Abstract
Insulin resistance is a risk factor for Alzheimer’s disease (AD), although its role in AD etiology is unclear. We assessed insulin resistance using fasting and insulin-stimulated measures in 51 elderly subjects with no dementia (ND; n=37) and with cognitive impairment (CI; n=14). CI subjects exhibited either Mild Cognitive Impairment or AD. Fasting insulin resistance was measured using the homeostatic model assessment of insulin resistance (HOMA-IR). Insulin-stimulated glucose disposal was assessed using the hyperinsulinemic-euglycemic clamp to calculate glucose disposal rate into lean mass (GDRLM), the primary site of insulin-stimulated glucose disposal. Because insulin crosses the blood brain barrier, we also assessed whether insulin infusion would improve verbal episodic memory compared to baseline. Different but equivalent versions of cognitive tests were administered in counterbalanced order in the basal and insulin-stimulated state. Groups did not differ in age or body mass index. Cognitively impaired subjects exhibited greater insulin resistance as measured at fasting (HOMA-IR; ND: 1.09 [1.1] vs CI: 2.01 [2.3] p=0.028) and during the hyperinsulinemic clamp (GDRLM; ND: 9.9 (4.5) vs. AD 7.2 (3.2) p=0.040). Cognitively impaired subjects also exhibited higher fasting insulin compared to ND subjects, (CI: 8.7 [7.8] vs ND: 4.2 [3.8] µU/mL; p=0.023) and higher fasting amylin (CI: 24.1 [39.1] vs. 8.37 [14.2]; p=0.050) with no difference in fasting glucose. Insulin infusion elicited a detrimental effect on one test of verbal episodic memory (Free and Cued SRT) in both groups (p<0.0001) and no change in performance on an additional task (delayed logical memory). In this study, although insulin resistance was observed in cognitively impaired subjects compared to ND controls, insulin infusion did not improve memory. Furthermore, a significant correlation between HOMA-IR and GDR was present only in ND (p=0.0002) but not in cognitively impaired (p=0.884) subjects, indicating potentially important physiological differences between these cohorts.
Introduction
Insulin resistance is linked to increased risk for both Alzheimer’s Disease (AD) (Cheng et al., 2011; Ott et al., 1999; Xu et al., 2009) and cognitive decline (Hishikawa et al., 2015; Young et al., 2006). Although the mechanistic relationship between insulin resistance and cognitive decline is unclear, insulin signaling has been linked to neurotransmission (Man et al., 2003; Skeberdis et al., 2001) and is impaired in AD brain postmortem. (Lee et al., 2009; Liu et al., 2011; Moloney et al., 2010; Steen et al., 2005) Insulin signaling also affects intracellular trafficking, excocytosis, and cell survival, providing molecular rationale for an epidemiological link (reviewed in (Morris and Burns, 2012)). Insulin can directly access neuronal tissues, as it freely crosses the blood brain barrier from the peripheral circulation through a saturable, receptor-mediated transport mechanism. (Banks, 2004; Baura et al., 1993)
The majority of clinical literature that addresses the role of insulin resistance in neurodegeneration uses fasting metabolic measures, primarily glucose and insulin, to characterize insulin resistance. A commonly-used calculation that uses fasting glucose and insulin values is the homeostatic model assessment of insulin resistance (HOMA-IR). In young, cognitively-intact cohorts, HOMA-IR correlates well with the “gold standard” measure of insulin resistance, the hyperinsulinemic-euglycemic clamp (Emoto et al., 1999; Katsuki et al., 2001; Yokoyama et al., 2003). However, no studies have addressed whether HOMA-IR is a viable surrogate measure for hyperinsulinemic clamp-derived estimate of insulin resistance in elderly or cognitively-impaired populations. Furthermore, no studies have assessed insulin resistance using the gold standard outcome, glucose disposal rate normalized to lean mass (the primary site of insulin-mediated glucose disposal) calculated from the hyperinsulinemic-euglycemic clamp.
There is also a line of evidence that intravenously or intranasally administered insulin improves memory in cognitively-impaired individuals (Craft et al., 2012; Reger et al., 2006; Reger et al., 2008b). However, not all studies show a beneficial effect of insulin in AD; for instance, Apolipoprotein E (APOE) ε4 carriers generally do not exhibit improved cognition (Rosenbloom et al., 2014). Furthermore, our group has shown that the relationship between circulating insulin, cognition, and brain structure differs between individuals with normal cognition and AD (Burns et al., 2007; Burns et al., 2012). Thus, the goals of this study were to 1) characterize insulin resistance in ND and CI subjects using both fasting and gold-standard methods, 2) assess the relationship between these methods, and 3) characterize the effect of insulin on memory function. We hypothesized that CI subjects would exhibit greater insulin resistance than healthy elderly, that HOMA-IR would correlate well with the hyperinsulinemic clamp across the whole cohort, and that insulin stimulation would improve performance on memory tests in all subjects.
Methods
This study was approved by the University of Kansas Medical Center’s Institutional Review Board. All participants in this study provided informed consent according to institutional guidelines and in accordance with the Declaration of Helsinki. KU Alzheimer’s Disease Center clinical cohort participants who were age 60 or older, post-menopausal, on stable medication doses, and exhibited the ability to provide informed consent were invited to participate in the study. Exclusion criteria included moderate to severe AD (CDR>1), Type 1 Diabetes, poorly controlled diabetes (recent hospitalization for hyperglycemia, HbA1C>10), neurodegenerative disorders other than AD with the potential to impair cognition, and clinically significant depression.
Clinical Assessment and Diagnosis
Diagnosis of no dementia or cognitive impairment was determined following a thorough clinical exam using the Clinical Dementia Rating (CDR) scale, (Morris, 1993) focusing on intrasubject change rather than deviation from group norms (Morris, 1993; Morris, 1997; Rockwood et al., 2000). Subjects with a CDR score of 0 without clinical evidence of cognitive impairment were recruited into this study as controls (no dementia; ND). Cognitively impaired subjects exhibited CDR scores of 0.5 or 1 and were diagnosed with either Mild Cognitive Impairment or AD. Diagnoses from a single clinician were confirmed at a consensus diagnosis conference.
Study visit
The study required each subject to complete one (approximately 6 hour) visit to the KU Clinical and Translational Science Unit for the hyperinsulinemic-euglycemic clamp procedure. Subjects arrived following an overnight fast. Vital signs (heart rate, blood pressure, temperature) were measured and two IV lines placed, one for infusion of dextrose, saline, and insulin, and another in the opposite arm for blood sampling. Plasma samples were acquired at baseline. Prior to the start of infusion, a staff psychometrician administered approximately 30 minutes of memory testing, including 2 tests of verbal episodic memory; Delayed Logical Memory (DLM) and Free and Cued Selective Reminding Test (SRT). The cognitive outcome measures were units recalled (DLM) and total free recall (SRT). To eliminate recall bias, a different version of the memory tests were administered beginning at 90 minutes after infusion began. Test versions were counterbalanced with each study visit.
Hyperinsulinemic-euglycemic clamp
Upon insertion of peripheral venous catheters, blood was drawn from an arm that was heated in a hand-warming box (50°C) to arterialize venous blood prior to measurement of baseline metabolic outcomes. Regular human insulin (100U/mL) was mixed with saline and the subject’s blood for infusion. 5mL of blood was drawn and immediately injected into a 250mL bag of sterile saline and inverted to mix before immediate addition of 1.25mL insulin. During the procedure, a primed continuous infusion of insulin based on body size (40mU·m−2·min−1) and variable infusion of 20% dextrose occurred through the catheter placed in the opposite arm. Glucose was measured every 5 minutes during the procedure and adjustments to dextrose infusion made accordingly to achieve euglycemia (90–100mg/dL) over the course of 3 hours, with the exception of during memory tests, when glucose measurements were performed between cognitive tests. Insulin sensitivity was determined based on the glucose disposal rate (GDR) during the steady state (minutes 150–180 of the procedure). After 3 hours, the insulin infusion was stopped and glucose infusion doubled and allowed to continue for an additional 30 minutes while the participant was provided a meal.
Anthropometric measures
Total body mass was determined prior to the hyperinsulinemic-euglycemic clamp using a digital scale accurate to 0.1kg (Seca Platform Scale, model 707). After the procedure, subjects were asked to void and evaluated using dual energy x-ray absorptiometry (DEXA, Lunar Prodigy, version 11.2068) to determine lean mass, fat mass, and bone mineral density.
Metabolic measures
Glucose was measured in arterialized blood every 5 minutes over the course of the procedure using a YSI-2300 Glucose and Lactate Analyzer, with the exception of during cognitive testing, when measurements were performed between cognitive tests. Additionally, plasma samples taken at 0, 60, 90, and 180 minutes were frozen at −80°C and stored until analysis of insulin and C-peptide using ELISA assays (Alpco). Plasma amylin was measured at the fasting using ELISA (Millipore). Plasma cortisol was measured at both fasting and at the 90 minute timepoint using ELISA (Alpco).
Statistical analyses
Demographic characteristics were compared using the two sample t-test for continuous measures or Pearson’s chi-square test statistic for categorical measures. For continuous measures that violated underlying assumptions based on visual inspection of residual analyses, Satterthwaite’s degrees of freedom or the nonparametric Wilcoxon signed rank test was used instead as indicated; for categorical measures we used Fisher’s exact test when the expected cell count assumptions were not met. Continuous baseline measures and change scores were similarly compared between groups using the two-sample t-test or nonparametric Wilcoxon rank sum test if indicated as described above. Continuous measures were correlated and compared between groups using ordinary least squares regression including interaction with group with model assessment by visual inspection of residual plots. Longitudinal measures were compared between groups using linear mixed models, with model assessment by visual inspection of residual plots.
We conducted sensitivity analyses by adjusting for age and sex. For single time point and change score continuous measures we used ordinary least squares regression. For APOE status we used unconditional logistic regression. For longitudinal continuous measures we used linear mixed models and tested the group by time interaction for longitudinal between group differences. Since this was a pilot study, no multiplicity adjustments were made to the analyses at this time. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, 2002–2012).
Results
The compared groups (n=37 non-demented; n=14 with MCI or AD) did not differ based on age (p=0.46), years of education (p=0.90) or proportion of APOE ε4 carriers (p=0.653); Table 1. Although there was a trend for more males in the CI group, (p=0.06), this missed statistical significance.
Table 1.
Mean (standard deviation) for demographic and clinical characteristics
| Nondemented (n=37) | MCI or AD (n=14) | p-value | |
|---|---|---|---|
| Age* (y) | 72.4 (6.6) | 71.2 (4.0) | 0.458 |
| Education (y) | 16.9 (2.8) | 16.8 (2.9) | 0.905 |
| Sex (#male, %) | 13 (35.1) | 9 (64.3) | 0.061 |
| APOE carriers (#male, %)‡ | 12 (35.3) | 4 (28.6) | .653 |
| GDR (mg/kg lean mass/min) | 9.9 (4.5) | 7.2 (3.2) | 0.040* |
| HOMA-IR | 1.09 (1.1) | 2.01 (2.3) | 0.028* |
| Weight (kg) | 76.8 (18.4) | 80.1 (16.0) | 0.561 |
| BMI | 27.0 (3.9) | 27.8 (5.0) | 0.538 |
| Fat mass (kg) | 29.7 (10.3) | 27.8 (6.7) | 0.514 |
| Lean mass (kg) | 44.6 (9.4) | 49.1 (11.7) | 0.154 |
| BMD | 1.11 (0.14) | 1.17 (0.09) | 0.124 |
| Systolic blood pressure (mmHg) | 127.0 (16.5) | 131.2 (13.8) | 0.404 |
| Diastolic blood pressure (mmHg) | 75.6 (7.8) | 74.1 (9.9) | 0.579 |
| CDR-SB | 0.0 (0) | 3.1 (2.2) | <0.001* |
| Amylin (fasting)‡‡ | 8.37 (14.2) | 24.1 (39.1) | 0.050 |
| Cortisol (fasting) | 21.4 (8.2) | 25.3 (13.1) | 0.345 |
| Cortisol (90 min) | 15.6 (8.6) | 16.3 (6.4) | 0.437 |
Satterthwaite’s approximation to the degrees of freedom.
Genotyping failed on three nondemented subjects; Fisher’s exact test used. All anthropometric measures were controlled for age and sex.
Wilcoxon rank sum test used due to violated assumptions for two sample t-test. Values reflect means ± SD.
The primary fasting markers of metabolic dysfunction in CI subjects were higher insulin (p=0.02; table 2) and amylin (p=0.05; table 1). Fasting glucose and C-peptide (a marker of endogenous insulin production) were not different between groups. Over the course of the hyperinsulinemic clamp, insulin levels were increased over the fasting levels as expected in both groups. Insulin levels did not differ between groups over the course of the clamp, although CI subjects exhibited slightly higher glucose at 60 and 90 minutes before levels equilibrated during the steady state. Groups did not differ based on anthropometric measures including fat mass (p=0.51), lean mass (p=0.15), body weight (p=0.56), body mass index (p=0.54), or blood pressure (p=0.40 (systolic) and p=0.58 (diastolic)); Table 1. No subjects exhibited hypertension (>140/90mmHg)
Table 2.
Metabolic biomarker levels over the course of the hyperinsulinemic-euglycemic clamp.
| Measure | Group | 0tp | 60min | 90min | 180min | p-value (fasting) |
p-value (clamp) ‡‡ |
|---|---|---|---|---|---|---|---|
| Glucose (mg/dL) | ND | 102.6 (17.5) | 110.1 (19.4) | 103.3 (13.7) | 96.7 (7.9) | 0.948‡ | 0.008 |
| MCI/AD | 102.8 (9.1) | 119.3 (21.3) | 113.6 (16.5) | 93.7 (4.8) | |||
| Insulin (μU/mL) | ND | 4.19 (3.8) | 52.4 (31.3) | 54.0 (38.2) | 50.3 (43.4) | 0.023* | 0.139 |
| MCI/AD | 8.73 (7.8) | 46.6 (19.7) | 38.6 (17.7) | 43.8 (15.9) | |||
| C-peptide (pM) | ND | 430.4 (224) | 568.6 (370) | 522.4 (320) | 367.2 (221) | 0.255 | 0.439 |
| MCI/AD | 468.8 (162) | 529.6 (222) | 525.4 (248) | 317.4 (148) |
Metabolic biomarkers were assessed both at fasting and over the course of the hyperinsulinemic-euglycemic clamp.
Satterthwaite’s approximation to the degrees of freedom.
Test of group by time interaction (linear mixed models used all available time points).
Wilcoxon rank sum test used due to violated assumptions for the two sample t-test.
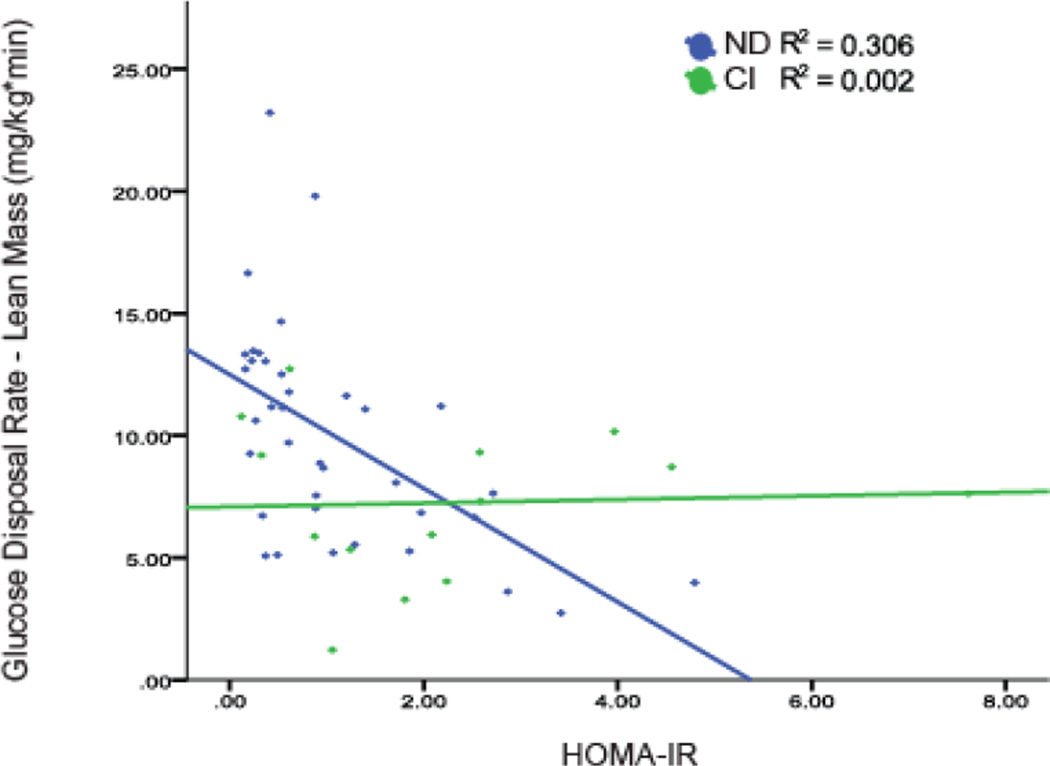
Greater insulin resistance was observed in individuals with CI compared to ND subjects using both the fasting measure HOMA-IR (p=0.03), and the insulin-stimulated measure GDRLM (p=0.04). As a secondary analysis, we investigated the effect of APOE genotype on these results. We observed a significant interaction effect between APOE ε4 genotype and diagnosis group on GDRLM (p=0.003), but not between APOE ε4 genotype and diagnosis group on HOMA-IR (p=0.906). Interestingly, the HOMA-IR (fasting) and GDRLM (insulin-stimulated) measures of insulin resistance were highly correlated in ND elderly (p=0.0002) but not CI subjects (p=0.884), potentially indicating important physiological differences in the metabolism of these groups.
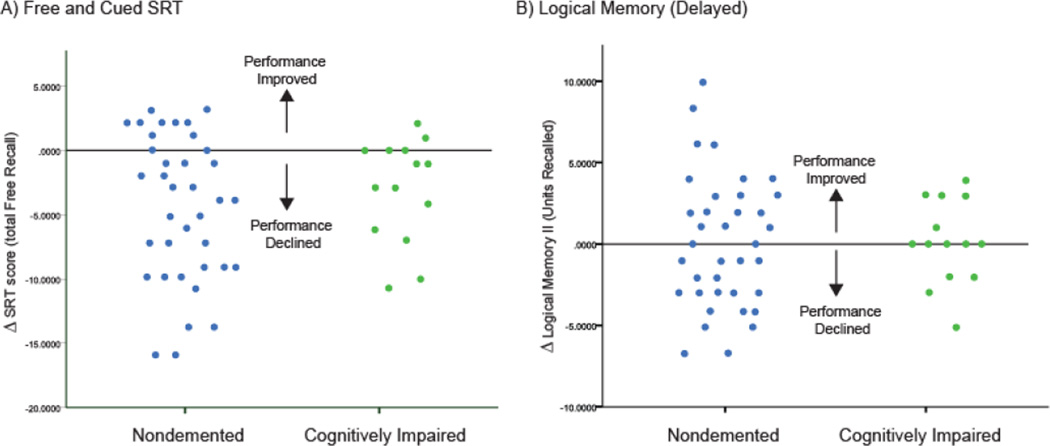
During memory testing, both ND and CI subjects exhibited a significant decline in performance on SRT (Total Free Recall) in the insulin-stimulated state (p<0.0001; Figure 2A). Neither diagnosis group showed change in cognitive performance on story recall (DLM) in the insulin-stimulated state compared to baseline (p=0.65; Figure 2B).
Figure 2.
Change in cognitive performance in hyperinsulinemic state
Discussion
In this study, we found that cognitively impaired subjects exhibited greater insulin resistance using both fasting (HOMA-IR) and insulin-stimulated (GDR-LM) measures. The HOMAIR and GDR assessments of insulin resistance were highly correlated in ND elderly, but not in cognitively impaired subjects. Tests of verbal episodic memory administered in the insulin-stimulated state failed to show an improvement in cognitive performance, in conflict with prior work. Performance on one test (SRT) significantly declined in both groups following insulin infusion.
There is strong evidence for a relationship between insulin resistance and cognitive impairment. For instance, diabetes increases AD risk (Cheng et al., 2011; Leibson et al., 1997; Ohara et al., 2011; Ott et al., 1999; Xu et al., 2009). Moreover, cognitively impaired subjects exhibit high fasting glucose (Carantoni et al., 2000), high fasting insulin (Carantoni et al., 2000; Craft et al., 1998), elevated HOMA (Morris et al., 2014), impaired insulin response to glucose (Craft et al., 1993), and increased frequency of diabetes (Garcia-Lara et al., 2010) compared to controls. However, only one prior study has assessed insulin resistance using the gold-standard measure, the hyperinsulinemic-euglycemic clamp, in nondemented and cognitively impaired subjects.(Craft et al., 1999b) This study did not compare the groups directly but split each group based on apolipoprotein ε4 genotype and found that AD subjects negative for the APOE ε4 allele exhibited the greatest insulin resistance. We included APOE ε4 was included as a factor in our GDRLM analyses as a secondary outcome measure. Consistent with the prior work, we observed an interaction effect. In cognitively impaired individuals, APOE e4 negative individuals exhibited more insulin resistance (GDRLM: 5.9 [2.6]) than APOE e4 positive subjects (GDRLM: 10.6 [1.7]). However, nondemented APOE e4 negative individuals exhibited less insulin resistance (GDRLM: 11.6 [4.7]) compared to APOE e4 positive individuals (7.9 [2.9]). This supports the notion that insulin resistance is an independent risk factor for cognitive decline, especially in individuals not at genetic risk for Alzheimer’s disease.
As expected, we found good concordance between our two estimates of insulin resistance (HOMA-IR and the GDR of the hyperinsulinemic clamp) in our ND group, consistent with reports in younger and cognitively-normal groups, (Emoto et al., 1999; Katsuki et al., 2001; Yokoyama et al., 2003). However, these two IR estimates were not correlated in cognitively impaired subjects, suggesting potential differences in underlying IR-relevant biological processes measured with the procedures. HOMA-IR is more reflective of hepatic insulin resistance given it is based on glucose and insulin values collected during the fasting state, when the liver is primarily responsible for glucose output. Because the hyperinsulinemic clamp procedure largely suppresses hepatic glucose secretion, the GDR primarily reflects glucose uptake into skeletal muscle and is the gold standard for measuring muscle insulin resistance. The discordance we observed between these two measures in cognitively impaired subjects suggests a possible disconnect between hepatic and skeletal muscle insulin resistance. The CNS plays a modifying role in regulating peripheral insulin resistance through hypothalamic output and vagal inputs.(Rojas and Schwartz, 2014) Aβ impairs insulin signaling (Lee et al., 2009; Xie et al., 2002) and insulin resistance is observed post-mortem in AD brain. (Moloney et al., 2010; Steen et al., 2005; Talbot et al., 2012) Thus varying levels of neuropathology in cognitively-impaired individuals may affect the relationship between HOMA-IR and GDR. No tracer studies analyzing tissue-specific differences in insulin resistance have been done in cognitively-impaired subjects, but such work may help clarify the complex relationship between peripheral insulin resistance and AD etiology.
We also measured levels of additional peptides secreted from the pancreas, including C-peptide and amylin. While insulin is cleared quickly by the liver, and differences in insulin clearance rates by the liver can be influenced by factors such as fatty liver, C-peptide (secreted in equimolar amounts) has a longer half-life. (Lebowitz and Blumenthal, 1993; Polonsky and Rubenstein, 1986) Thus, fasting C-peptide provides an estimate of insulin secretion, and the ratio of insulin to C-peptide is indicative of the rate of insulin clearance. In our study, fasting C-peptide was not significantly different between groups. However, the ratio of insulin to C-peptide was higher in cognitively-impaired subjects (ND=0.06 (0.5) vs. CI=0.10 (0.6), p=0.03). This may suggest that insulin is degraded more slowly in cognitively-impaired individuals, which is in line with prior studies that have noted decreased insulin degrading enzyme activity in AD brain (Perez et al., 2000) and decreased peptidase activity in AD serum (Liu et al., 2012). Amylin is another potentially important and understudied peptide in AD. Amylin is both stored and secreted from β-cells with insulin (Charge et al., 1995; Lukinius et al., 1989), influences energy balance, (Rushing et al., 2001) and exhibits neuroprotective properties.(Adler et al., 2014) Amylin also forms oligomeric deposits in the pancreas, which may contribute to β-cell dysfunction and T2D(Clark et al., 1990; Lorenzo et al., 1994) and CNS amylin aggregates are also observed in AD brain (Jackson et al., 2013), which may be of interest as we observed increased amylin in cognitively-impaired individuals.
We examined the effect of intravenous insulin infusion on memory performance (as assessed using both the SRT and story recall tasks) and found no effect on story recall performance and a detrimental effect on SRT performance. Although no prior studies have assessed the impact of insulin on SRT, these findings conflict with prior studies demonstrating improvement in story recall performance in cognitively impaired subjects with insulin infusion (Craft et al., 1999a; Craft et al., 1996). Other studies have examined the effect of intranasal insulin on memory, particularly in cognitively impaired subjects, and the results have been mixed. For instance, intranasal insulin improved memory performance in some studies (Craft et al., 2012; Reger et al., 2008b) while other studies found insulin’s effects on memory are dependent on ApoE genotype with reports of beneficial effects (Reger et al., 2006; Reger et al., 2008a), no effect (Rosenbloom et al., 2014), or a negative effect (Claxton et al., 2015) in APOE ε4 noncarriers. Another study found effects only when sex and ApoE genotype were considered.(Claxton et al., 2013) These conflicting findings may be explained in part due to key study differences in the sample studied, method of insulin administration (intranasal vs. intravenous), and type of insulin and thus more work will be necessary to more precisely define insulin’s cognitive effects. It may also be important to assess memory using multiple tasks. Nevertheless, our study using intravenous insulin did not replicate prior work demonstrating a beneficial effect of insulin on memory (as assessed by story recall) and we unexpectedly observed a decline in memory performance using the SRT. It is of note that cortisol levels can affect memory performance.(Het et al., 2005) Because of the invasive nature of our procedure, coupled with the fact that insulin infusion can affect the hypothalamus-pituitary-adrenal axis,(Fruehwald-Schultes et al., 2001) we measured cortisol levels at fasting and immediately prior to memory testing (after 90 minutes of insulin infusion). Both the fasting and insulin-stimulated measures were collected in the morning for all subjects. Cortisol levels were significantly decreased across all subjects after 90 minutes (22.5 [9.8] (fasting) vs. 15.8 [8.0] (90 min)). We hypothesize that participants were anxious about the procedure and the IV placement initially, and were more relaxed 90 minutes following insulin infusion. In any case, increased cortisol levels did not contribute the observed decline in memory performance during this study, as they were actually lower during memory testing. Our findings of lower cortisol are consistent with a prior study in healthy men showing a decrease in cortisol levels with a low-rate insulin infusion comparable to that used in the current study.(Fruehwald-Schultes et al., 2001) Interestingly, that study showed the opposite effect (increased cortisol) with a high rate insulin infusion.
The present study is subject to limitations. First, the sample size was small, which may have limited our ability to detect an association between HOMA-IR and glucose disposal rate in the cognitively-impaired group. The number of APOE ε4 carriers in our study was also lower than expected, thus differences in memory performance in that subgroups of individuals may not be detectible due to insufficient power. We also cannot rule out a potential effect of medication use. Eight cognitively-impaired subjects were taking acetylcholinesterase inhibitors, and two cognitively-impaired subjects were taking an NMDA receptor agonist. The other 4 cognitively-impaired subjects were not yet on these medications at the time of the study visit, and no cognitively-normal subjects were on either medication type.
Conclusion
In conclusion, this study supports previous studies indicating an impaired glucose metabolism in cognitively impaired individuals. Specifically, elderly subjects diagnosed with either MCI or AD exhibited significantly greater levels of insulin resistance compared to elderly subjects with normal cognition. These observed metabolic differences occurred independently of significant differences in body composition or vascular risk factors (i.e. hypertension). Infused insulin did not improve memory, and resulted in poorer performance on one memory test. Further research regarding the complex relationship between metabolic function and memory is warranted.
Figure 1.
Correlation between fasting (HOMA-IR) and euglycemic clamp measures of insulin resistance differs between groups
Highlights.
Cognitively impaired subjects exhibit insulin resistance
HOMA-IR and the hyperinsulinemic clamp only correlate in healthy elderly
Insulin infusion does not improve memory
Acknowledgments
This research was supported by NIH grant P30 AG035982 through the National Institute on Aging. The University of Kansas Clinical and Translational Research Center (UL1 TR000001) provided essential space, expertise, and nursing support and the Kansas Intellectual and Developmental Disabilities Center (NICHD HD02528) provided assay support. J.K.M. is supported by F32 AG044953, E.D.V is supported by KL2 TR000119, JPT is supported by R01DK088940 and J.M.B. is supported by R01AG043962. The authors would also like to thank Michelle Winter for technical support and Henry and Janet Hyndman for their generous donation in support of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler BL, et al. Neuroprotective effects of the amylin analogue pramlintide on Alzheimer's disease pathogenesis and cognition. Neurobiol Aging. 2014;35:793–801. doi: 10.1016/j.neurobiolaging.2013.10.076. [DOI] [PubMed] [Google Scholar]
- Banks WA. The source of cerebral insulin. European Journal of Pharmacology. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Baura GD, et al. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RG, et al. Degradation of amylin by insulin-degrading enzyme. J Biol Chem. 2000;275:36621–36625. doi: 10.1074/jbc.M006170200. [DOI] [PubMed] [Google Scholar]
- Burns JM, et al. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69:1094–1104. doi: 10.1212/01.wnl.0000276952.91704.af. [DOI] [PubMed] [Google Scholar]
- Burns JM, et al. Insulin is differentially related to cognitive decline and atrophy in Alzheimer's disease and aging. Biochim Biophys Acta. 2012;1822:333–339. doi: 10.1016/j.bbadis.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carantoni M, et al. Alzheimer disease and vascular dementia: relationships with fasting glucose and insulin levels. Dement Geriatr Cogn Disord. 2000;11:176–180. doi: 10.1159/000017232. [DOI] [PubMed] [Google Scholar]
- Charge SB, et al. Effect of pH and insulin on fibrillogenesis of islet amyloid polypeptide in vitro. Biochemistry. 1995;34:14588–14593. doi: 10.1021/bi00044a038. [DOI] [PubMed] [Google Scholar]
- Cheng D, et al. Type 2 diabetes and late-onset Alzheimer's disease. Dement Geriatr Cogn Disord. 2011;31:424–430. doi: 10.1159/000324134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, et al. Islet amyloid polypeptide in diabetic and non-diabetic Pima Indians. Diabetologia. 1990;33:285–289. doi: 10.1007/BF00403322. [DOI] [PubMed] [Google Scholar]
- Claxton A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis. 2015;44:897–906. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- Claxton A, et al. Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer's disease. J Alzheimers Dis. 2013;35:789–797. doi: 10.3233/JAD-122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, et al. Enhancement of Memory in Alzheimer Disease With Insulin and Somatostatin, but Not Glucose. Archives of General Psychiatry. 1999a;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. Insulin metabolism in Alzheimer's disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology. 1999b;70:146–152. doi: 10.1159/000054469. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, et al. Effects of hyperglycemia on memory and hormone levels in dementia of the Alzheimer type: a longitudinal study. Behav Neurosci. 1993;107:926–940. doi: 10.1037//0735-7044.107.6.926. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- Craft S, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- Cui W, et al. Insulin is a kinetic but not a thermodynamic inhibitor of amylin aggregation. FEBS J. 2009;276:3365–3371. doi: 10.1111/j.1742-4658.2009.07061.x. [DOI] [PubMed] [Google Scholar]
- Emoto M, et al. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care. 1999;22:818–822. doi: 10.2337/diacare.22.5.818. [DOI] [PubMed] [Google Scholar]
- Fruehwald-Schultes B, et al. Hyperinsulinemia causes activation of the hypothalamus-pituitaryadrenal axis in humans. Int J Obes Relat Metab Disord. 2001;25(Suppl 1):S38–S40. doi: 10.1038/sj.ijo.0801695. [DOI] [PubMed] [Google Scholar]
- Garcia-Lara JM, et al. The metabolic syndrome, diabetes, and Alzheimer's disease. Rev Invest Clin. 2010;62:343–349. [PubMed] [Google Scholar]
- Het S, et al. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hishikawa N, et al. Cognitive and affective functions in diabetic patients associated with diabetes-related factors, white matter abnormality and aging. Eur J Neurol. 2015;22:313–321. doi: 10.1111/ene.12568. [DOI] [PubMed] [Google Scholar]
- Jackson K, et al. Amylin deposition in the brain: A second amyloid in Alzheimer disease? Ann Neurol. 2013;74:517–526. doi: 10.1002/ana.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki A, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- Kimura R, et al. Beta amyloid-induced depression of hippocampal long-term potentiation is mediated through the amylin receptor. J Neurosci. 2012;32:17401–17406. doi: 10.1523/JNEUROSCI.3028-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz MR, Blumenthal SA. The molar ratio of insulin to C-peptide. An aid to the diagnosis of hypoglycemia due to surreptitious (or inadvertent) insulin administration. Arch Intern Med. 1993;153:650–655. [PubMed] [Google Scholar]
- Lee HK, et al. The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid. Mol Biol Cell. 2009;20:1533–1544. doi: 10.1091/mbc.E08-07-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, et al. Risk of dementia among persons with diabetes mellitus: a population- based cohort study. American Journal of Epidemiology. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. Deficient brain insulin signalling pathway in Alzheimer's disease and diabetes. J Pathol. 2011;225:54–62. doi: 10.1002/path.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, et al. Characterization of insulin degrading enzyme and other amyloid-beta degrading proteases in human serum: a role in Alzheimer's disease? J Alzheimers Dis. 2012;29:329–340. doi: 10.3233/JAD-2011-111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, et al. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Lukinius A, et al. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia. 1989;32:240–244. doi: 10.1007/BF00285291. [DOI] [PubMed] [Google Scholar]
- Man HY, et al. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Moloney AM, et al. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [see comment] [DOI] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9:173–176. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- Morris JK, Burns JM. Insulin: an emerging treatment for Alzheimer's disease dementia? Curr Neurol Neurosci Rep. 2012;12:520–527. doi: 10.1007/s11910-012-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, et al. Insulin resistance and gray matter volume in neurodegenerative disease. Neuroscience. 2014;270:139–147. doi: 10.1016/j.neuroscience.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara T, et al. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011;77:1126–1134. doi: 10.1212/WNL.0b013e31822f0435. [DOI] [PubMed] [Google Scholar]
- Ott A, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Perez A, et al. Degradation of soluble amyloid beta-peptides 1–40, 1–42, and the Dutch variant 1–40Q by insulin degrading enzyme from Alzheimer disease and control brains. Neurochem Res. 2000;25:247–255. doi: 10.1023/a:1007527721160. [DOI] [PubMed] [Google Scholar]
- Polonsky KS, Rubenstein AH. Current approaches to measurement of insulin secretion. Diabetes Metab Rev. 1986;2:315–329. doi: 10.1002/dmr.5610020306. [DOI] [PubMed] [Google Scholar]
- Reger MA, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiology of Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reger MA, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008a;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger MA, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008b;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- Rockwood K, et al. Interrater reliability of the Clinical Dementia Rating in a multicenter trial. J Am Geriatr Soc. 2000;48:558–559. doi: 10.1111/j.1532-5415.2000.tb05004.x. [DOI] [PubMed] [Google Scholar]
- Rojas JM, Schwartz MW. Control of hepatic glucose metabolism by islet and brain. Diabetes Obes Metab. 2014;16(Suppl 1):33–40. doi: 10.1111/dom.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MH, et al. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer's disease. CNS Drugs. 2014;28:1185–1189. doi: 10.1007/s40263-014-0214-y. [DOI] [PubMed] [Google Scholar]
- Rushing PA, et al. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology. 2001;142:5035. doi: 10.1210/endo.142.11.8593. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, et al. Insulin promotes rapid delivery of N-methyl-D- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci U S A. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen E, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Xu W, et al. Mid- and late-life diabetes in relation to the risk of dementia: a population-based twin study. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, et al. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care. 2003;26:2426–2432. doi: 10.2337/diacare.26.8.2426. [DOI] [PubMed] [Google Scholar]
- Young SE, et al. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care. 2006;29:2688–2693. doi: 10.2337/dc06-0915. [DOI] [PubMed] [Google Scholar]