Abstract
We investigated whether: 1) serum levels of 25-hydroxyvitamin D [25(OH)D]; and 2) single nucleotide polymorphisms (SNPs) in the group-specific component (GC) gene regulating serum 25(OH)D levels are associated with cognition in older individuals; and 3) whether causal relationships exist between 25(OH)D and cognition during aging.
Data from 1207 participants in the Baltimore Longitudinal Study of Aging were analyzed (mean follow-up 10.4 years) to test associations between serum 25(OH)D and cognition. Two GC SNPs were used to derive a composite genetic risk score associated with lower 25(OH)D concentrations.
Lower serum 25(OH)D and higher GC composite scores were associated with lower executive function at baseline. Mendelian randomization analyses suggested a causal relationship between lower serum 25(OH)D and poorer executive function and psychomotor speed. The SNP score was also associated with lower performance on measures of visuospatial abilities at baseline but with attenuated declines over time in visuospatial abilities and executive function.
Widespread associations between vitamin-D regulatory SNPs and cognition suggest a mechanistic basis for the relationship between serum 25(OH)D levels and cognition during aging.
Keywords: Vitamin D, cognitive performance, Mendelian randomization
1. Introduction
Both state (i.e. serum/plasma concentrations) and trait (i.e., polymorphic variation in genes) markers of vitamin-D are associated with several adverse health outcomes including hypertension, common cancers, and autoimmune diseases (Holick and Chen, 2008) as well as lower cognitive performance and an increased risk of cognitive decline (Balion et al., 2012, van der Schaft et al., 2013). Epidemiological and observational studies report associations between low serum levels of vitamin-D and age-related cognitive decline and dementia risk (Annweiler et al., 2011, Balion et al., 2012, Lee et al., 2009, Littlejohns et al., 2014, Llewellyn et al., 2010, Oudshoorn et al., 2008, Slinin et al., 2012, van der Schaft et al., 2013). However, these findings have been inconsistent (Breitling et al., 2012, McGrath et al., 2007, Slinin et al., 2010, Tolppanen et al., 2011). Furthermore, evidence from randomized controlled trials evaluating the effects of vitamin-D supplementation on cognition and dementia risk is scarce. Reverse causation or uncontrolled confounding may be important considerations in observational studies of vitamin-D. For example, given the role sunlight plays in the synthesis of vitamin-D, lower levels of serum vitamin-D could be the result of less exposure to sunlight due to an underlying disease rather than its cause (Berry et al., 2012).
The most widely accepted biomarker for vitamin-D is 25-hydroxyvitamin D (25(OH)D) (Ahn et al., 2010). Approximately 25% of variability in serum 25(OH)D concentrations can be explained by such factors as diet, exposure to sunlight, and dietary supplements (Snellman et al., 2009). Genetic factors also contribute significantly to variability in vitamin-D with heritability estimates ranging from 23% to 80% (Karohl et al., 2010, Shea et al., 2009). A number of studies have identified genetic polymorphisms associated with vitamin-D concentrations, including common variants in the group-specific component (GC) gene (i.e., Vitamin-D binding protein) (Ahn et al., 2010, Berry et al., 2012, Nissen et al., 2014, Wang et al., 2010) which play an important role in 25(OH)D distribution (Speeckaert et al., 2006).
GC is a key 25(OH)D carrier protein and determines bioavailability of vitamin-D metabolites to key target cells. Variants in GC may define genetic susceptibility to vitamin-D deficiency in certain individuals (Speeckaert et al., 2006). Protein-bound vitamin-D metabolites have a longer half-life in circulation which suggests that an important function of the vitamin-D binding protein (VDBP) is the stabilization and maintenance of 25(OH)D concentrations (Zella et al., 2008). There are three common isoforms of VDBP based on the combination of alleles from GC SNPs rs7041 and rs4588 (Fu et al., 2009; Speeckaert et al., 2006). In samples of European ancestry, the minor alleles of these SNPs are consistently associated with lower 25(OH)D levels (Ahn et al., 2010, Fu et al., 2009; Wang et al., 2010). Furthermore, healthy individuals with different VDBP genotypes have varying responses to the same vitamin-D dose (Fu et al., 2009) which may have important implications for randomized controlled trials of vitamin-D supplementation. It must be noted however, that although several genome-wide association studies in diverse populations have consistently demonstrated a relationship between polymorphisms in the GC and 25(OH)D concentrations, the precise interplay between GC SNPs, 25(OH)D concentrations, and binding/bioavailability of 25(OH)D are unclear (Ahn et al., 2010).
In the current study, we used data from the Baltimore Longitudinal Study of Aging (BLSA) to explore whether: 1) serum levels of 25(OH)D are associated with cognition during aging; 2) polymorphic variation in the GC gene regulating serum 25(OH)D levels are associated with cognition during aging; and 3) causal relationships exist between 25(OH)D levels and cognitive performance during aging.
2. Methods
2.1. Subjects
Written informed consent was obtained from participants at each visit, and the study was approved by local Institutional Review Board and the National Institute on Aging.
2.1.1. Baltimore Longitudinal Study of Aging
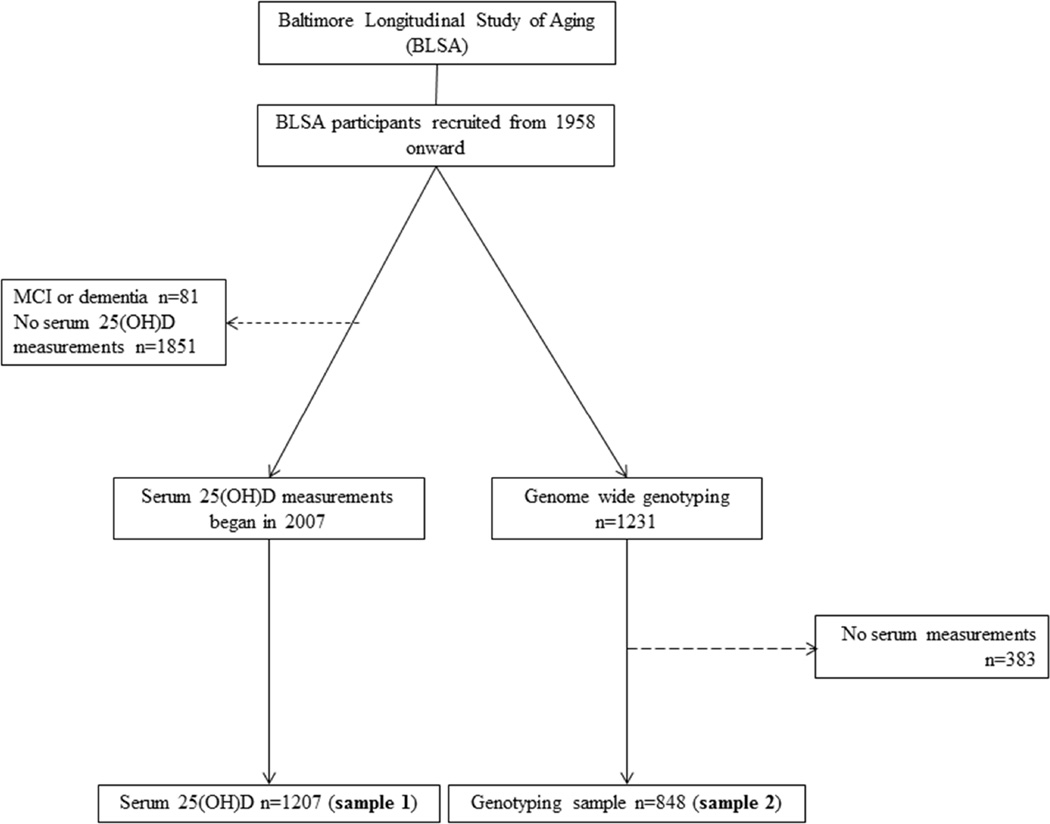
The BLSA is a prospective cohort study of community-dwelling adult volunteer participants in Baltimore, MD which began in 1958 (Figure 1) (Shock et al., 1984). Data from BLSA participants who did not develop dementia or mild cognitive impairment at any point during follow-up were used in the current analyses (n=1207, mean follow-up interval 10.4 years). All participants in this study sample were Caucasian. Participants received detailed examinations, including neurological, neuropsychological, and laboratory assessments every two years. Starting in 2003, participants aged 80 and older were seen annually.
Figure 1.
Schematic summary of the samples used in the present analyses from the Baltimore Longitudinal Study of Aging. Dashed lines indicate excluded participants.
2.2. Measurement of serum 25(OH)D
Concentration of 25(OH)D was measured in serum samples from BLSA participants collected annually over a 7 year period (2007–2014; n= 2411). Samples were stored at −80°C until analysis. Concentrations of 25(OH)D were assessed by liquid chromatography-mass spectrometry at Mayo Clinic laboratories (Rochester, MN, USA). The inter-assay CV was 10% while the lower limit of detection was 4 ng/mL.
2.3. Cognitive performance
Neuropsychological testing was performed by an experienced tester using a standardized protocol and battery. Mental status was measured with the Mini-Mental State Examination (Folstein et al., 1975). Memory was assessed using the California Verbal Learning Test (Delis et al., 1987). The Trail Making Test Parts A and B assessed attention and executive function respectively (Reitan, 1958), and the Clock Drawing to command (Rouleau et al., 1992) measured executive functioning. Letter and category fluency measured phonetic and semantic fluency (Benton, 1968). The Boston Naming Test (BNT) assessed confrontation naming. Two subtests from the Wechsler Adult Intelligence Scale-Revised (WAIS-R), Digit Span backwards and Similarities, assessed working memory and verbal concept formation and reasoning. The Wide Range Achievement Test Letter and Word Reading sub-test (WRAT) (Wilkinson, 1993) measured verbal abilities. The Purdue Pegboard assessed psychomotor speed (Tiffin and Asher, 1948), while the Rey-Osterrieth Complex Figure (Rey-O) (Rey, 1941) measured visuospatial abilities and figural memory.
2.4. SNP selection and genotyping
Blood samples were collected for DNA extraction, and genome-wide genotyping was completed for 1231 subjects using the Illumina 550K platform. The analysis was restricted to Caucasian participants and each analysis was further adjusted for the top two principal components derived from an EIGENSTRAT analysis utilizing ~10,000 randomly selected SNPs from the 550K SNP panel (Price et al., 2006). Genotyping was completed for 848 participants of European ancestry using a call rate of >98.5% without sex discrepancy based on X-chromosome homozygosity rates (Purcell et al., 2007). 501,704 autosomal SNPs that passed quality control (completeness ≥99%, MAF ≥1%, HWE ≥10−4) were used for imputation (Tanaka et al., 2009). In the BLSA, ~2.5 million HapMap SNPs were imputed using CEU sample (Phase II, release 22, build 36) as a reference using MACH (Li et al., 2010).
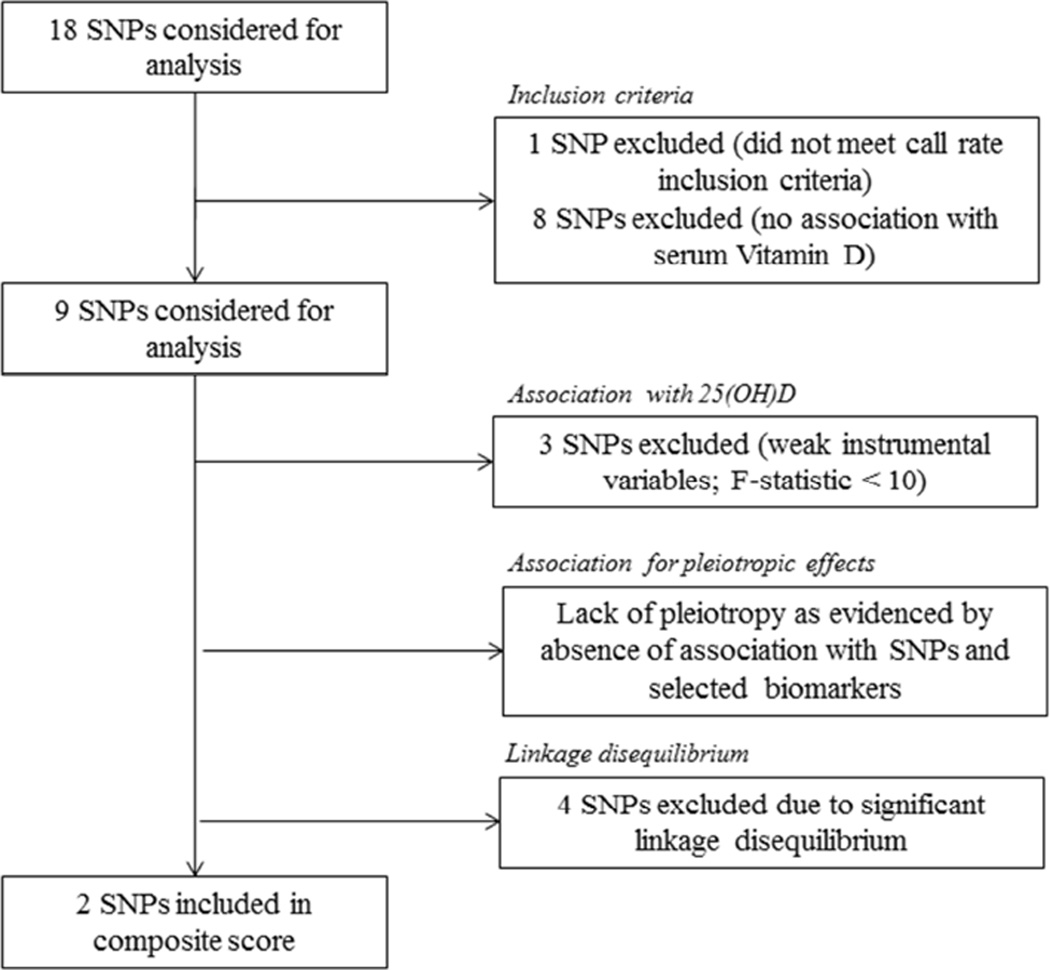
SNPs selected for analyses in the current report were identified from two large genome-wide association studies (GWAS) of circulating vitamin-D levels performed in participants of European ancestry (Ahn et al., 2010, Wang et al., 2010). SNPs significantly associated with circulating vitamin-D levels in these prior studies included those in genes encoding vitamin-D binding protein; DBP (i.e. group-specific component gene GC), CYP2R1, encoding the enzyme catalyzing C-25 hydroxylation of vitamin D3 and DHCR7, encoding the enzyme 7-dehydrocholesterol (7-DHC) reductase, which converts 7-DHC, a precursor of 25-hydroxyvitamin D3 to cholesterol (Ahn et al., 2010, Wang et al., 2010). Eighteen SNPs (see Table A.1) were identified based on these previous GWAS of circulating vitamin-D levels (Ahn et al., 2010, Wang et al., 2010). Figure 2 summarizes the exclusion criteria we applied to these 18 SNPs to further identify the most suitable SNPs in our current analyses. Briefly, we excluded one SNP that did not meet our call rate inclusion criteria and eight SNPs that were not significantly associated with serum vitamin-D levels in our study sample. Of the remaining nine SNPs, three were excluded because they were judged to be weak instrumental variables for Mendelian Randomization analyses, as estimated by an F-statistic <10.0 (Burgess et al., 2013) (please see Section 2.6). Of the six SNPs that remained, four (rs17467825, rs3755967, rs4588, and rs2298850) were in significant linkage disequilibrium and were not further considered (Broad Institute SNP Annotation and Proxy Search; SNAP, https://www.broadinstitute.org/mpg/snap/ldsearch.php; HapMap-3, Release-2, CEU sample). The two remaining SNPs (rs2282679 and rs7041) were judged to be appropriate for our current analyses as they were not in linkage disequilibrium (r2<0.3) and were within the GC gene. The F-statistic for the composite SNP was >10.0 (F-statistic = 10.6) after adjustment for age and sex.
Figure 2.
Selection of 25(OH)D single nucleotide polymorphisms for Mendelian randomization analysis
2.5. Linear mixed models analyses
Linear mixed models were used to determine the association between serum 25(OH)D and cognition as well as to examine the association of the composite GC SNP score with cognition (both at baseline and rates of change over time).
Age, sex (male=1, female=0), years of education, significant depressive symptoms (1=yes, 0=no), body mass index (BMI), and APOE ε4 status were included as covariates in all analyses. Because obesity has been associated with decreased bioavailability of vitamin-D (Wortsman et al., 2000), BMI was included as a covariate in all analyses. A cut-off score of ≥16 on the Center for Epidemiological Studies Depression Scale (CESD) (Radloff and Teri, 1986) was used to determine significant depressive symptomatology (Lewinsohn et al., 1997). Season of serum vitamin-D collection was included as a covariate in all analyses with serum 25(OH)D. Time was treated as a continuous variable in all models. For all analyses, outcome variables were standardized (Mean=0, SD=1).
2.6. Mendelian Randomization analysis
To examine the causal relationship between vitamin-D and cognition, a Mendelian randomization (MR) analysis was used. First, we regressed serum 25(OH)D on the GC SNP score, adjusting for the previously mentioned covariates. The strength of the association was assessed using the F-statistic, with values <10 considered weak instrumental variables not suitable for MR analyses (Burgess et al., 2013). Next, we regressed the GC SNP score on each cognitive outcome adjusting for the previously mentioned covariates.
A MR analysis was used by adopting a two-stage least-squares (2SLS) (Leong et al., 2014) estimator that regressed each outcome against predicted values of 25(OH)D level per composite GC SNP score using the command “ivreg2” in the Stata SE13.1 software package. This method allows for the estimation of the unconfounded association of genetically predicted concentrations of 25(OH)D with cognition. The Durbin-Wu-Hausman chi-square test for endogeneity in a regression estimated using instrumental variables was computed using the “ivendog” command in which the null hypothesis is that an ordinary least squares estimator of the same equation would yield consistent estimators (Baum et al., 2003). The MR approach thus controls for unmeasured confounders and reverse causality that may distort the directly assessed association between outcome and the exposure of interest (i.e., serum vitamin-D).
To determine whether the relationship between the GC SNPs and 25(OH)D was caused by other factors or genetic confounding, we first included several other available biomarkers (HbA1c, triglycerides, low density lipoproteins, high density lipoproteins, total cholesterol, systolic and diastolic blood pressure, D-dimer, CRP, IL-6, and IGF1) as covariates in a linear regression model examining the associations between GC SNPs and 25(OH)D (Berry et al., 2012).
Next, we tested for pleiotropy by examining the associations between GC SNPs and the biomarkers listed above after adjustment for 25(OH)D. If pleiotropy is present, the association between the GC SNPs and biomarkers should be strong and should not be affected by 25(OH)D adjustment. Finally, interactions between 25(OH)D and GC SNPs with other biomarkers mentioned above were also explored. A Bonferroni corrected p-value was used for the analyses (0.05/11 = <0.004; where the denominator is the number of biomarker tests for each SNP; Table A.2).
2.7. GC SNP composite score
Minor allele frequencies of GC SNPs in the BLSA were 0.25 for rs17467825, 0.25 for rs2282679, 0.25 for rs3755967, 0.28 for rs4588, 0.44 for rs7041, and 0.25 for rs2298850 which are consistent with previous studies (Wang et al., 2010). A composite GC SNP score was created using the two GC SNPs that passed MR requirements (rs2282679, rs7041) by summing the minor alleles in each SNP (Figure 1; sample 2).
3. Results
3.1. Demographic characteristics
Table 1 shows the demographic characteristics. In the BLSA sample examining associations between serum 25(OH)D and cognition, participants were 52.6 years of age (SD=16.0) and attained 17.1 (SD=2.6 years) years of education.
Table 1.
Demographic characteristics of BLSA sample
| n=1,207 | |
|---|---|
| Age at baseline, years M±SD, range | 52.6±16.0, 18–91 |
| Male, % | 49.8 |
| Education in years, M±SD, range | 17.1±2.6, 7–30 |
| 25(OH)D, M±SD, range | 32.9±12.3, 6.4–201.0 |
| CESD score ≥16, % | 5.9 |
| APOE ε4 status, % | 21.6 |
| Body mass index, M±SD, range | 26.9±4.9, 16.8–53.5 |
Abbreviations: CESD: Center for Epidemiological Studies Depression Scale
3.2. Aim 1: Serum 25(OH)D and cognition
On average, serum 25(OH)D concentrations were 32.9 ng/mL (SD=12.3, range 6.4–201) at the initial measurement (Figure 1; sample 1). At baseline, higher concentrations of 25(OH)D were associated with better performance on the clock drawing test (clock 3:25: β=0.02, 95% Confidence Interval (CI): 0.01,0.04, p=.007 and clock 11:10:β=0.02 95% CI: −0.001, 0.03, p=.056). Serum 25(OH)D was not significantly associated with any other cognitive measures (Table 2).
Table 2.
Observational and Mendelian randomization analyses for the causal association of the composite GC SNP score with 25-hydroxy-Vitamin D and cognition
| Observational Regression Analysis | Mendelian randomization analysis | ||||
|---|---|---|---|---|---|
| Outcome | Effect estimate* (95% CI) | p-value | Effect estimate* (95% CI) | p-value | Endogeneity p-value |
| CVLT total recall | 0.00 (−0.01,0.01) | .80 | 0.004 (−0.03, 0.03) | .82 | .98 |
| CVLT short delay | 0.00 (−0.01,0.01) | .85 | −0.001 (−0.03, 0.03) | .96 | .71 |
| CVLT long delay | 0.00 (−0.01,0.01) | .83 | −0.005 (−0.03, 0.02) | .73 | .62 |
| Digit span forward | −0.01 (−0.02,0.01) | .22 | 0.02 (−0.007, 0.05) | .16 | .22 |
| Digit span backward | −0.01 (−0.02,0.01) | .34 | 0.007 (−0.02, 0.03) | .67 | .81 |
| Digit symbol substitution | 0.01 (−0.0003,0.02) | .06 | 0.02 (−0.001, 0.04) | .07 | .12 |
| Similarities | −0.01 (−0.02,0.00) | .06 | 0.01 (−0.01, 0.03) | .35 | .68 |
| WRAT | 0.00 (−0.01,0.01) | .92 | 0.006 (−0.006, 0.05) | .15 | .44 |
| Mini-Mental State Exam | 0.00 (−0.01,0.01) | .87 | 0.01 (−0.01, 0.06) | .41 | .18 |
| Trail Making Test - A | 0.00 (−0.01,0.02) | .38 | 0.007 (−0.01, 0.02) | .51 | .19 |
| Trail Making Test - B | 0.01 (0.00,0.02) | .14 | 0.04 (0.01, 0.08) | .006 | .001 |
| Category fluency | 0.00 (−0.01,0.01) | .72 | 0.02 (−0.005, 0.03) | .14 | .08 |
| Letter fluency | 0.00 (−0.01,0.01) | .78 | −0.01 (−0.04, 0.01) | .25 | .45 |
| Boston naming test | 0.00 (−0.01,0.01) | .50 | −0.04 (−0.07, −0.01) | .004 | .56 |
| Rey-O copy | 0.01 (−0.01,0.03) | .45 | 0.01 (−0.01, 0.04) | .35 | .62 |
| Rey-O short delay | 0.00 (−0.02,0.02) | .87 | 0.02 (−0.01, 0.06) | .17 | .29 |
| Rey-O long delay | −0.01 (−0.03,0.02) | .60 | 0.02 (−0.02, 0.08) | .30 | .42 |
| Clock 3:25 | 0.02 (0.01,0.04) | .007 | 0.05 (0.01, 0.08) | .002 | .001 |
| Clock 11:10 | 0.02 (−0.001,0.03) | .06 | 0.03 (0.006, 0.06) | .02 | .03 |
| Clock copy | 0.00 (−0.01,0.02) | .49 | 0.008 (−0.01, 0.03) | .46 | .67 |
| Pegboard dominant hand | 0.00 (−0.01,0.01) | .99 | 0.02 (0.006, 0.05) | .01 | .003 |
| Pegboard nondominant hand | 0.00 (−0.01,0.01) | .54 | 0.04 (0.01, 0.06) | .003 | .009 |
Adjusted for age, sex, education, APOEε4, depressive symptoms, body mass index, and season of 25(OH)D collection.
Abbreviations: CVLT - California Verbal Learning Test; Rey-O: Rey-Osterrieth Complex Figure Test; WRAT - Wide Range Achievement Test
3.3. Aim 2: Composite GC SNP score and cognition
A one unit increase in the GC composite SNP score was associated with a reduction in serum Vitamin-D levels of approximately 0.61 ng/mL (95% CI −0.93, −0.40; p<.0001) after adjustment for age and sex.
There was no clear evidence for pleiotropic effects of the selected SNPs since they were not significantly associated with any of the variables examined at baseline (Table S2). This illustrates that the GC SNP score can be used largely as an unconfounded instrument to assess causality between serum 25(OH)D and cognition. Prior studies have used these GC SNPs as instrumental variables in MR analyses (Berry et al., 2012, Vimaleswaran et al., 2013).
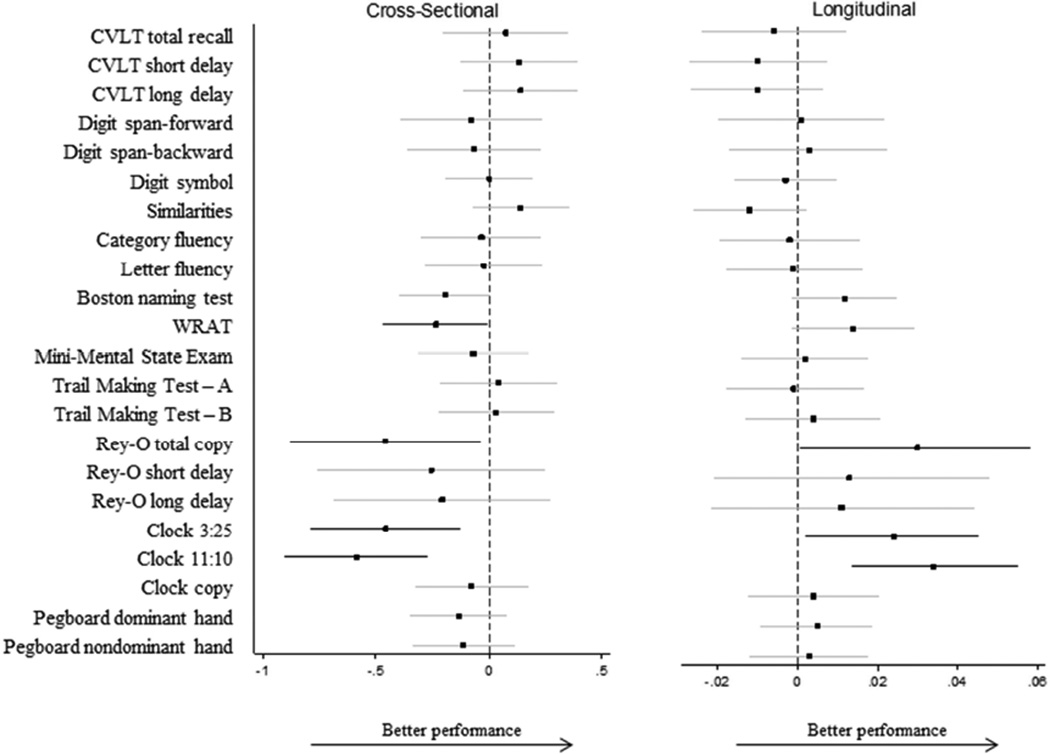
At baseline, higher composite GC SNP score (i.e., more risk alleles) was associated with lower performance on the Clock Drawing tests [3:25 (β=−0.47; 95% CI: −0.81, −0.13; p=.007); 11:10 (β=−0.58; 95% CI: −0.89, −0.26; p=.003; Figure 3)]; however a higher GC composite SNP score was associated with attenuated declines over time on the Clock drawing test [3:25 (β=0.03; 95% CI: 0.01, 0.05; p=.0001)]. The composite GC score was also associated with lower scores on the WRAT (β=−0.23; 95% CI −0.46, −0.002; p=.04) and the Rey-O total copy (β=−0.46; 95% CI −0.87, −0.03; p=.03) at baseline, but with attenuated declines on the Rey-O total copy over time (β=0.03; 95% CI 0.001, 0.06; p=.04).
Figure 3. Association of composite GC SNP score with cognitive performance.
Effect estimates are adjusted for age, sex, education, APOEε4, body mass index, and depressive symptoms. Bold lines are significant at p<.05. All cognitive outcomes have been standardized with M=0, SD=1. Abbreviations: CVLT – California Verbal Learning Test; Rey-O: Rey-Osterrieth Complex Figure Test; WRAT – Wide Range Achievement Test
3.4. Aim 3: Evaluation of causal association between serum Vitamin-D and cognition using MR
In MR analyses to examine a causal association between serum 25(OH)D concentrations and cognition (Figure 1, samples 1 and 2) we observed a causal association between 25(OH)D and the Clock Drawing task (3:25 β=0.05; 95% CI 0.01, 0.08; p=.002; test for endogeneity p=.001; 11:10 β=0.03; 95% CI 0.006, 0.06; p=.02; test for endogeneity p=.03; Table 2) as well as the Trail Making Test–Part B (β=0.04; 95% CI 0.01, 0.08; p=.006; test for endogeneity p=.001). The MR results also showed a significant causal association between 25(OH)D and psychomotor speed (pegboard dominant hand β=0.02; 95% CI 0.006, 0.05; test for endogeneity p=.003; pegboard nondominant hand β=0.04; 95% CI 0.01, 0.06; test for endogeneity p=.01).
3.5. Sensitivity analyses
We performed several sensitivity analyses to explore potential explanations for attenuated declines noted in longitudinal analyses of the GC SNP score and cognitive performance. We first tested whether extreme values of serum vitamin-D, GC SNP scores, or age influenced these results. In none of the cases did extreme values affect significant findings. We also tested whether age, sex, APOE, or BMI affected the relationship between vitamin-D (serum and GC SNP score) and the outcome by adding an interaction term as an additional covariate (e.g., BMI×serum 25(OH)D, BMI×GC SNP score). In none of the cases did the interaction term add significantly to the amount of variance explained. To determine if results were due to regression to the mean, a linear mixed effects model was used with intercept and time as both fixed and random effects using an unstructured correlation matrix. If the correlation between the random intercept and random slope is positive, higher baseline values are associated with slower rates of decline. We did not observe a significant positive correlation between the random intercept (baseline cognitive values) and random slope in the mixed effects models.
4. Discussion
The aim of the current study was to examine the influence of both state and trait markers of vitamin-D on cognition during aging in non-demented older individuals. We asked whether: 1) serum 25(OH)D concentrations are associated with cognition; 2) a composite GC SNP risk score related to lower serum 25(OH)D concentrations was associated with cognition; and 3) causal relationships exist between serum 25(OH)D and cognition.
We find that low levels of serum vitamin-D are associated with lower performance on measures of executive function (i.e. clock drawing to command). SNPs within the GC gene that are associated with lower serum concentrations of vitamin-D are also associated with lower performance in executive function, visuospatial and verbal abilities. Furthermore, using Mendelian randomization, we are able to demonstrate that lower concentrations of serum vitamin-D may causally mediate lower performance in executive function and psychomotor speed during aging. We did not find any associations with serum vitamin-D or the GC composite SNP score on memory performance.
Our results indicate domain-specific effects of vitamin-D on cognition during aging suggesting that executive function, visuospatial and verbal abilities, and psychomotor speed may be especially susceptible to perturbations in vitamin-D physiology. The effects of genetic variation in vitamin-D related SNPs on specific cognitive domains appear to be more extensive compared to the effects of serum vitamin-D levels alone. While we observed a significant detrimental effect of GC SNPs on cognition at baseline, suggesting that genetic predisposition to lower serum vitamin-D levels is associated with lower cognitive performance, these individuals show attenuated declines in cognition during follow-up. It is worth noting that the mean age of our sample at baseline was 52 years with participants having achieved an average of 17 years of education. Given these analyses were performed in a high functioning, middle-aged, and well educated sample, it is plausible that greater cognitive reserve in these individuals may attenuate longitudinal detrimental effects of both genetic and environmental risk factors for cognitive decline. It is plausible that longer follow-up periods may capture differential rates of cognitive decline as our sample entered the highest risk periods for cognitive impairment and dementia. We also suggest that in this sample of healthy older individuals who maintain cognitive health throughout follow-up, these findings may point to compensatory mechanisms recruited in those ‘at-risk’ to preserve cognitive function over time. These may include as yet unidentified gene×environment interactions that overcome the effects of genetic vulnerability to lower serum vitamin-D levels during aging. The eventual failure of such compensatory mechanisms in older individuals at genetic risk for lower vitamin-D levels may predispose them to accelerated cognitive decline during aging. While the precise mechanisms responsible for the attenuated declines in longitudinal cognitive performance associated with genetic risk for lower vitamin-D levels in our sample are unclear, our sensitivity analyses suggest that neither extreme values in serum vitamin-D concentrations nor age were driving these findings. Furthermore, regression to the mean was ruled out as there was not a significant positive correlation between the random intercept (baseline cognitive values) and random slope in the mixed effects models.
Despite accumulating evidence linking vitamin-D deficiency with various diseases, a recent report from the Institute of Medicine (IOM, 2011) on ‘dietary reference intakes for calcium and vitamin D’ concluded that the evidence about the potential benefits of vitamin-D supplementation is unreliable. Similarly, while several previous observational studies have consistently shown an association between low serum vitamin-D levels and lower cognitive performance (Annweiler et al., 2011, Balion et al., 2012, Lee et al., 2009, Littlejohns et al., 2014, Llewellyn et al., 2010, Oudshoorn et al., 2008, Slinin et al., 2012, van der Schaft et al., 2013), they do not establish a causal relationship between peripheral vitamin-D concentrations and cognition. Equally importantly, such observational studies may be confounded by reverse causality wherein it is impossible to determine whether lifestyle factors such as a poor diet or lower exposure to sunlight in cognitively impaired subjects, relative to healthy individuals may account for their lower serum vitamin-D concentration. The power of Mendelian randomization relies upon the use of genetic variants associated with serum vitamin-D levels as unconfounded instrumental variables to establish a causal relationship between peripheral vitamin-D concentrations and cognitive performance. For this reason, the MR approach has been referred to as nature’s randomized clinical trial in the post-genome era (Thanassoulis et al., 2009).
The molecular mechanisms mediating a protective role of vitamin-D on cognition are diverse and may include neurotrophic effects mediated through nerve growth factor and glial cell line derived neurotrophic factor (Wrzosek et al., 2013). Moreover, vitamin-D has been shown to protect against both age-related inflammatory changes within key memory circuits as well as excitotoxic neuronal damage (Wrzosek et al., 2013).
The strengths of our study include a well characterized and longitudinally followed cohort of older individuals with both serial cognitive and neuroimaging assessments. Furthermore, the availability of genetic data on GC SNPs, together with biochemical measures of analytes related to potentially confounding biological pathways, allowed us to apply a robust MR strategy to examine causal relationships between vitamin-D levels and cognition. Some limitations and methodological considerations must be acknowledged. As serum measurements of vitamin-D levels were only available over a 7 year period, our sample size was limited to participants who had cognitive assessments performed during this interval. Another potential limitation of our study is that our analyses only used participants of European ancestry that were highly educated, which may limit the generalizability of our results.
5. Conclusion
In conclusion, we demonstrated that vitamin-D exerts both state and trait-dependent effects on brain function during aging. The widespread associations between vitamin-D regulatory SNPs and cognition as well as results from the Mendelian randomization analyses, suggest a mechanistic basis for the relationship between vitamin-D and cognition during aging.
Highlights.
Lower serum 25(OH)D is associated with lower executive function during aging.
GC gene variants are associated with lower serum 25(OH)D concentrations.
GC risk allele carriers show lower executive function, visuospatial and verbal abilities.
Mendelian randomization tested causality between serum 25(OH)D and cognition.
Low serum 25(OH)D may be causally linked to poorer executive function and psychomotor speed during aging.
Acknowledgments
We are grateful to the Baltimore Longitudinal Study of Aging participants and staff for their dedication to these studies. This work was supported entirely by the Intramural Research Program of the National Institute of Health (NIH), National Institute on Aging (NIA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix
Table A.1.
Single nucleotide polymorphisms identified in previous genome-wide association studies16, 20 and their association with serum Vitamin D in the Baltimore Longitudinal Study of Aging
| Nearest gene | Role in Vitamin D | SNP | MAF | Association with serum Vitamin D‡ (β; p-value) |
|---|---|---|---|---|
| GC | Metabolism | rs17467825 | 0.22 | −3.44; p<.0001 |
| rs3755967 | 0.22 | −3.44; p<.0001 | ||
| rs2298850 | 0.20 | −3.48; p<.0001 | ||
| rs7041 | 0.39 | −2.72; p<.0001 | ||
| rs4588 | 0.22 | −3.41; p<.0001 | ||
| rs842999 | 0.36 | −2.51; p<.0001 | ||
| rs2282679 | 0.22 | −3.44; p<.0001 | ||
| rs12512631 | 0.30 | −1.48; p<.0001 | ||
| rs16846876 | 0.26 | −2.34; p<.0001 | ||
| CYP24A1 | Metabolism | rs73913757 | 0.18 | * |
| rs6013897 | 0.23 | 0.72; p>.05 | ||
| CYP27B1 | Metabolism | rs10877012 | 0.35 | 0.17; p>.05 |
| CYP2R1 | Metabolism | rs2060793 | 0.36 | −1.11; p>.05 |
| rs1993116 | 0.34 | −1.06; p>.05 | ||
| rs12794714 | 0.48 | −1.32; p>.05 | ||
| rs10741567 | 0.33 | 0.55; p>.05 | ||
| rs2060793 | 0.36 | −1.11; p>.05 | ||
| DHCR7 | Synthesis | rs12785878 | 0.47 | 0.77; p>.05 |
Poor imputation quality;
Models adjusted for age and sex; MAF: minor allele frequency
Table A.2.
Association between GC SNPs and other biomarkers adjusted for 25(OH)D and sex
| Biomarker | SNP | Coefficient (95% CI) | p-value | Interaction p-value† |
|---|---|---|---|---|
| Lipid markers | ||||
| Triglycerides | rs7041 | 0.02 (−0.05,0.08) | 0.58 | 0.25 |
| rs2282679 | 0.02 (−0.05,0.08) | 0.55 | 0.25 | |
| LDL | rs7041 | −0.02 (−0.06,0.03) | 0.43 | 0.36 |
| rs2282679 | −0.02 (−0.06,0.03) | 0.43 | 0.38 | |
| HDL | rs7041 | 0.00 (−0.04,0.04) | 0.84 | 0.33 |
| rs2282679 | 0.00 (−0.04,0.04) | 0.92 | 0.33 | |
| Cholesterol | rs7041 | 0.00 (−0.03,0.03) | 0.95 | 0.10 |
| rs2282679 | 0.00 (−0.03,0.03) | 0.89 | 0.10 | |
| Cardiovascular disease related markers | ||||
| Diastolic BP | rs7041 | 0.00 (−0.01,0.02) | 0.78 | 0.33 |
| rs2282679 | 0.00 (−0.02,0.02) | 0.75 | 0.33 | |
| Systolic BP | rs7041 | 0.01 (−0.01,0.02) | 0.34 | 0.79 |
| rs2282679 | 0.01 (−0.01,0.02) | 0.34 | 0.79 | |
| HbA1c | rs7041 | 0.00 (−0.01,0.02) | 0.76 | 0.92 |
| rs2282679 | 0.00 (−0.01,0.02) | 0.76 | 0.92 | |
| IGF-1 | rs7041 | 0.01 (−0.05,0.07) | 0.77 | 0.90 |
| rs2282679 | 0.01 (−0.06,0.07) | 0.86 | 0.89 | |
| Inflammatory markers | ||||
| IL-6 | rs7041 | −0.34 (−0.67,−0.00) | 0.05 | 0.03 |
| rs2282679 | −0.34 (−0.67,−0.00) | 0.05 | 0.03 | |
| CRP | rs7041 | −0.43 (−1.08,0.22) | 0.19 | 0.39 |
| rs2282679 | −0.43 (−1.08,0.22) | 0.19 | 0.39 | |
| Coagulation markers | ||||
| D-dimer | rs7041 | −0.02 (−0.14,0.10) | 0.71 | 0.56 |
| rs2282679 | −0.02 (−0.13,0.10) | 0.78 | 0.57 | |
| Fibrogen | rs7041 | −0.16 (−0.39, 0.06) | 0.16 | 0.02 |
| rs2282679 | −0.16 (−0.39, 0.06) | 0.16 | 0.02 | |
Bonferroni corrected p-value 0.05/11 = 0.004;
- Where the biomarkers have been log transformed to achieve a normal distribution
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors report no disclosures.
References
- Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Beauchet O. Serum vitamin D deficiency as a predictor of incident non-Alzheimer dementias: a 7-year longitudinal study. Dement Geriatr Cogn Disord. 2011;32:273–278. doi: 10.1159/000334944. [DOI] [PubMed] [Google Scholar]
- Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, Llewellyn DJ, Raina P. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79 doi: 10.1212/WNL.0b013e31826c197f. 1397-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum CF, Schaffer ME, Stillman S. Instrumental variables and GMM: Estimation and testing. Stata Journal. 2003;3:1–31. [Google Scholar]
- Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Berry DJ, Vimaleswaran KS, Whittaker JC, Hingorani AD, Hyppönen E. Evaluation of Genetic Markers as Instruments for Mendelian Randomization Studies on Vitamin D. PLoS ONE. 2012;7:e37465. doi: 10.1371/journal.pone.0037465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling LP, Perna L, Muller H, Raum E, Kliegel M, Brenner H. Vitamin D and cognitive functioning in the elderly population in Germany. Exp Gerontol. 2012;47:122–127. doi: 10.1016/j.exger.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–665. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, Research edition. New York, NY: The Psychological Corporation; 1987. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fu L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response to serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42:1174–1177. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press; 2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK56070/ [PubMed] [Google Scholar]
- Karohl C, Su S, Kumari M, Tangpricha V, Veledar E, Vaccarino V, Raggi P. Heritability and seasonal variability of vitamin D concentrations in male twins. Am J Clin Nutr. 2010;92:1393–1398. doi: 10.3945/ajcn.2010.30176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DM, Tajar A, Ulubaev A, Pendleton N, O'Neill TW, O'Connor DB, Bartfai G, Boonen S, Bouillon R, Casanueva FF, Finn JD, Forti G, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Lean ME, Punab M, Silman AJ, Vanderschueren D, Wu FC EMAS study group. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80:722–729. doi: 10.1136/jnnp.2008.165720. [DOI] [PubMed] [Google Scholar]
- Leong A, Rehman W, Dastani Z, Greenwood C, Timpson N, Langsetmo L, Berger C, METASTROKE. Fu L, Wong BY, Malik S, Malik R, Hanley DA, Cole DE, Goltzman D, Richards JB. The causal effect of vitamin D binding protein (DBP) levels on calcemic and cardiometabolic diseases: a Mendelian randomization study. PLoS Med. 2014;11:e1001751. doi: 10.1371/journal.pmed.1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PHM, Fried L, Kestenbaum BR, Kuller LH, Langa KM, Lopez OL, Kos K, Soni M, Llewellyn DJ. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83:920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn DJ, Lang IA, Langa KM, Muniz-Terrera G, Phillips CL, Cherubini A, Ferrucci L, Melzer D. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Scragg R, Chant D, Eyles D, Burne T, Obradovic D. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007;29:49–54. doi: 10.1159/000108918. [DOI] [PubMed] [Google Scholar]
- Nissen J, Rasmussen LB, Ravn-Haren G, Andersen EW, Hansen B, Andersen R, Mejborn H, Madsen KH, Vogel U. Common Variants in CYP2R1 and GC Genes Predict Vitamin D Concentrations in Healthy Danish Children and Adults. PLoS ONE. 2014;9:e89907. doi: 10.1371/journal.pone.0089907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudshoorn C, Mattace-Raso FU, van der Velde N, Colin EM, van der Cammen TJ. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;25:539–543. doi: 10.1159/000134382. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS, Teri L. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clin Gerontol. 1986;5:119–136. [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encéphalopathie traumatique. (Les problems.). The psychological examination in cases of traumatic encepholopathy. Archives de Psychologie. 1941;28:215–285. [Google Scholar]
- Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K. Quantitative and qualitative analyses of clock drawings in Alzheimer's and Huntington's disease. Brain Cogn. 1992;18:70–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D'Agostino RBS, Ordovas JM, O'Donnell CJ, Dawson-Hughes B, Vasan RS, Booth SL. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2009;63:458–464. doi: 10.1038/sj.ejcn.1602959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shock NW, Gruelich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD. Washington, DC: US Government Printing Office; 1984. Normal Human Aging: The Baltimore Longitudinal Study of Aging. [Google Scholar]
- Slinin Y, Paudel M, Taylor BC, Ishani A, Rossom R, Yaffe K, Blackwell T, Lui LY, Hochberg M, Ensrud KE Study of Osteoporotic Fractures Research Group. Association between serum 25(OH) vitamin D and the risk of cognitive decline in older women. J Gerontol A Biol Sci Med Sci. 2012;67:1092–1098. doi: 10.1093/gerona/gls075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinin Y, Paudel ML, Taylor BC, Fink HA, Ishani A, Canales MT, Yaffe K, Barrett-Connor E, Orwoll ES, Shikany JM, Leblanc ES, Cauley JA, Ensrud KE. Osteoporotic Fractures in Men (MrOS) Study Research Group. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology. 2010;74:33–41. doi: 10.1212/WNL.0b013e3181c7197b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman G, Melhus H, Gedeborg R, Olofsson S, Wolk A, Pedersen NL, Michaelsson K. Seasonal genetic influence on serum 25-hydroxyvitamin D levels: a twin study. PLoS One. 2009;4:e7747. doi: 10.1371/journal.pone.0007747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeckaert M, Huang G, Delanghe JR, Taes YE. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372:33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Scheet P, Giusti B, Bandinelli S, Piras MG, Usala G, Lai S, Mulas A, Corsi AM, Vestrini A, Sofi F, Gori AM, Abbate R, Guralnik J, Singleton A, Abecasis GR, Schlessinger D, Uda M, Ferrucci L. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am J Hum Genet. 2009;84:477–482. doi: 10.1016/j.ajhg.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanassoulis G, O'Donnell CJ. Mendelian randomization: Nature's randomized trial in post-genome era? JAMA. 2009;301:2386–2388. doi: 10.1001/jama.2009.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–237. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- Tolppanen AM, Williams DM, Lawlor DA. The association of serum ionized calcium and vitamin D with adult cognitive performance. Epidemiology. 2011;22:113–117. doi: 10.1097/EDE.0b013e3181f74683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaft J, Koek HL, Dijkstra E, Verhaar HJ, van der Schouw YT, Emmelot-Vonk MH. The association between vitamin D and cognition: a systematic review. Ageing Res Rev. 2013;12:1013–1023. doi: 10.1016/j.arr.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, Cooper JD, Dastani Z, Li R, Houston DK, Wood AR, Michaelsson K, Vandenput L, Zgaga L, Yerges-Armstrong LM, McCarthy MI, Dupuis J, Kaakinen M, Kleber ME, Jameson K, Arden N, Raitakari O, Viikari J, Lohman KK, Ferrucci L, Melhus H, Ingelsson E, Byberg L, Lind L, Lorentzon M, Salomaa V, Campbell H, Dunlop M, Mitchell BD, Herzig KH, Pouta A, Hartikainen AL, Genetic Investigation of Anthropometric Traits-GIANT Consortium. Streeten EA, Theodoratou E, Jula A, Wareham NJ, Ohlsson C, Frayling TM, Kritchevsky SB, Spector TD, Richards JB, Lehtimaki T, Ouwehand WH, Kraft P, Cooper C, Marz W, Power C, Loos RJ, Wang TJ, Jarvelin MR, Whittaker JC, Hingorani AD, Hypponen E. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test 3. Wilmington, DE: Wide Range, Inc.; 1993. [Google Scholar]
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- Wrzosek M, Lukaszkiewicz J, Wrzosek M, Jakubczyk A, Matsumoto H, Piatkiewicz P, Radziwon-Zaleska M, Wojnar M, Nowicka G. Vitamin D and the central nervous system. Pharmacol Rep. 2013;65:271–278. doi: 10.1016/s1734-1140(13)71003-x. [DOI] [PubMed] [Google Scholar]
- Zella LA, Shevde NK, Hollis BW, Cooke NE, Pike JW. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinol. 2008;149:3656–3667. doi: 10.1210/en.2008-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]