Abstract
Due to the increasing prevalence of diabetes mellitus, demand for diabetic retinopathy (DR) screening platforms is steeply increasing. Early detection and treatment of DR are key public health interventions that can greatly reduce the likelihood of vision loss. Current DR screening programs typically employ retinal fundus photography, which relies on skilled readers for manual DR assessment. However, this is labor-intensive and suffers from inconsistency across sites. Hence, there has been a recent proliferation of automated retinal image analysis software that may potentially alleviate this burden cost-effectively. Furthermore, current screening programs based on 2-dimensional fundus photography do not effectively screen for diabetic macular edema (DME). Optical coherence tomography is becoming increasingly recognized as the reference standard for DME assessment and can potentially provide a cost-effective solution for improving DME detection in large-scale DR screening programs. Current screening techniques are also unable to image the peripheral retina and require pharmacological pupil dilation; ultra-widefield imaging and confocal scanning laser ophthalmoscopy, which address these drawbacks, possess great potential. In this review, we summarize the current DR screening methods using various retinal imaging techniques, and also outline future possibilities. Advances in retinal imaging techniques can potentially transform the management of patients with diabetes, providing savings in health care costs and resources.
Keywords: diabetic retinopathy, imaging, retina, screening
The prevalence of diabetes mellitus (DM) is projected to approach 600 million people worldwide by 2035 and there are concerns about a potential diabetes epidemic in Asia.1 Diabetic retinopathy (DR), a complication of DM, afflicts a third of diabetics and is the principal cause of vision loss among the working age group in developed countries2 and remains a leading cause of preventable blindness.3 The proportion of visual impairment worldwide caused by DR has increased.4 Diabetic macular edema (DME), characterized by increased vascular permeability and the deposition of hard exudates at the central retina, can develop at any stages of DR and afflicts 21 million people globally.5
Through regular eye examinations and adequate DM management, the diabetes-related vision loss can be prevented in 98% of cases.6,7 Primary interventions, such as intensive glycemic and blood pressure control, can reduce the incidence and progression of DR and DME, while secondary interventions, such as laser photocoagulation and injections of anti–vascular endothelial growth factor drugs, may prevent development and progression of vision loss.8-13 Therefore, early detection of DR and DME through screening programs and appropriate referral for therapy are essential to preserving vision in individuals with diabetes. DR screening has been shown to be a cost-effective method of preventing diabetes-related vision loss.14,15 Since blindness from DR and DME is preventable from both public health screening and clinical management perspectives, it is important to precisely identify persons who have DR/DME for early intervention before they progress to severe vision-threatening stages.16
Current DR screening programs typically employ retinal fundus photography and manual assessment of DR,17 but this approach requires highly skilled readers which is labor-intensive and costly. Moreover, fundus photography as a technique is 2-dimensional, not 3-dimensional, making it difficult to assess for DME. New retinal image processing methods and imaging technologies can potentially provide solutions for detecting DR and DME18 in a more efficient and cost-effective manner. In this review, we will summarize the current retinal imaging technologies for DR screening and discuss future possibilities.
Fundus Photography
The UK National Institute for Clinical Excellence (NICE) guidelines19 states that a DR screening test should have sensitivity and specificity of at least 80% and 95% respectively, with a technical failure rate of less than 5%. The gold standard photography method for the detection of DR is stereoscopic color fundus photography in 7 standard fields (30°) as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) group.20 It is also useful for identifying DME and subtle retinal neovascularization.21,22 However, from the patient’s perspective, taking 7 fields is time-consuming, tedious and uncomfortable.
Several studies have compared the use of 1-field, 2-fields and 3-fields fundus photography with the standard 7-fields ETDRS fundus photography.17,23-31 Single-field fundus photography had significantly lower sensitivity (78%) and specificity (86%) for detection of referable DR29 compared to 2-fields (sensitivity 96%, specificity 89%)30 and 3-fields fundus photography (sensitivity 92%, specificity 97%).26 Thus, 2 or 3-fields fundus photography are currently commonly used for screening25,31 as they represent a good compromise with reasonable sensitivity and good comfort favoring patient compliance with screening.26
Mydriatic fundus photography, with further use of ophthalmoscopy for ungradable cases, has been shown to be an effective DR screening strategy.32 It offers a sensitivity of at least 80% in the detection of any grade of DR, and sensitivity and specificity of 97% and 92% respectively for vision-threatening DR (VTDR).19 Despite the significant improvement in the detection rate of DR for mydriatic compared with nonmydriatic fundus photography, the safety of pharmacological pupil dilation remains a concern among primary eye care physicians. The incidence of mydriasis-induced acute angle-closure attack33 has been reported as 6 in 20 000 Caucasians, but this figure may be higher among Asian populations due to differences in eye anatomy.
Nonmydriatic fundus photography (Figure 1) has thus become a popular screening tool for DR at the primary care level.17 However, it faces drawbacks including a higher technical failure rate resulting from media opacities, small pupils, and difficulty in obtaining stereoscopic views. Reassuringly, its sensitivity has been reported to be between 78% and 98%, with a specificity between 86% and 90%, in the detection for VTDR requiring referrals.29,34
Figure 1.
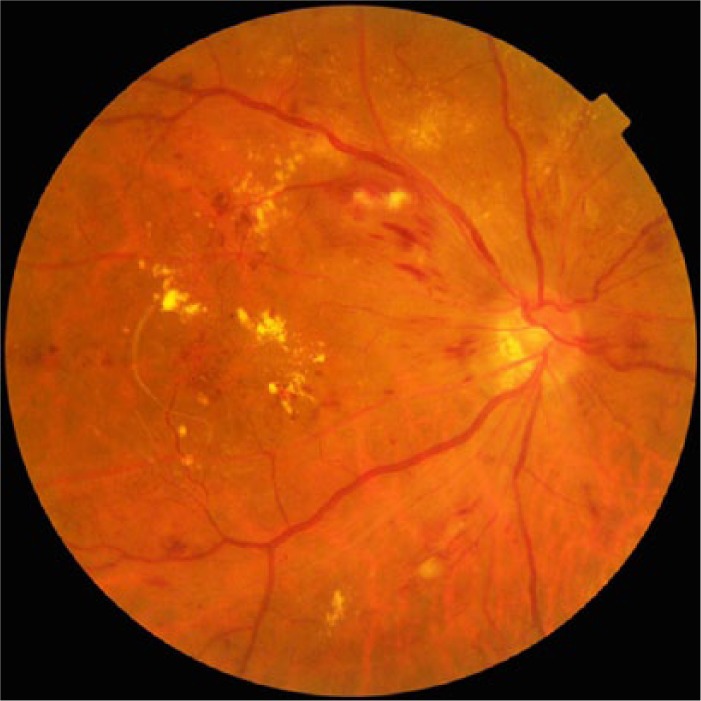
Nonmydriatic digital retinal photography of a patient with severe nonproliferative diabetic retinopathy. Dot, blot, and flame hemorrhages associated with hard exudates are seen. This image was obtained using TRC-NW8 (Topcon Medical Systems, Oakland, NJ).
In Scotland, a 3-tiered screening approach has been implemented which involves obtaining only 1 macula-centered digital fundus photograph per eye without mydriasis (tier 1) and if unsuccessful then mydriasis is used (tier 2) and finally biomicroscopy with a slit lamp if photography remains unsuccessful (tier 3).35,36
The use of digital imaging systems has reduced the technical failure rate associated with previous nondigital film photography, and the electronic image facilitates easy storage and cataloguing.37 Modern digital systems for retinal photography have been shown to achieve sensitivities and specificities of approximately 90% in detecting referable DR.38 Comparisons between film and digital fundus photographs found agreement to be substantial to almost perfect for DR severity level and moderate to substantial for DME and CSME severity levels, respectively.39
Recently, several articles have been published reporting the use of telemedicine for the screening of DR using nonmydriatic fundus photography14,15,40 in the primary care setting.41 Based on remote assessment of fundus photographs by a centralized team of trained and accredited technicians, telemedicine has been shown to have a pooled sensitivity exceeding 80% and a pooled specificity exceeding 90% for DR.42 Other ocular conditions can also be detected at a high rate, a collateral benefit of DR screening programs that may be underappreciated.43 Telemedicine has been shown to be cost-effective for patient populations of >3500, patients aged <80 years, and all racial groups.44
In addition, there are still some details in fundus photographs, such as retinal vessel caliber,45 tortuosity46 and fractal dimensions,47 that have not been fully exploited which may improve DR screening and risk stratification. For example, retinal arteriolar caliber has been shown to widen with increasing glucose and HbA1C levels.48 Several population-based cross-sectional studies have consistently reported that wider retinal venular caliber is associated with more severe DR.49 Wider retinal venular caliber has also been shown to predict progression of DR in few prospective studies,50 independent of other known risk factors.It is noted that there is still no fully automated algorithm to measure these retinal vascular parameters.
DR assessment using nonmydriatic digital fundus photography still requires specially trained highly skilled readers (a resource in limited supply globally)51 and it is labor-intensive. The DR grading time is about 1 to 10 minutes for each patient with DM even by a well-trained reader (grading time varies with DR severity and image quality). Given the projection of 600 million people living with diabetes globally by the year 2035, the current DR screening approach may not be able to keep pace with the demand for DR screening services.1 In addition, the manual nature of DR severity grading can result in inconsistency and variability between readers and across screening sites.52 Moreover, fundus photography as a technique is 2-dimensional, not 3-dimensional, making it difficult to accurately assess detailed morphological abnormalities including intraretinal cystic changes and subretinal fluid accumulation, and identify DME, which is best assessed with a 3-dimensional view. As a result, graders assess for surrogates of DME such as hard exudates near the fovea from fundus photographs, leading to a high false positive rate and high number of unnecessary referrals to tertiary centers.
DR Lesion Detection by Automated Retinal Image Analysis
In view of the shortcomings of current DR screening programs that rely heavily on manual readers, numerous software for automated detection of DR lesions from fundus photographs have been developed.51 These systems have the potential to substantially improve DR screening by reducing the burden on readers and therefore improving efficiency. Table 1 summarizes these automated DR lesion detection systems.
Table 1.
Summary of Current Automated Diabetic Retinopathy (DR) Lesion Detection Systems.
| System | Company | Location | Grading details | Algorithm |
|---|---|---|---|---|
| DR-RACS™ | Vision Quest Biomedical LLC | Vision Quest Biomedical LLC, Albuquerque, NM | Low risk/high risk for DR | Amplitude modulation-frequency modulation (AM-FM), k-means clustering, and a partial least square classifier |
| EyeArt | Eyenuk Inc | Woodland Hills, CA | Refer/no refer recommendation; microaneurysm turnover | Machine learning; morphology-inspired filter bank descriptors |
| IDx-DR | IDx, LLC | University of Iowa, USA | Diabetic retinopathy index; referable/nonreferable disease | Fusion algorithm produces a DR index |
| iGradingM | Medalytix LLC; Digital Healthcare | University of Aberdeen, Scotland, UK | Presence/absence of DR | Local contrast, normalization and local vessel detection |
| RetinaLyze A/S | RetinaLyze A/S | Denmark | Presence/absence of DR based on microaneurysm and hemorrhage detection | Automated red lesion detection, including microaneurysm and hemorrhage using vector based algorithm. |
| Retmarker DR | Retmarker Ltd | Coimbra University, Portugal | Presence/absence of DR; microaneurysm turnover | Longitudinal analysis by comparing with baseline image |
| Singapore Eye Lesion Analyzer (SELENA) | – | Singapore Eye Research Institute and National University of Singapore, Singapore | Grade of DR and referable/nonreferable | Deep learning technology using convolutional neural network and region extraction algorithms |
| RetinaVue (formerly The TRIAD Network) | Welch Allyn, Inc (Hubble Telemedicine Inc) | University of Tennessee Health Science Center and the Oak Ridge National Laboratory, USA | Presence/absence of DR; grade of DR | Content-based image retrieval techniques for automated diagnosis |
Many systems are commercially available currently, including iGradingM (Medalytix LLC; Digital Healthcare, Scotland, UK),53 RetmarkerDR (Retmarker Ltd, Coimbra, Portugal),54 IDx-DR (IDx LLC, Iowa City, IA, USA),56 RetinaLyze System A/S (RetinaLyze A/S, Horsholm, Denmark)57 and EyeArt (Eyenuk Inc, Woodland Hills, CA, USA).58 Systems that are under active development and not yet commercially available are Singapore Eye Lesion Analyser (SELENA),59 the TRIAD Network (Hubble Telemedicine Inc),55 and DR-RACS (VisionQuest Biomedical LL).60 It is noteworthy that the TRIAD Network has been acquired by Welch Allyn and the technology is now rebranded as RetinaVue.
Most of these systems, or more specifically iGradingM, RetinaLyze A/S and Retmarker DR, make a decision on the presence or absence of DR. Others, such as EyeArt and SELENA, further provide a recommendation regarding the need for referral (ie, referable DR). On the other hand, DR-RACS classifies cases as “high risk” or “low risk” for DR, which translates to a not referable/referable outcome.60 Iowa Detection Program (IDx-DR) utilizes a fusion algorithm to produce a DR index,56 which will be suggestive of referable or nonreferable disease.
Some of these systems have additional functions. For example, both EyeArt and Retmarker DR can measure microaneurysm turnover (ie, the sum of the microaneurysm formation and disappearance rates), which has been shown to be associated with DR progression.54,61 iGradingM also possesses an image quality assessment function.53
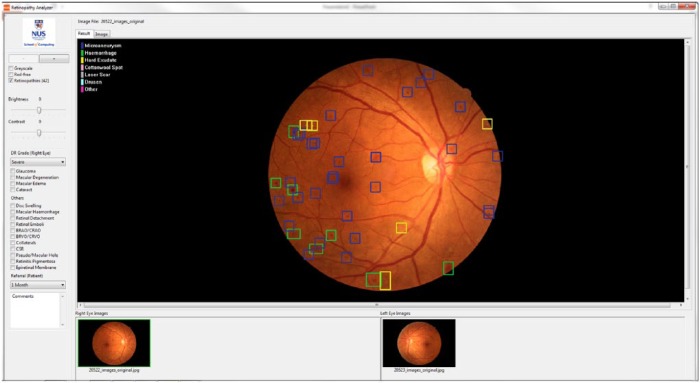
The Singapore Eye Lesion Analyzer (SELENA), based on deep learning technology using convolutional neural network and region extraction algorithms, is the first Asian automated detection system for DR (Figure 2). In addition, both SELENA and the TRIAD Network can provide specific DR severity level59 and classify DR lesions but they are both not commercially available yet. There are currently various methods employed to classify DR lesions with good accuracy, such as bag-of-visual-words algorithm.62,63 A drawback of commercially available systems is the lack of integration of recent published work such as automated detection of neovascularization based on fractal and texture analysis.64,65
Figure 2.
Singapore Eye Lesion Analyzer (SELENA) is a cloud-based automated imaging program for diabetic retinopathy screening developed by Singapore Eye Research Institute and National University of Singapore. SELENA is able to automatically detect various DR lesions (colored boxes) from fundus photographs and provide DR severity grade and referable/nonreferable DR recommendation.
The emergence of automated DR detection systems is a response to the need for an effective DR screening tool as a result of the increase prevalence of DR. Although many systems are under development, it is difficult to determine which system is more superior to the other. These systems use different algorithms and are tested in different patient populations, making direct comparison almost impossible at present. However, these systems do share a similar primary aim: to ease the burden of trained manual grader by screening out images that exhibit no disease or are nonreferable, with the objective to relieve health care costs and burden by providing real-time evaluation to expedite diagnosis, and referral if necessary. Already, Scotland has implemented a 2-tier strategy by replacing first level manual grading in their National Screening Program with an automated system developed to assess image quality and detect the presence of any retinopathy, saving £201 600 per year in grading and quality assurance costs to the NHS.66 This strategy was found to safely53 reduce manual grading workload by 36.3%.67 Automated grading is likely to be a cost-effective alternative to manual grading because compared with manual grading, automated grading saves between £1727 and £3834 per case missed in grading costs.68
Optical Coherence Tomography
A major limitation of many current DR screening programs based on fundus photography is the inability to accurately identify DME on 2-dimensional fundus photographs. Optical Coherence Tomography (OCT) has emerged as a useful DR screening tool as it provides direct detailed 2- and 3-dimensional visualization of histological changes in the layered retinal structures and precise quantitative assessment with ultra-high scan speed and resolution.69 OCT uses low-coherence interferometry to provide noncontact and noninvasive optical biopsy of the tissue morphology of the retina (including macula, optic nerve head and retinal nerve fiber layer).70,71 In clinical practice, OCT has been widely used as an objective tool to measure the volume and total thickness of the retina, along with structural changes of the various cellular layers of the retina with the aid of segmentation algorithms.72
OCT is capable of quantifying retinal thickness, identify intra/subretinal pathology (eg, subretinal fluid/hemorrhage secondary to choroidal neovacularization), and monitor for disease complications (eg, cystoid macular edema secondary to central retinal vein occlusion) in various retinal diseases, making it a useful tool for detecting and managing retinal abnormalities. The role of OCT in the assessment and management of diabetic eye disease has aided our understanding of the internal architecture of the retina in diabetes.73-75 OCT has greatly improved DR and mostly DME management by enabling the direct evaluation of retinal thickness and the quantitative follow-up of retinal thickness changes that may greatly influence therapeutic decisions.76,77 For example, in an observational study of subclinical DME, it has been shown that the cumulative probability of meeting an increase in OCT central point thickness of at least 50 microns from baseline and a central point thickness of at least 300 microns, or treatment for DME was 27% by 1 year and 38% by 2 years.78
Specifically, OCT is increasingly being used to diagnose DME in patients with diabetes.79,80 Clinicians often request OCT imaging when a diabetic patient has a fundus photograph suspicious for DME because OCT allows for an objective evaluation of DME with effectiveness in particular for the identification of macular thickening. OCT compares favorably to other methods of DME assessment, such as slit-lamp biomicroscopy and fundus photography.81 In addition to central subfield mean retinal thickness that has most commonly been used to identify macular thickening in clinical research trials,80 spectral-domain-OCT (SD-OCT), a newer generation of OCT technology, can evaluate detailed morphological abnormalities (Figure 3) that occur in DME, including intraretinal cystic changes, subretinal fluid accumulation, and vitreo-retinal interface at the macula in differentiating vitreo-retinal attachment from vitreo-retinal traction, such as vitreo-macular traction.82,83 In a recent Cochrane systematic review, OCT was found to have a pooled sensitivity of 78% and specificity of 86% for detecting DME,84 using diagnosis by means of fundus biomicroscopy or stereophotography by ophthalmologists or other trained personnel as reference.
Figure 3.
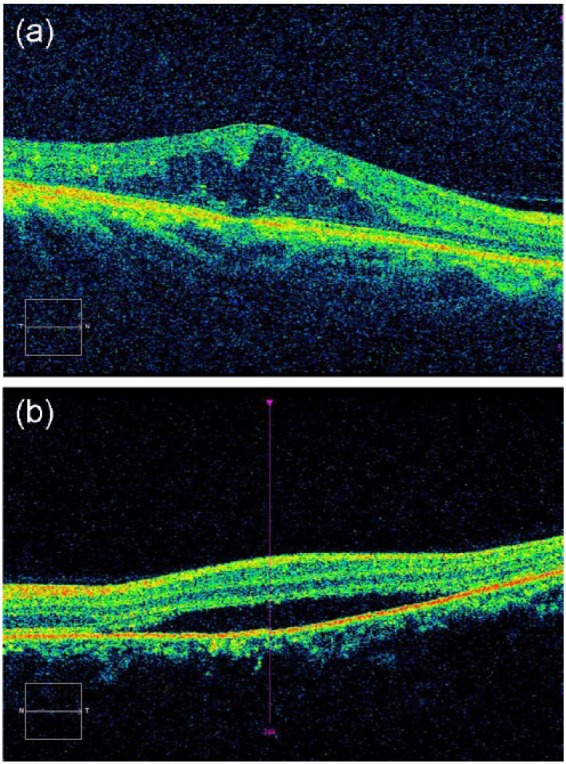
Optical coherence tomography (OCT) technology can evaluate detailed morphological abnormalities that occur in diabetic macular edema (DME), including (A) intraretinal cystic changes and (B) subretinal fluid accumulation.
As OCT becomes increasingly available in clinical practice,83 it has become widely recognized as the new reference standard for assessment of DME.84 However, the role of OCT is still unclear in DR screening in addition to fundus photography. There is only a moderate correlation between OCT and FP assessments of retinal thickening in patients with DME.86 OCT imaging can be considered in cases suspicious for DME on fundus photography because OCT is especially useful in ruling out DME, thereby reducing unnecessary referrals (Figure 4). A DR screening protocol using SD-OCT has been tested in UK, in which Dodson found that in patients with referable DME, as defined by FP alone, only 26% of patients showed macular thickening on SD-OCT. This suggests that OCT is potentially an effective method of reducing the unnecessary referrals to hospital eye clinics.87 OCT can also be used to follow-up patients whose DR is above the screening referral threshold but does not actually require treatment.88 SD-OCT can serve as a valuable adjunct in evaluating intraretinal microvasular abnormalities and neovascularization elsewhere, since it can verify the histopathologic correlate and provide subtle anatomic insights.89
Figure 4.
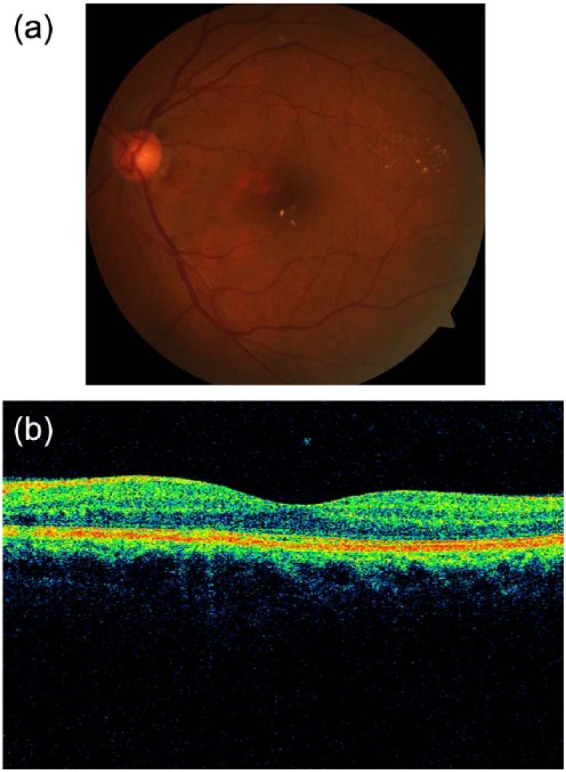
An example of a left retina suspicious for diabetic macular edema (DME) on (A) fundus photography (using hard exudates as surrogate for DME), but negative for DME on (B) optical coherence tomography (OCT). The fovea contour is preserved, with no retinal thickening or intraretinal cysts.
Promisingly, in a recent publication in Scotland and the UK, economic modeling suggested that combining SD-OCT with FP resulted in cost savings without reducing health benefits. This is likely to be cost-effective as the estimated marginal cost of including SD-OCT within the existing screening program was low (£31.96 per patient) compared with the cost of a tertiary referral, treatment and consequent monitoring in the outpatient setting (£420).90 Olsen et al reported that the addition of OCT prior to referral, results in a reduction in costs to the health service with no decrement in the number of DME cases detected.91 Most recently, a virtual SD-OCT clinic has been audited as an alternative way of managing a large cohort of referred patients with relatively few resources whilst ensuring that appropriate treatment is provided when necessary.92 Together, these results indicate that OCT shows promise to transform from being a diagnostic tool to a screening tool in the primary care office.93
Biomarkers that can be extracted from OCT imaging in the context of early detection of DR lesions include the volume and total thickness of the retina, and central subfield mean retinal thickness.94 These measurements can be provided by automated OCT software and it has been shown that the error involved is sufficiently small that results are not likely to be affected if scans are not routinely sent to a reading center.85 Subclinical macular edema identified by OCT appear to be a good candidate as an organ-specific biomarker of DR.95 However, a recent study showed that that there is no advantage in performing OCT routinely in patients with type 1 DM without minimal DR because OCT did not show changes in retinal thickness in those patients compared to controls.96
Scanning Laser Ophthalmoscopy
Scanning laser ophthalmoscopy (SLO) is an imaging technology that uses laser light instead of a bright flash of white light to illuminate the retina. Image quality is essential for accurate DR assessment in conventional fundus photography, necessitating pharmacological pupil dilation. Confocal SLO imaging (cSLO) is the process of scanning an object point by point by a focused laser beam and then capturing the reflected light through a small aperture (a confocal pinhole). The confocal pinhole suppresses light reflected or scattered from outside of the focal plane, providing a sharp, high-contrast image without the need for pharmacological pupil dilation. Other advantages of using cSLO over traditional fundus photography include improved patient comfort through less bright light, video capability, and effective imaging of patients who do not dilate well. Since diabetics typically do not dilate well and account for a large number of patients with vision problems,97 cSLO imaging is a valuable tool for eye care providers especially in the context of DR screening.
Current screening techniques based on fundus photography suffer from an inability to image the peripheral retina where DR lesions may be missed. Ultra-widefield (UWF) fundus imaging technology uses laser light and the principles of confocal laser scanning microscopy to yield images with high resolution.98 By combining a SLO with an ellipsoidal mirror, this technology has the ability to capture a widefield image (200°) in a single photograph (Figure 5) in comparison to the typical 45° or 50° of standard fundus photography. Current devices taking advantage of UWF imaging capability are the Optos retinal imaging devices (eg, Optomap 200Tx, Daytona and California Imaging Systems by Optos, Marlborough, MA) and Heidelberg HRA (Heidelberg Engineering, Carlsbad, CA), which uses a noncontact UWF module that attaches easily to any Spectralis or HRA2 camera head.
Figure 5.
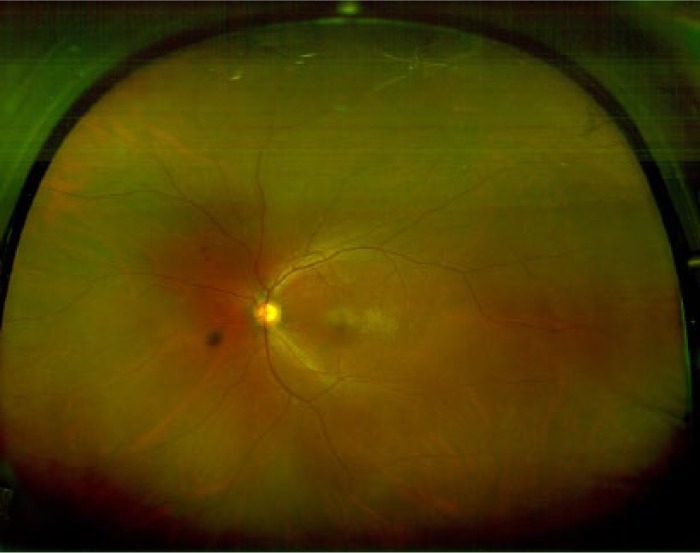
Ultra-widefield (UWF) image obtained using Optos P200MA (Optos, Marlborough, MA).
In a published telemedicine screening program, the use of Optomap UWF imaging was shown to increase the identification of DR by 17%, with lesions documented in the periphery suggesting greater disease severity in 9% of cases compared with nonmydriatic fundus photography.99 Real-time UWF image evaluation in a telemedicine program had a sensitivity and specificity for identifying more than minimal DR of 95% and 84%, respectively.100 Less than 0.1% of patients with referable DR would be missed and reading center image grading burden would be reduced by 60%.100 Compared with nonmydriatic fundus photography, UWF imaging99 reduced the ungradable rate by 71% (to <3%) and reduced image evaluation time per patient by 28%. Other studies have also shown that nonmydriatic UWF imaging compared favorably101 with dilated ETDRS photography and dilated fundus examination in determining DR and DME severity;102,103 furthermore, UWF image acquisition time was less than half that of dilated ETDRS photography.104 Comparing to gold standard 7 ETDRS views, the use of Optomap increased the DR severity grade in 15% of images with substantial agreement.105
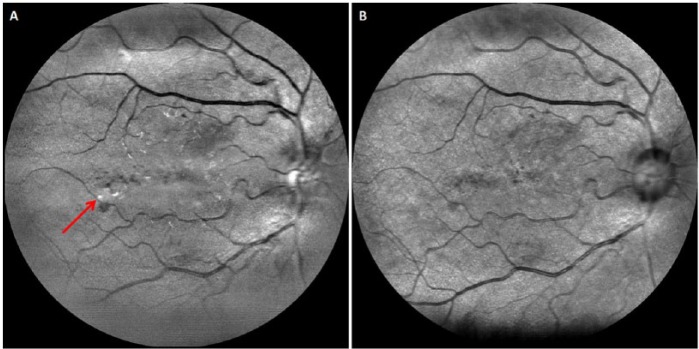
EasyScan (i-Optics, the Hague, the Netherlands) is another cSLO device that allows retinal imaging through undilated pupils and media opacities such as cataracts. As EasyScan possesses 2 lasers (1 green and 1 infrared) which penetrate to different depths of the retina, it can provide greater details such as the approximate depth at which a lesion is located (Figure 6), in comparison with fundus photography. In a cross-sectional study to evaluate the diagnostic performance of EasyScan in nonmydriatic DR assessment, it was found that gradability of EasyScan images was significantly higher than undilated fundus photography (CR-DGi 10D, Canon, Tokyo, Japan), and the diagnostic performance between cSLO and undilated fundus photography was similar (unpublished data). A study comparing cSLO with fundus camera found improved sensitivity and equivalent specificity for DR detection.106 Therefore, cSLO is a potential tool for nonmydriatic DR screening. However, more data to replicate and validate such imaging device is needed before the true potential of cSLO in DR screening can be ascertained.
Figure 6.
Images obtained using EasyScan (i-Optics, the Hague, the Netherlands): (A) green laser and (B) infrared laser. This diabetic patient’s right eye exhibits signs of diabetic retinopathy, with blot hemorrhages associated with hard exudates (arrow) located inferotemporal to the fovea.
Future Possibilities
New retinal imaging technologies have been recently developed to image the retina with more details and provide retinal functional assessment, which may potentially improve our DR screening practices in the future. Newer technologies such as portable handheld nonmydriatic cameras,107 enhanced depth imaging OCT,108-110 swept source OCT,111 OCT angiography, Doppler OCT, adaptive optics, retinal function assessment, retinal oximetry, metabolic imaging, and fundus autofluorescence112 are beginning to find applications in DR management and have the potential to change the landscape further in the future. However, more large-scale studies on the diagnostic performance and cost-effectiveness of these imaging technologies in the primary care setting are required before widespread recommendations for their use in routine DR screening can be made. Furthermore, as the use of these technologies in the context of DR is fairly new, standardized protocols have not been well established. In the meantime, these imaging technologies (except portable handheld nonmydriatic cameras) may be useful in the secondary or tertiary care model. We introduce several of these imaging technologies below.
OCT Angiography
OCT angiography113 can be used both to analyze blood flow quantitatively and to provide high-contrast images of the retinal vascular bed immediately and without the need for dye injection.114,115 Recent studies have demonstrated the potential of this technique in assessing retinal vasculature abnormalities and to confirm neovascularization in retinal diseases such as DR.116-118
Doppler OCT
Doppler OCT imaging has recently demonstrated usefulness in assessing blood flow changes119,120 in patients with DR, as well as in analyzing the 3-dimensional architecture of neovascularization in proliferative DR.121 Blood flow parameters in diabetics can be monitored longitudinally over the course of treatment, providing possible insights into endothelial function and response to therapy.119
Adaptive Optics
Adaptive optics (AO) technology is able to reduce the effects of wavefront distortions, compensating for astigmatism and higher-order aberrations caused by imperfections in the cornea and lens of the eye, thereby facilitating the lateral resolution of ophthalmoscopes to the microscopic scale122 and even reduce motion artifacts123 without the use of contrast.124 AO systems have been coupled with flood-illuminated cameras,125 scanning laser ophthalmoscopes126 and OCT.127 AO imaging have shown great potential in improving the noninvasive evaluation of the retina and retinal capillary network in early forms of DR128,129 because it can detect preclinical abnormalities of retinal microcirculation in patients with diabetes.130 For example, the retinal parafoveal capillary network has been found to be disrupted in type 2 diabetes, even before the onset of DR.131 Lombardi et al have demonstrated that the lumen of parafoveal capillaries is narrower in patients with Type 1 diabetes and nonproliferative DR than in healthy subjects.129 Recently, evidence of extensive capillary remodeling was found in subjects with only mild or moderate nonproliferative DR.132 Considering that existing DR classifications are based on lower-resolution retinal imaging techniques, AO has the potential to drastically improve clinical classification of diabetic individuals by detecting minute yet important microvascular changes among them.132 AO imaging may also help elucidate the pathophysiology of DR and evaluate the effects of clinical interventions.133 However, at present, the small field of view in AO devices limits its application in routine clinical practice. In addition, high cost and system complexity hinder the wide adoption of AO technology in clinical practice. Most AO retinal cameras have been designed and constructed for the best imaging performance possible, with the exclusion of all other factors, including size, cost, complexity, ease-of-use, time required to obtain, process, and analyze the retinal image.130 Fortunately, advances in automated methods to evaluate the retinal micro-structures, including cell photoreceptors, vessels, and nerve fiber bundles demonstrate potential in reducing the time required for image processing and analysis.128
Retinal Function Assessment
Imaging functional changes in the retina before anatomic consequences arise and before irreversible cell death becomes visible holds promise as a powerful technique for early detection of retinal diseases such as DR. The Retinal Function Imager (RFI) by Optical Imaging (Rehovot, Israel) enables direct in vivo, noninvasive functional assessment of 4 key parameters: retinal blood flow, blood oximetry, metabolic state, and hidden vasculature (particularly capillaries). These 4 functional parameters of the retina have been known to be affected by retinal diseases.134 Already, studies using RFI have shown that in early diabetes without DR, there is increased velocity in the retinal arterioles and venules;135 while in DR, the velocities decrease in both.134 The noninvasive qualitative and quantitative imaging of blood flow in the secondary and tertiary vessels of the retinal vasculature using a stroboscopic fundus camera136 could aid us in elucidating pathological changes other than the typical morphological changes that can be detected by other assessment techniques.
Metabolic Imaging
The OcuMet Beacon by OcuSciences (Ann Arbor, MI) is a novel device that allows noninvasive assessment of retinal mitochondrial dysfunction, which is a marker of metabolic stress and tissue damage seen in retinal diseases such as DR, macular degeneration, and glaucoma. Based on the intensity flavoprotein fluorescence in retinal tissues, it possesses potential for DR screening and monitoring for progression and improvement.137 Field et al have shown that individuals with DR in at least 1 eye had significantly greater mitochondria flavoprotein autofluorescence activity than diabetic subjects without retinopathy in either eye.138
Retinal Oximetry
Some DR lesions are secondary to disturbances in retinal blood flow, which may influence the oxygen supply for retinal metabolism. Using retinal oximetry, it has been shown that the oxygen saturation is increased in retinal arterioles and venules of diabetic patients with retinopathy.139 The oxygen saturation in retinal vessels from diabetic patients was found to be dependent on the severity and type of vision-threatening retinopathy.140 Recently, Man et al found that in patients with diabetes, eyes with DR were associated with increased venular oxygen saturation and decreased arteriovenous difference compared with eyes without DR, suggesting an altered metabolic state in DR.141
Conclusion
Detection and treatment of DR are key public health interventions because they can greatly reduce the likelihood of vision loss.6,7 The demand for DR screening platform using new ocular imaging techniques is steeply increasing due to the increase of prevalence of DM.5 Current DR screening programs based on fundus photography rely heavily on skilled graders,17 yet have difficulty detecting DME.142 Computer-assisted and automated retinal image analysis platforms can potentially reduce the reliance on manual graders54 but more studies are required to define their role in existing DR screening programs. Advanced retinal imaging technologies such as OCT and SLO are at the forefront of a new wave of solutions for more efficient DR screening.84,100 However, data on the validity and cost-effectiveness of DR assessment and risk prediction using newer ocular imaging techniques and automated analysis for these imaging technologies still need to be shown. This will eventually transform the management of patients with diabetes, providing savings in health care costs and resources.
Footnotes
Abbreviations: AO, adaptive optics; cSLO, confocal scanning laser ophthalmoscopy; DM, diabetes mellitus; DME, diabetic macular edema; DR, diabetic retinopathy; OCT, optical coherence tomography; RFI, Retinal Function Imager; SD-OCT, spectral-domain-OCT; SLO, scanning laser ophthalmoscopy; UWFI, ultra-widefield fundus imaging
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: TYW received funding support from National Medical Research Council STaR Investigator Award Singapore.
References
- 1. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129-2140. [DOI] [PubMed] [Google Scholar]
- 2. Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290(15):2057-2060. [DOI] [PubMed] [Google Scholar]
- 3. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124-136. [DOI] [PubMed] [Google Scholar]
- 4. Bourne RR, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Global Health. 2013;1(6):e339-e349. [DOI] [PubMed] [Google Scholar]
- 5. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rohan TE, Frost CD, Wald NJ. Prevention of blindness by screening for diabetic retinopathy: a quantitative assessment. BMJ. 1989;299(6709):1198-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferris FL., III How effective are treatments for diabetic retinopathy? JAMA. 1993;269(10):1290-1291. [PubMed] [Google Scholar]
- 8. Kiire CA, Porta M, Chong V. Medical management for the prevention and treatment of diabetic macular edema. Surv Ophthalmol. 2013;58(5):459-465. [DOI] [PubMed] [Google Scholar]
- 9. Pershing S, Enns EA, Matesic B, Owens DK, Goldhaber-Fiebert JD. Cost-effectiveness of treatment of diabetic macular edema. Ann Intern Med. 2014;160(1):18-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Fong DS, Strauber SF, et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125(4):469-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diabetic Retinopathy Clinical Research Network,Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells JA, Glassman AR, Jampol LM, et al. Association of baseline visual acuity and retinal thickness with 1-year efficacy of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema [published online ahead of print November 25, 2015]. JAMA Ophthalmol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kroenke K. Telemedicine screening for eye disease. JAMA. 2015;313(16):1666-1667. [DOI] [PubMed] [Google Scholar]
- 15. Chasan JE, Delaune B, Maa AY, Lynch MG. Effect of a teleretinal screening program on eye care use and resources. JAMA Ophthalmol. 2014;132(9):1045-1051. [DOI] [PubMed] [Google Scholar]
- 16. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298(8):902-916. [DOI] [PubMed] [Google Scholar]
- 17. Williams GA, Scott IU, Haller JA, Maguire AM, Marcus D, McDonald HR. Single-field fundus photography for diabetic retinopathy screening: a report by the American Academy of Ophthalmology. Ophthalmology. 2004;111(5):1055-1062. [DOI] [PubMed] [Google Scholar]
- 18. Bragge P, Gruen RL, Chau M, Forbes A, Taylor HR. Screening for presence or absence of diabetic retinopathy: a meta-analysis. Arch Ophthalmol. 2010;129(4):435-444. [DOI] [PubMed] [Google Scholar]
- 19. National Institute for Clinical Excellence. Management of Type 2 Diabetes: Retinopathy—Screening and Early Management. London, UK: Inherited Clinical Guideline; 2002. [Google Scholar]
- 20. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):786-806. [PubMed] [Google Scholar]
- 21. Rudnisky CJ, Hinz BJ, Tennant MT, de Leon AR, Greve MD. High-resolution stereoscopic digital fundus photography versus contact lens biomicroscopy for the detection of clinically significant macular edema. Ophthalmology. 2002;109(2):267-274. [DOI] [PubMed] [Google Scholar]
- 22. Cavallerano JD, Aiello LP, Cavallerano AA, et al. Nonmydriatic digital imaging alternative for annual retinal examination in persons with previously documented no or mild diabetic retinopathy. Am J Ophthalmol. 2005;140(4):667-673. [DOI] [PubMed] [Google Scholar]
- 23. Murgatroyd H, Ellingford A, Cox A, et al. Effect of mydriasis and different field strategies on digital image screening of diabetic eye disease. Br J Ophthalmol. 2004;88(7):920-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ting DS, Tay-Kearney ML, Kanagasingam Y. Light and portable novel device for diabetic retinopathy screening. Clin Experiment Ophthalmol. 2012;40(1):e40-e46. [DOI] [PubMed] [Google Scholar]
- 25. Vujosevic S, Benetti E, Massignan F, et al. Screening for diabetic retinopathy: 1 and 3 nonmydriatic 45-degree digital fundus photographs vs 7 standard early treatment diabetic retinopathy study fields. Am J Ophthalmol. 2009;148(1):111-118. [DOI] [PubMed] [Google Scholar]
- 26. Aptel F, Denis P, Rouberol F, Thivolet C. Screening of diabetic retinopathy: effect of field number and mydriasis on sensitivity and specificity of digital fundus photography. Diabetes Metab. 2008;34(3):290-293. [DOI] [PubMed] [Google Scholar]
- 27. Pugh JA, Jacobson JM, Van Heuven WA, et al. Screening for diabetic retinopathy. The wide-angle retinal camera. Diabetes Care. 1993;16(6):889-895. [DOI] [PubMed] [Google Scholar]
- 28. Ku JJ, Landers J, Henderson T, Craig JE. The reliability of single-field fundus photography in screening for diabetic retinopathy: the Central Australian Ocular Health Study. Med J Australia. 2013;198(2):93-96. [DOI] [PubMed] [Google Scholar]
- 29. Lin DY, Blumenkranz MS, Brothers RJ, Grosvenor DM. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: a comparison with ophthalmoscopy and standardized mydriatic color photography. Am J Ophthalmol. 2002;134(2):204-213. [DOI] [PubMed] [Google Scholar]
- 30. Olson JA, Strachan FM, Hipwell JH, et al. A comparative evaluation of digital imaging, retinal photography and optometrist examination in screening for diabetic retinopathy. Diabet Med. 2003;20(7):528-534. [DOI] [PubMed] [Google Scholar]
- 31. Boucher MC, Gresset JA, Angioi K, Olivier S. Effectiveness and safety of screening for diabetic retinopathy with two nonmydriatic digital images compared with the seven standard stereoscopic photographic fields. Can J Ophthalmol. 2003;38(7):557-568. [DOI] [PubMed] [Google Scholar]
- 32. Hutchinson A, McIntosh A, Peters J, et al. Effectiveness of screening and monitoring tests for diabetic retinopathy—a systematic review. Diabet Med. 2000;17(7):495-506. [DOI] [PubMed] [Google Scholar]
- 33. Liew G, Mitchell P, Wang JJ, Wong TY. Fundoscopy: to dilate or not to dilate? BMJ. 2006;332(7532):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fransen SR, Leonard-Martin TC, Feuer WJ, Hildebrand PL, Inoveon Health Research Group. Clinical evaluation of patients with diabetic retinopathy: accuracy of the Inoveon diabetic retinopathy-3DT system. Ophthalmology. 2002;109(3):595-601. [DOI] [PubMed] [Google Scholar]
- 35. Facey K, Cummins E, Macpherson K. Organisation of Services for Diabetic Retinopathy Screening. Health Technology Assessment Report 1 Glasgow, UK: Health Technology Board for Scotland; 2002. [Google Scholar]
- 36. Thomas RL, Dunstan FD, Luzio SD, et al. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol. 2015;99(1):64-68. [DOI] [PubMed] [Google Scholar]
- 37. Ryder RE. Screening for diabetic retinopathy in the 21st century. Diabet Med. 1998;15(9):721-722. [DOI] [PubMed] [Google Scholar]
- 38. Fransen SR, Leonard-Martin TC, Feuer WJ, Hildebrand PL. Clinical evaluation of patients with diabetic retinopathy: accuracy of the Inoveon diabetic retinopathy-3DT system. Ophthalmology. 2002;109(3):595-601. [DOI] [PubMed] [Google Scholar]
- 39. Gangaputra S, Almukhtar T, Glassman AR, et al. Comparison of film and digital fundus photographs in eyes of individuals with diabetes mellitus. Invest Ophthalmol Vis Sci. 2011;52(9):6168-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romero-Aroca P, Sagarra-Alamo R, Pareja-Rios A, Lopez M. Importance of telemedicine in diabetes care: Relationships between family physicians and ophthalmologists. World J Diabetes. 2015;6(8):1005-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garg S, Jani PD, Kshirsagar AV, King B, Chaum E. Telemedicine and retinal imaging for improving diabetic retinopathy evaluation. Arch Intern Med. 2012;172(21):1677-1678. [DOI] [PubMed] [Google Scholar]
- 42. Shi L, Wu H, Dong J, Jiang K, Lu X, Shi J. Telemedicine for detecting diabetic retinopathy: a systematic review and meta-analysis. Br J Ophthalmol. 2015;99(6):823-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Owsley C, McGwin G, Jr, Lee DJ, et al. Diabetes eye screening in urban settings serving minority populations: detection of diabetic retinopathy and other ocular findings using telemedicine. JAMA Ophthalmol. 2015;133(2):174-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kirkizlar E, Serban N, Sisson JA, Swann JL, Barnes CS, Williams MD. Evaluation of telemedicine for screening of diabetic retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120(12):2604-2610. [DOI] [PubMed] [Google Scholar]
- 45. Ikram MK, Cheung CY, Lorenzi M, et al. Retinal vascular caliber as a biomarker for diabetes microvascular complications. Diabetes Care. 2013;36(3):750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sasongko MB, Wong TY, Nguyen TT, Cheung CY, Shaw JE, Wang JJ. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia. 2011;54(9):2409-2416. [DOI] [PubMed] [Google Scholar]
- 47. Broe R, Rasmussen ML, Frydkjaer-Olsen U, et al. Retinal vascular fractals predict long-term microvascular complications in type 1 diabetes mellitus: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Diabetologia. 2014;57(10):2215-2221. [DOI] [PubMed] [Google Scholar]
- 48. Tsai AS, Wong TY, Lavanya R, et al. Differential association of retinal arteriolar and venular caliber with diabetes and retinopathy. Diabetes Res Clin Pract. 2011;94(2):291-298. [DOI] [PubMed] [Google Scholar]
- 49. Cheung CY, Lamoureux E, Ikram MK, et al. Retinal vascular geometry in Asian persons with diabetes and retinopathy. J Diabetes Sci Technol. 2012;6(3):595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ding J, Ikram MK, Cheung CY, Wong TY. Retinal vascular calibre as a predictor of incidence and progression of diabetic retinopathy. Clin Exp Optom. 2012;95(3):290-296. [DOI] [PubMed] [Google Scholar]
- 51. Sim DA, Keane PA, Tufail A, Egan CA, Aiello LP, Silva PS. Automated retinal image analysis for diabetic retinopathy in telemedicine. Curr Diab Rep. 2015;15(3):14. [DOI] [PubMed] [Google Scholar]
- 52. Wu H, Zhang X, Geng X, Dong J, Zhou G. Computer aided quantification for retinal lesions in patients with moderate and severe non-proliferative diabetic retinopathy: a retrospective cohort study. BMC Ophthalmol. 2014;14:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Philip S, Fleming AD, Goatman KA, et al. The efficacy of automated “disease/no disease” grading for diabetic retinopathy in a systematic screening programme. Br J Ophthalmol. 2007;91(11):1512-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Haritoglou C, Kernt M, Neubauer A, et al. Microaneurysm formation rate as a predictive marker for progression to clinically significant macular edema in nonproliferative diabetic retinopathy. Retina. 2014;34(1):157-164. [DOI] [PubMed] [Google Scholar]
- 55. Chaum E, Karnowski TP, Govindasamy VP, Abdelrahman M, Tobin KW. Automated diagnosis of retinopathy by content-based image retrieval. Retina. 2008;28(10):1463-1477. [DOI] [PubMed] [Google Scholar]
- 56. Niemeijer M, van Ginneken B, Russell SR, Suttorp-Schulten MS, Abramoff MD. Automated detection and differentiation of drusen, exudates, and cotton-wool spots in digital color fundus photographs for diabetic retinopathy diagnosis. Invest Ophthalmol Vis Sci. 2007;48(5):2260-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hansen AB, Hartvig NV, Jensen MS, Borch-Johnsen K, Lund-Andersen H, Larsen M. Diabetic retinopathy screening using digital non-mydriatic fundus photography and automated image analysis. Acta Ophthalmol Scand. 2004;82(6):666-672. [DOI] [PubMed] [Google Scholar]
- 58. Solanki K, Ramachandra C, Bhat S, Bhaskaranand M, Nittala MG, Sadda SR. EyeArt: automated, high-throughput, image analysis for diabetic retinopathy screening. Invest Ophthamol Vis Sci. 2015;56(7):1429-1429. [Google Scholar]
- 59. Tan PC, Cheung CYL, Lamoureux E, Hsu W, Lee ML, Wong TY. Cloud-based imaging program for diabetic retinopathy screening and monitoring [ARVO abstract]. Invest Ophthamol Vis Sci. 2015;56(7):1430-1430. [Google Scholar]
- 60. VisionQuest Biomedical. DR-RACS™. DR Screening and Image Quality 2015. Available at: http://visionquest-bio.com. Accessed August 31, 2015.
- 61. Ribeiro ML, Nunes SG, Cunha-Vaz JG. Microaneurysm turnover at the macula predicts risk of development of clinically significant macular edema in persons with mild nonproliferative diabetic retinopathy. Diabetes Care. 2013;36(5):1254-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pires R, Jelinek HF, Wainer J, Valle E, Rocha A. Advancing bag-of-visual-words representations for lesion classification in retinal images. PLOS ONE. 2014;9(6):e96814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Usman Akram M, Khalid S, Tariq A, Khan SA, Azam F. Detection and classification of retinal lesions for grading of diabetic retinopathy. Comput Biol Med. 2014;45:161-171. [DOI] [PubMed] [Google Scholar]
- 64. Hassan SS, Bong DB, Premsenthil M. Detection of neovascularization in diabetic retinopathy. J Digital Imaging. 2012;25(3):437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee J, Zee BC, Li Q. Detection of neovascularization based on fractal and texture analysis with interaction effects in diabetic retinopathy. PLOS ONE. 2013;8(12):e75699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Scotland GS, McNamee P, Philip S, et al. Cost-effectiveness of implementing automated grading within the national screening programme for diabetic retinopathy in Scotland. Br J Ophthalmol. 2007;91(11):1518-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fleming AD, Goatman KA, Philip S, Prescott GJ, Sharp PF, Olson JA. Automated grading for diabetic retinopathy: a large-scale audit using arbitration by clinical experts. Br J Ophthalmol. 2010;94(12):1606-1610. [DOI] [PubMed] [Google Scholar]
- 68. Scotland GS, McNamee P, Fleming AD, et al. Costs and consequences of automated algorithms versus manual grading for the detection of referable diabetic retinopathy. Br J Ophthalmol. 2010;94(6):712-719. [DOI] [PubMed] [Google Scholar]
- 69. Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27(1):45-88. [DOI] [PubMed] [Google Scholar]
- 70. Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Costa RA, Skaf M, Melo LA, Jr, et al. Retinal assessment using optical coherence tomography. Prog Retin Eye Res. 2006;25(3):325-353. [DOI] [PubMed] [Google Scholar]
- 72. Stanga PE, Bird AC. Optical coherence tomography (OCT): principles of operation, technology, indications in vitreoretinal imaging and interpretation of results. Int Ophthalmol. 2001;23(4-6):191-197. [DOI] [PubMed] [Google Scholar]
- 73. Schaudig UH, Glaefke C, Scholz F, Richard G. Optical coherence tomography for retinal thickness measurement in diabetic patients without clinically significant macular edema. Ophthalmic Surg Lasers. 2000;31(3):182-186. [PubMed] [Google Scholar]
- 74. Oshitari T, Hanawa K, Adachi-Usami E. Changes of macular and RNFL thicknesses measured by Stratus OCT in patients with early stage diabetes. Eye (Lond). 2009;23(4):884-889. [DOI] [PubMed] [Google Scholar]
- 75. Asefzadeh B, Fisch BM, Parenteau CE, Cavallerano AA. Macular thickness and systemic markers for diabetes in individuals with no or mild diabetic retinopathy. Clin Experiment Ophthalmol. 2008;36(5):455-463. [DOI] [PubMed] [Google Scholar]
- 76. Goebel W, Kretzchmar-Gross T. Retinal thickness in diabetic retinopathy: a study using optical coherence tomography (OCT). Retina. 2002;22(6):759-767. [DOI] [PubMed] [Google Scholar]
- 77. Bressler NM, Edwards AR, Antoszyk AN, et al. Retinal thickness on Stratus optical coherence tomography in people with diabetes and minimal or no diabetic retinopathy. Am J Ophthalmol. 2008;145(5):894-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Diabetic Retinopathy Clinical Research Network, Bressler NM, Miller KM, et al. Observational study of subclinical diabetic macular edema. Eye (Lond). 2012;26(6):833-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Krzystolik MG, Strauber SF, Aiello LP, et al. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114(8):1520-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Browning DJ, Glassman AR, Aiello LP, et al. Optical coherence tomography measurements and analysis methods in optical coherence tomography studies of diabetic macular edema. Ophthalmology. 2008;115(8):1366-1371, 1371 e1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Massin P, Girach A, Erginay A, Gaudric A. Optical coherence tomography: a key to the future management of patients with diabetic macular oedema. Acta Ophthalmol Scand. 2006;84(4):466-474. [DOI] [PubMed] [Google Scholar]
- 82. Chan A, Duker JS. A standardized method for reporting changes in macular thickening using optical coherence tomography. Arch Ophthalmol. 2005;123(7):939-943. [DOI] [PubMed] [Google Scholar]
- 83. Virgili G, Menchini F, Murro V, Peluso E, Rosa F, Casazza G. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2011(7):CD008081. [DOI] [PubMed] [Google Scholar]
- 84. Virgili G, Menchini F, Casazza G, et al. Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy. Cochrane Database Syst Rev. 2015;1:CD008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Glassman AR, Beck RW, Browning DJ, Danis RP, Kollman C, Diabetic Retinopathy Clinical Research Network Study Group. Comparison of optical coherence tomography in diabetic macular edema, with and without reading center manual grading from a clinical trials perspective. Invest Ophthalmol Vis Sci. 2009;50(2):560-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Davis MD, Bressler SB, Aiello LP, et al. Comparison of time-domain OCT and fundus photographic assessments of retinal thickening in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49(5):1745-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dodson P, Quany L, Leigh R, Wharton H. Ophthalmic Photographic Diabetic Review (OPDR): a virtual clinic approach for management of referable diabetic maculopathy, 2012. Available at: http://www.retinalscreening.co.uk/wp-content/uploads/2015/06/OPDR-poster.pdf. Accessed August 31, 2015.
- 88. Manjunath V, Papastavrou V, Steel DH, et al. Wide-field imaging and OCT vs clinical evaluation of patients referred from diabetic retinopathy screening. Eye (Lond). 2015;29(3):416-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lee CS, Lee AY, Sim DA, et al. Reevaluating the definition of intraretinal microvascular abnormalities and neovascularization elsewhere in diabetic retinopathy using optical coherence tomography and fluorescein angiography. Am J Ophthalmol. 2015;159(1):101-110 e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Prescott G, Sharp P, Goatman K, et al. Improving the cost-effectiveness of photographic screening for diabetic macular oedema: a prospective, multi-centre, UK study. Br J Ophthalmol. 2014;98(8):1042-1049. [DOI] [PubMed] [Google Scholar]
- 91. Olson J, Sharp P, Goatman K, et al. Improving the economic value of photographic screening for optical coherence tomography-detectable macular oedema: a prospective, multicentre, UK study. Health Technol Assess. 2013;17(51):1-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kanji A, Jojo V, Schmermer S, Connor C, SS M. Managing patients with early diabetic maculopathy via virtual SD-OCT clinics. Diabetic Eye J. 2015;March:34-38. Available at: http://www.eyescreening.org.uk/userFiles/File/DiabeticEyeJournal/DEJ4-1a.pdf
- 93. Shelton RL, Jung W, Sayegh SI, McCormick DT, Kim J, Boppart SA. Optical coherence tomography for advanced screening in the primary care office. J Biophotonics. 2014;7(7):525-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Oshitari T, Mitamura Y. Optical coherence tomography for complete management of patients with diabetic retinopathy. Curr Diabetes Rev. 2010;6(4):207-214. [DOI] [PubMed] [Google Scholar]
- 95. Cunha-Vaz J, Ribeiro L, Lobo C. Phenotypes and biomarkers of diabetic retinopathy. Prog Retin Eye Res. 2014;41:90-111. [DOI] [PubMed] [Google Scholar]
- 96. Elhabashy SA, Elbarbary NS, Nageb KM, Mohammed MM. Can optical coherence tomography predict early retinal microvascular pathology in type 1 diabetic adolescents without minimal diabetic retinopathy? A single-centre study. J Pediatr Endocrinol Metab. 2015;28(1-2):139-146. [DOI] [PubMed] [Google Scholar]
- 97. Trinavarat A, Pituksung A. Effective pupil dilatation with a mixture of 0.75% tropicamide and 2.5% phenylephrine: a randomized controlled trial. Indian J Ophthalmol. 2009;57(5):351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Soliman AZ, Silva PS, Aiello LP, Sun JK. Ultra-wide field retinal imaging in detection, classification, and management of diabetic retinopathy. Semin Ophthalmol. 2012;27(5-6):221-227. [DOI] [PubMed] [Google Scholar]
- 99. Silva PS, Cavallerano JD, Tolls D, et al. Potential efficiency benefits of nonmydriatic ultrawide field retinal imaging in an ocular telehealth diabetic retinopathy program. Diabetes Care. 2014;37(1):50-55. [DOI] [PubMed] [Google Scholar]
- 100. Silva PS, Cavallerano JD, Tolson AM, et al. Real-time ultrawide field image evaluation of retinopathy in a diabetes telemedicine program. Diabetes Care. 2015;38(9):1643-1649. [DOI] [PubMed] [Google Scholar]
- 101. Rasmussen ML, Broe R, Frydkjaer-Olsen U, et al. Comparison between Early Treatment Diabetic Retinopathy Study 7-field retinal photos and non-mydriatic, mydriatic and mydriatic steered widefield scanning laser ophthalmoscopy for assessment of diabetic retinopathy. J Diabetes Complications. 2015;29(1):99-104. [DOI] [PubMed] [Google Scholar]
- 102. Kiss S, Berenberg TL. Ultra widefield fundus imaging for diabetic retinopathy. Curr Diab Rep. 2014;14(8):514. [DOI] [PubMed] [Google Scholar]
- 103. Kernt M, Hadi I, Pinter F, et al. Assessment of diabetic retinopathy using nonmydriatic ultra-widefield scanning laser ophthalmoscopy (Optomap) compared with ETDRS 7-field stereo photography. Diabetes Care. 2012;35(12):2459-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol. 2012;154(3):549-559 e542. [DOI] [PubMed] [Google Scholar]
- 105. Price LD, Au S, Chong NV. Optomap ultrawide field imaging identifies additional retinal abnormalities in patients with diabetic retinopathy. Clin Ophthalmol. 2015;9:527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Etten PGv, Brouwere DD, Westers P, Ciriano JPM. Zero dilation ophthalmoscopy. J Ophthalmic Photography. 2014;36(2):55-62. [Google Scholar]
- 107. Yogesan K, Constable IJ, Barry CJ, Eikelboom RH, McAllister IL, Tay-Kearney ML. Telemedicine screening of diabetic retinopathy using a hand-held fundus camera. Telemedicine J. 2000;6(2):219-223. [DOI] [PubMed] [Google Scholar]
- 108. Querques G, Lattanzio R, Querques L, et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53(10):6017-6024. [DOI] [PubMed] [Google Scholar]
- 109. Xu J, Xu L, Du KF, et al. Subfoveal choroidal thickness in diabetes and diabetic retinopathy. Ophthalmology. 2013;120(10):2023-2028. [DOI] [PubMed] [Google Scholar]
- 110. Rayess N, Rahimy E, Ying GS, et al. Baseline choroidal thickness as a predictor for response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol. 2015;159(1):85-91 e81-83. [DOI] [PubMed] [Google Scholar]
- 111. Adhi M, Badaro E, Liu JJ, et al. Three-dimensional enhanced imaging of vitreoretinal interface in diabetic retinopathy using swept-source optical coherence tomography. Am J Ophthalmol. 2016;162:140-149.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yoshitake S, Murakami T, Uji A, et al. Clinical relevance of quantified fundus autofluorescence in diabetic macular oedema. Eye (Lond). 2015;29(5):662-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Makita S, Hong Y, Yamanari M, Yatagai T, Yasuno Y. Optical coherence angiography. Opt Express. 2006;14(17):7821-7840. [DOI] [PubMed] [Google Scholar]
- 114. Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Opt Express. 2007;15(7):4083-4097. [DOI] [PubMed] [Google Scholar]
- 115. Yasuno Y, Hong Y, Makita S, et al. In vivo high-contrast imaging of deep posterior eye by 1-microm swept source optical coherence tomography and scattering optical coherence angiography. Opt Express. 2007;15(10):6121-6139. [DOI] [PubMed] [Google Scholar]
- 116. Moult E, Choi W, Waheed NK, et al. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):496-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Thorell MR, Zhang Q, Huang Y, et al. Swept-source OCT angiography of macular telangiectasia type 2. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):369-380. [DOI] [PubMed] [Google Scholar]
- 118. Huang Y, Zhang Q, Thorell MR, et al. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lee JC, Wong BJ, Tan O, et al. Pilot study of Doppler optical coherence tomography of retinal blood flow following laser photocoagulation in poorly controlled diabetic patients. Invest Ophthalmol Vis Sci. 2013;54(9):6104-6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wang Y, Fawzi A, Tan O, Gil-Flamer J, Huang D. Retinal blood flow detection in diabetic patients by Doppler Fourier domain optical coherence tomography. Opt Express. 2009;17(5):4061-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Miura M, Hong YJ, Yasuno Y, Muramatsu D, Iwasaki T, Goto H. Three-dimensional vascular imaging of proliferative diabetic retinopathy by Doppler optical coherence tomography. Am J Ophthalmol. 2014;159(3):528-538 e523. [DOI] [PubMed] [Google Scholar]
- 122. Kim JE, Chung M. Adaptive optics for retinal imaging: current status. Retina. 2013;33(8):1483-1486. [DOI] [PubMed] [Google Scholar]
- 123. An L, Shen TT, Wang RK. Using ultrahigh sensitive optical microangiography to achieve comprehensive depth resolved microvasculature mapping for human retina. J Biomed Optics. 2011;16(10):106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Nelson DA, Burgansky-Eliash Z, Barash H, et al. High-resolution wide-field imaging of perfused capillaries without the use of contrast agent. Clin Ophthalmol. 2011;5:1095-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rha J, Jonnal RS, Thorn KE, Qu J, Zhang Y, Miller DT. Adaptive optics flood-illumination camera for high speed retinal imaging. Opt Express. 2006;14(10):4552-4569. [DOI] [PubMed] [Google Scholar]
- 126. Roorda A, Romero-Borja F, Donnelly Iii W, Queener H, Hebert T, Campbell M. Adaptive optics scanning laser ophthalmoscopy. Opt Express. 2002;10(9):405-412. [DOI] [PubMed] [Google Scholar]
- 127. Zawadzki RJ, Cense B, Zhang Y, Choi SS, Miller DT, Werner JS. Ultrahigh-resolution optical coherence tomography with monochromatic and chromatic aberration correction. Opt Express. 2008;16(11):8126-8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wang Q, Kocaoglu OP, Cense B, et al. Imaging retinal capillaries using ultrahigh-resolution optical coherence tomography and adaptive optics. Invest Ophthalmol Vis Sci. 2011;52(9):6292-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lombardo M, Parravano M, Serrao S, Ducoli P, Stirpe M, Lombardo G. Analysis of retinal capillaries in patients with type 1 diabetes and nonproliferative diabetic retinopathy using adaptive optics imaging. Retina. 2013;33(8):1630-1639. [DOI] [PubMed] [Google Scholar]
- 130. Lombardo M, Serrao S, Devaney N, Parravano M, Lombardo G. Adaptive optics technology for high-resolution retinal imaging. Sensors. 2013;13(1):334-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tam J, Dhamdhere KP, Tiruveedhula P, et al. Disruption of the retinal parafoveal capillary network in type 2 diabetes before the onset of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(12):9257-9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Burns SA, Elsner AE, Chui TY, et al. In vivo adaptive optics microvascular imaging in diabetic patients without clinically severe diabetic retinopathy. Biomed Opt Express. 2014;5(3):961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bek T. Fine structure in diabetic retinopathy lesions as observed by adaptive optics imaging. A qualitative study. Acta Ophthalmologica. 2014;92(8):753-758. [DOI] [PubMed] [Google Scholar]
- 134. Burgansky-Eliash Z, Nelson DA, Bar-Tal OP, Lowenstein A, Grinvald A, Barak A. Reduced retinal blood flow velocity in diabetic retinopathy. Retina. 2010;30(5):765-773. [DOI] [PubMed] [Google Scholar]
- 135. Burgansky-Eliash Z, Barak A, Barash H, et al. Increased retinal blood flow velocity in patients with early diabetes mellitus. Retina. 2011;32(1):112-119. [DOI] [PubMed] [Google Scholar]
- 136. Nelson DA, Krupsky S, Pollack A, et al. Special report: Noninvasive multi-parameter functional optical imaging of the eye. Ophthalmic Surg Lasers Imaging. 2005;36(1):57-66. [PubMed] [Google Scholar]
- 137. OcuSciences. OcuMet Beacon. 2015. Available at: http://www.ocumet.com/. Accessed August 25, 2015.
- 138. Field MG, Elner VM, Puro DG, et al. Rapid, noninvasive detection of diabetes-induced retinal metabolic stress. Arch Ophthalmol. 2008;126(7):934-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Khoobehi B, Firn K, Thompson H, Reinoso M, Beach J. Retinal arterial and venous oxygen saturation is altered in diabetic patients. Invest Ophthalmol Vis Sci. 2013;54(10):7103-7106. [DOI] [PubMed] [Google Scholar]
- 140. Jorgensen CM, Hardarson SH, Bek T. The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision-threatening retinopathy. Acta Ophthalmologica. 2014;92(1):34-39. [DOI] [PubMed] [Google Scholar]
- 141. Man RE, Sasongko MB, Xie J, et al. Associations of retinal oximetry in persons with diabetes. Clin Experiment Ophthalmol. 2015;43(2):124-131. [DOI] [PubMed] [Google Scholar]
- 142. Cunha-Vaz J, Faria de, Abreu JR, Campos AJ. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975;59(11):649-656. [DOI] [PMC free article] [PubMed] [Google Scholar]