Treg were labeled with CFSE or VPD450, equal numbers of labeled cells mixed together and infused into an IS monkey (CM118). At the indicated time points, 1ml blood was tested for the presence of infused cells and end Teff and end Treg, as described in Figure 2. (B) dot plots and plot of #/ml blood vs. time post-infusion are shown. (C) MFI of Foxp3, CD25, CD127 in/on infused CFSE-labeled or VPD450-labeled exogenous Treg were compared with those in/on end Teff and end Treg at the indicated time points.
Persistence of ex vivo-expanded auto versus non-auto cynomolgus Treg after their adoptive transfer to normal recipients
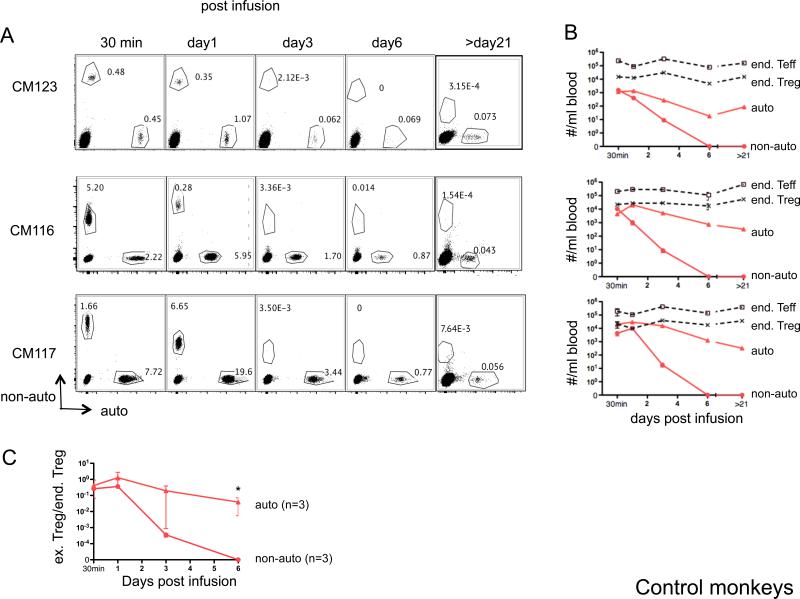
To constitute effective therapeutic agents, adoptively-transferred Treg and their progeny need to survive and function long enough in vivo, and in the presence of IS agents, to regulate adverse immune responses. Even though ex vivo-expanded ‘third-party’ (non-auto) Treg have been tested clinically in hematopoietic stem cell transplantation (30, 55, 56), potential elimination of infused non-autologous cells by host innate or adaptive defense mechanisms may limit their clinical utility. To compare the persistence of ex vivo-expanded auto- versus non-auto-Treg in vivo, we first infused normal control monkeys with differentially-labeled cells (CFSE or VPD450), and monitored their presence in the blood at different times post-infusion. As shown in Figure 3, both labeled auto- and non-auto-Treg could be readily detected 30 min post-infusion. The number of infused auto-Treg that could be detected per ml of peripheral blood at 30 min was approx 10 × 103/ml; numbers peaked on day 1 (approx 20 × 103/ml), then declined to 8.3 and 0.8 × 103/ml on days 3 and 6 post-infusion, respectively (Figure 3 A, B). The monkey that received the largest dose of auto-Treg (M117) displayed the highest number of exogenous dye-labeled Treg in the circulation. Longer-term follow-up analysis (>21 days) revealed a mean of 0.33 × 103/ml dye-labeled auto-Treg (CD25hiCD127loFoxp3+) 7 weeks post-infusion. By contrast, a much more rapid and consistent decline in non-auto-Treg was observed, from a mean of 6.2 × 103/ml at 30 min, to 4.5 × 103, 12 and <10/ml on days 1, 3 and 6 post-infusion, respectively. No dye-labeled non-auto-Treg were detected at later time points. The more rapid loss of non-auto-Treg from the circulation was accompanied by a more pronounced overall decline in the ratio of infused to endogenous Treg after day 1 (Figure 3C). These results were consistent with the high degree of MHC disparity between the non-auto-Treg and their recipients, as demonstrated by deep sequencing of MHC class I and DRB amplicons from these animals (Table 1 and Supplementary Table 1). MHC disparity was essentially complete for this cohort, with the exception of single, shared Mafa-DRB haplotypes between two donor/recipient pairs, as well as a more distantly related Mafa-B and Mafa-DRB haplotype with several distinct allelic variants that was shared between recipient and his non-auto-Treg donor animals (Supplementary Table 1).
Figure 3. Persistence of ex vivo-expanded Treg in peripheral blood of control monkeys.
Auto- or non-auto-Treg were labeled with either CFSE or VPD450, respectively, then infused i.v. into healthy control monkeys. At the days indicated post-Treg infusion, 1ml blood was tested for the presence of the infused cells and endogenous (end) Teff and Treg by flow cytometry. Counting beads were added before running flow analysis. Cell numbers per ml blood were calculated as the number of cell events/number of bead events × total number of beads added. Duplicate samples were tested at each time-point. (A) Flow cytometric profiles of CD4-gated PBMC from 3 monkeys showing the percentages of infused, labeled auto-and non-auto-Treg. Endogenous (unlabeled) CD4+ T cells are also evident (lower left quadrant). (B) Kinetics of the number of infused Treg (auto- and non-auto-Treg) and endogenous (end) Teff and Treg numbers in the peripheral blood after labeled Treg infusion in the 3 control monkeys. (C) Kinetics of the ratio of infused (ex) Treg to endogenous (end) Treg in the peripheral blood after labeled Treg infusion in these monkeys (n=3). *, The ratio of auto-Treg/end Treg was significantly greater than that of non-auto-Treg/end Treg, p<0.05.
Persistence of ex vivo-expanded auto versus non-auto cynomolgus Treg after their adoptive transfer to immunosuppressed recipients
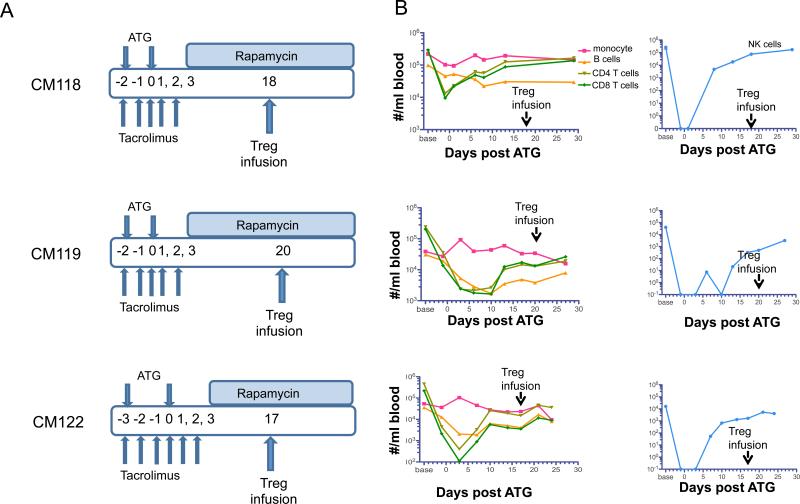
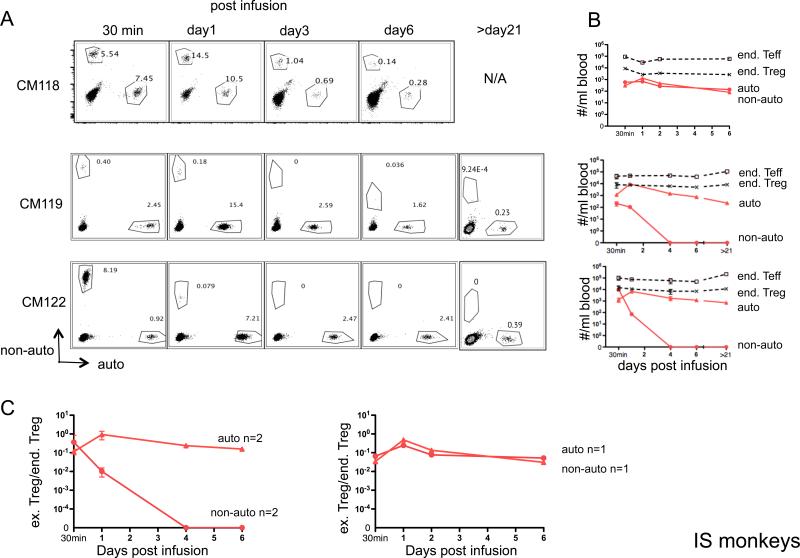
To assess the influence of a clinically-relevant T cell-depleting and rapamycin maintenance protocol (Figure 4A) on these cells in vivo, we infused IS monkeys with ex vivo-expanded auto- and non-auto-Treg (Table 2) labeled with CFSE or VPD450. As shown in Figure 4B, the IS drug regimen induced profound, reversible depletion of endogenous T cells and other lymphocyte subsets, including NK cells. To avoid any potential deleterious effects of ATG on the survival of the infused Treg, they were administered, initially, during the lymphopenia recovery phase, 17 to 20 days after the last dose of ATG. In IS monkeys (Figure 5A, B), the overall decline in auto-Treg and in the ratio of infused to endogenous Treg (Figure 5C) was similar to that observed without IS during the first week post-infusion. However, in one IS monkey (CM118) out of 3 infused, the decline in infused non-auto-Treg was markedly attenuated compared to that observed in non-IS monkeys and similar levels of auto- and non-auto-Treg, together with enhanced non-auto-Treg/endogenous Treg ratios were observed for the duration of the experiment. However, in the other two monkeys (CM119 and CM122), as in the absence of IS, non-auto-Treg declined rapidly. Longer follow-up analysis (>21 days) of these monkeys maintained on rapamycin monotherapy revealed a mean of 0.47 × 103/ml dye-labeled auto-Treg 4 weeks post infusion. Thus, in 5 out of 6 monkeys exhibiting a uniform high degree of MHC disparity with their non-auto-Treg donor, there was rapid disappearance of non-auto-Treg, whether or not the animals received lymphopenic IS conditioning followed by mTOR inhibition.
Figure 4. Protocol for infusion of Treg in monkeys treated with ATG, tacrolimus and rapamycin.
(A) Immunosuppressive (IS) drug regimen and Treg infusion time points (days after last ATG infusion) in 3 monkeys. (B) Kinetics of host T cell, B cell and NK cell depletion and recovery.
Figure 5. Persistence of ex vivo-expanded Treg in peripheral blood of immunosuppressed (IS) monkeys.
Auto- or non-auto-Treg were labeled with either CFSE or VPD450, respectively and infused (3.106 to 3.107/kg) i.v. into 3 IS monkeys. At the days indicated post-Treg infusion, 1ml blood was tested for the presence of the infused cells and endogenous (end) Teff and Treg by flow cytometry. Counting beads were added before running flow analysis. Cell numbers per ml blood were calculated as the number of cell events/number of bead events × total number of beads added. Duplicate samples were tested at each time-point. (A) Flow cytometric profiles of CD4-gated PBMC from 3 IS monkeys, showing the percentages of infused, labeled auto-and non-auto-Treg. Endogenous (unlabeled) CD4+ T cells are also evident (lower left quadrant). Two IS monkeys were monitored beyond 21 days. (B) Kinetics of the number of infused Treg (auto- and non-auto-Treg) and endogenous (end) Teff and Treg numbers in the peripheral blood after labeled Treg infusion in 3 IS monkeys for 30 min to day 6 and in 2 of these monkeys beyond day 21. (C) Kinetics of the ratio of infused (ex) Treg to endogenous (end) Treg in the peripheral blood after labeled Treg infusion in the IS monkeys (n=3).
Infused non-auto-Treg display similar levels of Ki67 expression to auto-Treg in vivo
Given that the fluorescent dyes used to track the exogenous Treg dilute with cell proliferation, we considered whether the apparent overall more rapid disappearance of non-auto-Treg might reflect the proliferation of these cells in response to recipient Ags. To compare the proliferation of exogenous auto- vs. non-auto-Treg in vivo, we stained the cells for expression of the proliferation marker Ki67. As shown in Supplementary Figure 2, in those IS monkeys (CM119 and CM122) with lower non-auto-Treg numbers compared with auto-Treg, the non-auto-Treg exibited only slightly higher expression of Ki67 early after their infusion.
Both infused auto- and non-auto-Treg can be detected in host peripheral lymph nodes
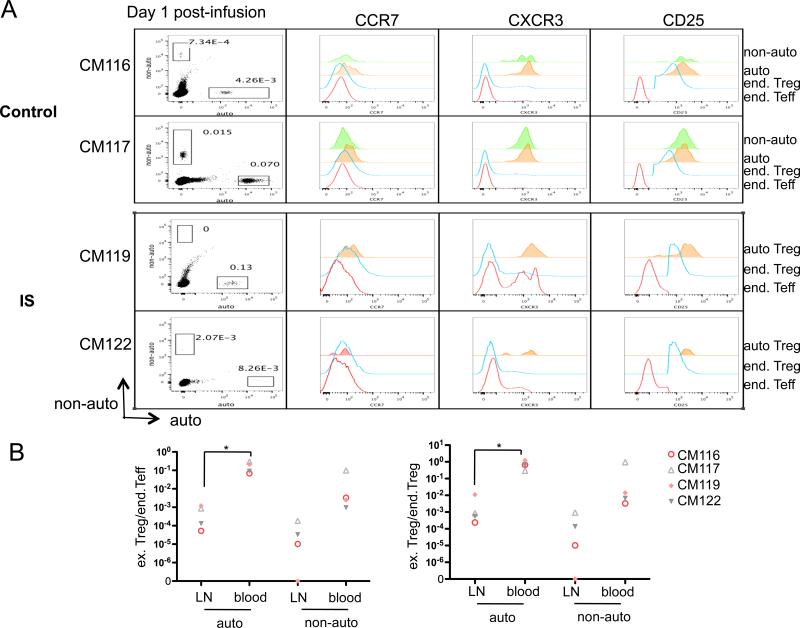
Inguinal LN were removed from 2 control monkeys and 2 IS monkeys on day 1 post-infusion, when non-auto-Treg began to decline more rapidly than auto-Treg in the blood. Figure 6A shows that, as in the blood, and although rare, more auto-Treg than non-auto-Treg could be detected in both control and IS monkey LN. The ratio of infused auto- or non-auto-Treg to endogenous CD4 Teff (Figure 6B, left panel) or Treg (Figure 6B, right panel) was consistently and significantly lower in LN than in the blood. These data suggest that greater accumulation of non-auto-Treg in LN does not account for their more rapid decline in the blood.
Figure 6. Detection of ex vivo-expanded Treg in host lymph nodes after infusion.
Ex vivo-expanded auto-and non-auto-Treg were labeled with CFSE or VPD450 before infusion. On day 1 post-infusion, inguinal LN were dissected and assessed for the presence of infused Treg. (A) Presence of exogenous Treg shown as dot plots. Expression of CCR7, CXCR3 and CD25 (offset histogram) by endogenous (end) Teff, end Treg, and exogenous auto-Treg in all 4 monkeys examined and non-auto-Treg in 2 of these monkeys. (B) Ratio of infused (ex) Treg and endogenous (end) Teff (left) or end Treg (right) in 2 control and 2 IS monkeys. *, P<0.05.
Preservation of Treg characteristics following their adoptive transfer
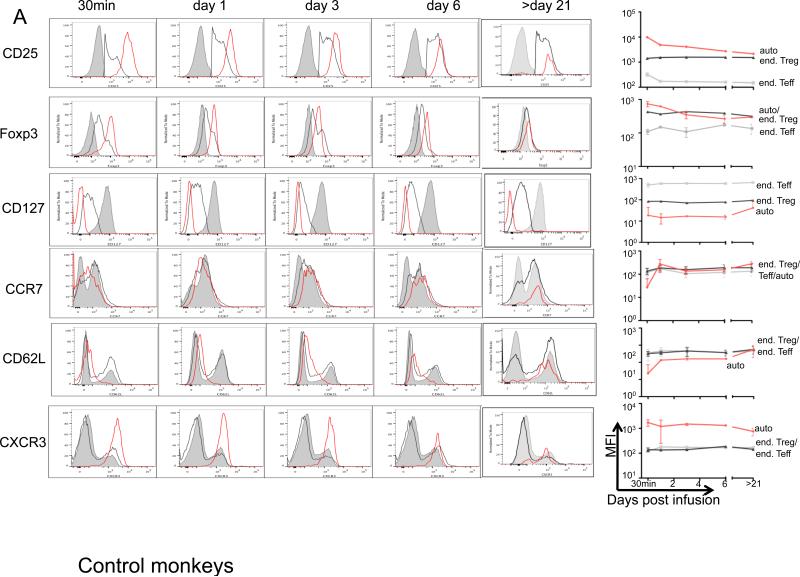
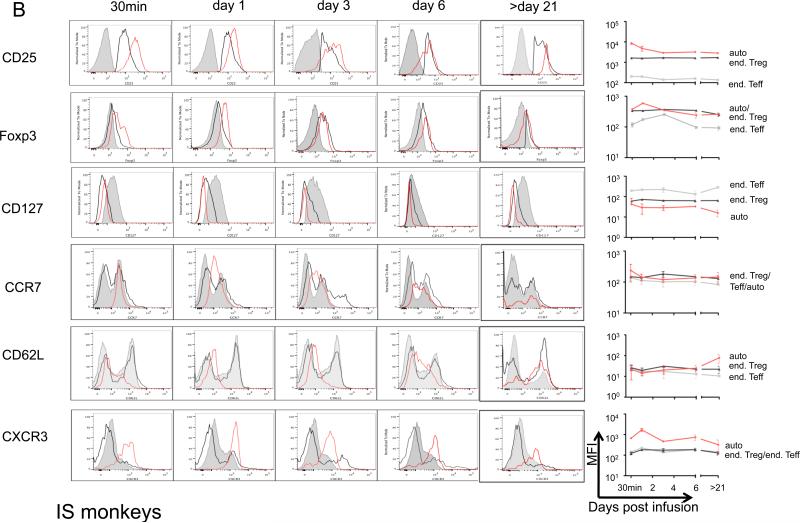
Sequential monitoring of infused expanded auto-Treg in blood revealed that they maintained a phenotype with similarly high Foxp3 and CD25 and low CD127 when compared with endogenous Treg for at least 3 weeks post-infusion, although expression of Foxp3 diminished over time compared with immediate post-infusion levels in both the control and IS monkeys (Figure 7).
Figure 7. Expression of Treg signature and activation and tissue-homing markers by ex vivo-expanded auto-Treg after their infusion.
Ex vivo-expanded auto-Treg were labeled with CFSE or VPD450 before infusion. Expression (mean fluorescence intensity; MFI) of Foxp3, CD25, CD127, CXCR3, CCR7 and CD62L at the times indicated post-Treg infusion is shown as flow histogram overlays for endogenous (end) Teff (grey solid), end Treg (black line), and exogenous auto-Treg (red line). Representative histogram overlays and pooled MFI from (A) 3 control monkeys and (B) 3 IS-monkeys, with 2 IS monkeys beyond day 21, respectively are shown.
Discussion
CD4+CD25+Foxp3+ Treg have attracted considerable attention as they promote tolerance to self Ags (57-59) or alloAgs (60, 61) in preclinical mouse models. Recent progress in the ex vivo-expansion of these otherwise rare cells has made their prospective clinical application feasible. Although a limited number of recent clinical trials have tested the safety and potential efficacy of ex vivo-expanded non-auto-Treg (30, 55, 56), many questions and challenges remain (62). These include the optimal protocol to generate adequate numbers of effective Treg, the type and number of Treg (polyclonal versus alloreactive; auto versus non-autologous) most feasible/appropriate for therapy, and the frequency, fate, phenotype and function of infused Treg. The current study demonstrates that ex vivo expansion of NHP CD4+CD25+CD127−Foxp3+Treg using, for the first time to our knowledge, aAPCs to promote their growth, provides an efficient means to generate substantial numbers of stable Treg for in vivo testing. An additional important question is how IS drugs likely to be used in conjunction with Treg in the clinic, impact of these cells’ in vivo stability and survival. Here we show that, in the absence of to organ transplantation, auto-Treg persist much longer than MHC-mismatched non-auto-Treg in 3/3 control monkeys and in 2/3 IS monkeys. Although numbers of these fluorescent-labeled cells declined considerably in the blood early post-infusion, as reported recently for labeled rhesus auto-Treg [in Abstract form (63)], they retained key Treg phenotypic characteristics in vivo.
In the past decade, considerable success has been achieved in devising methods to generate adequate numbers of Treg via ex vivo-expansion (26, 31, 57, 64-66). The concept of ‘off-the-shelf’, non-auto (or ‘third party’) Treg (Treg ‘banks’) has several significant clinical implications, since mass production and quality control/standardization of these regulatory cells may enhance their use as therapeutic agents. Previously, we and others (22, 24) have demonstrated the ex vivo-expansion of NHP Treg using stimulatory anti-CD3/CD28 mAb and rhu IL-2. In this study, using aAPCs, we have significantly improved the expansion of these cells from purified, flow-sorted peripheral blood Treg over a 3-wk period. A further advantage of aAPCs is that their use eliminates (potential) contamination of the regulatory immune cell product with potent T cell stimulatory beads. Significantly, the adhesive property of the L-32 cells (67) promotes their elimination from expanded Treg preparations.
One concern regarding use of non-auto-Treg compared with auto-Treg is their attenuated survival after infusion. On the one hand, infused non-auto-Treg could be depleted by the host's innate or adaptive immune mechanisms. On the other, they could conceivably avoid rejection due to their inherent immunoregulatory/suppressive capacity. In this study, non-auto-Treg infused in numbers similar to those that have been used in hematopoietic stem cell transplantation, and that are envisaged for Treg use in solid organ transplantation (62), could be detected consistently in the blood of control monkeys for only 3 days post-infusion, whereas auto-Treg persisted in greater numbers and for longer (at least 7 weeks). This finding suggests that the inherent suppressive capacity of the infused non-auto-Treg alone (that may be inferior to that of auto-Treg) is not sufficient to protect them from destruction/elimination by normal host protective mechanisms.
A less consistent result was observed in IS monkeys. In one out of three monkeys, IS prolonged survival/persistence of MHC-mismatched non-auto-Treg similar to that seen with auto-Treg was observed. However, in the other two IS monkeys, as in control animals, non-auto-Treg numbers fell rapidly to undetectable levels in the blood within 4 days post-infusion. All non-auto-Treg were MHC-mismatched at multiple haplotypes and by the time of Treg infusion, host T and NK cells had partially recovered in IS recipients. Recently, Issa et al (68) have reported in abstract form that, as in our monkey model, adoptively-transferred (HLA-mismatched) human Treg are lost in humanized mouse recipients, an effect they ascribed to alloimmune-mediated killing. Immunosuppression, however, promoted their survival. Based on the current findings, careful evaluation of immunosuppressive protocols will be necessary to ascertain the persistence and biodistribution of MHC-mismatched non-auto-Treg in NHP and humans. A concern with the use of non-auto-Treg, as with other regulatory immune cells from non-autologous donors e.g. regulatory macrophages or regulatory dendritic cells, is the potential risk of host sensitization (69). However, we found no evidence of significant anti-donor alloAb production determined by flow cytometric analysis of serum from 2 IS monkeys that had received 3 infusions of non-auto-Treg (Zhang H et al, unpublished observation).
In order to inhibit alloimmune responses, it is thought that Treg need to be activated and to migrate to sites of T cell priming (draining LNs) (70, 71) or effector function (e.g., an allograft) (72). Zhang et al (72) have shown that, in mice, Treg migrate first to (islet) allografts, where dendritic cell migration is inhibited, and then to the draining LNs, where they prevent alloreactive T cell priming and migration. Our ex vivo-expanded cynomolgus Treg exhibited high expression of CXCR3 and low expression of CD62L and CCR7. This expression pattern is consistent with Treg activation and potential to accumulate in vivo at sites of inflammation, e.g. an organ allograft rather than secondary lymphoid tissue after infusion. Tracking and resolution of infused immune cells in tissues other than blood and LN, particularly in living recipients, is however challenging, especially in large animals and humans. Only limited success has been achieved to date in these species using radio-labeled cells and with a short follow-up time (30 h) (73). In this study, in the absence of inflammation, we detected rare/dye-labeled Treg shortly after their infusion in host peripheral LN. This suggests that these ex vivo-expanded Treg may have limited ability to migrate to secondary lymphoid tissue. However, much more needs to be learned about the biodistribution adoptively-transferred Treg. Our future research will determine their ability to migrate to sites of inflammation, in particular the extent to which they migrate to an organ allograft.
Overall, our observations demonstrate the retention of a Treg signature similar to endogenous Treg by ex-vivo expanded auto-Treg following their infusion in a clinically-relevant NHP model. Foxp3 expression did diminish progressively compared with pre-infusion levels in these monkeys in the absence of inflammation or allostimulation in vivo. It will therefore be of interest to ascertain Foxp3 expression by these ex vivo-expanded Treg following their adoptive transfer in allograft recipients. Our findings also indicate that ex vivo-expanded, MHC-mismatched non-auto-Treg do not persist as long as auto-Treg in the peripheral circulation of normal NHP, or as consistently as auto-Treg in non-transplanted IS NHP treated with ATG and rapamycin. This may represent a potential limitation to their potential use as therapeutic agents.
Supplementary Material
Acknowledgments
The study was supported by National Institutes of Health (NIH) grant U01 AI91197, part of the NIH NHP Transplantation Tolerance Cooperative Study group and sponsored by the NIAID and NIDDK. MHC genotyping was supported by NIAID contract HHSN272201100013C. HZ was in receipt of an American Society of Transplantation Basic Science Fellowship and is a non-concurrent NIH T32 Training Grant Fellowship. We thank Miriam Freeman for skillful administrative support and Dr. Qizhi Tang (University of California, San Francisco) for valuable discussion and constructive suggestions.
Abbreviations
- APC(s)
antigen-presenting cell(s)
- ATG
anti-thymocyte globulin
- CFSE
carboxyfluorescein succinimidyl ester
- Foxp3
forkhead box P3
- IS
immunosuppression/immunosuppressive/immunosuppressed
- MFI
mean fluorescence intensity
- NHP
non-human primate
- Teff
effector T cells
- Treg
regulatory T cells
- TSDR
Treg-specific demethylation region
- VPD450
violet proliferation dye 450
Footnotes
Disclosure
The authors have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7(8):650–654. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- 3.June CH, Blazar BR. Clinical application of expanded CD4+25+ cells. Semin Immunol. 2006;18(2):78–88. doi: 10.1016/j.smim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12(6):417–430. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Powrie F, Ransohoff RM. Re-establishing immunological self-tolerance in autoimmune disease. Nat Med. 2012;18(1):54–58. doi: 10.1038/nm.2622. [DOI] [PubMed] [Google Scholar]
- 6.Torrealba JR, Katayama M, Fechner JH, Jr., Jankowska-Gan E, Kusaka S, Xu Q, et al. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates. J Immunol. 2004;172(9):5753–5764. doi: 10.4049/jimmunol.172.9.5753. [DOI] [PubMed] [Google Scholar]
- 7.Bashuda H, Kimikawa M, Seino K, Kato Y, Ono F, Shimizu A, et al. Renal allograft rejection is prevented by adoptive transfer of anergic T cells in nonhuman primates. J Clin Invest. 2005;115(7):1896–1902. doi: 10.1172/JCI23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma A, Qi S, Song L, Hu Y, Dun H, Massicotte E, et al. Adoptive transfer of CD4+CD25+ regulatory cells combined with low-dose sirolimus and anti-thymocyte globulin delays acute rejection of renal allografts in Cynomolgus monkeys. Int Immunopharmacol. 2011;11(5):618–629. doi: 10.1016/j.intimp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Taflin C, Nochy D, Hill G, Frouget T, Rioux N, Verine J, et al. Regulatory T cells in kidney allograft infiltrates correlate with initial inflammation and graft function. Transplantation. 2010;89(2):194–199. doi: 10.1097/TP.0b013e3181c3ca11. [DOI] [PubMed] [Google Scholar]
- 10.Bestard O, Cruzado JM, Rama I, Torras J, Goma M, Seron D, et al. Presence of FoxP3+ regulatory T Cells predicts outcome of subclinical rejection of renal allografts. J Am Soc Nephrol. 2008;19(10):2020–2026. doi: 10.1681/ASN.2007111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Llordella M, Puig-Pey I, Orlando G, Ramoni M, Tisone G, Rimola A, et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant. 2007;7(2):309–319. doi: 10.1111/j.1600-6143.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 12.Tokita D, Mazariegos GV, Zahorchak AF, Chien N, Abe M, Raimondi G, et al. High PDL1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation. 2008;85:369–377. doi: 10.1097/TP.0b013e3181612ded. [DOI] [PubMed] [Google Scholar]
- 13.Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16(7):809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu DC, Hester J, Nadig SN, Zhang W, Trzonkowski P, Gray D, et al. Ex vivo expanded human regulatory T cells can prolong survival of a human islet allograft in a humanized mouse model. Transplantation. 2013;96(8):707–716. doi: 10.1097/TP.0b013e31829fa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 16.Levings MK, Sangregorio R, Roncarolo MG. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193(11):1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, et al. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98(9):2736–2744. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 18.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193(11):1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, et al. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104(2):453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 22.Anderson A, Martens CL, Hendrix R, Stempora LL, Miller WP, Hamby K, et al. Expanded nonhuman primate Tregs exhibit a unique gene expression signature and potently downregulate alloimmune responses. Am J Transplant. 2008;8(11):2252–2264. doi: 10.1111/j.1600-6143.2008.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh K, Kozyr N, Stempora L, Kirk AD, Larsen CP, Blazar BR, et al. Regulatory T cells exhibit decreased proliferation but enhanced suppression after pulsing with sirolimus. Am J Transplant. 2012;12(6):1441–1457. doi: 10.1111/j.1600-6143.2011.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dons EM, Raimondi G, Zhang H, Zahorchak AF, Bhama JK, Lu L, et al. Ex vivo-expanded cynomolgus macaque regulatory T cells are resistant to alemtuzumab-mediated cytotoxicity. Am J Transplant. 2013;13(8):2169–2178. doi: 10.1111/ajt.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kean LS, Gangappa S, Pearson TC, Larsen CP. Transplant tolerance in non-human primates: progress, current challenges and unmet needs. Am J Transplant. 2006;6(5 Pt 1):884–893. doi: 10.1111/j.1600-6143.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 26.Dons EM, Raimondi G, Cooper DK, Thomson AW. Non-human primate regulatory T cells: current biology and implications for transplantation. Transplantation. 2010;90(8):811–816. doi: 10.1097/TP.0b013e3181ebf782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant. 2013;13(8):1989–2005. doi: 10.1111/ajt.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tchorsh-Yutsis D, Zlotnikov Klionsky Y, Bachar-Lustig E, Aronovich A, Feine I, Shezen E, et al. Embryonic pig pancreatic tissue for the treatment of diabetes: potential role of immune suppression with “off-the-shelf” third-party regulatory T cells. Transplantation. 2011;91(4):398–405. doi: 10.1097/TP.0b013e318204be15. [DOI] [PubMed] [Google Scholar]
- 29.Steiner D, Brunicki N, Blazar BR, Bachar-Lustig E, Reisner Y. Tolerance induction by third-party “off-the-shelf” CD4+CD25+ Treg cells. Exp Hematol. 2006;34(1):66–71. doi: 10.1016/j.exphem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo xpanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11(6):1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari N, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111(7):3675–3683. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boenisch O, Lopez M, Elyaman W, Magee CN, Ahmad U, Najafian N. Ex vivo expansion of human Tregs by rabbit ATG is dependent on intact STAT3-signaling in CD4(+) T cells and requires the presence of monocytes. Am J Transplant. 2012;12(4):856–866. doi: 10.1111/j.1600-6143.2011.03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Q, Leung J, Melli K, Lay K, Chuu EL, Liu W, et al. Altered balance between effector T cells and FOXP3+ HELIOS+ regulatory T cells after thymoglobulin induction in kidney transplant recipients. Transpl Int. 2012;25(12):1257–1267. doi: 10.1111/j.1432-2277.2012.01565.x. [DOI] [PubMed] [Google Scholar]
- 35.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. Am J Transplant. 2010;10(9):2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raimondi G, Sumpter TL, Matta BM, Pillai M, Corbitt N, Vodovotz Y, et al. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. J Immunol. 2010;184(2):624–636. doi: 10.4049/jimmunol.0900936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hester J, Schiopu A, Nadig SN, Wood KJ. Low-dose rapamycin treatment increases the ability of human regulatory T cells to inhibit transplant arteriosclerosis in vivo. Am J Transplant. 2012;12(8):2008–2016. doi: 10.1111/j.1600-6143.2012.04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiseman RW, Karl JA, Bohn PS, Nimityongskul FA, Starrett GJ, O'Connor DH. Haplessly hoping: macaque major histocompatibility complex made easy. ILAR J. 2013;54(2):196–210. doi: 10.1093/ilar/ilt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol. 2013;190(5):2001–2008. doi: 10.4049/jimmunol.1201379. [DOI] [PubMed] [Google Scholar]
- 40.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177(12):8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 41.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3(83):83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9(2):83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 43.Marco MR, Dons EM, van der Windt DJ, Bhama JK, Lu LT, Zahorchak AF, et al. Post-transplant repopulation of naive and memory T cells in blood and lymphoid tissue after alemtuzumab-mediated depletion in heart-transplanted cynomolgus monkeys. Transpl Immunol. 2013;29(1-4):88–98. doi: 10.1016/j.trim.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 47.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10(11):2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10(7):689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 50.Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM, et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184(6):2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 51.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199(3):303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187(2):205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99(1):23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 54.Burrell BE, Nakayama Y, Xu J, Brinkman CC, Bromberg JS. Regulatory T cell induction, migration, and function in transplantation. J Immunol. 2012;189(10):4705–4711. doi: 10.4049/jimmunol.1202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133(1):22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 57.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169(9):4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Podojil JR, Chang J, Luo X, Miller SD. TGF-beta-induced myelin peptide-specific regulatory T cells mediate antigen-specific suppression of induction of experimental autoimmune encephalomyelitis. J Immunol. 2010;184(12):6629–6636. doi: 10.4049/jimmunol.0904044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joffre O, Gorsse N, Romagnoli P, Hudrisier D, van Meerwijk JP. Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood. 2004;103(11):4216–4221. doi: 10.1182/blood-2004-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14(1):88–92. doi: 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Juvet SC, Whatcott AG, Bushell AR, Wood KJ. Harnessing regulatory T cells for clinical use in transplantation: the end of the beginning. Am J Transplant. 2014;14(4):750–763. doi: 10.1111/ajt.12647. [DOI] [PubMed] [Google Scholar]
- 63.Singh K, Stempora L, Garrett A, Kirk AD, Larsen CP, Blazar BR, et al. In-vivo persistence, trafficking and phenotypic stability of autologous, ex-vivo expanded rhesus macaque natural regulatory T cells. Am J Transplant. 2013;13(5):77. Abstract #155. [Google Scholar]
- 64.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108(8):2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Putnam AL, Safinia N, Medvec A, Laszkowska M, Wray M, Mintz MA, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant. 2013;13(11):3010–3020. doi: 10.1111/ajt.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3(83):83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MacDonald KG, Han JM, Himmel ME, Huang Q, Kan B, Campbell AI, et al. Response to comment on “helios+ and helios-cells coexist within the natural FOXP3+ T regulatory cell subset in humans”. J Immunol. 2013;190(9):4440–4441. doi: 10.4049/jimmunol.1390019. [DOI] [PubMed] [Google Scholar]
- 68.Issa F, Hester J, Milward R, Goto R, Wood K. Evaluation and optimisation of nonautologous human regulatory T cell therapy for transplantation. Am J Transplant. 2014;14(s3):25. Abstract 744. [Google Scholar]
- 69.Hutchinson JA, Ahrens N, Riquelme P, Walter L, Gruber M, Boger CA, et al. Clinical management of patients receiving cell-based immunoregulatory therapy. Transfusion. 2014 doi: 10.1111/trf.12641. [DOI] [PubMed] [Google Scholar]
- 70.Lee MKt, Moore DJ, Jarrett BP, Lian MM, Deng S, Huang X, et al. Promotion of allograft survival by CD4+CD25+ regulatory T cells: evidence for in vivo inhibition of effector cell proliferation. J Immunol. 2004;172(11):6539–6544. doi: 10.4049/jimmunol.172.11.6539. [DOI] [PubMed] [Google Scholar]
- 71.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107(2):827–834. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30(3):458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, et al. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187(5):2072–2078. doi: 10.4049/jimmunol.1100762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.