Abstract
Background:
We assessed the impact of “almost daily” use of continuous glucose monitoring (CGM) in adults with type 1 diabetes who had at least 1 year of CGM experience.
Methods:
In this single-center survey, we utilized a 16-item questionnaire to assess changes hypoglycemia fear, incidence of emergency medical treatment and utilization of self-monitoring of blood glucose (SMBG) before and after 1 year of CGM use. Participation was restricted to individuals who used the same brand of CGM system to avoid confounding responses due to differences between commercial devices. Participants were recruited on an “as-seen” basis from a major, urban internal medicine clinic and associated diabetes education center. The questionnaire was completed during the clinic visit. Responses to the survey were analyzed by standard descriptive statistics.
Results:
Seventy-four patients completed the survey: 42.9 years (range: 23-71 years), 38 (51%) female, 59 current insulin pump users. Most (84%) reported wearing their devices “almost daily” (n = 58) or 3 weeks per month (n = 4). “Almost daily” users reported an 86% reduction in incidence of emergency medical treatment events (P = .0013) and >50% reduction in daily SMBG frequency (P < .0001). Reductions in hypoglycemia fear were apparent but not statistically significant (P = .7359).
Conclusions:
“Almost daily” use of CGM with the Dexcom G4 system reduced incidence of emergency treatment events and daily SMBG utilization among survey respondents and a trend toward reduced hypoglycemia fear. This may indicate cost savings in reduction of emergency medical intervention and likely improved quality of life without increasing safety concerns related to hypoglycemia.
Keywords: CGM, continuous glucose monitoring, hypoglycemia, SMBG, emergency
Large clinical trials have shown that frequent, consistent use of continuous glucose monitoring (CGM) can significantly improve glycemic control in insulin-treated diabetes.1-3 However, in many CGM studies, clinical benefit was primarily seen in those patients who regularly wore their devices for approximately 6 days or more per week.3-9
Current evidence suggests that frequency and persistence of CGM use is strongly associated with users’ perceptions of the accuracy and reliability of their devices, and that users’ trust in their devices is linked to more aggressive insulin adjustments, improvements in quality of life and less reliance on data from self-monitoring of blood glucose (SMBG).10 In a survey of 877 current CGM users, Polonsky and Hessler reported that satisfaction with device accuracy was an independent predictor of 3 quality-of-life benefits associated with CGM use: perceived control over diabetes, hypoglycemic safety, and interpersonal support.11 Unpublished findings from the same data set revealed that satisfaction with accuracy was also associated with more frequent CGM use and with perceiving their device as more helpful in avoiding hypoglycemia and hyperglycemia, and in achieving better overall glycemic control.10
Although the current generation of CGM devices is now even more accurate and reliable than previously, there still appear to be sizeable differences in accuracy between the current manufacturers. In a recent study that evaluated whether perception of accuracy and patient treatment satisfaction differ between the Enlite sensor (Medtronic MiniMed, Inc, Northridge, CA) and Dexcom G4 sensor (Dexcom, San Diego, CA), the Dexcom G4 sensor was associated with greater accuracy (overall and within the hypoglycemic range) than the Enlite sensor. Patients also reported a significantly more positive experience using the Dexcom G4 than the Enlite.12
We recently reported findings from a survey we conducted that assessed satisfaction and frequency of using earlier generation CGM among 87 adult respondents with type 1 diabetes.13 In that survey, 43 participants used the Medtronic MiniLink Real-Time CGM system and 38 used the Dexcom Seven Plus system. Survey results showed that 76% of Dexcom device users reported wearing their devices “almost daily” compared with 19% of Medtronic device users. Among the 35 Medtronic device users who indicated less frequent wear; 28 reported wearing their device less than 1 week per month; device inaccuracy was the most commonly reported reason.
Differences between commercial CGM devices can influence user perceptions of accuracy and utility. This can impact of CGM use on users’ clinical outcomes, and attitudes and behaviors can be confounded when assessments include multiple CGM devices. We conducted a survey of adults with type 1 diabetes to assess the impact of frequent and persistent use of a single CGM system on hypoglycemia fear, emergency medical treatment, and SMBG frequency.
Research Design and Methods
In this single-center survey, we utilized a 16-item questionnaire (Figure 1) to assess changes in fear of hypoglycemia, SMBG utilization, and incidence of emergency medical treatment and fear of hypoglycemia among individuals with type 1 diabetes who were treated with intensive insulin regimens and had used their current CGM device for at least 1 year. Because differences between the various commercial CGM devices can impact user perceptions of performance and usability, we restricted participation in the survey to individuals who were using the same CGM brand/model (Dexcom G4 Continuous Glucose Monitoring System, Dexcom, Inc, San Diego, California) to avoid confounding responses. The survey was approved by the institutional review board that governed the medical center.
Figure 1.
Survey instrument.
Participants were recruited on an “as-seen” basis from a major, urban internal medicine clinic that sees between 700 and 800 patients per year on both an inpatient and outpatient basis and an associated diabetes education center that sees between 500 and 600 outpatients per year. The average HbA1c level among clinic patients was 7.4%.
Once written consent was obtained, the questionnaire was administered and completed during the clinic visit. Responses to the survey were collated and analyzed by standard descriptive statistics using Excel (Microsoft Corp, Redmond, WA).
We assessed changes in hypoglycemia fear, daily SMBG testing frequency and emergency medical treatment, comparing the year prior to CGM to 1 year after CGM use in respondents who reported “almost daily” wear of their CGM device. Results are presented as either number or percentage of patients.
Survey Results
A total of 74 patients were invited to participate in the survey, which was conducted from June 2014 to March 2015. All of the patients completed the survey. The average age of participants was 42.9 years (range: 23-71 years) and 38 (51%) were female. Fifty-nine participants had 10-25+ years duration of diabetes, and 59 were currently using an insulin pump. Approximately 76% (n = 56) of participants reported participating in at least 1 formal training session with a trainer.
Survey results showed that 84% of respondents reported wearing their devices “almost daily” (n = 58) or 3 weeks per month (n = 4) (Table 1). Among frequent users, “improved glycemic control” and “knowing glucose at all times” were most commonly reported as primary reasons for frequent use. Among less frequent CGM users (≤3 weeks per month) the most common reasons reported were “tired of wearing 2 devices” and “sensor did not remain attached.” Seventy (94.6%) respondents indicated that they would purchase the Dexcom G4 again.
Table 1.
Frequency and Reasons for Persistent CGM Use.
| Frequency of CGM wear (n = 74) | ||||||
|---|---|---|---|---|---|---|
| Almost daily | 3 weeks/month | 2 weeks/month | ≤1 week/month | |||
| 58 (78.3%) | 4 (5.4%) | 4 (5.4%) | 8 (10.8%) | |||
| Primary reason for frequent CGM wear (almost daily to 3 weeks/month), n = 62a | ||||||
| Improved glucose | Avoid high glucose | Avoid low glucose | Directional arrows | Knowing glucose at all times | Felt safer | Othera |
| 21 (36.2%) | 1 (1.7%) | 9 (15.5%) | 6 (10.3%) | 19 (32.8%) | 1 (1.7%) | 1 (1.7%) |
| Primary reason for less frequent CGM wear (≤3 weeks/month), n = 16 | ||||||
| Cost | Perceived inaccuracy | Frequent alarms | Tired of wearing 2 devices | Sensor errors | Otherb | |
| 1 (6.3%) | 1 (6.3%) | 1 (6.3%) | 2 (12.5%) | 1 (6.2%) | 10 (62.5%) | |
1 reported “provider wanted me to wear it.”
3 reported that “sensors did not stay attached at site”; 1 reported “order issues”; 1 reported “not wanting to wear sensor all the time”; however, 2 reported “feeling safer”; 2 reported “improved control”; 1 reported “directional arrows were valuable.”
Change in Hypoglycemia Fear
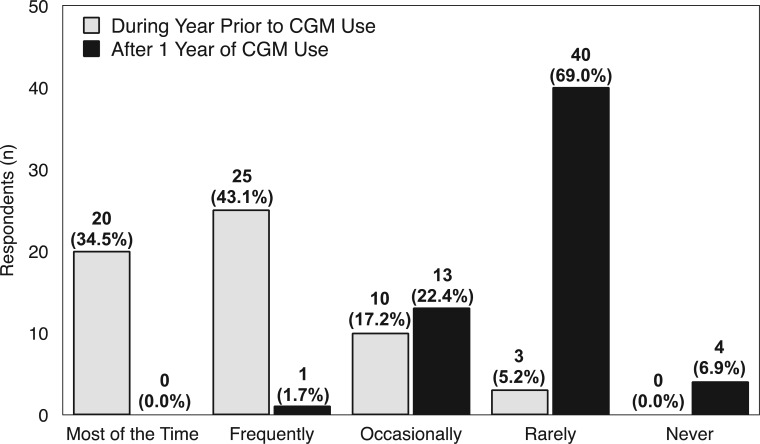
Among respondents who indicated “almost daily” CGM use, 45 (78%) reported worrying about hypoglycemia “most of the time” or “frequently” prior to CGM use. After 1 year of CGM use, no respondents reported worrying about hypoglycemia “most of the time” and 1 (2.0%) reported frequent worry, a 98% decrease in significant hypoglycemia fear (Figure 2). Although there was an apparent trend toward reduced hypoglycemia worry, Fisher exact test P value was not statistically significant (P = .7359).
Figure 2.
Hypoglycemia fear in year prior to CGM use compared with 1 year after CMG use (n = 58). Overall changes were apparent but not statistically significant (P = .7359).
Change in Emergency Medical Care Frequency
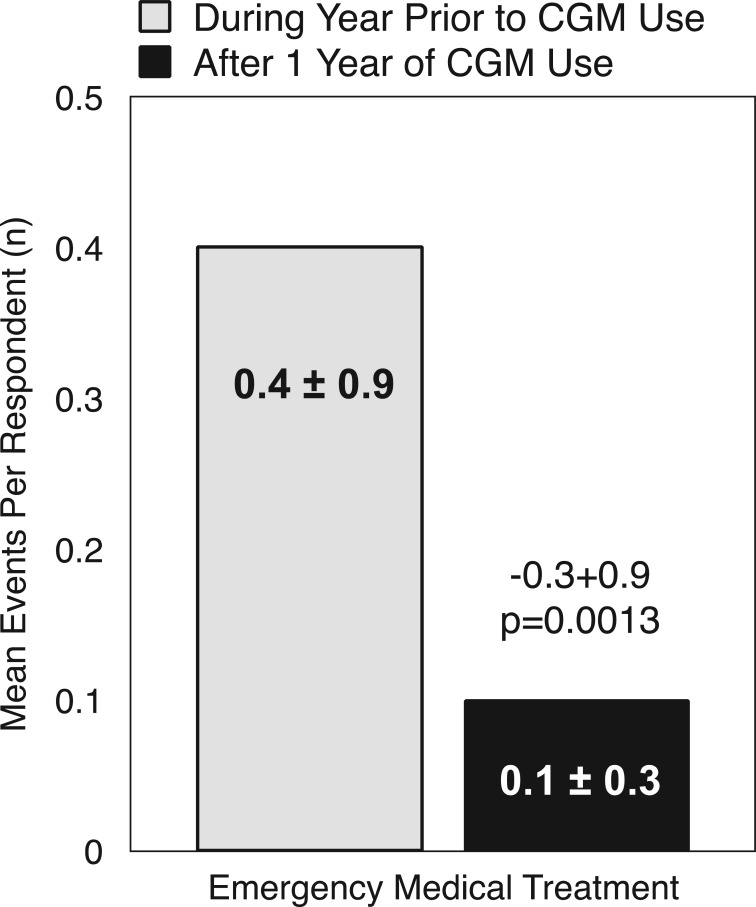
“Almost daily” CGM users reported an 86% reduction in the number of events requiring emergency medical treatment after 1 year of CGM use compared to the year prior to CGM use (P = .0013) (Figure 3).
Figure 3.
Paired t test for mean number of emergency visits per respondent before and after CGM (n = 58). The mean (SD) number of emergency treatment events per respondent was significantly reduced after 1 year of CGM use compared with no CGM in prior year among all respondents.
Change in SMBG Frequency
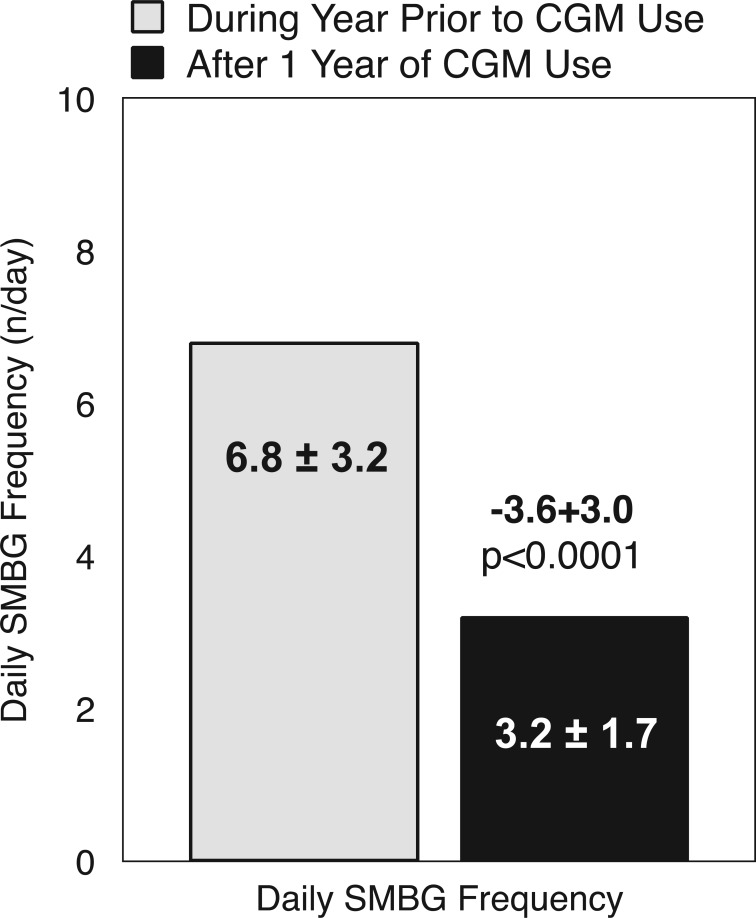
“Almost daily” CGM users reported a significant reduction in daily frequency of SMBG after 1 year of CGM use compared with the prior year of no CGM use (Figure 4).
Figure 4.
Paired t test for the mean daily SMBG frequency before and after CGM (n = 58).
Discussion
Our survey showed that the vast majority of respondents persistently used their CGM devices at frequencies associated with significant glycemic improvements in large clinical trials.3-9 After 1 year of CGM use, high-frequency users reported notable reductions in SMBG utilization, incidence of emergency medical treatment/hospitalizations, and hypoglycemia fear.
Although CGM is indicated as an adjunctive monitoring method to SMBG, our findings were not unexpected. In a recent survey of 222 individuals with type 1 diabetes, most respondents indicated that their dosage decisions were based on the “rate-of-change” (ROC) arrows on their CGM device; only 11% of respondents stated they did not use the ROC arrows to adjust their dose.14 This suggests that respondents had become less reliant on SMBG for dosage calculations. Interestingly, respondents also reported making significantly larger adjustments to their insulin dose than they would using SMBG alone, and that many lowered their glycemic goals,14 which suggests that CGM use may have reduced their fear of hypoglycemia.
Fear of hypoglycemia is widely recognized as a major barrier to achieving and sustaining optimal glycemic control.15 This fear is often exacerbated by the development of hypoglycemic unawareness in many well-controlled patients,16 and it is strongly associated with poor adherence to prescribed insulin regimens.17 A recent retrospective analysis found that in patients with optimized therapy, CGM use was significantly associated with improvements in HbA1c and reduced rates of severe hypoglycemia but did not restore hypoglycemia awareness.18 Although the investigators did not assess hypoglycemia fear among the study population, the reductions seen in HbA1c suggest that CGM use supported treatment adherence.
A significant limitation of our survey was the use of self-reported data, which may not accurately reflect participants’ actual SMBG utilization or history incidence of emergency medical treatment. Lack of objective measurements of clinical and financial outcomes (eg, change in HbA1c, insurance data regarding emergency room visits, SMBG data) further limit the interpretation of our findings. Another limitation was the small sample size; a larger number of participants would likely have increased the generalizability of our findings, particularly if we had found a larger number less frequent CGM users.
Nevertheless, our findings of decreased events requiring emergency medical treatment and decreased SMBG utilization provide additional evidence for the benefits of frequent and persistent CGM use in individuals treated with intensive insulin regimens. Although not statistically significant, the apparent trend toward reduced hypoglycemia fear also suggests a quality of life benefit.
However, because of the demonstrated differences between commercially available CGM devices that can influence user frequency and persistence of use, it is important that clinicians become familiar with the strengths and weaknesses of each of the CGM systems they recommend to their patients. Larger studies are needed.
Acknowledgments
The authors thank Christopher G. Parkin, MS, CGParkin Communications, Inc, Boulder City, NV, USA, for his editorial assistance.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; ROC, rate of change; SMBG, self-monitoring of blood glucose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JJC is a speaker for Sanofi, Janssen, and BMS-AZ. DD is a certified insulin pump trainer for Animas, Medtronic, Roche Diabetes Care, Insulet, and Tandem. EG is a certified pump trainer for Animas, Medtronic, Roche Diabetes Care, Insulet, and Tandem.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dexcom, Inc provided funding to support for the writing of the article. This was an investigator-initiated study and was designed and executed independently by the investigators without industry support.
References
- 1. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33(1):17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29(12):2730-2732. [DOI] [PubMed] [Google Scholar]
- 6. Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabetes Care. 2009;32(12):2245-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250-1257. [DOI] [PubMed] [Google Scholar]
- 8. Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9(3):203-210. [DOI] [PubMed] [Google Scholar]
- 9. Frontino G, Bonfanti R, Scaramuzza A, et al. Sensor-augmented pump therapy in very young children with type 1 diabetes: an efficacy and feasibility observational study. Diabetes Technol Ther. 2012;14(9):762-764. [DOI] [PubMed] [Google Scholar]
- 10. Polonsky WH, Hessler D. Perceived accuracy in continuous glucose monitoring: understanding the impact on patients. J Diabetes Sci Technol. 2015;9(2):339-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Polonsky WH, Hessler D. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther. 2013;15(4):295-301. [DOI] [PubMed] [Google Scholar]
- 12. Matuleviciene V, Joseph JI, Andelin M, et al. A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 system) and Enlite sensor (guardian REAL-time system) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol Ther. 2014;16(11):759-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chamberlain J, Dopita D, Gilgen E. Persistence of continuous glucose monitoring use in a community setting 1 year after purchase. Clin Diabetes. 2013;31:106-109. [Google Scholar]
- 14. Pettus J, Price D, Edelman S. How patients with type 1 diabetes translate continuous glucose monitoring data into diabetes management decisions. Endocr Pract. 2015;21:613-620. [DOI] [PubMed] [Google Scholar]
- 15. Cryer PE. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14(6):750-756. [DOI] [PubMed] [Google Scholar]
- 16. Fanelli CG, Porcellati F, Pampanelli S, Bolli GB. Insulin therapy and hypoglycaemia: the size of the problem. Diabetes Metab Res Rev. 2004;20(suppl 2):S32-S42. [DOI] [PubMed] [Google Scholar]
- 17. Smith CB, Choudhary P, Pernet A, Hopkins D, Amiel SA. Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Diabetes Care. 2009;32(7):1196-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choudhary P, Ramasamy S, Green L, et al. Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care. 2013;36(12):4160-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]