Abstract
Complications of diabetes mellitus, namely diabetic retinopathy and diabetic maculopathy, are the leading cause of blindness in working aged people. Sufferers can avoid blindness if identified early via retinal imaging. Systematic screening of the diabetic population has been shown to greatly reduce the prevalence and incidence of blindness within the population. Many national screening programs have digital fundus photography as their basis. In the past 5 years several techniques and adapters have been developed that allow digital fundus photography to be performed using smartphones. We review recent progress in smartphone-based fundus imaging and discuss its potential for integration into national systematic diabetic retinopathy screening programs. Some systems have produced promising initial results with respect to their agreement with reference standards. However further multisite trialling of such systems’ use within implementable screening workflows is required if an evidence base strong enough to affect policy change is to be established. If this were to occur national diabetic retinopathy screening would, for the first time, become possible in low- and middle-income settings where cost and availability of trained eye care personnel are currently key barriers to implementation. As diabetes prevalence and incidence is increasing sharply in these settings, the impact on global blindness could be profound.
Keywords: diabetic retinopathy, fundoscopy, mHealth, ophthalmoscopy, smartphone, telemedicine
Diabetic retinopathy (DR) and diabetic maculopathy (DMac) are the leading causes of blindness in high-income settings for those aged between 20 and 74 years.1 As the most common microvascular complications of diabetes mellitus (DM), they are also increasingly becoming a major cause of blindness in low- and middle-income countries (LMICs) as DM prevalence and incidence in these settings have risen sharply in recent years.2
Although the development of sight-threatening complications of DM can be delayed by appropriate treatment of systemic diseases such as DM itself, high blood pressure and lipid metabolism abnormalities,3 nearly all type 1 DM patients and 60% type 2 patients develop DR or DMac.4 If the disease progresses to a stage where direct intervention is necessary, laser treatment or intravitreal injection of steroid or antivascular endothelial growth factor (anti-VEGF) agents are often successful in preserving vision.4 In each case, early diagnosis is crucial to the success of the treatment.5
Diagnosis of DR and DMac is commonly achieved by imaging the fundus either by retinal photography, by direct or indirect ophthalmoscopy, or by slit lamp biomicroscopy.6 Given the criticality of early diagnosis, opportunistic diagnosis of DR during routine eye examinations is insufficient. Consequently, many countries have adopted systematic screening programs within their DM populations to reduce the numbers of people developing blinding disease.7-11
Development of the Diabetic Retinopathy Screening Workflow
In 1980 Iceland became the first country to introduce nationwide, systematic retinal screening amongst patients with DM.7 Approximately 90% of the country’s several hundred insulin-treated diabetic patients were examined on an annual or biennial basis, at Iceland’s single diabetes clinic.12 During each patient visit eye and general health histories were first reviewed, followed by visual acuity measurements. An examination of the posterior segment would then be conducted by an ophthalmologist specializing in retina with the posterior pole being examined by slit lamp biomicroscopy and the peripheral retina being examined by indirect ophthalmoscopy. Color photographs of the fundus would also be taken during each visit. Macular laser treatment or panretinal photocoagulation would then be performed as appropriate.13 The result of this program has been the reduction of the prevalence of blindness within the diabetic population from 2.4% to 0.5%.14
In the United Kingdom, every DM patient over the age of 12 is offered an annual retinal examination. As the UK has a diabetic population of close to 2 million people,8 a different screening approach to that of the Icelandic model has been adopted. During each patient visit a medical history is taken (although this is not used for referral decisions), visual acuity is assessed and 45° field digital images of each fundus are photographed under dilation.15 The fundus images are then forwarded to a grading center for grading by an experienced grader holding the appropriate vocational qualification.16 All images graded abnormal and 10% of those graded normal are independently graded by a second grader. Only if the graders disagree is the image subsequently forwarded to an ophthalmologist specializing in retina. Patients who are found to have no visible maculopathy and either no visible or only background retinopathy are asked to return the following year for rescreening while unclassifiable patients and those with other grades of disease are referred to an eye clinic.15 In contrast to the Icelandic model, this workflow does not require each patient to be examined by an ophthalmologist nor does it involve biomicroscopy or indirect ophthalmoscopy during the initial screening stage. The model therefore more readily lends itself to the deployment of non-hospital-based clinics, such as mobile clinics or eye screening clinics based within primary care centers. These have been shown to increase screening effectiveness in rural and remote settings as well as being a more cost-effective means of detecting DR compared to classical techniques.17 Predominantly as a result of nationwide screening, DR is no longer the leading cause of blindness amongst working-age adults in the UK, having been so for at least 50 years.18 Similar screening workflows have been adopted in several other European nations also keen to reduce DR-related blindness.9-11
In addition, automated screening algorithms have begun to be incorporated into the national DR screening program in Scotland, UK.19 These offer the potential to screen out the bulk of healthy, and most time consuming to assess, images before a human retinal screener needs to be involved.20 This requires a workflow where digital fundus photography is used during the first stage of screening.
It should be noted that although the majority of diabetes suffers live in LMICs, where nationwide DR screening and care has thus far not been effectively implemented, with only rudimentary detection and management existing in many countries.21 Barriers to effective DR care implementation include: few ophthalmologists trained in DR management, lack of fundoscopy training for eye health workers including opticians and ophthalmic clinical officers, poorly functioning referral systems from primary to secondary care, little access to imaging technology, lack of treatment infrastructure such as properly maintained lasers, and a lack of relevant national policies.21
Diabetic Retinopathy Screening Requirements for Digital Photography
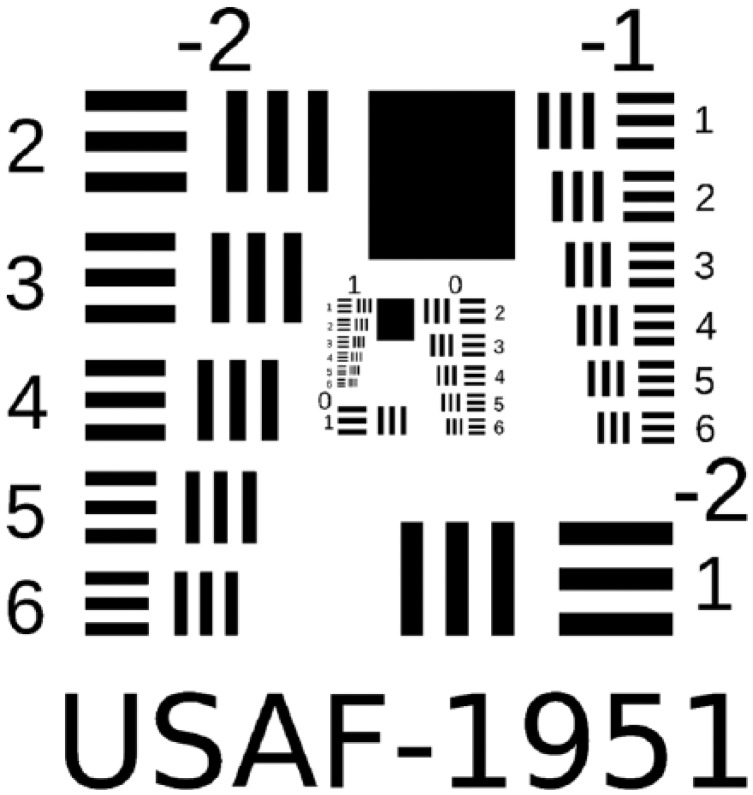
The advancement of DR grading using digital fundus images has been reviewed in detail elsewhere.22 Modern digital camera sensors have exceeded the resolution of traditional 35-mm film23 and digital fundus cameras have superseded their film predecessors. However, the quality of the image formed on the detector is also affected by aberrations within the camera optics, distortion, field curvature and is ultimately limited by diffraction. It is such factors that limit the image quality of modern digital cameras rather than the sensor resolution (pixel count—“megapixels”), which is nevertheless frequently provided as a sole measure of digital camera quality by manufacturers and in some peer-reviewed literature. Appropriate assessment of a fundus camera’s optical quality is achieved by using a specially designed test target (USAF 1951 resolution test chart, shown in Figure 1) to determine its resolving power, measured in line pairs per mm (lp/mm). This involves finding the minimum resolvable separation of a set of 3 parallel black lines, with a width equal to their separation, on a white background.24 The current international standard for fundus cameras specifies a lower limit of 80 lp/mm in the image center to 40 lp/mm at the periphery for a field of view (FoV) of 30° or less and 60 lp/mm to 25 lp/mm for a FoV greater than 30°.25
Figure 1.
USAF 1951 resolution test chart (not to scale). The resolving power of an optical imaging system is found according to the group and element of the smallest 3-line pattern that can be resolved, according to the equation: . For example the bottom-left target corresponds to 0.445 lp/mm and the top-right target corresponds to 0.500 lp/mm. Reproduced with permission from Buam.48
The continued increase in the quality of smartphones’ embedded cameras is therefore of interest, with recent handsets capturing images with quality comparable to those of compact digital cameras. Today’s handsets can also record high-quality videos at 1080p (1920 × 1080 pixels per frame) high-definition resolution, and even 4K (4096 × 2160 pixels per frame) ultra-high-definition resolution, in the case of some high-end devices.
However, as noted above, image quality relies on a host of parameters besides sensor pixel count. The engineering challenges in building quality camera optics within a mobile phone are substantial. The requirement to be low-cost and capture a wide variety of scenes necessitates plastic lenses suitable for mass production and an aperture with a fixed diameter. Also the whole camera module needs to be thin, limiting the allowed space between optical components. Finally, as with any digital camera, how the image is encoded and compressed after acquisition can also degrade the image quality.
Nevertheless, in recent years the advancement of smartphone imaging technology has been such that fundoscopy systems have begun to emerge using smartphone cameras as the imaging component.
Smartphone Prevalence
The introduction of smartphones has had a profound impact on mobile connectivity, combining the simple voice and text communication capabilities of their predecessors with more powerful computer processing, an operating system allowing application installation and upgrade, global positioning systems, high-resolution cameras, and a variety of other sensors such as accelerometers and fingerprint scanners.26 Such is the appeal of this technologically rich resource that in 2013 it was reported that 65% of US adults owned a smartphone.27 Medical professionals are certainly no exception with it being reported as early as 2010 that 80% of medical doctors in the UK owned a smartphone.28 Furthermore, the phenomenon is not confined to high-income settings. For example it was reported in 2014 that in China there is 95% mobile phone ownership and 37% smartphone ownership while in Kenya 82% own mobile phones with 1 in 4 of these being a smartphone.29
Using such a universally available consumer device as the basis of fundus imaging systems may offer a means of removing many of the aforementioned barriers to timely detection and treatment of DR where nationwide screening does not currently exist and significantly lower costs where it does. For this to be realized, the technology must be of sufficient quality and also be capable of integration into the DR workflow. In this article we review recent progress in smartphone-based fundus imaging and discuss its potential for integration into national systematic DR screening programs.
Review of Smartphone Retinal Imaging Technology
Smartphone Slit Lamp Adapters
The simplest means of introducing the imaging capabilities of smartphones into retinal imaging workflows is to simply “bolt-on” handsets to existing retinal imaging equipment. Barsam et al have shown that it is possible to capture good quality anterior segment images by manually aligning the optic of a smartphone (iPhone 3G, Apple Inc, Cupertino, CA, USA) with either eyepiece of a biomicroscope slit lamp (BM 900, Haag-Streit, Köniz, Switzerland).30 Although the authors reported only anterior segment images, it was later shown by Gurram that it is possible to capture retinal images by inserting a 90D condensing lens between the patient’s eye and the biomicroscope and aligning the light source with the optical axis.31
Monocular Indirect Ophthalmoscopy
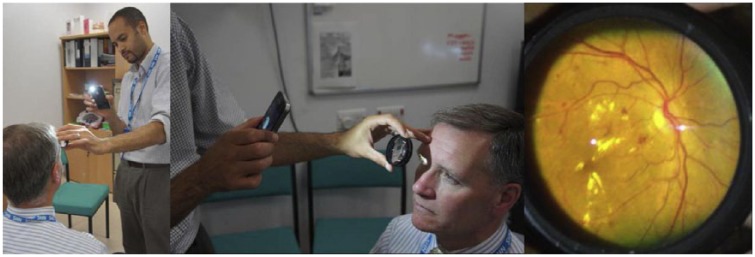
In 2010 Lord et al reported a simple method for capturing retinal images similar to classical indirect ophthalmoscopy.32 This involved holding a 20D lens in-front of the patient’s eye and holding a pen torch and smartphone (iPhone, Apple Inc) at a distance using the other hand. They found that the smartphone camera would then autofocus onto the retinal image formed by the lens allowing a digital image to be captured using the phone’s stock camera application. As smartphone technology matured and integrated LED flashes became commonplace, the smartphone could itself deliver the necessary coaxial retinal illumination, dispensing with the need to balance a torch alongside the phone, a technique coined “smartphone fundoscopy” and shown in Figure 2.33 Although this reduced the complexity of the procedure, finding and holding the lens at the correct distance from the eye nevertheless requires a level of skill generally only exhibited by ophthalmologists or trained eye-care personnel.
Figure 2.
Imaging the fundus can be achieved by positioning a 20D lens between the eye and the smartphone optic (left and center). Although diabetic macular edema, for example, can be imaged using this technique (right), it has been reported that insufficient sensitivity is achieved when the technique is integrated into the screening workflow.34 Left and center panel reproduced with permission from Bastawrous33; right panel reproduced with permission from Ryan et al.34
Ryan et al compared using this technique with an iPhone 5 (Apple Inc) to standard 3-field nonmydriatic and 7-field mydriatic retinal photography on 300 pharmacologically dilated diabetic patients.34 The authors found the sensitivity of the smartphone images to be 81% and 50% compared to each standard respectively and the specificity to be 94% against each standard. They therefore concluded that 20D lens-assisted smartphone photography lacks sufficient sensitivity for detection of DR.
Myung et al used 3D printing technology to simplify the above procedure. The authors designed the plastic arm shown in Figure 3 which holds the lens at a fixed distance from the camera, allowing the entire system to be held and moved as a single unit.35 Hong reported a similar, publically available design allowing anyone with access to a 3D printer to build the system.36 However the distance between the lens and the eye is not fixed and therefore a degree of skill is still required to form the retinal image.
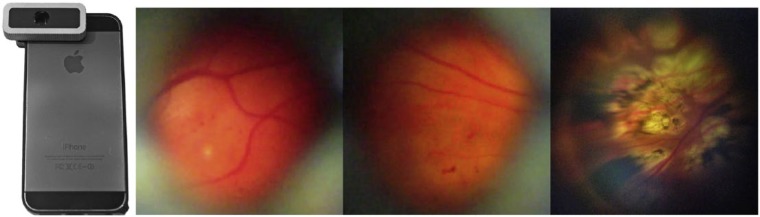
Figure 3.
The 3D printed smartphone retinal adapter for monocular indirect ophthalmoscopy developed by Myung et al35 on an iPhone 5 (Apple Inc) (left). Diabetic macular edema can be photographed using this lens-to-phone mount (right). Reproduced with permission from Myung et al.35
Ophthalmoscope manufacturer Welch Allyn Inc (Skaneateles Falls, NY, USA) has released a commercially available means of acquiring smartphone images. The iExaminer adapter for the PanOptic allows retinal images with a 25° subtended angle to be captured using an iPhone 4 or 4S (Apple Inc) while keeping all optical dimensions fixed.37 Although this is the only such device to achieve US Food and Drug Administration approval to date, the system, excluding smartphone, retails at over US$1000 and has not been updated for current handset models, meaning it is now out of date with respect to phone models actively on the market.38
Direct Ophthalmoscopy
Two adapters that allow smartphones to capture retinal images through direct ophthalmoscopy have since been the developed. Peek Retina uses a prism to closely align a light source with the optical axis of the smartphone camera39 and D-Eye (D-Eye Srl, Padova, Italy) inserts a beam splitter into the optical path of the camera optics to provide coaxial illumination of the eye using the LED flash.40
The inventor of D-Eye, shown in Figure 4, and colleagues have reported results from the examination of 240 eyes in 120 out-patients with either type I or type II DM at an ophthalmic diabetic center (Spedali Civili di Brescia, Italy).41 They reported a sensitivity of .90 (95% CI .82-.94) and a specificity of .96 (95% CI .90-.98) for detection of DR by a retinal specialist when compared to biomicroscopy by a retinal specialist. For grading of DR a simple κ of .78 (95% CI .71-.84; P < .001) was reported and 3.75% of eyes were ungradable with D-Eye compared to 1.7% with biomicroscopy. With respect to detecting significant cystoid macular edema a sensitivity of .81 (95% CI .57-.94), a specificity of .98 (95% CI .95-.99), and a simple κ of .79 (95% CI .65-.93) were reported when compared to biomicroscopy. In each case examinations and assessments were made by retinal specialists only.
Figure 4.
Example mydriatic retinal images taken with the D-Eye on iPhone 6 (far left). Mild nonproliferative diabetic retinopathy (second from left), moderate nonproliferative diabetic retinopathy (second from right), and panretinal photocoagulation scars on a retina with proliferative diabetic retinopathy (far right). Reproduced with permission from Russo et al.41
To the authors’ knowledge, there is presently no peer-reviewed comparison of D-Eye to conventional digital fundus photography, the gold standard and the only means used for the detection in the first stage of many national screening programs.
Peek Retina has been used in validation studies in LMICs. The device’s performance was first compared to a DRS retinal camera (Haag-Streit) nested within a population-based cohort study of eye disease in Nakuru, Kenya.42 Fundus images of both eyes were taken for 1,328 participants by non-health-care-trained, lay examiners using Peek Retina and by a specialist technician using the retinal camera. These images were then sent to Moorfields Eye Hospital Reading Centre (London, UK) for independent grading. The authors reported a weighted kappa of .71 when comparing lay examiners using Peek Retina with an ophthalmic technician using the reference desktop camera for optic nerve examination. Bland-Altman analysis demonstrated an average difference of –.02 with 95% limits of agreement between –.21 and .16 for vertical cup-to-disc ratio assessment, suggesting good agreement between the lay-operated Peek Retina and the reference standard.43
Peek Retina preproduction prototypes, shown in Figure 5, are presently being trialled alongside standard digital retinal cameras (Topcon NRW6, Topcon Corp, Tokyo, Japan) in an 18-site DR screening implementation and evaluation in Moshi, Tanzania. Although this study is still in progress, interim data has indicated good agreement between DR grading of images acquired by general clinical staff with a conventional fundus camera and Peek Retina when both are performed under dilation (Mwanansao C, et al, unpublished manuscript).
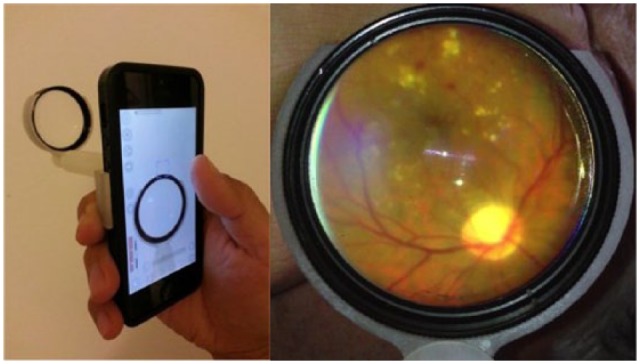
Figure 5.
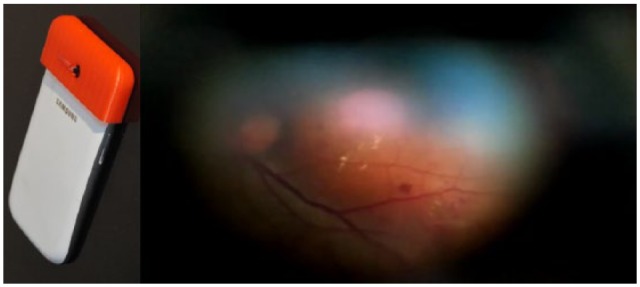
The preproduction prototype of Peek Retina on a Samsung S3 (left) uses a prism to project a light beam that is closely aligned to camera optical axis. It is currently being trialled alongside reference standards in a diabetic retinopathy study in Moshi, Tanzania (frame of captured video, right).
Given that systematic DR screening in sub-Saharan Africa is presently rarely available, the prospect of implementing an effective program in a highly challenging environment such as the Kilimanjaro region is most exciting. If the full results are to replicate those initially being indicated, then this will lend strong supporting evidence for the effectiveness of smartphone-based systematic DR screening.
Discussion
Despite their immense potential, thus far a very low proportion of the relatively numerous mHealth technology pilot studies conducted have gone on to achieve full integration into health care systems.44 A variety of reasons for this have been noted including failure to keep pace with the rapidly developing mobile phone sector, failure to recognize design decisions which affect the workflow and the need for better coordination between technologists, clinicians and policy makers in developing the common standards and frameworks necessary.45-47 In these respects smartphone retinal imaging technology is no exception.
With regard to keeping pace with the broader mobile phone market, a functioning screening program will inevitably require replacement devices during its life. If the technology in use is only compatible with obsolete, and no longer manufactured, handsets then the program will begin to break down as handsets age and require replacing. Ideally the device would therefore be independent of its host handset. Nevertheless, constant attention will have to be paid by smartphone imaging adapter manufacturers to ensure that their attachments’ quality, ease-of-use or even safety are not degraded by developments and trends within the mobile phone sector, or risk becoming obsolete in as little as 2 to 3 years after product launch.
The existing published literature relating to smartphone-based DR screening has thus far mostly consisted of ophthalmologists’ single-site comparisons to reference standards. This is a vital first step in validating the clinical usefulness of the technology and we commend the robust comparison of smartphone indirect ophthalmoscopy to standard retinal photography by Ryan et al34 in particular. However unless the technology can be effectively integrated into the appropriate clinical workflows, where there are nonclinical operators and graders, it cannot be adopted. In addition, new ways of structuring clinical workflows around the technologies have been postulated but there is little peer-reviewed literature showing the impact of these new workflows on patient outcomes. Thus, at present, policy makers are lacking the evidence they need to implement national screening programs based around mHealth.
To this end, the on-going multisite trial of Peek Retina and a referral system in Tanzania and its planned expansion to other similar studies in other countries are critically important in establishing the evidence base necessary for smartphone-based systematic DR screening. Developers of smartphone retinal imaging technologies should investigate the effectiveness of these tools within implementable DR screening workflows, with the rigor and scale appropriate for the trialling of any such medical device.
Conclusions
Pilot studies and single-site trials have produced promising results for the validation of smartphone-based DR assessment versus reference standards. However, by nature, the implementation of national, systematic screening programs is top-down. Continued collaboration across medicine, engineering, health care policy and other disciplines in adapting to local standards and filling gaps in the current literature is required to shape national DR strategies. Specifically, more literature describing multisite trialling, the impact of the technology on the whole clinical workflow, and ultimately the impact on the health outcomes of the screened population are required to shape national DR strategies.
Footnotes
Abbreviations: DM, diabetes mellitus; DMac, diabetic maculopathy; DR, diabetic retinopathy; FoV, field of view; LMIC, low- and middle-income country; pixel, picture element.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors are named inventors on pending patents relating to Peek Retina.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors are in receipt of research funding from the Queen Elizabeth Diamond Jubilee Trust.
References
- 1. Klein R, Klein BEK. Diabetes in America. 2nd ed Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 1995. [Google Scholar]
- 2. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311-321. [DOI] [PubMed] [Google Scholar]
- 3. Wright A, Dodson P. Medical management of diabetic retinopathy: fenofibrate and ACCORD Eye studies. Eye. 2011;25(7):843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124-136. [DOI] [PubMed] [Google Scholar]
- 5. Lerch C, Richter B, Bergerhoff K, Joussen AM. Digital retinal imaging for diagnosing diabetic retinopathy. The Cochrane Library 2011;5:1-15. [Google Scholar]
- 6. Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):s84-s87. [DOI] [PubMed] [Google Scholar]
- 7. Danielsen R, Jonasson F, Helgason T. Prevalence of retinopathy and proteinuria in type 1 diabetics in Iceland. Acta medica Scandinavica. 1982;212(5):277-280. [DOI] [PubMed] [Google Scholar]
- 8. Scanlon PH. The English national screening programme for sight-threatening diabetic retinopathy. J Med Screen. 2008;15(1):1-4. [DOI] [PubMed] [Google Scholar]
- 9. Lowe J. Screening for diabetic retinopathy. Nurs Gen Pract. 2014;7(6):34-38. [Google Scholar]
- 10. Hautala N, Aikkila R, Korpelainen J, et al. Marked reductions in visual impairment due to diabetic retinopathy achieved by efficient screening and timely treatment. Acta Ophthalmologica. 2014;92(6):582-587. [DOI] [PubMed] [Google Scholar]
- 11. Agardh E, Tababat-Khani P. Adopting 3-year screening intervals for sight-threatening retinal vascular lesions in type 2 diabetic subjects without retinopathy. Diabetes Care. 2011;34(6):1318-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olafsdottir E, Stefansson E. Biennial eye screening in patients with diabetes without retinopathy: 10-year experience. Br J Ophthalmol. 2007;91(12):1599-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kristinsson JK, Stefánsson E, Jónasson F, et al. Systematic screening for diabetic eye disease in insulin dependent diabetes. Acta ophthalmologica. 1994;72(1):72-78. [DOI] [PubMed] [Google Scholar]
- 14. Kristinsson JK, Hauksdóttir H, Stefánsson E, et al. Active prevention in diabetic eye disease. Acta Ophthalmologica Scandinavica. 1997;75(3):249-254. [DOI] [PubMed] [Google Scholar]
- 15. Peto T, Tadros C. Screening for diabetic retinopathy and diabetic macular edema in the United Kingdom. Curr Diabetes Rep. 2012;12(4):338-345. [DOI] [PubMed] [Google Scholar]
- 16. City & Guilds. Diabetic retinopathy screening. Available at: http://www.cityandguilds.com/qualifications-and-apprenticeships/health-and-social-care/health/7360-diabetic-retinopathy-screening. Accessed August 16, 2015.
- 17. Leese GP, Ahmed S, Newton RW, et al. Use of mobile screening unit for diabetic retinopathy in rural and urban areas. BMJ. 1993;306:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16-64 years) 1999-2000 with 2009-2010. BMJ Open. 2014;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fleming AD, Goatman KA, Philip S, et al. Automated grading for diabetic retinopathy: a large-scale audit using arbitration by clinical experts. Br J Ophthalmol. 2010;94(12):1606-1610. [DOI] [PubMed] [Google Scholar]
- 20. Philip S, Fleming AD, Goatman KA, et al. The efficacy of automated “disease/no disease” grading for diabetic retinopathy in a systematic screening programme. Br J Ophthalmol. 2007;91(11):1512-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burgess PI, Msukwa G, Beare NA. Diabetic retinopathy in sub-Saharan Africa: meeting the challenges of an emerging epidemic. BMC Med. 2013;11(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernardes R, Serranho P, Lobo C. Digital ocular fundus imaging: a review. Ophthalmologica. 2011;226(4):161-181. [DOI] [PubMed] [Google Scholar]
- 23. Prasad S, Roy B. Digital photography in medicine. J Postgrad Med. 2003;49(4):332. [PubMed] [Google Scholar]
- 24. Armed Forces Supply Support Center. Military Standard Photograph Lenses. Washington, DC: U.S. Government Printing Office; 1959. [Google Scholar]
- 25. International Organization for Standardization. Ophthalmic Instruments: Fundus Cameras. Geneva: International Organization of Standardization; 2009. [Google Scholar]
- 26. Bastawrous A, Armstrong MJ. Mobile health use in low- and high-income countries: an overview of the peer-reviewed literature. J Royal Soc Med. 2013;106(4):130-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nielsen Company. The Digital Consumer Report Feb 2014. New York: The Nielsen Company; 2014. [Google Scholar]
- 28. d4. A Survey of Mobile Phone Usage by Health Professionals in the UK. London: Devices 4 Limited; 2010. [Google Scholar]
- 29. Pew Research Center. Emerging Nations Embrace Internet, Mobile Technology. Washington, DC: Pew Research Center; 2014. [Google Scholar]
- 30. Barsam A, Bhogal M, Morris S, Little B. Anterior segment slitlamp photography using the iPhone. J Cataract Refract Surg. 2010;36(7):1240-1241. [DOI] [PubMed] [Google Scholar]
- 31. Gurram MM. Ophthalmic cell-phone imaging system: a costless imaging system. Can J Ophthalmol. 2013;48(5):e135-e139. [DOI] [PubMed] [Google Scholar]
- 32. Lord RK, Sha VA, San Filippo AN, Krishna R. Novel uses of smartphones in ophthalmology. Ophthalmology. 2010;117(6):1274-1274e3. [DOI] [PubMed] [Google Scholar]
- 33. Bastawrous A. Smartphone fundoscopy. Ophthalmology. 2012;119(2):433-433e2. [DOI] [PubMed] [Google Scholar]
- 34. Ryan ME, Rajalakshmi R, Prathiba V, et al. Comparison Among Methods of Retinopathy Assessment (CAMRA) Study: smartphone, nonmydriatic, and mydriatic photography. Ophthalmology. 2015;122(10):2038-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Myung D, Jais A, He L, et al. 3D printed smartphone indirect lens adapter for rapid, high quality retinal imaging. J Mobile Tech Med. 2014;3(1):9-15. [Google Scholar]
- 36. Hong SC. 3D printable retinal imaging adapter for smartphones could go global. Graefe’s Arch Clin Exp Ophthalmol. 2015;253(10):1831-1833. [DOI] [PubMed] [Google Scholar]
- 37. Allyn Welch. iExaminer—eye imaging on your iPhone. Available at: http://www.welchallyn.com/en/microsites/iexaminer.html. Accessed August 18, 2015.
- 38. MediSave. Welch Allyn iExaminer adaptor for iPhone 4 & 4S. Available from: http://www.medisave.co.uk/welch-allyn-iexaminer-iphone-adaptor.html. Accessed August 18, 2015.
- 39. Giardini ME, Livingstone AT, Jordan S, et al. A smartphone based ophthalmoscope. In: Proceedings of the 36th Annual International Conference IEEE Engineering in Medicine and Biology Society Red Hook, NY: Curran Associates; 2014:2177-2180 [DOI] [PubMed] [Google Scholar]
- 40. Russo A, Civili PS. A novel device to exploit the smartphone camera for fundus photography. J Ophthalmology. 2015;2015:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Russo A, Morescalchi F, Costagliola C, et al. Comparison of smartphone ophthalmoscopy with slit-lamp biomicroscopy for grading diabetic retinopathy. Am J of Ophthalmology. 2015;159(2):360-364e1. [DOI] [PubMed] [Google Scholar]
- 42. Bastawrous A, Mathenge W, Peto T, et al. The Nakuru eye disease cohort study: methodology & rationale. BMC Ophthalmol. 2014;14(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bastawrous A, Giardini ME, Bolster NM, et al. Agreement of a smartphone based ophthalmoscope (Peek Retina) with standard fundus cameras for optic nerve imaging in a large Kenyan cohort. JAMA Ophthalmol. In press; 2015. [Google Scholar]
- 44. Qiang CZ, Yamamichi M, Hausman V, et al. Mobile Applications for the Health Sector. Washington, DC: World Bank; 2012. [Google Scholar]
- 45. Kay M, Santos J, Takane M. mHealth: New Horizons for Health Through Mobile Technologies. Vol. 3 Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 46. Kumar S, Nilsen WJ, Abernethy A, et al. Mobile health technology evaluation: the mHealth evidence workshop. Am J Prev Med 2013;45(2):228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomlinson M, Rotheram-Borus MJ, Swartz L, Tsai AC. Scaling up mHealth: where is the evidence? PLOS Med. 2013;10(2):e1001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buam I. USAF-1951. Available at: https://commons.wikimedia.org/wiki/File:USAF-1951.svg. Accessed August 30, 2015.