Abstract
Aims:
The aim was to analyze the changes in a German type 2 diabetes population prior to (2006) and after (2010, 2014) launch of new drugs.
Methods:
Patients with T2DM in 2006, 2010, and 2014 were recruited for the study. Demographic data included age, gender, and health insurance type (private/statutory). Drug prescription, mean costs per patient, HbA1c levels, macrovascular complications, and time before first insulin prescription were analyzed.
Results:
In all, 64 098, 77 219, and 85 004 T2DM patients were included for 2006, 2010, and 2014, respectively. The mean age (65.9-66.9 years), proportion of men (50.8%-53.8%), and proportion of patients with private health insurance (6.6%-7.2%) differed significantly for each of the 3 years. There was a 1.25-fold increase in the total costs per patient, linked with an increase in the costs associated with the use of new drugs and a decrease in those associated with the use of old drugs, respectively. HbA1c levels were slightly better regulated in 2014 than in 2006 and 2010. The share of macrovascular complications decreased significantly over time, dropping from 27.4% in 2006 to 24.6% in 2014. The mean duration before first insulin treatment increased from 1225 days in 2006 to 1406 days in 2014.
Conclusions:
The new drugs analyzed in this study had positive effects on HbA1c levels, macrovascular complications, and mean time before first insulin treatment.
Keywords: type 2 diabetes, new drugs, DPP-4, insulin
Diabetes is one of the most common chronic diseases with an estimated worldwide prevalence of around 8.3%.1 There are around 60 million people with diabetes in Europe (10.3% of men and 9.6% of women aged 25 years and over).2 Type 2 diabetes mellitus (T2DM) is present in 90% of diabetic patients.3
T2DM has a serious impact on health and the economy in Europe. This disease is known to be associated with several other disorders, such as retinopathy,4 nephropathy,5 neuropathy,6 atherosclerosis,7 cardiovascular disease,8 stroke,9 and cerebrovascular disease.10 Recently, a meta-analysis of 35 studies conducted by Nwaneri and colleagues has shown that T2DM and its complications, in particular stroke, cause a 2-fold increase in mortality rates.11
Numerous drugs are available for the treatment of patients with T2DM. Metformin and sulfonylureas have been used for decades, either as monotherapies or as part of combination therapies.12-14 Since the mid-2000s, several new drugs presenting fewer side effects than metformin and sulfonylureas have emerged for T2DM treatment: dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, and sodium-glucose cotransporter-2 (SGLT2) inhibitors. Their use was approved in Europe by the European Medicines Agency (EMA) in 2007, 2009 and 2013, respectively.15-17 Whereas DPP-4 inhibitors and GLP-1 stimulate insulin release and inhibit glucagon release, SGLT2 inhibitors focus on the renal reabsorption of glucose.18-20 While these 3 families of molecules display different mechanisms of action, they all prevent hyperglycemia and regulate HbA1c levels in patients with T2DM.18,19,21 Although such drugs have proved their efficacy, the success of these therapies depends not only on the medications themselves, but also on compliance,22 diet,23 management, and therapeutic strategies.24,25
Therefore, the goal of this study was to analyze the changes in a German T2DM population prior to (2006) and after (2010, 2014) launch of these new drugs to gain a better understanding of their effects.
Patients and Methods
Database
The Disease Analyzer database (IMS HEALTH) compiles drug prescriptions, diagnoses, basic medical and demographic data obtained directly and in anonymous format from computer systems used in the practices of general practitioners.26 Diagnoses (ICD-10), prescriptions (Anatomical Therapeutic Chemical [ATC] Classification System), and the quality of reported data have been monitored by IMS based on a number of criteria (eg, completeness of documentation, linkage between diagnoses, and prescriptions).
In Germany, the sampling methods used for the selection of physicians’ practices were appropriate to obtain a representative database of primary care practices.26 Prescription statistics for several drugs were very similar to data available from pharmaceutical prescription reports.26 The age groups for given diagnoses in Disease Analyzer also agreed well with those in corresponding disease registries.26
Study Population
Patients with T2DM (ICD 10: E11) in 2006, 2010, and 2014 were included in the study. These were identified by 561 general practitioner practices in the IMS Disease Analyzer database. In all, 64 098, 77 219, and 85 004 subjects were available for 2006, 2010, and 2014, respectively.
Study Variables
Demographic data included age, gender, and health insurance type (private or statutory). Prescriptions were determined for each patient and classified into 3 different groups: (1) insulin treatment, (2) old drugs (insulin human base and insulin human isophane), and (3) new drugs (insulin glargin, insulin detemir, insulin degludec, insulin glulisin, insulin lispro, and insulin aspart). Therapy costs per patient were calculated as the sum of pharmacy sale prices for each prescription in the year. Mean HbA1C values were determined and their distribution established for 2006, 2010, and 2014. The proportion of patients displaying macrovascular complications was also analyzed. Macrovascular complications included the following diseases: myocardial infarction (I21, I22, I23, I25.2), angina pectoris (I20), coronary heart disease (I24, I25), stroke (I63, I64), transient ischemic attack (TIA: G45), and peripheral arterial occlusive disease (I73.9, E11.5). All complications had been documented at least once before the year of interest. Complications after T2DM diagnosis were also included. Finally, patients with a first insulin prescription in 2006, 2010, and 2014 were analyzed and the time between initial oral antidiabetic (OAD) therapy and the first insulin prescription calculated.
Results
Patient Characteristics
Patient characteristics are displayed in Table 1. In all, 64 098, 77 219, and 85 004 T2DM patients were included for 2006, 2010, and 2014, respectively. The mean age (between 65.9 and 66.9 years), the proportion of men (between 50.8% and 53.8%), and the proportion of patients with private insurance (between 6.6% and 7.2%) differed significantly for each of the 3 years of interest (P < .0001 for the 3 variables).
Table 1.
Characteristics of T2DM Patients in German Primary Care Practices.
| Variable | 2006 | 2010 | 2014 | P value |
|---|---|---|---|---|
| N | 64098 | 77219 | 85004 | |
| Age, mean (SD) | 65.9 (13.4) | 66.4 (13.6) | 66.9 (14.0) | <.0001 |
| Male (%) | 50.8 | 52.5 | 53.8 | <.0001 |
| Private insurance (%) | 6.6 | 6.9 | 7.2 | <.0001 |
Changes in T2DM Patients
The proportions of new OAD drugs, old OAD drugs and insulin treatment in T2DM patients in 2006, 2010, and 2014 are shown in Figure 1. The proportion of new OAD drugs increased significantly over time (from 0% to 17.6%, P < .0001), with the proportion of old OAD drugs decreasing in parallel (from 61.4% to 44.4%, P < .001). The share of patients treated with insulin did not change substantially between 2006 and 2014, remaining between 36.5% and 38.6%. Interestingly, 6.9%, 7.7%, and 0.2% of patients received glitazone in 2006, 2010, and 2014, respectively.
Figure 1.
Shares of insulin, old and new oral antidiabetics (OAD) prescribed in primary care practices in Germany in 2006, 2010, and 2014.
Total and drug-specific costs per patient are displayed in Figure 2. Total costs increased significantly from €351.70 to €440.60 (1.25-fold increase, P < .001) between 2006 and 2014. New OAD therapy costs increased significantly from €0 to €144.60, while there was a substantial decrease in old OAD therapy costs, which dropped from €112.20 to €45.60 (P < .001 in both cases). Costs associated with insulin treatment did not change notably, remaining between €238.3 and €250.40. Finally, 9.7%, 7.7%, and 0.3% of total costs were related to glitazone prescriptions.
Figure 2.
Therapy costs per patient in 2006, 2010, and 2014.
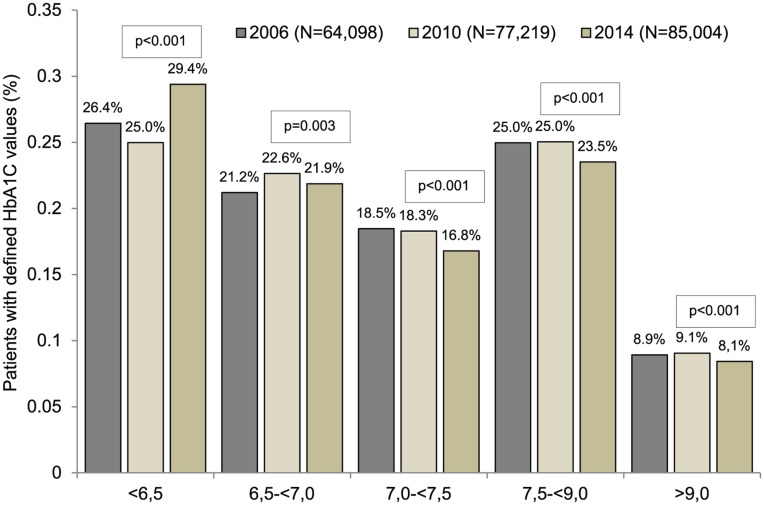
Distributions of HbA1c levels in T2DM patients in 2006, 2010, and 2014 are represented in Figure 3. Five different groups were considered: HbA1c < 6.5, 6.5 <= HbA1c < 7.0, 7.0 < =HbA1c < 7.5, 7.5 <= HbA1c < 9.0, and 9.0 <= HbA1c. There were significant differences between 2006 and 2014 for each group (all P values lower than .003), particularly that associated with HbA1c levels lower than 6.5 (26.4% in 2006 versus 29.4% in 2014).
Figure 3.
Distribution of patient HbA1c values in 2006, 2010, and 2014. P values are retained for comparison of HbA1C values between 2010 and 2014. HbA1c means = 2006: 7.3%; 2010: 7.3%; 2014: 7.2%.
The proportions of T2DM patients displaying macrovascular complications are shown in Figure 4. The share of macrovascular complications decreased over time, from 27.4% in 2006 to 25.7% in 2010 (P < .001), and from 25.7% in 2010 to 24.6% in 2014 (P < .001).
Figure 4.
Proportions of type 2 diabetes patients with macrovascular complications in 2006, 2010, and 2014.
Figure 5 represents the mean duration between the first OAD therapy and the first insulin prescription. This duration was longer in 2010 (mean = 1298 days, SD = 1126 days) and 2014 (mean = 1406 days, SD = 1190 days) than in 2006 (mean = 1225 days, SD = 1120 days) (P = .0037).
Figure 5.
Mean duration before first insulin therapy in type 2 diabetes patients in 2006, 2010, and 2014.
Discussion
This German database study showed that prescriptions for new T2DM drugs increased between 2006 and 2014. The more frequent use of these medications was associated with an increase in costs per patient, a slight decrease in HbA1c values, a decrease in the proportion of subjects with macrovascular complications, and an increase in the mean duration before the first insulin treatment.
The impact of DPP-4 inhibitors and GLP-1 receptor agonists on HbA1c blood levels has already been studied in several trials lasting in general several weeks (up to more than 52 weeks). These works analyzed the effects of these molecules, both as monotherapies and as part of combination therapies including other OAD drugs. Davidson reviewed numbers of these studies in 2009 and demonstrated that the use of DPP-4 inhibitors is associated with consequent decreases of HbA1c levels in blood at 12, 18, 24, 30 and 52 weeks.27 Interestingly, DPP-4 inhibitors have no significant effect on weight, unlike sulfonylureas28,29 and thiazolidinediones,30 the deleterious effects of which are visible after just a few months of treatment. Like DPP-4 inhibitors, GLP-1 receptor agonists reduce HbA1c levels, effecting a reduction of up to 1.5% at 30 weeks.27 In addition, the use of GLP-1 receptor agonists, particularly exendin-4 agonists, leads to weight loss of between -3.8 kg at 15 weeks and -3.7 kg at 30 weeks where exenatide is taken once a week. As SGLT2 inhibitors were commercialized after DPP-4 inhibitors and GLP-1 receptor agonists, evidence of their effects on HbA1c values only came several years after, most notably in the work of Rosenstock and his colleagues.31 They analyzed the effects of dapagliflozin, a member of the SGLT2 inhibitor family, and demonstrated that, when prescribed with thiazolidinedione, this new drug leads to a −0.97% reduction in HbA1c levels at 24 weeks and prevents the weight gain induced by thiazolidinedione to some extent.31
Before going any further, it is essential to bear in mind that we considered the whole T2DM population available in the Disease Analyzer database, and not just those T2DM patients who were treated with new drugs. Nonetheless, as the use of new drugs became more common between 2006 and 2014, we were able to analyze the changes associated with the prescription of such medications. Having said that, our results are in line with the literature, although decreases in HbA1c levels observed in our study were only moderate (29.4% of patients with HbA1c values lower than 6.5 in 2014 compared with 26.4% in 2006), suggesting that older drugs used in 2006, mainly metformin and sulfonylureas, already had good efficacies.
One other important result of our work is that the proportion of macrovascular complications decreased over time, although a causal link cannot be proved because of the study design. Several authors have analyzed the beneficial effects of DPP-4 inhibitors on the heart and cardiovascular system. Indeed, it was demonstrated in a mouse model of cardiovascular disorder that the use of 1 DPP-4 inhibitor, sitagliptin, can reduce the infarct area.32 The same year, these results were confirmed in diabetes patients with coronary heart disease, as sitagliptin improved their ventricular performance in response to stress and partially prevented postischemic stunning.33 Similarly, Mistry et al showed in 2008 that sitagliptin causes a 2 to 3 mm Hg reduction in 24-hour ambulatory blood pressure.34 Interestingly, such effects on heart rate and blood pressure are also found with GLP-1 receptor agonists35,36 and SGLT2 inhibitors.37,38 By contrast, a controversial study has demonstrated that the unadjusted risk of cardiovascular mortality with sulfonylureas monotherapy is 3.71 as compared with metformin monotherapy.39 Although some limitations have been pointed out in this work,40 which has not been confirmed by more recent clinical trials and meta-analyses,41,42 the use of sulfonylureas and its risk-benefice balance need to be reanalyzed.
It is well known that macrovascular complications may be caused by hypoglycemia.43 Indeed, Zhao and colleagues have shown in 44 261 T2DM patients that hypoglycemia is associated with a 2-fold-increased risk of macrovascular complications.44 As T2DM involves insulin deficiency and insulin resistance, which favor hyperglycemia, several antidiabetic drugs, such as insulin (long- and rapid-acting), sulfonylureas or repaglinide, may cause hypoglycemia.45 By contrast, the use of DPP-4 inhibitors and GLP-1 receptor agonists does not lead to low blood glucose.45 Surprisingly, a direct comparison between these old and new drugs has been lacking for years. In 2013, Rathmann et al were the first authors to try to compare DPP-4 inhibitors and sulfonylureas effects on glycemia.46 With 19 184 DPP-4 inhibitors and 31 110 sulfonylureas users, they demonstrated that after 2 years of treatment, hypoglycemia is significantly more common in patients treated with sulfonylureas (1.00% versus 0.18% in patients with DPP-4 inhibitors, OR = 4.8, 95% CI = 1.8-12.5). The authors, in line with the previous literature, also showed that hypoglycemia is associated with incident macrovascular complications (OR = 1.6, 95% CI = 1.1-2.2). Finally, they found that the risk of macrovascular events was 26% lower in subjects using DPP-4 inhibitors than in sulfonylureas users. Therefore, although our work did not focus on the evolution of hypoglycemia over time, it is reasonable to assume that the decreasing proportion of macrovascular complications is linked to a decreasing proportion of patients with hypoglycemia. Finally, it is important to remember that variations in HbA1c levels do not necessarily reflect hyper-, normo-, or hypoglycemia. In other words, the reduction of HbA1c levels is compatible with normoglycemia.
We also observed that the use of these new drugs was associated with an increased mean duration before the first insulin treatment. Although delaying insulin therapy may accelerate the development of T2DM long-term effects, in particular cardiovascular diseases, our result may suggest that the requirement for insulin was less pronounced than in patients using old drugs and that patients were satisfied with their treatment. As a matter of fact, compliance is an important aspect of T2DM management because of its chronicity. Nevertheless, a meta-analysis published in 2004 showed that the overall rate of adherence for OAD drugs is between 36% and 93% and decreases when therapies are combined or in the case of multiple doses, and that around 25% of patients do not take the medications prescribed by their general practitioner.47 In addition to the fact that DPP-4 inhibitors do not give rise to any significant side effects, such as hypoglycemia, they are oral drugs, which may help increase compliance and improve patient management.46 Even if we were not able to estimate the cost savings related to the use of these new drugs in Germany, it is already known that the prescription of these antidiabetes medications leads to lower costs for patients and health systems in other countries.48,49
Retrospective primary care database analyses are generally limited by the validity and completeness of the data on which they are based. The present study included several limitations, which should be mentioned. First, no valid information was provided on diabetes duration. In addition, the assessment of complications and comorbidity relied solely on ICD codes entered by primary care physicians. Data on socioeconomic status (eg, education, income) and lifestyle-related risk factors (eg, smoking, alcohol, physical activity) were also lacking. Unfortunately, the documentation of hypoglycemia was also insufficient and could not be used, although the reduction of hypoglycemia is one of the most important effects of innovative antihyperglycemic drugs. Finally, we used for the calculation of therapy costs the sum of the pharmacy sale prices, which can differ significantly from the actual reimbursed prices due to contracts between statutory sickness funds and manufacturers, resulting in lower costs.
In conclusion, our findings suggest that new T2DM drugs have positive effects on HbA1c levels, macrovascular complications, and mean time before the first insulin treatment. Although long-term data are still needed to further substantiate these findings, such medications, and DPP-4 inhibitors in particular, may represent good alternatives to sulfonylureas as second-choice treatments in the future.
Footnotes
Abbreviations: ATC, Anatomical Therapeutic Chemical Classification System; DPP-4, dipeptidyl peptidase 4; EMA, European Medicines Agency; GLP-1, glucagon-like peptide 1; OAD, oral antidiabetic; SGLT2, sodium-glucose cotransporter-2; T2DM, type 2 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. International Diabetes Federation. Diabetes: facts and figures. 2015. Available at: www.idf.org. Accessed July 10, 2015.
- 2. World Health Organization. Data and statistics. 2015. Available at: www.euro.who.int. Accessed July 10, 2015.
- 3. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. 1999. [Google Scholar]
- 4. Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004;27:s84-s87. [DOI] [PubMed] [Google Scholar]
- 5. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176. [DOI] [PubMed] [Google Scholar]
- 6. Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956-962. [DOI] [PubMed] [Google Scholar]
- 7. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570-2581. [DOI] [PubMed] [Google Scholar]
- 8. Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med. 2001;249:225-235. [DOI] [PubMed] [Google Scholar]
- 9. Sander D, Sander K, Poppert H. Review: stroke in type 2 diabetes. Br J Diabetes Vasc Dis. 2008;8:222-229. [Google Scholar]
- 10. Dalal PM, Parab PV. Cerebrovascular disease in type 2 diabetes mellitus. Neurol India. 2002;50:380-385. [PubMed] [Google Scholar]
- 11. Nwaneri C, Cooper H, Bowen-Jones D. Mortality in type 2 diabetes mellitus: magnitude of the evidence from a systematic review and meta-analysis. Br J Diabetes Vasc Dis. 2013;13:192-207. [Google Scholar]
- 12. Haupt E, Knick B, Koschinsky T, Liebermeister H, Schneider J, Hirche H. Oral antidiabetic combination therapy with sulphonylureas and metformin. Diabète Métabolisme. 1991;17:224-231. [PubMed] [Google Scholar]
- 13. Zimmerman BR. Sulfonylureas. Endocrinol Metab Clin North Am. 1997;26:511-522. [DOI] [PubMed] [Google Scholar]
- 14. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25-33. [DOI] [PubMed] [Google Scholar]
- 15. European Medicines Agency. Galvus (vildagliptin). 2015. Available at: http://www.ema.europa.eu.
- 16. European Medicines Agency. Victoza (liraglutide). 2015. Available at: http://www.ema.europa.eu.
- 17. European Medicines Agency. Invokana (canagliflozin). 2015. Available at: http://www.ema.europa.eu.
- 18. Chao EC, Henry RR. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551-559. [DOI] [PubMed] [Google Scholar]
- 19. Dicker D. DPP-4 inhibitors impact on glycemic control and cardiovascular risk factors. Diabetes Care. 2011;34:S276-S278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728-742. [DOI] [PubMed] [Google Scholar]
- 21. Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rombopoulos G, Hatzikou M, Athanasiadis A, Elisaf M. Treatment compliance with fixed-dose combination of vildagliptin/metformin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: a 24-week observational study. Int J Endocrinol. 2015;2015:251485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jonasson L, Guldbrand H, Lundberg AK, Nystrom FH. Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann Med. 2014;46:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cauch-Dudek K, Victor JC, Sigmond M, Shah BR. Disparities in attendance at diabetes self-management education programs after diagnosis in Ontario, Canada: a cohort study. BMC Public Health. 2013;13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Becher H, Kostev K, Schröder-Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47:617-626. [DOI] [PubMed] [Google Scholar]
- 27. Davidson JA. Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors. Cleve Clin J Med. 2009;76(suppl 5):S28-S38. [DOI] [PubMed] [Google Scholar]
- 28. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837-853. [PubMed] [Google Scholar]
- 29. Nichols GA, Gomez-Caminero A. Weight changes following the initiation of new anti-hyperglycaemic therapies. Diabetes Obes Metab. 2007;9:96-102. [DOI] [PubMed] [Google Scholar]
- 30. Fonseca V. Effect of thiazolidinediones on body weight in patients with diabetes mellitus. Am J Med. 2003;115(suppl 8A):42S-48S. [DOI] [PubMed] [Google Scholar]
- 31. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012;35:1473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ye Y, Keyes KT, Zhang C, Perez-Polo JR, Lin Y, Birnbaum Y. The myocardial infarct size-limiting effect of sitagliptin is PKA-dependent, whereas the protective effect of pioglitazone is partially dependent on PKA. Am J Physiol Heart Circ Physiol. 2010;298:H1454-1465. [DOI] [PubMed] [Google Scholar]
- 33. Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3:195-201. [DOI] [PubMed] [Google Scholar]
- 34. Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592-598. [DOI] [PubMed] [Google Scholar]
- 35. Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234-2243. [DOI] [PubMed] [Google Scholar]
- 36. Okerson T, Yan P, Stonehouse A, Brodows R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens. 2010;23:334-339. [DOI] [PubMed] [Google Scholar]
- 37. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420-428. [DOI] [PubMed] [Google Scholar]
- 39. Evans JMM, Ogston SA, Emslie-Smith A, Morris AD. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia. 2006;49:930-936. [DOI] [PubMed] [Google Scholar]
- 40. Raccah D. Comment on: Evans JMM, Ogston SA, Emslie-Smith A, Morris AD. (2006) Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 49:930-936. Diabetologia. 2007;50:1109-1110; author reply 1111. [DOI] [PubMed] [Google Scholar]
- 41. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443. [DOI] [PubMed] [Google Scholar]
- 42. Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394. [DOI] [PubMed] [Google Scholar]
- 43. Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao Y, Campbell CR, Fonseca V, Shi L. Impact of hypoglycemia associated with antihyperglycemic medications on vascular risks in veterans with type 2 diabetes. Diabetes Care. 2012;35:1126-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010;33:428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rathmann W, Kostev K, Gruenberger JB, Dworak M, Bader G, Giani G. Treatment persistence, hypoglycaemia and clinical outcomes in type 2 diabetes patients with dipeptidyl peptidase-4 inhibitors and sulphonylureas: a primary care database analysis. Diabetes Obes Metab. 2013;15:55-61. [DOI] [PubMed] [Google Scholar]
- 47. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218-1224. [DOI] [PubMed] [Google Scholar]
- 48. Genovese S, Tedeschi D. Effects of vildagliptin/metformin therapy on patient-reported outcomes: work productivity, patient satisfaction, and resource utilization. Adv Ther. 2013;30:152-164. [DOI] [PubMed] [Google Scholar]
- 49. Sicras-Mainar A, Navarro-Artieda R. Healthcare costs of the combination of metformin/dipeptidyl peptidase-4 inhibitors compared with metformin/other oral antidiabetes agents in patients with type 2 diabetes and metabolic syndrome. Diabetes Technol Ther. 2014;16:722-727. [DOI] [PubMed] [Google Scholar]