Abstract
Background:
The Bio-inspired Artificial Pancreas (BiAP) is a closed-loop insulin delivery system based on a mathematical model of beta-cell physiology and implemented in a microchip within a low-powered handheld device. We aimed to evaluate the safety and efficacy of the BiAP over 24 hours, followed by a substudy assessing the safety of the algorithm without and with partial meal announcement. Changes in lactate and 3-hydroxybutyrate concentrations were investigated for the first time during closed-loop.
Methods:
This is a prospective randomized controlled open-label crossover study. Participants were randomly assigned to attend either a 24-hour closed-loop visit connected to the BiAP system or a 24-hour open-loop visit (standard insulin pump therapy). The primary outcome was percentage time spent in target range (3.9-10 mmol/l) measured by sensor glucose. Secondary outcomes included percentage time in hypoglycemia (<3.9 mmol/l) and hyperglycemia (>10 mmol/l). Participants were invited to attend for an additional visit to assess the BiAP without and with partial meal announcements.
Results:
A total of 12 adults with type 1 diabetes completed the study (58% female, mean [SD] age 45 [10] years, BMI 25 [4] kg/m2, duration of diabetes 22 [12] years and HbA1c 7.4 [0.7]% [58 (8) mmol/mol]). The median (IQR) percentage time in target did not differ between closed-loop and open-loop (71% vs 66.9%, P = .9). Closed-loop reduced time spent in hypoglycemia from 17.9% to 3.0% (P < .01), but increased time was spent in hyperglycemia (10% vs 28.9%, P = .01). The percentage time in target was higher when all meals were announced during closed-loop compared to no or partial meal announcement (65.7% [53.6-80.5] vs 45.5% [38.2-68.3], P = .12).
Conclusions:
The BiAP is safe and achieved equivalent time in target as measured by sensor glucose, with improvement in hypoglycemia, when compared to standard pump therapy.
Keywords: closed-loop insulin delivery, Bio-inspired Artificial Pancreas, type 1 diabetes, diabetes technology
A closed-loop insulin delivery system, also known as an artificial pancreas (AP) system, may improve the lives of people with type 1 diabetes. Diabetes technology is an increasingly important component of type 1 diabetes management with continuous subcutaneous insulin infusion (CSII, pump therapy) supported by a NICE technology appraisal1 and evidence supporting subcutaneous continuous glucose sensor usage.2,3 The combination of sensor and CSII in sensor-augmented pump therapy with the low-glucose suspend feature has been reported to improve HbA1c and reduce hypoglycemia.4,5 The next step in diabetes technology is the artificial pancreas, comprising a subcutaneous glucose sensor, control algorithm to calculate insulin infusion from dynamic glucose changes, and an insulin pump. The primary focus of the artificial pancreas is to reduce hypoglycemia, especially overnight, and to increase percentage time spent in target. Improvements in glycemia have the potential to reduce the frequency and severity of hypoglycemia, and to improve HbA1c, and quality of life.
AP systems currently being evaluated use 3 main control strategies: proportional integral derivative (PID) control, model predictive control (MPC), and fuzzy logic (FL). Since the first AP study in 2006,6 a number of clinical trials have assessed feasibility of closed-loop systems using PID, MPC and FL in a controlled clinical environment.6-12 Some AP systems integrate glucagon in a bi-hormonal configuration.12-14 Progress has been rapid with clinical evaluation of AP systems in supervised out-of-hospital settings14-18 and unsupervised (without remote monitoring system) in the home.19,20 To date the AP algorithm has been integrated into tablet computers, which may not be practical for daily use, or in smartphones where limited battery life and concerns over security remain. A Bio-inspired Artificial Pancreas (BiAP) system, using a novel algorithm based on a mathematical model of beta-cell physiology,21,22 has been developed using semiconductor microchip technology.23 The device (Supplementary Figure 1) offers advantages of miniaturization and low power.
The feasibility and safety of the BiAP system has been evaluated during fasting, overnight and postbreakfast conditions.24 The results were promising and here we present the results from a randomized controlled crossover study comparing the BiAP system (closed-loop) with standard insulin pump therapy (open-loop) over 24 hours.
At present capillary blood glucose is measured in type 1 diabetes self-management. Measurement of capillary blood ketone bodies (3-hydroxybutyrate) is encouraged at times of hyperglycemia to guide management, prevent diabetic ketoacidosis and assess the need for hospital admission. Rising glucose values in the context of falling ketone concentrations suggest a postmeal state, while rising glucose with rising ketones suggests insulin deficiency. In an AP system these scenarios require different action from the controller. In this study we measured 3-hydroxybutyrate to assess its potential value in an AP system and also measured lactate and nonesterified fatty acids (NEFA) to establish patterns of these analytes in closed-loop and open-loop therapy.
Proving safety of any AP algorithm in the absence of meal announcement is important and in this article we also present data from evaluation of the BiAP system with partial and no meal announcement.
Methods
Participants and Study Design
This is a prospective randomized controlled open-label crossover study. Regulatory approvals by the regional ethics committee and by the MHRA were obtained. Adult participants with type 1 diabetes were recruited from the diabetes clinics at Imperial College Healthcare NHS Trust. Inclusion criteria were age 18-75 years, duration of diabetes >1 year, fasting c-peptide <0.2 nmol/l, treatment with CSII for >6 months, and HbA1c <8.5% (69 mmol/mol). Exclusion criteria were recurrent severe hypoglycemia, pregnancy or planning pregnancy, breastfeeding, enrolment in other clinical studies, active malignancy, or being under investigation for malignancy. Informed written consent was obtained. As part of screening 5 days of blinded retrospective continuous glucose monitoring (CGM) data were collected from participants to optimize their insulin pump treatment.
Randomization
Participants were randomly assigned the order of the closed-loop and open-loop study visit using computer-generated allocation numbers, by the study investigators. A 1-week minimum washout period occurred between visits. Participants were blinded to the sensor and blood glucose.
After completion of both the closed-loop and open-loop visit all participants were invited to attend an additional visit to participate in a substudy without randomization to evaluate the safety of the BiAP algorithm without and with partial meal announcement.
Closed-Loop Visit
Two subcutaneous glucose sensors (Enlite, Medtronic, Northridge, CA, USA) were inserted in the abdomen the day before the study. The first sensor was designated as the primary sensor for the closed-loop system and the second sensor as reserve in case of primary sensor failure. Participants attended the Clinical Research Facility at 16:00 hours on the day of the study. Capillary blood glucose was measured on arrival and the participant’s basal insulin infusion rate was adjusted if required. The handheld BiAP unit was connected to 1 of the sensors. The participant’s own insulin pump was replaced with the study pump (Accu-Chek Spirit Combo pump, Roche, Basel, Switzerland) and basal insulin infusion continued until closed-loop insulin delivery was commenced. Rapid-acting insulin aspart (Novorapid, Novo Nordisk, Bagsværd, Denmark) was used throughout. The sensor signal was transmitted to the handheld unit by cable and communication between the handheld unit and the laptop with a graphical user interface was achieved via Bluetooth. The insulin infusion instruction was transmitted to the pump by Bluetooth communication protocol. Following sensor calibration to venous glucose at the start of closed-loop control, the control algorithm recommended an insulin dose and insulin was automatically delivered. Every 30 minutes a venous blood sample was analyzed for glucose and lactate using a YSI 2300 bedside analyzer (Yellow Springs Instrument, Yellow Springs, OH, USA). Three standardized meals were provided: dinner containing 80 g of carbohydrate (CHO) at 19:00 hours, breakfast (40 g of CHO) at 07:00 hours, and lunch (50 g of CHO) at 12:00 hours. Participants preselected their meal choices from a limited hospital canteen menu prior to the study. All the meals were announced to the algorithm based on the participant’s insulin:carbohydrate ratio. In the event of hypoglycemia (defined as symptomatic blood glucose <3.9 mmol/l or asymptomatic blood glucose <3.5 mmol/l) requiring carbohydrate rescue participants were informed of their venous glucose. A total of 15 g of quick-acting CHO was given to correct the hypoglycemia. Participants were allowed to drink water throughout.
Open-Loop Visit
This visit followed as for the closed-loop visit except the participant used their own insulin pump preset with their normal basal rates. The insulin infusion set was replaced with a new one on arrival to the clinical research facility. Rapid-acting insulin aspart (Novorapid, Novo Nordisk, Bagsværd, Denmark) was used throughout the open-loop study too. One of the sensors was connected to the BiAP handheld unit for CGM. Sampling and meals were the same as during the closed-loop visit. Participants were asked to measure their capillary blood glucose premeal, calculate the meal insulin bolus and administer it. Participants were permitted to do additional capillary testing between meals and administer correction boluses if desired.
Closed-Loop Without and With Partial Meal Announcement Visit
This visit followed as for the closed-loop visit, with the exception of the meal announcement strategy. The dinner was not announced to the controller, 100% of the breakfast carbohydrate was announced, and lunch was partially announced (50% of carbohydrate content announced).
Control Algorithm
We use a bio-inspired control algorithm based on a mathematical model of beta-cell physiology.21,22 The glucose target was set to 6.5 mmol/l. The algorithm is initialized with a personalized gain based on the participant’s preexisting correction factor. The gain is dynamic and automatically adapts based on the sensor glucose. In our previously published closed-loop study we employed a meal announcement strategy of 70% of the carbohydrate announced to the controller. To improve postprandial hyperglycemia 100% meal announcement was used in this study. Hypoglycemia prevention strategies including insulin infusion reduction and suspension were employed for forecasted glucose concentrations below predefined thresholds.
Sample Analysis
Blood glucose and lactate were analyzed using the YSI 2300 analyzer. Serum samples were stored at −80°C and analyzed for insulin, NEFAs and ketones (3-hydroxybutyrate). Serum insulin concentrations were analyzed using a chemilluminescent immunoassay (ARCHITECT i2000 immunoassay, Abbott Laboratories). Serum 3-hydroxybutyrate and NEFAs were analyzed using an automated analyzer (COBAS Mira S, Roche Diagnostics). 3-hydroxybutyrate were analyzed using a kinetic enzymatic reaction assay (RANDOX RABNUT kit) and NEFAs were analyzed using enzymatic colorimetric assay (RANDOX NEFA kit).
Outcomes
The primary outcome was percentage time spent in sensor glucose target range (3.9-10.0 mmol/l). Secondary outcomes were percentage time in euglycemia (3.9-7.8 mmol/l), hypoglycemia (<3.9 mmol/l) and hyperglycemia (>10.0 mmol/l), mean sensor glucose, insulin dose delivered and glycemic risk measures of low blood glucose index (LBGI) and high blood glucose index (HBGI). We calculated all glycemic outcomes for the whole time period and the overnight period. Other secondary outcomes included intercorrelations between metabolic analytes during closed-loop and open-loop control.
Statistical Analysis
The primary and secondary glycemic outcomes presented are calculated using the sensor glucose as this is the input to the control algorithm. The equivalent glycemic outcomes based on venous blood glucose have also been analyzed and are presented for comparison. The study start time was 17:00, but we encountered up to 45 minutes delay in starting in some participants due to hypoglycemia on arrival, late arrival, and technical issues. The first hour of the study (between 17:00 hours and 18:00 hours) has therefore been excluded from the data analysis.
LBGI and HBGI were calculated using the EasyGV version 9.0 software.25 Normally distributed data were compared using the paired t-test and nonnormally distributed data with the Wilcoxon matched-pairs signed-rank test. All outcomes are reported as mean (SD) or median (interquartile range [IQR]), unless stated otherwise. P values below .05 were considered statistically significant. Sensor accuracy was evaluated by calculating the median absolute relative difference (MARD). Data were analyzed using Stata/SE version 13.1.
Results
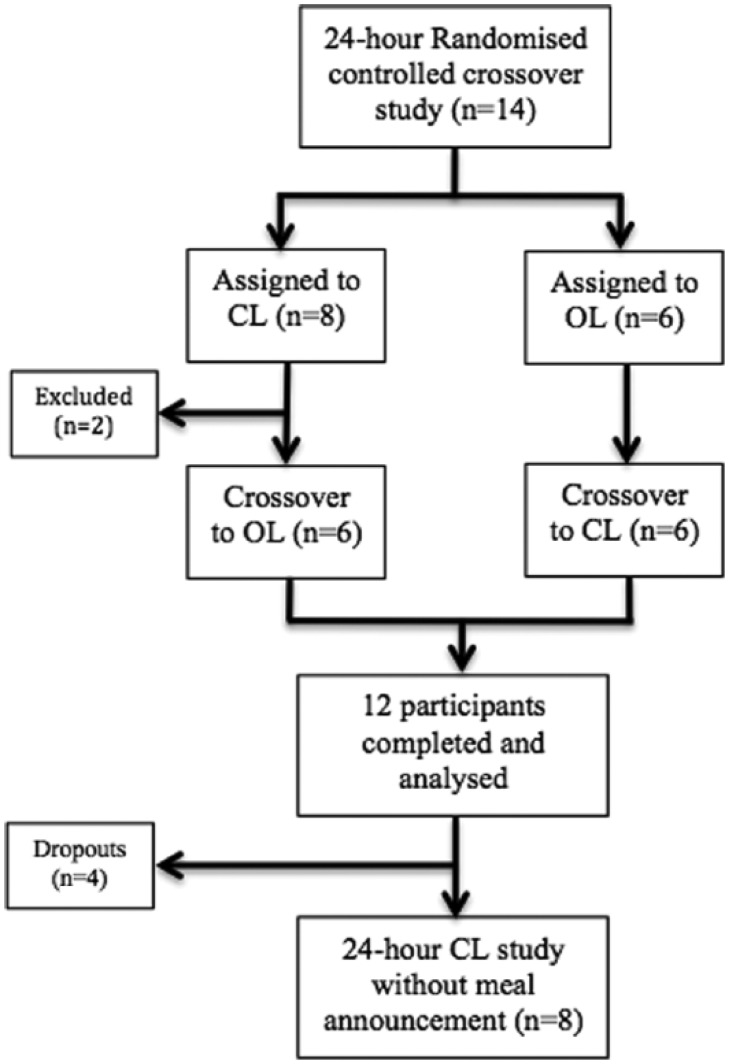
Fourteen participants were screened and included in the 24-hour randomized controlled crossover study of which 2 participants were unable to complete both study visits (recurrent venous cannula failures in 1 participant and illness unrelated to diabetes in another) and were excluded from the analysis (Figure 1). Table 1 outlines the baseline characteristics for the 12 participants included. Eight of these participants agreed to participate in the closed-loop visit without and with partial meal announcement.
Figure 1.
Flowchart of trial design. The overall trial design of the 24-hour randomized controlled crossover closed-loop study, followed by the substudy evaluating the BiAP algorithm without and with partial meal announcement assessment as outlined in this article.
Table 1.
Demographics and Baseline Characteristics (n = 12).
| Characteristic | Results, mean (SD) |
|---|---|
| Age (years) | 45 (10) |
| Sex | Male 42%, female 58% |
| BMI (kg/m2) | 25 (4) |
| Duration of diabetes (years) | 22 (12) |
| Duration of insulin pump therapy (years) | 3.4 (4) |
| Insulin requirements (units/kg) | 0.5 (0.1) |
| HbA1c (%) | 7.4 (0.7) |
| mmol/l | 58 (8) |
Outcomes From the 24-Hour Study Comparing Closed-Loop With Open-Loop (18:00 Hours-18:00 Hours)
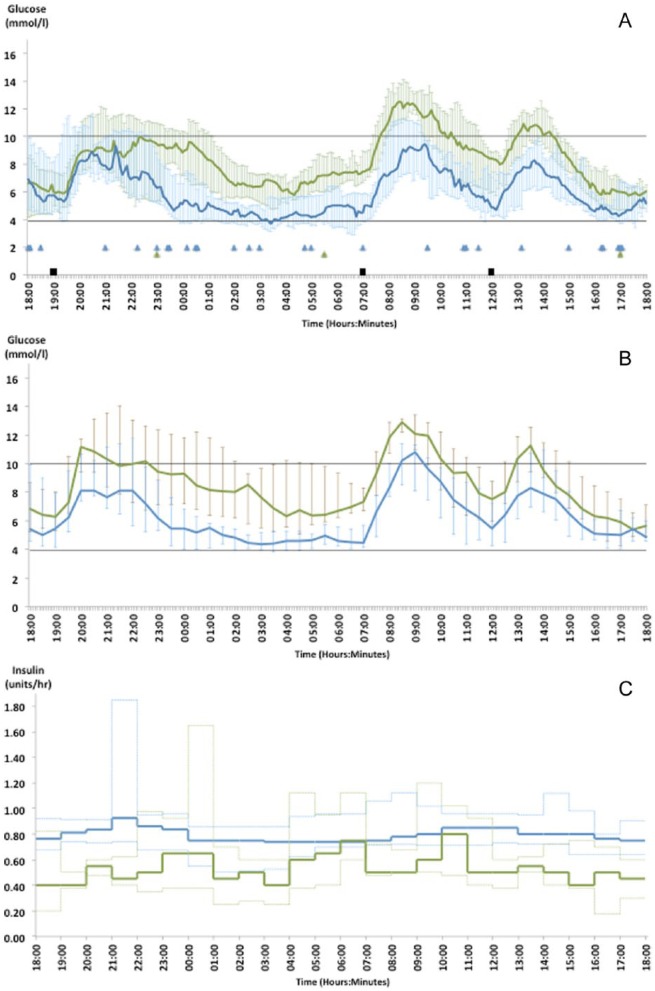
There was no significant difference in time spent in glucose target (3.9-10 mmol/l) between closed-loop and open-loop, 71% vs 66.9% respectively (P = .9). The median sensor glucose, blood glucose and insulin delivered during closed-loop and open-loop for all 12 participants throughout the 24-hour study is displayed in Figure 2. More insulin was delivered over 24 hours of open-loop compared to closed-loop (38.0 [5.7] vs 32.2 [6.4] units, P < .01), however no difference was observed over night (5.2 [1.6] vs 5.2 [2.2], P = .9). Similarly the mean rate of basal insulin delivery (defined as insulin delivered per hour excluding the meal boluses) was higher over 24 hours of open-loop (0.9 [0.2] units/hour vs 0.7 [0.3] units/hour, P = .01) and again no difference was found over night (0.7 [0.2] units/hour vs 0.7 [0.3] units/hour, P = .9). Circulating insulin concentrations were higher in the open-loop group compared to closed-loop (26.57 [12.5] mU/l vs 23.08 [10.17] mU/l, P = .05). The median (IQR) meal boluses given during closed-loop and open-loop were 8.0 (7.1-8.1) units vs 8.0 (7.6-8.8) units at 19:00 hours, 4.0 (3.6-4.2) units vs 3.8 (3.4-4.3) units at 07:00 hours, and 5.0 (4.5-5.1) units vs 5.0 (4.7-6.3) units at 12:00 hours.
Figure 2.
Median (IQR) sensor glucose (A), median (IQR) blood glucose (B), and median (IQR) insulin (excluding the meal boluses) (C) delivered for all 12 subjects during the 24-hour closed-loop (green line) and open-loop (blue line) study. Standardized meals (black squares) were provided at 19:00 hours (80 g CHO), 07:00 hours (40 g CHO), and 12:00 hours (50 g CHO). Triangles denote episodes of rescue CHO (10-15 g CHO) for hypoglycemia.
Glycemic outcome measures based on sensor and blood glucose from the full 24-hour study are outlined in Table 2. Although open-loop achieved a lower mean glucose compared to closed-loop (8.4 [1.1] versus 6.4 [0.8] mmol/l, P < .01), this was at the expense of significantly increased % time spent in hypoglycemia (<3.9 mmol/l) with open-loop (17.9% vs 3.0%, P < .01). The difference in % time spent in hypoglycemia for each individual participant over 24-hours and overnight is illustrated in the supplement (Supplementary Figure 2). With <3.5 mmol/l or <3.3 mmol/l as cutoff points for hypoglycemia, the significant reduction in hypoglycemia with closed-loop remains. Using an even lower hypoglycemia cutoff point (<2.8 mmol/l), hypoglycemia was completely eliminated with closed-loop whereas a small proportion (1.7%) of time was spent at <2.8 mmol/l with open-loop. The risk of hypoglycemia, as measured by LBGI was much lower in the closed-loop group (3.0 [0.9-4.9] vs 5.9 [3.8-10.8], P = .01). Rescue carbohydrates for hypoglycemia were required on 28 occasions overall (2.3 [2.5] times/24 hours) during open-loop compared to 3 occasions (0.3 [0.5] times/24 hours) during closed-loop. In addition to the meal boluses, 13 correction boluses were taken during open-loop (1.1 [1.0] correction bolus/24 hours). The correction bolus doses ranged from 0.05 units to 3.4 units. An increase in time spent in hyperglycemia (>10 mmol/l) was seen with closed-loop (10% vs 28.9%, P = .01), mostly attributed to the postprandial glucose excursions seen, particularly following the evening meal.
Table 2.
Glycemic Outcome Measures of Closed-Loop Versus Open-Loop Using Sensor and Venous Blood Glucose (n = 12).
| Time 18:00-18:00 (next day) (total of 24 hours) | ||||||
|---|---|---|---|---|---|---|
| Glycemic outcome measure | Closed-loop (n = 12) sensor glucose | Open-loop (n = 12) sensor glucose | P value | Closed-loop (n = 12) blood glucose | Open-loop (n = 12) blood glucose | P value |
| % time in target range, 3.9-10.0 mmol/l | 71 (61.0 -73.8) | 66.9 (55.4-82.5) | .9 | 68.4 (50.6-74.5) | 76.5 (70.2 - 79.4) | .05 |
| % time in euglycemia, 3.9-7.8 mmol/l | 41.9 (27.0-59.5) | 60.4 (40.2- 65.5) | .1 | 40.8 (25.8 - 51.0) | 60.2 (46.9 - 73.5) | .02 |
| % time in hypoglycemia, <2.8 mmol/l | 0.0 (0.0-0.0) | 1.7 (0.0-10.2) | .02 | 0.0 (0.0 - 0.0) | 0.0 (0.0 - 1.0) | .08 |
| % time in hypoglycemia, <3.3 mmol/l | 0.0 (0.0-1.9) | 4.4 (0.0-14.8) | .02 | 0.0 (0.0 - 0.0) | 2.0 (1.0 - 2.1) | .01 |
| % time in hypoglycemia, <3.5 mmol/l | 0.0(0.0-4.0) | 6.6(1.7-18.1) | .01 | 0.0 (0.0 - 0.1) | 4.0 (1.0 - 6.1) | .01 |
| % time in hypoglycemia, <3.9 mmol/l | 3.0 (0.0-7.0) | 17.9 (8.4-33.8) | <.01 | 0.0 (0.0 - 2.1) | 12.2 (3.1 - 18.4) | .01 |
| % time in hyperglycemia, >10 mmol/l | 28.9 (23.6 - 36.8) | 10.1 (7.7-15.1) | .01 | 31.6 (23.5-49.4) | 12.5 (6.2-17.3) | .01 |
| % time in hyperglycemia, >15 mmol/l | 1.4 (0.0-5.0) | 0.0 (0.0-1.2) | .3 | 0.0 (0.0 - 0.0) | 0.0 (0.0 - 0.0) | .95 |
| Glucose (mmol/l) | 8.4 (1.1) | 6.4 (0.8) | <.01 | 8.8 (1.2) | 6.6 (0.9) | <.01 |
| Low blood glucose index (0.0-6.9) | 3.0 (0.9-4.9) | 5.7 (3.8- 10.8) | .01 | 1.4 (0.4-2.6) | 4.4 (3.0-5.8) | <.01 |
| High blood glucose index (0.0-7.7) | 6.9 (6.1-9.4) | 5.0 (3.2-9.2) | .27 | 7.3 (5.5-11.4) | 4.8 (3.3-6.8) | .01 |
| No of CHO rescue in total | 3 | 28 | <.01 | 3 | 27 | .01 |
| Time 00:00-07:00 (total of 7 hours overnight) | ||||||
| % time in target range, 3.9-10.0 mmol/l | 82.7 (69.0-100) | 79.2 (29.0- 85.7) | .1 | 75.0 (52.8-100.0) | 78.6 (60.2-100) | .84 |
| % time in euglycemia, 3.9-7.8 mmol/l | 59.5 (47.0-90.2) | 66.7 (29.0-78.3) | .4 | 46.4 (28.6-98.2) | 78.6 (54.7-100.0) | .07 |
| % time in hypoglycemia, <2.8 mmol/l | 0.0 (0.0-0.0) | 0.0 (0.0-4.5) | .08 | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | .32 |
| % time in hypoglycemia, <3.3 mmol/l | 0.0 (0.0-0.0) | 0.0 (0.0-19.6) | .03 | 0.0 (0.0-0.0) | 0.0 (0.0-7.1) | .05 |
| % time in hypoglycemia, <3.5 mmol/l | 0.0 (0.0-0.0) | 4.2 (0.3-31.0) | <.01 | 0.0 (0.0-0.0) | 3.6 (0.0-7.6) | .02 |
| % time in hypoglycemia, <3.9 mmol/l | 0.0 (0.0 - 0.0) | 20.8 (14.3-71.0) | <.01 | 0.0 (0.0-0.0) | 21.4 (0.0-39.8) | .01 |
| % time in hyperglycemia, >10 mmol/l | 6.5 (0.0-31.0) | 0.0 (0.0-0.0) | .01 | 25.0 (0.0-48.2) | 0.0 (0.0-0.0) | .01 |
| % time in hyperglycemia, >15 mmol/l | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | .3 | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | — |
| Glucose (mmol/l) | 7.5 (1.3) | 4.8 (1.5) | <.01 | 8.1 (1.9) | 5.0 (0.9) | <.01 |
| Low blood glucose index (0.0-6.9) | 0.8 (0.1-1.8) | 5.8 (4.2- 9.4) | <.01 | 0.1 (0.0-1.7) | 5.2 (1.9-7.0) | <.01 |
| High blood glucose index (0.0-7.7) | 2.8 (1.0- 6.9) | 0.1 (0.0-2.0) | .01 | 5.1 (0.5-9.2) | 0.1 (0.0-1.0) | <.01 |
Outcomes are expressed as median (IQR) unless stated otherwise.
An overview of the median (IQR) of insulin concen-tration, lactate concentration, NEFA concentration and 3-hydroxybutyrate concentration throughout the 24-hour study is displayed in the supplement (Supplementary Figure 3). There was no significant difference between mean concentration of lactate (0.60 [0.09] mmol/l vs 0.62 [0.12] mmol/l and NEFA (0.30 [0.11] mmol/l vs 0.28 [0.1] mmol/l) between closed-loop and open-loop. The mean concentration of 3-hydroxybutyrate was higher with closed-loop (0.14 [0.07] mmol/l vs 0.09 [0.06] mmol/l, P = .03). There were significant intercorrelations between glucose, lactate, 3-hydroxybutyrate, NEFA, and insulin levels for both open- and closed-loop studies (Supplementary Table 1). The strongest positive correlations were between 3-hydroxybutyrate and NEFA (r = .7, P < .01), but there were no consistent differences in strengths of association between open- and closed-loop. NEFA and 3-hydroxybutyrate were suppressed postmeals.
Outcomes From the Overnight Time Period Comparing Closed-Loop With Open-Loop (00:00 Hours-07:00 Hours)
The percentage time in target (3.9-10 mmol/l) overnight did not significantly differ between closed-loop and open-loop (82.7% vs 79.2% respectively, P = .1). However there was a significant reduction in hypoglycemia (<3.9 mmol/l) with closed-loop (20.8% vs 0.0%, P < .01). There was a small increase in percentage time spent in hyperglycemia with closed-loop compared to open-loop, but severe hyperglycemia was not observed in either group. Mean glucose with open-loop (4.8 mmol/l) is significantly lower than with closed-loop (7.5 mmol/l), in accord with the increased time spent in hypoglycemia during open-loop. Glycemic outcome measures from the overnight study period are outlined in the latter section of Table 2.
Sensor Accuracy
The sensor was calibrated at the beginning of each study and was recalibrated if the discrepancy between sensor glucose and blood glucose was >30% over 1 hour or as determined by the supervising physician. A total of 0.8 recalibrations/24 hours were required during closed-loop and 1.0 recalibration/24 hours during open-loop. Overall 0.9 recalibrations/24 hours were carried out, consistent with the manufacturer’s guidance. The MARD between the venous blood glucose and the sensor glucose was 11.0% (7.0-26.0) and 15.0% (5.0-20.0) during closed-loop and open–loop respectively with an overall MARD of 12.6% (5.7-23.6).
Insulin Delivery Interruptions and Missing Data Points
During the 24-hour randomized controlled crossover study closed-loop insulin delivery was interrupted on 3 occasions overall (0.25 times per 24-hour closed-loop visit) after study start due to technical fault with the BiAP handheld device. Each interruption lasted for 20 minutes and was resolved by resetting the BiAP. We experienced 1 sensor failure during closed-loop, where the study was completed using the second sensor without any interruptions to the insulin delivery. There were no interruptions to open-loop insulin delivery. During open-loop we experienced 1 sensor failure and 2 technical faults with BiAP unit resulting in some gaps in the continuous monitoring of interstitial glucose.
Overall 0.9% of the closed-loop sensor glucose data and 3.7% of open-loop sensor glucose data were missing from the study period as a result of the above mentioned technical faults.
Closed-Loop Without and With Partial Meal Announcement
The median (IQR) sensor glucose during closed-loop without and with partial meal announcement, closed-loop (with meal announcement) and open-loop for the 8 participants who completed all three 24-hour study visits is displayed in the supplement (Supplementary Figure 4). The percentage time in target was higher when all meals were announced during closed-loop compared to no or partial meal announcement (65.7% [53.6-80.5] vs 45.5% [38.2-68.3], P = .12), but this was not statistically significant. The time spent in hypoglycemia (<3.9 mmol/l) was lower with closed-loop without meal announcement compared to closed-loop (0.4% [0.0-1.3] vs 5.8% [0.8-8.0], P = .03). The mean glucose was 9.8 mmol/l (1.3) with closed-loop without and with partial meal announcement compared to 8.4 (1.3) closed-loop with meal announcement (P = .06). Additional glycemic outcomes comparing closed-loop without and with partial meal announcement and closed-loop with meal announcement are summarized in the supplement (Supplementary Table 2).
Discussion
The results from this study demonstrate that the BiAP system significantly reduces time spent in hypoglycemia. It should be noted that this study was performed in a cohort of people with well-controlled type 1 diabetes (HbA1c 7.4%) and a high observed time spent in hypoglycemia in the open-loop arm. This may be explained, in part, by exposure to accurate CHO counting during the study leading to higher insulin doses, the impact of observation on participants, and differences in daily routine. Of nocturnal sensor glucose values, 10% were below 70 mg/dL in the control group of the ASPIRE study,5 compared with 20.8% in our study, emphasizing the challenges of short periods of open loop control in an unfamiliar environment. However, even when compared with a more pragmatic real-world frequency of nocturnal hypoglycemia the presented data represent an improvement.
The reduction in hypoglycemia during closed-loop was most prominent overnight when hypoglycemia poses the highest risk, as hypoglycemic awareness is reduced while sleeping.26 An increased time in hyperglycemia (>10 mmol/l) was observed with closed-loop compared to open-loop and one could argue that the reduction in hypoglycemia seen was at the expense of hyperglycemia resulting from less insulin delivered during closed-loop. However, insulin delivered overnight did not differ between the groups.
Data from the initial hours of closed-loop control demonstrate that the algorithm was able to safely control the glucose without knowledge of insulin on board, an important safety consideration, while the prolonged postprandial hyperglycemia seen after the evening meal is explained by some participants starting the study with lower glucose and minimal insulin being delivered over the first hours of the study. This resulted in low circulating insulin concentrations (to prevent imminent hypoglycemia) prior to challenging the controller with a meal.
This is the first time ketone bodies and lactate have been investigated in a closed-loop study. Previous measurement of NEFA6 showed, similarly to our study, suppression after meals. Although, the mean concentrations of lactate and NEFAs did not differ between open-loop and closed-loop the relationships between the analytes may prove useful in future as an additional input to AP systems. Circulating 3-hydroxybutyrate was higher with closed-loop compared to open-loop. However this difference is unlikely to be clinically significant at such low concentration levels.
At present, technology to measure analytes beyond interstitial glucose in the home setting is unavailable. Manual input of capillary blood glucose values is currently used for sensor calibration at least twice daily though newer sensors are capable of calibration free operation.27 Multiarray micro-needle sensors capable of multiple analyte sensing are in development,28 but the benefits of intermediary metabolites as an input to the closed-loop algorithm needs further investigation.
CGM technology senses glucose in interstitial fluid where glucose changes lag behind blood by around 5 to 7 minutes,29 while rapid acting insulin analogues delivered subcutaneously do not exert their peak effect for 60 minutes.30 These delays pose a challenge to AP systems around mealtimes and a meal announcement strategy, with meals announced to the controller, minimizes initial prandial hyperglycemia and delayed postprandial hypoglycemia. Without meal announcement a higher and longer hyperglycemic excursion postevening meal was observed compared to a meal announcement strategy. Reassuringly no delayed hypoglycemic episodes occurred after the unannounced meal. As expected, improved glycemic outcomes are achieved with meal announcement, an approach also adopted by other closed-loop groups.14,19 However, we have shown that the BiAP remains safe in the event that a meal is unannounced or the carbohydrate content is underestimated.
A major advantage of the BiAP system is the implementation of the bio-inspired algorithm in a microchip within a miniaturized low power device. With power consumption of under 2 mW guaranteeing 2 days of use, the BiAP system also has the potential to overcome the limited battery life issue seen with other platforms such as smartphones. The bio–inspired approach replicating the biphasic nature of insulin secretion by the beta cell may have additional advantages when used with intraperitoneal insulin or novel ultra-rapid insulins. Our study is limited by the small sample size, relatively short duration and that it took place in a controlled clinical environment. Participants were not randomized for the substudy evaluating the safety of the BiAP algorithm without and with partial meal announcement, and the bias of participant selection is a limitation of this part of the study. The blinding of the sensor glucose to participants during open-loop may be considered a limitation of the study since the state of the art in current care is sensor augmented pump therapy. However, sensor augmented pump therapy has not yet been widely adopted by people with type 1 diabetes in the UK as CGM currently requires self-funding. Work in progress includes assessing the BiAP system with glucagon, during exercise and mixed meals, followed by longer duration home studies, including hybrid studies of overnight closed-loop and daytime open-loop control.
Conclusions
We conclude that the BiAP system is safe and reduces hypoglycemia compared to standard pump therapy in a clinically controlled environment. The reduction in hypoglycemia was at the expense of an increase in time spent in hyperglycemia, which could be counteracted by using a less conservative approach in tuning the algorithm.
Supplementary Material
Acknowledgments
The authors wish to thank Dr Rochan Agha-Jaffar, Dr Shivani Misra, Miss Maria Xenou, MSc, Dr Ahmed El-Laboudi, and Miss Joanna Zaw-Linn (Division of Diabetes, Endocrinology and Metabolism, Imperial College London) for their contribution toward the clinical studies and most importantly all the study participants for their valuable time and commitment.
Footnotes
Abbreviations: AP, artificial pancreas; BiAP, Bio-inspired Artificial Pancreas; CGM, continuous glucose monitoring; CHO, carbohydrate; CSII, continuous subcutaneous insulin infusion; FL, fuzzy logic; HBGI, high blood glucose index; IQR, interquartile range; LBGI, low blood glucose index; MARD, median absolute relative difference; MDI, multiple daily injections; MPC, model predictive control; NEFA, nonesterified fatty acid; PID, proportional integral derivative.
Authors’ Note: An abstract outlining the results from this study was presented in an oral session (Abstract id ATTD-0377) at the Advanced Technology and Therapeutics in Diabetes (ATTD) Conference in Paris, February 18-21, 2015. The online data supplements is available at http://dst.sagepub.com/supplemental.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Wellcome Trust. The funding body was not involved in the design of the study, collection of data, data analysis, preparation of the manuscript or publication decisions. This article presents independent research funded by the Wellcome Trust and carried out at the NIHR/Wellcome Trust Imperial Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the Wellcome Trust, NHS, the NIHR or the Department of Health. Imperial College London is supported by the NIHR Diabetes Research Network and the Imperial NIHR Biomedical Research Centre.
References
- 1. Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus. July 2008. NICE technology appraisal guidance 151. Available at: http://www.nice.org.uk/guidance/ta151.
- 2. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 3. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Bode B, Beck RW, et al. Sustained benefit of continuous glucose monitoring on A1C, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Diabetes Care. 2009;32:2047-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;22;363:311-320. [DOI] [PubMed] [Google Scholar]
- 5. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369:224-232. [DOI] [PubMed] [Google Scholar]
- 6. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344-3350. [DOI] [PubMed] [Google Scholar]
- 7. Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ. 2011;342:doi: 10.1136/bmj.d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375:743-751. [DOI] [PubMed] [Google Scholar]
- 9. Dassau E, Zisser H, Harvey RA, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36:801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycaemia. Diabetes. 2012. 61:2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33:1072-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haidar A, Legault L, Messier V, Maria Mitre T, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3:17-26. [DOI] [PubMed] [Google Scholar]
- 13. Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for prevention of hypoglycaemia in type 1 diabetes. Diabetes Care. 2010;33:1072-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368:824-833. [DOI] [PubMed] [Google Scholar]
- 16. Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37:1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ly TT, Breton MD, Keith-Hynes P, et al. Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care. 2014;37:2310-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nimri R, Muller I, Atlas E, et al. MD-logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37:3025-3032. [DOI] [PubMed] [Google Scholar]
- 19. Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931-1937. [DOI] [PubMed] [Google Scholar]
- 20. Thabit H, Lubina-Solomon A, Stadler M, et al. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol. 2014;2:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herrero P, Georgiou P, Oliver N, Johnston D, Toumazou C. A bio-inspired glucose controller based on pancreatic β-cell physiology. J Diabetes Sci Technol. 2012;6:606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pedersen MG, Toffolo GM, Cobelli C. Cellular modeling: insight into minimal models of insulin secretion. Am J Physiol Endocrinol Metab. 2010;298:597-601. [DOI] [PubMed] [Google Scholar]
- 23. Georgiou P, Toumazou C. A silicon pancreatic beta cell for diabetes. IEEE Trans Biomed Circuits Systems. 2009;1:39-49. [DOI] [PubMed] [Google Scholar]
- 24. Reddy M, Herrero R, El Sharkawy M, et al. Feasibility study of a Bio-inspired Artificial Pancreas in adults with type 1 diabetes. Diabetes Technol Ther. 2014;16:10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13:921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52:1195-1203. [DOI] [PubMed] [Google Scholar]
- 27. Hoss U, Budiman ES, Liu H, Christiansen MP. Feasibility of factory calibration for subcutaneous glucose sensors in subjects with diabetes. J Diabetes Sci Technol. 2014;8:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El-Laboudi A, Oliver NS, Cass A, Johnston D. Use of microneedle array devices for continuous glucose monitoring: a review. Diabetes Technol Ther. 2013;15:101-115. [DOI] [PubMed] [Google Scholar]
- 29. Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes. 2013;62:4083-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Home PD. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes Metab. 2012;14:780-778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.