Abstract
Nanosilver has become one of the most widely used nanomaterials in consumer products because of its antimicrobial properties. Public concern over the potential adverse effects of nanosilver's environmental release has prompted discussion of federal regulation. In this paper, we assess several classes of consumer products for their silver content and potential to release nanosilver into water, air, or soil. Silver was quantified in a shirt, a medical mask and cloth, toothpaste, shampoo, detergent, a towel, a toy teddy bear, and two humidifiers. Silver concentrations ranged from 1.4 to 270,000 μg Ag g product−1. Products were washed in 500 mL of tap water to assess the potential release of silver into aqueous environmental matrices (wastewater, surface water, saliva, etc.). Silver was released in quantities up to 45 μg Ag g product−1, and size fractions were both larger and smaller than 100 nm. Scanning electron microscopy confirmed the presence of nanoparticle silver in most products as well as in the wash water samples. Four products were subjected to a toxicity characterization leaching procedure to assess the release of silver in a landfill. The medical cloth released an amount of silver comparable to the toxicity characterization limit. This paper presents methodologies that can be used to quantify and characterize silver and other nanomaterials in consumer products. The quantities of silver in consumer products can in turn be used to estimate real-world human and environmental exposure levels.
Silver has long been used in products as an antimicrobial agent. Particles of silver can be administered in products to release ionic silver (Ag-), which is often attributed with antimicrobial efficacy (Percival et al., 2005; Wijnhoven et al., 2009). Nanosilver, defined here as particles of silver having at least one dimension in the 1- to 100-nm size range, is increasingly being used in consumer products to control the growth of microorganisms on surfaces and in solutions. Properties of nanosilver, such as a low redox potential (Ivanova and Zamborini, 2009), could increase the capacity of smaller nanosilver particles to release Ag- compared with bulk silver. In addition, the generation of reactive oxygen species has been suggested as a mechanism for nanosilver toxicity (Kim et al., 2007). Regardless of the toxicity mechanism(s), increased antimicrobial behavior of nanosilver is often observed at particle sizes under 30 nm (Auffan et al., 2009) and allows manufacturers to minimize the total silver used in a product compared with other forms such as silver nitrate or microscale silver (Ki et al., 2007).
However, it is unclear whether the novel properties of nanosilver will lead to adverse human and/or environmental health effects. The production, use, and disposal of products containing nanosilver can lead to the release of increased amounts of silver into various environmental compartments (air, water, soil). For example, nanosilver released from clothing could enter the environment in the effluent and/or biosolids resulting from wastewater treatment (Benn and Westerhoff, 2008). The possible adverse effects of increased environmental exposure to nanosilver include the development of silver-resistant bacteria (Gupta et al., 1999; Percival et al., 2005, 2008; Silver 2003), the impairment of aquatic (Choi and Hu, 2008; Griffitt et al., 2008, 2009; Navarro et al., 2008) and soil (Roh et al., 2009) organisms, and the impairment of human health (El-Ansary and Al-Daihan, 2009; Greulich et al., 2009; Hussain et al., 2005; Ji et al., 2007; Mirsattari et al., 2004; Takenaka et al., 2001).
The possible adverse effects have prompted 14 organizations, led by the International Center for Technology Assessment, in 2008 to petition the USEPA to regulate all consumer products containing nanosilver. Any potential policy measures will have an impact on the commercial nanosilver market, which consisted of >230 products as of August 2008 (Woodrow Wilson International Center for Scholars, 2009). Therefore, the public, regulatory, and commercial arenas could benefit from a risk assessment of the use of nanosilver.
Quantifying human and environmental exposures to silver is a key component to determining the risk from an increased use of nanosilver. Environmental exposure levels have been modeled by estimating production, use, and release scenarios (Mueller and Nowack, 2008) and by probabilistic material flow analyses (Gottschalk et al., 2009). However, limited empirical data are available on the release of nanosilver from manufactured products to validate the results of these models. Additionally, potential human exposures resulting from the use of commercial products containing nanosilver have not yet been determined.
The increasing number of products containing nanosilver provides an opportunity to obtain data that can lend to quantification of human and environmental exposures. The total quantification of silver in products by acid digestion (Benn and Westerhoff, 2008) can provide an upper limit for human and environmental silver exposure estimates. The potential release of silver into complex aquatic systems (wastewater, surface water, saliva, landfill leachate, etc.) can be estimated by quantifying the release of silver into comparable water conditions. Quantification of nanosilver released from products into water samples can be obtained with electron microscopy and nanoscale filtration methods (Benn and Westerhoff, 2008). Confirming the presence of nanosilver in products and quantifying the potential release into water will be valuable for the risk assessment of nanosilver technologies.
This study focuses on quantifying and characterizing the silver currently being used in and potentially released from consumer products. Inductively coupled plasma—optical emission spectroscopy (ICP—OES) was used to quantify (i) silver in 10 consumer products (shirt, medical mask and cloth, toothpaste, shampoo, detergent, towel, toy teddy bear, and two humidifiers) and (ii) the release of this silver into tap water, airborne water droplets, and simulated landfill leachate. Scanning electron microscopy with energy dispersive X-ray (SEM/EDX) and filtration techniques were used to investigate the form, shape, and size of the silver. The flux of silver into landfills as a result of the disposal of wastewater treatment plant (WWTP) biosolids and consumer products is also discussed. These data will aid risk assessors in approximating levels of human and environmental exposure to silver as a result of consumer product use. This research also provides a methodology for manufacturers, researchers, and risk assessors to quantify and characterize the nanomaterials used in consumer products.
Materials and Methods
Products were purchased based on advertisements of the use of nanosilver, colloidal silver, and/or ionic silver (Table 1). Toothpaste, shampoo, and laundry detergent were purchased to determine if products advertised to use colloidal or ionic silver contain and release nanosilver as well. Puckskin (Macker International Apparel Inc., Furry Creek, BC, Canada) donated an athletic shirt and unfinished cloth (not yet tailored into shirts or other products) that contained Texcare, a nanosilver fabric. The toothpaste was the only product to advertise a specific concentration of silver, which was 100 ppm.
Table 1. Quantification of total silver in products†.
| Product | Company | Advertised form of silver | Silver content | Total mass of product | Estimated total silver in product |
|---|---|---|---|---|---|
| μg Ag g product−1 | g | mg | |||
| Athletic shirt | Puckskin | Nanosilver | 30 ± 5.4 | 178 | 5.3 ± 0.96 |
| Unfinished cloth fabric | Puckskin | Nanosilver | 44 ± 2.4 | Not applicable | Not applicable |
| Medical mask | Nanbabies | Silver | 270,000 ± 67,000 | 2.2 | 590 ± 150 |
| Medical cloth | Nanbabies | Silver | 230,000 ± 69,000 | 3.5 | 810 ± 240 |
| Toothpaste | SilvaFresh | Colloidal | 7.6 ± 9.8 | 110 | 0.8 ± 1.1 |
| Shampoo | Primos | Colloidal, ionic | 1.4 ± 0.02 | 462 | 0.65 ± 0.01 |
| Detergent | Primos | Colloidal | 3.4 ± 0.06 | 935 | 3.2 ± 0.06 |
| Yellow cloth (towel) | Good4U | Nanosilver | 270 ± 80 | 8.6 | 2.3 ± 0.69 |
| Teddy bear | Pure Plushy | Nanosilver | 70 ± 30‡ | 377 | 26 ± 11 |
| Small humidifier | Germguardian | Silver particles | 60 ± 4.6§ | 108 | 6.5 ± 0.50 |
| Large humidifier | Venta | Nanosilver | 1.2 ± 1.7¶ | 118 | 0.12 ± 0.20 |
The ± values represent standard deviations from digestions of three samples of each product.
Silver content was quantified for the interior foam of the teddy bear, and the total mass refers to the foam.
Silver content was quantified for the blue plastic of the water tank.
Silver content was quantified for the resin contained in the filter cartridge.
Samples of the products (0.1–0.5 g) were subjected to nitric acid digestion (Standard Method 3030 E) to determine the total silver content (Clesceri et al., 1998). Representative samples of the products were washed in a glass container with 500 mL of municipal tap water (Tempe, AZ; pH ≈ 7.6, conductance ≈ 1.0 mS) and mixed for 1 h at room temperature. Tempe, AZ, tap water conforms to USEPA drinking water standards and has typical values for chloride, chlorine residual, alkalinity, and hardness of 97 mg L−1 0.71 mg L−1, 135 mg L−1 as CaCO3, and 186 mg L−1 as CaCO3 respectively (unpublished data, 2010; City of Tempe, 2010). This washing protocol is designed to provide estimations of silver leached into more complex aqueous environments (wastewater, surface water, saliva, etc.) where differences in water conditions (temperature, pH, redox potential, particulate matter, etc.) may effect silver release. Three samples of the athletic shirt and two samples of the unfinished cloth were washed one time each to determine an average release of silver. One sample of each of the remaining products was washed one time. For size fractionation analysis, water samples were filtered through 450-nm-, 100-nm-, and 20-nm-pore-size filters (Whatman, Kent, UK) and acidified (pH <2) with Ultrapure Nitric Acid (JT Baker, Phillipsburg, NJ). There was no retention of ionic silver by any filters in control experiments. Silver was analyzed by ICP–OES (Thermo iCap 6000, Waltham, MA) with a method detection limit of 0.1 μgL−1. Inductively coupled plasma spectroscopy techniques are standard methods for quantifying total silver content in environmental samples. However, these techniques alone do not provide information on the shape, size, or form of the silver.
Scanning electron microscopy with energy dispersive X-ray (FEI-XL30, EFSEM/EDAX, FEI, Hillsboro, OR) was used for visual and elemental confirmation of silver in the samples. The SEM image resolution is approximately 3 nm and the electron beam for EDX analysis can be hundreds of nanometers wide and penetrate up to 1.3 μm into the sample. Solid samples (e.g., medical cloth and mask) were secured onto SEM stubs with carbon tape. For the tap water samples, the particulate matter was allowed to settle, then 15 mL of the supernatant was centrifuged onto a carbon-taped SEM stub at F = 10,000 × g for 15 min (Thermo IEC, Waltham, MA). Low-magnification images are provided as Supplementary Fig. SI—S4 to demonstrate the relative abundance of particles found in samples.
Nanopure water (MilliQ, Billerica, MA) was loaded into two humidifiers, and the mist was collected and analyzed for total silver content. The mist was pulled through a stainless steel ultraviolet lamp housing column (3.2-cm diam. by 31.5 cm, Millipore, Billerica, MA) packed with 5-mm-diam. borosilicate glass beads (VWR, West Chester, PA) by connecting a 1/4-HP vacuum pump (VWR, West Chester, PA) with Tygon R-3603 tubing (VWR, West Chester, PA). During vacuum pump operation, the airborne water droplets attached to the surface of the beads. Intermittent stopping of the vacuum pump allowed the water to fall out of the bottom of the column for collection. A schematic of the apparatus is supplied in the Supplementary Information (Supplementary Fig. S5). Eight mist samples (15 mL each) were collected from the small humidifier. A composite mist sample (500 mL) was collected from the large humidifier and concentrated to 50 mL for analysis because of low silver content.
Experiments based on the toxicity characterization leaching procedure (TCLP) (USEPA, 1992) were conducted with the medical mask, medical cloth, plastic tank of the small humidifier, and filter resin of the large humidifier to simulate the potential release of silver after disposal of the products in landfills. The TCLP evaluates the release of contaminants from wastes under water conditions intended to represent landfill leachate. For silver, the product is considered toxic if the TCLP solution contains >5 mg L−1 silver. The TCLP extraction solution was prepared by adding 5.7 mL of glacial acetic acid (JT Baker, Phillipsburg, NJ) and 64.3 mL of 1 M NaOH (ACS grade, Mallinckrodt, Phillipsburg, NJ) to nanopure water (Millipore, Billerica, MA) and diluting to 1 L for a final pH of 4.8 (USEPA, 1992). The extraction solution (20× the product mass) and products (1—2 g) were combined in 50-mL high-density polyethylene centrifuge vials (VWR, West Chester, PA) and rotated at 30 rpm on a TCLP rotary agitator (Analytical Testing Corporation, Warrington, PA) for 24 h. Samples were filtered (GF/F, Whatman, Kent, UK) before ICP–OES analysis.
Results
Quantification/Characterization of Silver in Products
Table 1 summarizes the silver content extracted from each product via nitric acid digestion. The silver content ranged from 1 μg Ag g product−1 to 270 mg Ag g product−1. In general, the personal care products (detergent, shampoo, and toothpaste) contained <10 μg Ag g product−1, while the clothing exhibited silver contents of approximately 30 to 45 μg Ag g product−1. The face mask and medical cloth contained the highest quantity of silver, approximately 25% by weight (270,000 and 230,000 μg Ag g product−1, respectively).
The silver content in several samples (medical mask and cloth, toothpaste, towel, teddy bear, and large humidifier) exhibited high variability. Microscale silver particles found in some of these products may result in a heterogeneous silver distribution throughout the material, which may prevent obtaining a reproducible sample for analysis. For example, the high standard deviation of the toothpaste silver content could be the result of unevenly distributed micrometer-sized silver. Figure 1 is an SEM image that confirms the release of microscale silver from the toothpaste. The size fractionation data on the silver released from the toothpaste into tap water also suggest that the majority of silver is retained by the 100-nm-pore-size filter (see Table 2 and next section). A heterogeneous distribution of silver in the toothpaste may explain the discrepancy between the advertised concentration (100 ppm) and the measured concentration (8 μg g−1 [8 ppm]). Figure 2a shows 500-nm-diam. agglomerates of nanosilver that are typical of the medical mask, and SEM at lower magnification shows them unevenly distributed over the mask surface (Supplementary Fig. S2). Furthermore, precipitates were visually identified at the bottom of the detergent bottle. The SEM analysis of these particulates showed micrometer-scale agglomerates comprised of nanoscale particles that EDX analysis suggests are silver (Fig. 3). These unique crystal-like structures may be formed during the evaporation of the detergent during SEM stub preparation. However, these agglomerates were not detected with SEM in the bulk of the detergent. The microscale silver that settled to the bottom of the bottle was probably not sampled during the acid digestion analysis, and the low variability in the silver content analysis of the detergent could be attributed to dissolved silver ions. Such heterogeneous silver distributions could result in varying levels of silver released during use of the product and/or impair product performance.
Fig. 1.
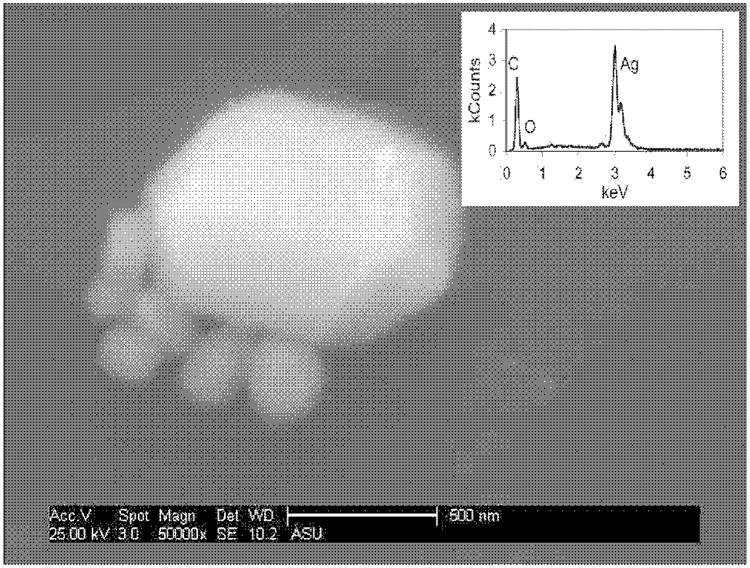
Scanning electron micrograph of silver particles in a tap water wash of toothpaste. Inset: Energy dispersive X-ray analysis of particles indicating presence of silver.
Table 2. Mass and size fractionation of silver released from consumer products washed in tap water for 1 h.
| Product | Mass of product washed | Size fraction of silver released into 500 mL of tap water | Total silver released per product mass | ||
|---|---|---|---|---|---|
|
| |||||
| Total | <100 nm | <20 nm | |||
| g | μg | μg Ag g product−1 | |||
| Athletic shirt† | 41 ± 9.6 | 27 ± 1.4 | 20 ± 0.5 | 11 ± 1.2 | 0.56 ± 0.01 |
| Unfinished cloth fabric‡ | 45 and 46 | 22 and 47 | 12 and 16 | 12 and 13 | 0.5 and 1.1 |
| Medical mask | 1.4 | 15.8 | 14.8 | 14.8 | 11 |
| Medical cloth | 0.3 | 13.8 | 13.3 | 13.3 | 46 |
| Toothpaste | 2.1 | 37.3 | 14.8 | 4.3 | 18 |
| Shampoo | 13.2 | 11.8 | 4.8 | 3.8 | 0.9 |
| Detergent | 23.9 | 43.3 | 6.8 | 1.7 | 1.8 |
| Yellow cloth (towel) | 5 | <5 | <5 | <5 | <1.0 |
| Teddy bear | 26 | <5 | <5 | <5 | <0.2 |
Three samples of the athletic shirt were washed and analyzed.
Two samples of the unfinished cloth fabric were washed and analyzed.
Fig. 2.
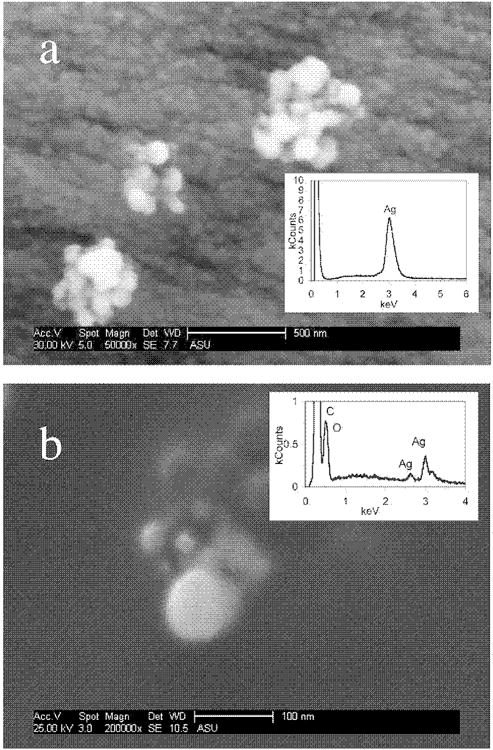
(a) Scanning electron micrograph (SEM) of three nanosilver agglomerations (∼200–500 nm diam.) on the fabric surface of a medical face mask that is also completely coated with nanosilver particles <20 nm in diameter (underlying particles). Inset: Energy dispersive X-ray (EDX) analysis indicating the agglomerations and surface of the mask contain silver. (b) SEM of a tap water wash of the face mask. Inset: EDX analysis showing silver. Carbon and oxygen peaks are attributed to the background carbon tape of the SEM stub.
Fig. 3.
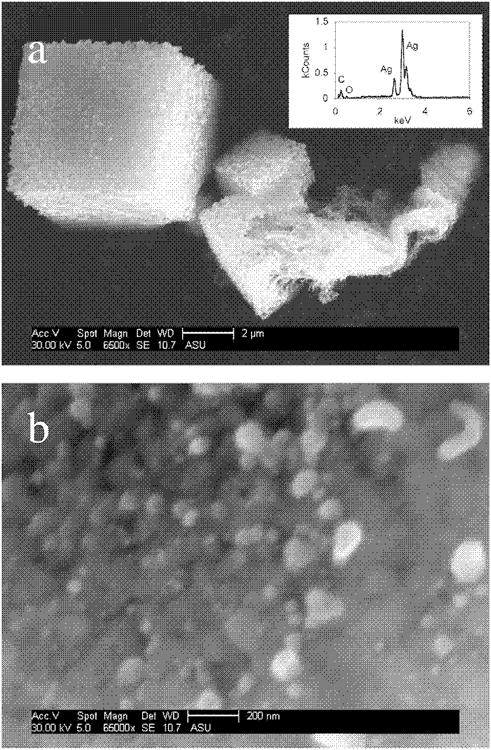
(a) Micron-sized, cubic agglomerates of nanosilver particles collected from the bottom of the detergent bottle. Inset: Energy dispersive X-ray analysis of the cubic structure showing dominant silver presence. (b) Surface of a cubic structure showing the arrangement of silver nanoparticles.
Release of Silver into Water
Table 2 summarizes the quantity and particle size fractionation of silver released from consumer products that were washed in tap water for 1 h. The products released highly variable amounts of their silver. For example, the face mask, which contained approximately 27% silver by weight, released <0.01% (15.8 μg) of its silver into the wash water. In contrast, the shirt, which contained 44 μg g−1 of silver, released about 2% (34 μg) of its silver. The toothpaste, shampoo, and detergent were assumed to release all of their silver into the wash water.
The 100-nm-pore-size filter removed the majority of the silver released by the toothpaste, shampoo, and detergent. This suggests that the silver is released as particles, or is associated with particles, larger than 100 nm. Conversely, the majority of the silver released from the shirt, mask, and medical cloth passed through the 20-nm filter. Scanning electron microscopy was used to confirm the size of the nanosilver released from the medical cloth and mask. Figure 2a shows three large agglomerates (∼500 nm in diam.) of silver nanoparticles on the surface of the face mask, which is completely coated with smaller nanoparticles (<20 nm in diam.). The small nanoparticles on the surface were confirmed with an EDX analysis at a location on the face mask surface away from the 500-nm agglomerates, which also yielded a dominant silver signal. This suggests that the silver passing through the 20-nm filter could be nanoparticles in addition to dissolved silver ions. Figure 2b shows agglomerations of particles with sizes <100 nm that were released into the tap water. These agglomerations are similar to those seen on the mask fabric. However, they could be an artifact of the centrifugation step during preparation of the SEM sample; forced settling during centrifugation may increase the probability of particles colliding and forming larger agglomerates.
The SEM analysis was also conducted on other products and their subsequent wash waters. The typical particles found in the wash water of the toothpaste (Fig. 1) are much larger in size (100–500 nm) than those from the medical cloth and mask. This concurs with the size fraction data in Table 2, in which only about 10% of the toothpaste silver passed through a 20-nm filter. Although not advertised to contain nanosilver, the toothpaste appears to release at least some nanosize silver particles into tap water. The SEM/EDX also confirmed the presence of silver nanoparticles in the wash water of the shirt (Supplementary Fig. S4).
A calculation of the silver released per capita from the use of daily household products (i.e., washing one 178-g shirt and using 5 g of shampoo, 100 g of detergent, and 1 g of toothpaste), assuming that all of the released silver reaches the sewer, suggests that one consumer could be responsible for releasing about 470 μg Ag into the sewer system per day. This calculation uses the wash water data for the shirt and toothpaste and assumes that the shampoo and detergent release 100% of their silver contents. This calculation can be used to comprehend the influx of silver to sewage as a result of the use of nanosilver in the home. If 10,000 people released 470 μg Ag per capita d−1 into a million-gallon-per-day capacity WWTP, the biosolids silver concentration might increase by 0.7 mg Ag kg−1 (using model from Benn and Westerhoff, 2008). This value is comparable to the 1.29 mg kg−1 sludge silver concentration predicted by a probability density function model (Gottschalk et al., 2009). Furthermore, a survey of U.S. WWTPs in 2006 to 2007 quantified silver in biosolids ranging from 1.94 to 856 mg kg−1 (USEPA, 2009b). Therefore, a worst-case scenario where everybody in a population leached 470 μg Ag d−1 might lead to increases in WWTP biosolids silver concentrations comparable to concentrations currently observed. This demonstrates that an increase in the use of nanosilver could noticeably increase the amount of silver in some wastewater systems. The environmental impact of this increase in wastewater silver is yet to be determined. It should be noted that other consumer products containing silver were not tested that could add to an estimation of silver released per capita per day. Also, the release of silver over product lifetimes could be investigated to justify this calculation.
Release of Silver from Humidifiers into Air
Silver was quantified in the water emitted from two humidifiers. The plastic tank of the small humidifier, which contained 60 μg Ag g plastic−1, released water containing 1.1 ± 0.4 μg Ag L− (± represents standard deviation of eight samples) into the air at a flow rate of 100 mL h−1. Therefore, the mass flow rate of silver released into the air is estimated to be 0.11 ± 0.04 μg Ag h−1. Similarly, the large humidifier released water containing 0.19 μg Ag L−1 at a flow rate of 420 mL h−, which is a release rate into the air of 0.08 μg Ag h−1. Because the silver concentrations in the collected water were low, filter fractionation and SEM analyses could not be conducted. The SEM/EDX analysis was attempted on the plastic of the small humidifier, but the presence of nanosilver could not be confirmed due to the instability of the plastic sample under the electron beam.
Release of Silver into Landfills
It is probable that most of the silver released from these products into wastewater will enter municipal and septic sewage systems. At municipal WWTPs most of the silver can be removed either by adsorption to particulates (Benn and Westerhoff, 2008; Wang et al., 2003) or precipitation with chloride (Wang et al., 2003), after which it is settled out within the biosolids to be disposed of as agricultural fertilizer, incinerated, or landfilled. Silver not removed at a WWTP will reenter the aquatic environment on discharge of the treated effluent. Although this amount of silver is estimated to be low (Benn and Westerhoff, 2008; Mueller and Nowack, 2008), it must not be neglected when considering fate and transport over long time scales and potential adverse environmental effects due to bioaccumulation.
Several products in this study will end up in landfills at the end of their useful life. The medical mask and cloth, towel, teddy bear, and humidifier did not release a significant portion of their silver in the tap water wash, so it is reasonable to assume that these products will still contain silver when they are disposed of in landfills. Moreover, if one assumes that a majority of the silver released into wastewater is collected in WWTP biosolids and eventually deposited in a landfill, the flux of silver entering landfills can be estimated as the total silver contained in the products. The total combined estimated silver in the consumer products investigated here is approximately 1450 mg (Table 1).
Since silver-containing products will be disposed of in landfills, TCLP experiments were performed on four products to simulate the release of silver within a landfill. Table 3 presents the results. The medical mask and cloth were chosen for TCLP experiments because of their high silver content, and the humidifiers were chosen because their low silver release rate suggested that the product might contain silver at the time of disposal. At 2900 μg L−1, the medical cloth TCLP silver concentration was on the same order as the toxicity characterization limit of 5000 μg L−1 (USEPA, 1992). The medical mask and cloth released 1.7 and 54 μg Ag g product−1, respectively. These values are similar to the mass of silver released into water (Table 2). Conversely, the humidifiers released silver faster into the TCLP solution than into water droplets during product use. The plastic and filter resin from the small and large humidifiers released 0.22 and 0.13 μg Ag g product−1, respectively. Therefore, based on a 24-h evaluation, the humidifiers would release silver in a landfill at a rate about 10 times faster than into the air during use. This example illustrates the complexity of understanding and predicting fluxes of nanomaterials into the environment.
Table 3. Silver released during toxicity characterization leaching procedure (TCLP)†.
| Sample‡ | Sample mass | TCLP solution volume | TCLP silver content | Mass of silver leached |
|---|---|---|---|---|
| g | mL | μg L−1 | μg Ag g product−1 | |
| Medical mask | 0.7 | 28 | 88 | 1.7 |
| Medical cloth | 0.9 | 17.5 | 2900 | 54 |
| Small humidifier (plastic) | 1.5–2.0 | 30–39 | 10 ± 0.9 | 0.2 ± 0.02 |
| Large humidifier (resin) | 1.9–2.4 | 39–48 | 7 ± 3.5 | 0.1 ± 0.07 |
The ± values represent standard deviations of three repetitions.
Medical mask and medical cloth were analyzed only once due to limited sample quantity.
Implications
The research presented here quantifies the silver that is currently being used in consumer products and could be released into sectors of the environment. These data, when coupled with future nanosilver toxicity data, can be used to estimate the environmental and human health risks resulting from the use of nanosilver. This work is thus useful for consumers, product manufacturers, and policymakers, although some limitations are worth consideration. The wash experiments were designed to investigate silver leaching into tap water as opposed to simulating “real-world” use scenarios. Silver leaching in real-world scenarios will be affected by water quality parameters such as pH, detergents, and oxidants (Geranio et al., 2009) as well as consumer behaviors (washing habits, disposal, use of product, etc.). Lower pH values, higher temperatures, mechanical stress, and oxidants such as bleaching agents would cause a general trend toward increased silver release rates compared with the conditions of these experiments. Despite higher pH values (∼10) in “real-world” washing conditions, silver release rates may be higher than those observed here if oxidizing agents such as bleach are used (Geranio et al., 2009). The quantities of silver passing 100- and 20-nm-pore-size filters most likely consist of both ionic and nanoparticle silver. Some of the silver retained by the 100-nm-pore-size filter may be ionic and/or nanoparticles associated with material larger than 100 nm. Detection methods specific to nanosilver are needed to provide a more accurate characterization of silver. Finally, the quantities of silver released from these products may be considered as an initial leaching characterization due to the lack of replication in the leaching experiments. The validity of assumptions made for WWTP and landfill influxes could be assessed by replicating leaching experiments and quantifying silver release rates over product life cycles.
Because government does not specifically regulate the use of nanosilver in products, the onus of protecting human and environmental health from potential adverse effects currently falls on individuals. This research demonstrates that consumers will subject themselves and/or the environment to some exposure of silver (nanoparticle, ionic, or microscale) by using and/or disposing of silver-containing products. Although many factors contribute to perceptions of nanotechnology risks (Kahan et al., 2008), these data allow individuals to conceptually weigh the potential adverse effects of these quantities of silver against the perceived benefits from use of these products.
It has been demonstrated that silver particles can agglomerate and settle out of some products, such as the detergent. This suggests that some silver products may not perform as designed. Manufacturers may want to consider validating the function of the nanosilver in their products using some of the methods described here. For example, these characterization techniques can verify the properties of nanosilver (e.g., concentration, size) being varied in a product while the impact on antimicrobial efficacy is monitored.
The silver being used in products clearly will be released into the environment at some point of the product life cycle. Knowing this, society can begin to take an earth systems engineering and management approach to the design of nanosilver products (Allenby, 2000, 2007). That is, we should recognize that by engineering a product to be antimicrobial, we are also engineering the concentration of silver in various environments. This allows for the potential human and environmental effects to be factored into an improved design or a regulatory framework for nanosilver products.
Environmental occurrence and toxicity data related to engineered nanomaterials are needed before a regulatory direction can be established (Morris and Willis, 2007). This research provides an example of occurrence and environmental release data for nanomaterials that the USEPA's voluntary Nanomaterial Stewardship Program was designed to produce (USEPA, 2009a). The efficiency of that program has been questioned (Maynard and Rejeski, 2009), and one possible explanation for the lack of participation is the cost, in time and money, of producing such data. The methods presented here indicate that gathering basic data regarding the environmental transport of nanomaterials from consumer products can be achieved with relative ease at the laboratory scale. But it will be a challenge to scale up this approach to acquire occurrence and environmental release data for all consumer products containing nanomaterials.
The uncertainty regarding the potential negative impacts from the use of nanosilver in consumer products makes it unclear whether the government should regulate the growing economic market for these items. The research presented here is evidence for consumers, product manufacturers, scientists, and policymakers that nanomaterials will enter our environment as a result of their use in consumer products. Because these data only specify quantities of silver in products and possible releases into the environment, not toxicity, the nanosilver regulation debate remains open.
Acknowledgments
Funding was provided by the NSF through the Urban Ecology IGERT program at Arizona State University (Award Abstract no. 0504248), the USEPA (Grant no. RD833322), and the Fulton Undergraduate Research Initiative at Arizona State University. Additional funding was provided from the Paul L. Busch Award for Innovation by the Water Environment Research Foundation. The authors gratefully acknowledge the use of facilities within the LeRoy Eyring Center for Solid State Science and the Goldwater Environmental Laboratory at Arizona State University. This work was supported in part by the Department of Energy (DE-FG02-08ER64613) with Daniel Drell as contract monitor. The authors would like to thank Mr. McMillan at Puckskin for his generous donation of fabrics as well as Jameson Wetmore and Brad Allenby at Arizona State University for their input on the implications of the research. The opinions expressed here are those of the authors.
Abbreviations
- EDX
energy dispersive X-ray
- ICP–OES
inductively coupled plasma–optical emission spectroscopy
- SEM
scanning electron microscopy
- TCLP
toxicity characterization leaching procedure
- WWTP
wastewater treatment plant
Contributor Information
Troy Benn, School of Sustainable Engineering and the Built Environment, Arizona State Univ., PO Box 875306, Tempe, AZ 85287-5306.
Bridget Cavanagh, School of Sustainable Engineering and the Built Environment, Arizona State Univ., PO Box 875306, Tempe, AZ 85287-5306.
Kiril Hristovski, College of Technology and Innovation, Arizona State Univ.-Polytechnic Campus, 6073. S. Backus Mall, Mesa, AZ 85212.
Jonathan D. Posner, Mechanical Engineering and Chemical Engineering Programs, Arizona State Univ., P.O. Box 876106, Tempe, AZ 85287-6106. Assigned to Associate Editor Joel Pedersen
Paul Westerhoff, School of Sustainable Engineering and the Built Environment, Arizona State Univ., PO Box 875306, Tempe, AZ 85287-5306.
References
- Allenby B. Earth systems engineering and management. IEEE Technol Soc Mag. 2000;19:10–24. doi: 10.1021/es072657r. [DOI] [PubMed] [Google Scholar]
- Allenby B. Earth systems engineering and management: A manifesto. Environ Sci Technol. 2007;41:7960–7965. doi: 10.1021/es072657r. [DOI] [PubMed] [Google Scholar]
- Auffan M, Rose J, Bottero J, Lowry G, Jolivet J, Wiesner M. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nature Nanotechnol. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol. 2008;42:4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- Choi O, Hu ZQ. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Tcchnol. 2008;42:4583–4588. doi: 10.1021/es703238h. [DOI] [PubMed] [Google Scholar]
- City of Tempe. City of Tempe; Tempe, AZ: 2010. Typical water quality values. Available at http://www.tempe.gov/waterquality/typical_values.htm (verified 15 June 2010) [Google Scholar]
- Clesceri LS, Greenberg AE, Eaton AD. Standard methods for the examination of water and wastewater. Am Public Health Assoc, Am Water Works Assoc, Water Environ Fed; Washington, DC: 1998. [Google Scholar]
- El-Ansary A, Al-Daihan S. On the toxicity of therapeutically used nanoparticles: An overview. J Toxicol. 2009 doi: 10.1155/2009/754810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geranio L, Heuberger M, Nowack B. The behavior of silver nanotextiles during washing. Environ Sci Technol. 2009;43:8113–8118. doi: 10.1021/es9018332. [DOI] [PubMed] [Google Scholar]
- Gottschalk F, Sonderer T, Scholz R, Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ Sci Technol. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- Greulich C, Kittler S, Epple M, Muhr G, Koller M. Studies on the biocompatibility and the interaction of silver nanoparticles with human mesenchymal stem cells (hMSCs) Langenbecks Arch Chir. 2009;394:495–502. doi: 10.1007/s00423-009-0472-1. [DOI] [PubMed] [Google Scholar]
- Griffitt RJ, Hyndman K, Denslow ND, Barber DS. Comparison of molecular and histological changes in zebrafish gills exposed to metallic nanoparticles. Toxicol Sci. 2009;107:404–415. doi: 10.1093/toxsci/kfn256. [DOI] [PubMed] [Google Scholar]
- Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chcm. 2008;27:1972–1978. doi: 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- Gupta A, Matsui K, Lo JF, Silver S. Molecular basis for resistance to silver cations in Salmonella. Nat Med. 1999;5:183–188. doi: 10.1038/5545. [DOI] [PubMed] [Google Scholar]
- Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19:975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Ivanova O, Zamborini F. Size-dependent electrochemical oxidation of silver nanoparticles. J Am Chcm Soc. 2009;132:70–72. doi: 10.1021/ja908780g. [DOI] [PubMed] [Google Scholar]
- Ji JH, Jung JH, Kim SS, Yoon JU, Park JD, Choi BS, Chung YH, Kwon IH, Jeong J, Han BS, Shin JH, Sung JH, Song KS, Yu IJ. Twenty-eight-day inhalation toxicity study of silver nanoparticles in sprague-dawley rats. Inhal Toxicol. 2007;19:857–871. doi: 10.1080/08958370701432108. [DOI] [PubMed] [Google Scholar]
- Kahan DM, Slovic P, Braman D, Gastil J, Cohen G, Kysar D. Woodrow Wilson Int Cent for Scholars. Washington, DC: 2008. Biased assimilation, polarization, and cultural credibility: An experimental study of nanotechnology risk perceptions. [Google Scholar]
- Ki HY, Kim JH, Kwon SC, Jeong SH. A study on multifunctional wool textiles treated with nano-sized silver. J Mater Sci. 2007;42:8020–8024. [Google Scholar]
- Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nano-med Nanotechnol Biol Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Maynard A, Rejeski D. Too small to overlook. Nature. 2009;460:174. doi: 10.1038/460174a. [DOI] [PubMed] [Google Scholar]
- Mirsattari SM, Hammond RR, Sharpe MD, Leung FY, Young GB. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408–1410. doi: 10.1212/01.wnl.0000120671.73335.ec. [DOI] [PubMed] [Google Scholar]
- Morris J, Willis J. US Environmental Protection Agency nanotechnology white paper. USEPA; Washington, DC: 2007. EPA 100/B-07/001. [Google Scholar]
- Mueller NC, Nowack B. Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol. 2008;42:4447–4453. doi: 10.1021/es7029637. [DOI] [PubMed] [Google Scholar]
- Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R. Toxicity of silver nanoparticlcs to Chlamydomonas reinhardlii. Environ Sci Technol. 2008;42:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect. 2005;60:1–7. doi: 10.1016/j.jhin.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Percival SL, Woods E, Nutekpor M, Bowler P, Radford A, Cochrane C. Prevalence of silver resistance in bacteria isolated from diabetic foot ulcers and efficacy of silver-containing wound dressings. Ostomy Wound Manage. 2008;54:30–40. [PubMed] [Google Scholar]
- Roh JY, Sim SJ, Yi J, Park K, Chung KH, Ryu DY, Choi J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabdilis elegans using functional ecotoxicogenomics. Environ Sci Technol. 2009;43:3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- Silver S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003;27:341–353. doi: 10.1016/S0168-6445(03)00047-0. [DOI] [PubMed] [Google Scholar]
- Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, Schramel P, Heyder J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Pcrspect. 2001;109:547–551. doi: 10.1289/ehp.01109s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. Test methods for evaluating solid waste: Physical/chemical methods. USEPA; Washington, DC: 1992. Test Method 1311: Toxicity characterization leaching procedure. SW-846. [Google Scholar]
- USEPA. USEPA Office of Pollution Prevention and Toxics; Washington, DC: 2009a. Nanoscalc Materials Stewardship Program interim report; p. 38. Available at http://www.epa.gov/oppt/nano/nmsp-interim-report-final.pdf (verified 15 June 2010) [Google Scholar]
- USEPA. Targeted National Sewage Sludge Survey sampling and analysis technical report. USEPA Office of Water (4301T); Washington, DC: 2009b. EPA-822-R-08-016. [Google Scholar]
- Wang J, Huang CP, Pirestani D. Interactions of silver with wastewater constituents. Water Res. 2003;37:4444–4452. doi: 10.1016/S0043-1354(03)00407-X. [DOI] [PubMed] [Google Scholar]
- Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van De Meent D, Dekkers S, De Jong WH, van Zijverden M, Sips AJAM, Geertsma RE. Nano-silver: A review of available data and knowledge gaps in human and environmental risk assessment. Nano-toxicology. 2009;3:109–138. [Google Scholar]
- Woodrow Wilson International Center for Scholars. Project on Emerging Nanotechnologies; Washington, DC: 2009. Nanotechnology consumer product inventory. Available at http://www.nanotechproject.org/inventories/consumer/ (verified 15 June 2010) [Google Scholar]


