Abstract
Serotonin from the descending pain modulatory pathway is critical to nociceptive processing. Its effects on pain modulation may either be inhibitory or facilitatory, depending on the type of pain and which receptors are involved. Little is known about the role of serotonergic systems in bladder nociceptive processing. These studies examined the effect of systemic administration of the serotonin precursor, 5-hydroxytryptophan (5-HTP), on normal bladder and somatic sensation in rats. ELISA was used to quantify peripheral and central changes in serotonin and its major metabolite following 5-HTP administration, and the potential role of the 5-HT3 receptor on changes in bladder sensation elicited by 5-HTP was investigated.
5-HTP produced bladder hypersensitivity and somatic analgesia. The pro-nociceptive effect of 5-HTP was attenuated by intrathecal, but not systemic, ondansetron. Peripheral increases in serotonin, its metabolism and rate of turnover were detectable within 30 min of 5-HTP administration. Significant enhancement of serotonin metabolism was observed centrally. These findings suggest that 5-HTP increases serotonin, which may then affect descending facilitatory systems to produce bladder hypersensitivity via activation of spinal 5-HT3 receptors.
Keywords: visceral, urinary bladder, hypersensitivity, 5-hydroxytryptophan, serotonin
Introduction
It is well-established that the descending serotonergic system from the rostral ventromedial medulla (RVM) is involved in the modulation of spinal nociceptive transmission[1]. While the earliest studies indicated an inhibitory role of this system in pain processing[2,3], later evidence revealed that descending serotonergic influences can be pronociceptive in a number of different models[4-6]. Little is known about the role of serotonergic systems in urinary bladder nociceptive processing. To date, the only study investigating serotonergic modulation of bladder pain was conducted by Randich and colleagues who demonstrated that inflammation-induced bladder hypersensitivity was significantly attenuated by intrathecal administration of non-specific and specific serotonergic receptor antagonists, including the 5-HT3 receptor[7]. The bidirectional effects of serotonin are likely a consequence of activation of the diverse family of receptor subtypes and the type of pain.
In the present studies, systemic administration of 5-HTP was employed to delineate the role of serotonin in somatic sensation and nociceptive processing related to the urinary bladder. The main effect of 5-HTP is an increase in serotonin levels, both peripherally and centrally, since it readily crosses the blood-brain barrier[8]. An antagonist to the 5-HT3 receptor was given both systemically and intrathecally to determine the role of 5-HT3 receptor mechanisms underlying serotonergic modulation of visceral pain.
Materials and Methods
Animals
Female Lewis rats 11-12 weeks of age were used. Female rats were chosen since urinary bladder disorders associated with pain are prevalent in and primarily affect the female population. Estrous cycle was not controlled for. Food and water were available on an ad libitum basis. A 12:12-h light-dark cycle was maintained. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Bladder Nociceptive Testing
Under isoflurane anesthesia, a 22-gauge polytetrafluoroethylene angiocatheter was placed into the bladder via the urethra and held in place by a tight suture around the distal urethral orifice. Electrodes (silver wire) were inserted into the external oblique musculature immediately superior to the inguinal ligament. Anesthesia was lowered until flexion reflexes were present in the hindlimbs, but spontaneous escape behaviors were absent (0.75-1%). Urinary bladder distensions (UBDs; 20 sec) were produced using compressed air and a previously described distension control device[9], and intravesical pressure was monitored using an in-line, low volume pressure transducer. Visceromotor responses (VMRs; contraction of abdominal and hindlimb musculature), recorded as EMG activity, were measured via the electrodes using standard differential amplification and rectification and saved on a computer. Body temperature was maintained at ~37°C by a heating pad.
Somatic Nociceptive Testing
The method described by Hargreaves et al.[10] was used in tests of thermal sensitivity. Rats were confined within a clear Plexiglas cage (11 × 23 × 16 cm3) placed on an elevated piece of glass. Rats were accommodated to the apparatus for two days prior to experimental trials. An infrared heat source was positioned under the glass floor so the beam struck the glabrous skin and toe pads of the hind paw. The latency to withdraw the hind paw was measured. Three trials (3 min inter-trial interval) were conducted on each hind paw and averaged to obtain the mean response latency for each hind paw. These values were averaged to obtain a single measure of thermal sensitivity.
Specific Experiments
Experiment 1
To determine the effect of 5-HTP on visceral nociceptive processing, rats were treated s.c. with 5-HTP (1, 10 or 100 mg/kg in distilled water) or vehicle and after 15 min, were anesthetized and instrumented for bladder nociceptive testing as described above. Thirty min after 5-HTP, three 20 s, constant-pressure air distensions of the urinary bladder at pressures of 60 mm Hg (3 min inter-trial intervals) were presented. This was followed by graded distensions, of the bladder (10, 20, 30, 40, 50, 60 mm Hg; 1 min inter-trial intervals). Two additional groups of rats received s.c. 5-HTP (10 mg/kg) or vehicle, and UBD-evoked VMRs were measured 2 h later.
The effect of 5-HTP on thermal somatic nociception was assessed separately. Hindpaw withdrawal responses to a thermal stimulus were obtained. Rats were treated with 5-HTP (10 mg/kg) or vehicle, and withdrawal responses to thermal stimulation were reassessed 30 min and 2 h later. The experimenter was blind as to whether rats received 5-HTP or vehicle. Antagonist effects were not examined.
Experiment 2
One set of rats was divided into 2 groups. 5-HTP (10 mg/kg) was administered s.c. to all rats, followed 15 min later by systemic administration of ondansetron (5 mg/kg in sterile saline) or saline. Responses to bladder distension, as described above, were determined 20, 40 and 60 min after 5-HTP administration. Responses obtained at each distending pressure were averaged across time.
The second set of rats was divided into 3 groups; two received 5-HTP and the other group received vehicle. Rats were anesthetized, a midline incision was made, and a polyethylene catheter (PE10 tubing; 7.5cm) was inserted through the atlanto-occipital membrane into the subarachnoid space. 5-HTP-injected animals received ondansetron (10 μg) or saline (7 μl) intrathecally 15 min later; vehicle-injected animals received saline intrathecally. Twenty, 40 and 60 min after 5-HTP or vehicle, UBD-evoked VMRs were determined. Responses at each distending pressure were averaged across time.
Experiment 3
Cardiac blood and CSF were obtained under isoflurane anesthesia at 30 min or 2 h after administration of 5-HTP or vehicle. ELISA kits (LDN, Nordhorn, Germany) were used to determine serotonin and 5-HIAA content. Absorbance optical density was read at 450 nm using a FLUOstar Omega microplate reader. Peptide concentrations were calculated from a standard curve using MARS data analysis software.
Statistical Analysis
All data are presented as group mean ± standard error of the mean (SEM). In studies involving graded UBD testing, EMG activity was quantified as a response (change) score which represents a signal-to-noise ratio, as in our previous studies[11,12]. EMG responses in Experiments 1 and 2 were analyzed using repeated measures ANOVAs, and when significant main effects or relevant interactions were obtained, post-hoc comparisons of means were performed using Holm's procedure[13]. Paired t-tests were used to analyze paw withdrawal latencies in Experiment 1. Statistical analysis of the ELISA data in Experiment 3 was performed via independent samples t-test. In all analyses, p<0.05 was considered statistically significant.
Results
Experiment 1
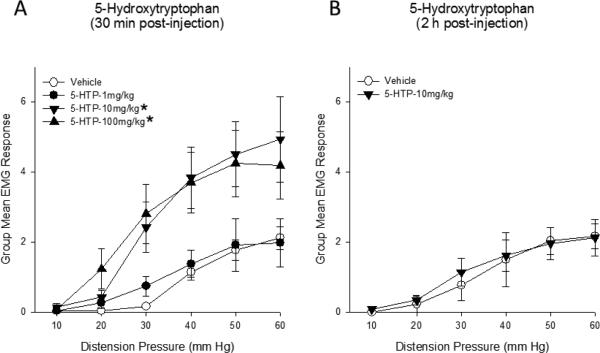
A significant increase in the vigor of the VMR induced by bladder distension was observed within 30 min of 5-HTP administration (Figure 1). Compared to s.c. saline administration, both 10 mg/kg and 100 mg/kg of 5-HTP significantly augmented bladder nociceptive responses. This enhanced bladder sensitivity had dissipated 2 h post-injection.
Figure 1.
(A) UBD-evoked visceromotor responses to bladder distension were significantly enhanced 30 min after 5-HTP administration. (B) Responses returned to baseline within 2 h. * p<0.05 compared to vehicle. N=6-8/group.
In contrast to its pro-nociceptive effects on bladder sensation, 5-HTP (10 mg/kg) produced anti-nociception to somatic thermal stimulation in the Hargreaves test. Significant somatic thermal analgesia was observed within 30 min of 5-HTP administration (Figure 2). Vehicle administration did not alter paw withdrawal latencies, and the analgesic effect of 5-HTP was no longer evident at 2 h.
Figure 2.
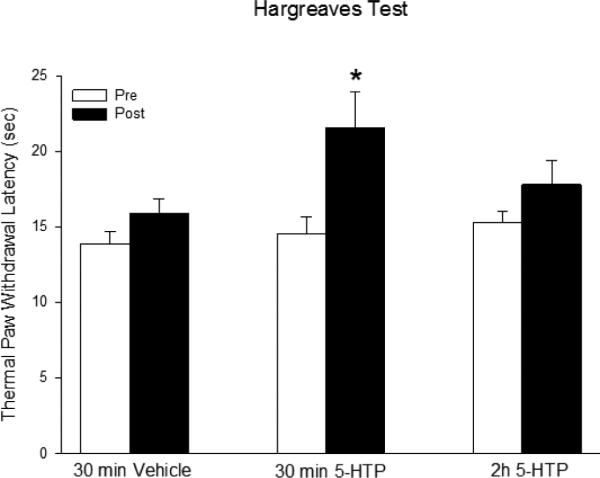
In contrast to its sensitizing effects on bladder nociception, 5-HTP (10 mg/kg) produced significant analgesia to thermal stimulation of the hindpaw. This effect dissipated by 2 h. * p<0.05 compared to vehicle. N=6-8/group.
Experiment 2
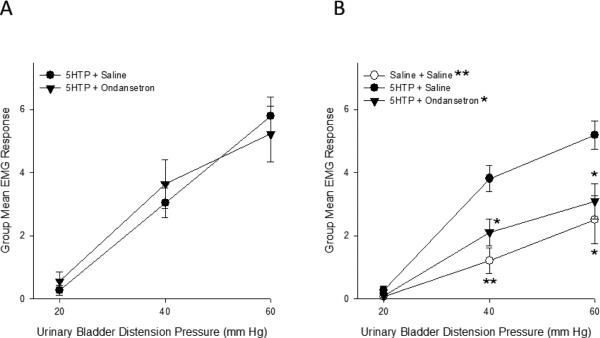
The effect of a systemically administered 5-HT3 receptor antagonist, ondansetron, on 5-HTP-induced bladder hypersensitivity was investigated (Figure 3A). An ANOVA revealed no significant effect of group (F(1,11)=0.015; p=0.906), a significant effect of distension pressure (F(2,22)=86.843; p>0.01) and no significant distension pressure x group interaction (F(2,22)=1.221; p=0.314). Evoked responses to bladder distension were virtually identical in animals that received ondansetron compared to those that received saline vehicle following 5-HTP. Thus, antagonism of peripheral 5-HT3 receptors did not attenuate bladder hypersensitivity induced by 5-HTP.
Figure 3.
(A) The enhanced EMG responses to bladder distension observed after administration of 5-HTP were still apparent after administration of a 5-HT3 receptor antagonist. (B) 5-HTP-induced bladder hypersensitivity was inhibited by intrathecal administration of ondansetron. * and ** indicate p<0.05 and p<0.01, respectively. N=5-8/group.
Ondansetron was also administered intrathecally (Figure 3B) to ascertain whether spinal 5-HT3 receptors are involved in bladder hypersensitivity induced by 5-HTP. An ANOVA revealed a significant effect of group (F(2,16)=7.338; p<0.01), a significant effect of distension pressure (F(2,32)=92.694; p>0.01) and a significant pressure x group interaction (F(4,32)=5.184; p<0.01). Animals that received intrathecal saline following 5-HTP demonstrated marked bladder hypersensitivity, as evidenced by enhanced UBD-evoked VMRs, relative to those that received intrathecal saline after s.c. vehicle. Intrathecal ondansetron significantly attenuated 5-HTP-induced hypersensitivity.
Experiment 3
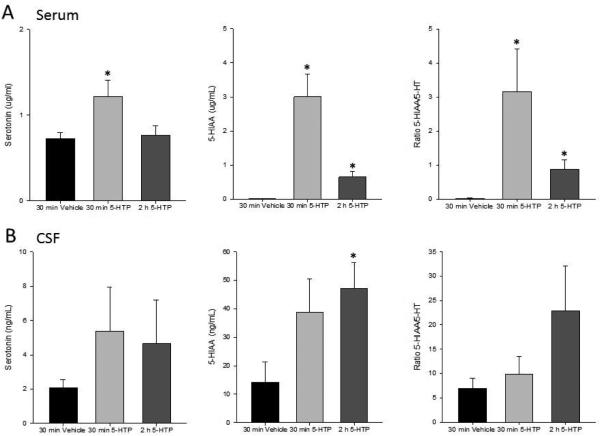
ELISA was used to quantify changes in peripheral levels of serotonin and 5-HIAA following administration of 5-HTP or vehicle (Figure 4A). An ANOVA of serum serotonin revealed a significant group effect (F(2,11)=9.374; p=0.021). Compared to vehicle administration, serum serotonin was significantly increased 30 min, but not 2 h, after 5-HTP. Significant between group differences in 5-HIAA were also evident after 5-HTP administration (F(2,11)= 9.374; p<0.01). Increases in peripheral serotonin metabolism were apparent within 30 min of 5-HTP administration and persisted for at least 2 h. The ratio of 5-HIAA:serotonin was compared to determine whether peripheral serotonin turnover was enhanced in response to 5-HTP. Turnover was significantly increased 30 min and 2 h after 5-HTP administration.
Figure 4.
(A) Serum serotonin was significantly increased 30 min after administration of 5-HTP (left). Levels returned to those of vehicle-treated animals by 2 h. 5-HIAA (middle) and 5-HIAA:5-HT (right) were also significantly elevated 30 min and 2 h after 5-HTP compared to vehicle-treated animals. (B) No significant changes in CSF levels of 5-HT were observed following administration of 5-HTP (left). Both 5-HIAA (middle) and 5-HIAA:5-HT (right) were significantly higher 2 h after 5-HTP than what was observed in vehicle-treated animals. * p<0.05 compared to vehicle. N=3-6/group.
Changes in CSF levels of serotonin and 5-HIAA were also quantified subsequent to 5-HTP administration. Although centrally, serotonin itself was not statistically altered in response to 5-HTP, an ANOVA revealed that serotonin metabolism was significantly enhanced (F(2,12)=3.224; p=0.038). Post-hoc contrasts indicated that compared to vehicle administration, 5-HIAA was significantly increased 2 h after 5-HTP. While an ANOVA also indicated between group differences in serotonin turnover (F(2,10)=2.962; p=0.049), post-hoc contrasts did not reveal significant effects.
Discussion
The RVM is a critical component of the descending modulatory system that both inhibits and facilitates pain at the level of the spinal cord. Early research indicated that serotonergic projections from the RVM to the spinal cord were involved in descending inhibition of nociception[1]. However recent studies revealed that serotonin may also be pro-nociceptive in some pain states[14-16]. The bidirectional effects of serotonin on nociceptive processing may depend on a number of factors including type (cutaneous, neuropathic, or visceral) and duration (acute or chronic) of pain. Furthermore, the existence of fourteen subtypes of serotonin receptors, each with different functions, makes it difficult to ascertain their precise role in descending modulation of pain. To date, few studies have investigated serotonergic modulation of visceral pain, and even less is known about its role in the urinary bladder. The purpose of the present study was to determine the role of serotonin in nociceptive processing related to the bladder and to examine a candidate receptor that may modulate the effect.
Here we demonstrated that systemic 5-HTP, the serotonin precursor, produces significant bladder hypersensitivity, suggesting facilitatory effects of serotonin on visceral nociceptive processing related to the bladder. These findings are consistent with studies showing that 5-HTP also enhances colorectal sensitivity[17,18]. Interestingly, nociceptive responses to a somatic thermal stimulus were actually attenuated following 5-HTP administration, such that animals exhibited somatic analgesia. Antinociceptive effects of serotonin on thermal sensitivity have been reported in other studies. Olfactory bulbectomized rats exhibit thermal hyperalgesia in tail flick tests, as well as profound alterations in serotonergic neurotransmission[19]. In this model, hyperalgesia was progressively attenuated by chronic administration of fluoxetine, and fluoxetine also increased tail-flick latencies in sham bulbectomized rats. Spinal cord injuery-induced thermal hyperalgesia was inhibited by a selective serotonin reuptake inhibitor[20]. In neuropathic mice, chronic treatment with resveratrol normalized thermal hyperalgesia, an effect that was abolished with chemical depletion of serotonin but potentiated with co-administration of 5-HTP[21]. On the other hand, intraplantar administration of serotonin has been reported to produce edema and thermal hyperalgesia[22,23]. However, these effects likely occur solely via peripheral mechanisms. Activation of 5-HT1 and 5-HT2 receptors on blood vessels enhances vascular permeability, leading to edema and release of pro-inflammatory and pro-nociceptive substances[24,25]. The presence of multiple subtypes of serotonergic receptors on primary afferent nociceptors indicates that serotonin may directly produce pain by activating receptors on Aδ- and C-fibers[26].
A facilitatory role for serotonin in visceral pain processing, as demonstrated in the present study, is supported by several studies[27,28]. With regard to the bladder Randich et al. showed that bladder hypersensitivity following acute inflammation was significantly attenuated by intrathecal administration of the non-specific serotonergic antagonist, methysergide[7]. A selective serotonin receptor antagonist was utilized in the present study to investigate a possible pharmacological mechanism underlying serotonergic facilitation of bladder nociception. Ondansetron was administered systemically and intrathecally to ascertain whether peripheral and/or spinal 5-HT3 receptors are responsible for the enhanced bladder sensitivity in response to 5-HTP.
The pro-nociceptive effect of 5-HTP was not blocked by systemic ondansetron, which selectively antagonizes peripheral 5-HT3 receptors when administered systemically. Coelho et al.[29] also reported a similar lack of effect of another 5-HT3 receptor antagonist, granisetron, on colorectal hypersensitivity. However, a number of studies suggest that activation of peripheral 5HT3 receptors induces pro-nociceptive effects. Local administration of 5HT3 receptor antagonists have been shown to be analgesic in acute and chronic pain[30], and in a model of persistent temporomandibular joint inflammation[31]. In humans, topical ondansetron attenuates the nociceptive and inflammatory effects of intradermal capsaicin[32]. Most of these studies investigated effects on inflammatory pain that is not of visceral origin, which could account for the discrepant findings.
Intrathecal ondansetron completely blocked 5-HTP-induced bladder hypersensitivity. The 5-HT3 receptor is the only ligand-gated ion channel with excitatory functions in the serotonin family, and ample evidence suggests that 5-HT3 receptor-dependent nociceptive facilitation is involved in persistent pain states. Thermal and mechanical sensitivity induced by spinal nerve ligation was blocked by intrathecal administration of the specific 5-HT3 receptor antagonist Y25130[33]. Intrathecal ondansetron attenuated mechanical hyperalgesia and allodynia[34] and inhibited mechanical and thermal evoked responses of spinal dorsal horn neurons in this same model. Spinal administration of ondansetron was also analgesic in models of spinal cord injury, cancer-induced bone pain and osteoarthritis[35-37]. Consistent with reports of pro-nociceptive effects of peripheral 5-HT3 receptors[38,39], inflammatory pain also appears to be mediated, in part, by spinal 5-HT3 receptors. Randich and colleagues have shown that intrathecal ondansetron eliminated the enhancement of the VMR response to urinary bladder distension after zymosan inflammation[7]. Similarly, blocking spinal 5-HT3 receptors attenuated inflammation-induced hypersensitivity[38], and responses to formalin were decreased in HT3A receptor knockout mice[38].
Peripheral and central levels of serotonin and turnover were quantified after administration of 5-HTP. Not surprisingly, serum serotonin, metabolism and turnover were augmented peripherally, consistent with systemic administration of the serotonin precursor. Central changes lagged behind those seen in the periphery; the greatest increases in serotonin metabolism and turnover were observed at 2 h. Since 5-HTP can readily cross the blood brain barrier[8], it was not unexpected to observe central changes in serotonin and metabolism, although these effects were not as robust as the peripheral changes. Interestingly, the time course of 5-HTP-induced hypersensitivity mimics the peripheral changes. Early studies indicated that intravenous administration of serotonin can exert actions on descending pain modulatory systems from the brainstem[40]. It is possible that in the present study, 5-HTP increases peripheral serotonin, which may then activate non-5-HT3 serotonin receptors and lead to activation of descending facilitatory systems that produced bladder hypersensitivity.
In the present studies, systemic 5-HTP produced visceral hypersensitivity and somatic analgesia, indicating that serotonin can have differential effects on nociceptive processing. The pro-nociceptive effect was prevented by intrathecal ondansetron, suggesting that in the urinary bladder, the last part of any nociceptive facilitation process involves activation of spinal 5-HT3 receptors.
Highlights.
5-HTP produces visceral hypersensitivity and somatic thermal analgesia.
Serotonin can have differential effects on nociceptive processing.
5-HTP induced bladder hypersensitivity occurs by a spinal 5-HT3 receptor mechanism.
Acknowledgements
Supported by NIH R00-DK080981(M.T.R.), R01-DK100904(M.T.R.), and R01-DK51413(T.J.N.).
Abbreviations
- 5-HTP
5-hydroxytryptophan
- 5-HIAA
5-hydroxyindoleacetic acid
- RVM
Rostral ventromedial medulla
- UBD
Urinary bladder distension
- VMR
Visceromotor response
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Millan MJ. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 2.Basbaum AI, Clanton CH, Fields HL. Opiate and stimulus-produced analgesia: functional anatomy of a medullospinal pathway. Proc. Natl. Acad. Sci. U.S.A. 1976;73:4685–4688. doi: 10.1073/pnas.73.12.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J. Neurophysiol. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- 4.Zhuo M, Gebhart GF. Spinal serotonin receptors mediate descending facilitation of a nociceptive reflex from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Brain Res. 1991;550:35–48. doi: 10.1016/0006-8993(91)90402-h. [DOI] [PubMed] [Google Scholar]
- 5.Géranton SM, Fratto V, Tochiki KK, Hunt SP. Descending serotonergic controls regulate inflammation-induced mechanical sensitivity and methyl-CpG-binding protein 2 phosphorylation in the rat superficial dorsal horn. Mol. Pain. 2008;4:35–44. doi: 10.1186/1744-8069-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, Guo W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J. Neurosci. 2010;30:8624–8636. doi: 10.1523/JNEUROSCI.5389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randich A, Shaffer AD, Ball CL, Mebane H. Serotonergic and noradrenergic facilitation of the visceromotor reflex evoked by urinary bladder distension in rats with inflamed bladders. Neurosci. Lett. 2008;442:253–256. doi: 10.1016/j.neulet.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birdsall TC. 5-Hydroxytryptophan: a clinically-effective serotonin precursor. Altern. Med. Rev. 1998;3:271–280. [PubMed] [Google Scholar]
- 9.Anderson RH, Ness TJ, Gebhart GF. A distension control device used for quantitative studies of hollow organ sensation. Physiol. Behav. 1988;41:635–638. doi: 10.1016/0031-9384(87)90322-2. [DOI] [PubMed] [Google Scholar]
- 10.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 11.Robbins MT, DeBerry J, Ness TJ. Chronic psychological stress enhances nociceptive processing in the urinary bladder in high anxiety rats. Physiol. Behav. 2007;91:544–550. doi: 10.1016/j.physbeh.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins MT, Ness TJ. Footshock-induced urinary bladder hypersensitivity: role of spinal corticotrophin-releasing factor receptors. J. Pain. 2008;9:991–998. doi: 10.1016/j.jpain.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Statistics. 1979;6:65–70. [Google Scholar]
- 14.Pertovaara A. Plasticity in descending pain modulatory systems. Prog. Brain Res. 2000;129:231–242. doi: 10.1016/S0079-6123(00)29017-1. [DOI] [PubMed] [Google Scholar]
- 15.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 16.Gebhart GF. Descending modulation of pain. Neurosci. Biobehav. Rev. 2004;27:729–737. doi: 10.1016/j.neubiorev.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Banner SE, Sanger GJ. Differences between 5-HT3 receptor antagonists in modulation of visceral hypersensitivity. Br. J. Pharm. 1995;114:558–562. doi: 10.1111/j.1476-5381.1995.tb13263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu CL, Hsieh JC, Dun NJ, Oprea TI, Wang PS, Luo JC, Lin HC, Chang FY, Lee SD. Estrogen rapidly modulates 5-hydroxytrytophan-induced visceral hypersensitivity via GPR30 in rats. Gastroenterol. 2009;137:1040–1050. doi: 10.1053/j.gastro.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Gaztelumendi A, Rojo ML, Pazos A, Díaz A. An altered spinal serotonergic system contributes to increased thermal nociception in an animal model of depression. Exp. Brain Res. 2014;232:1793–1803. doi: 10.1007/s00221-014-3871-7. [DOI] [PubMed] [Google Scholar]
- 20.Horiuchi H, Ogata T, Morino T, Takeba J, Yamamoto H. Serotonergic signaling inhibits hyperalgesia induced by spinal cord damage. Brain Res. 2003;963:312–320. doi: 10.1016/s0006-8993(02)04055-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Yu C, Wang C, Zhang J-F, Zhou W-H, Cui W-G, Ye F, Xu Y. Chronic resveratrol treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with mononeuropathy: Involvement of serotonergic system. Neuropharmacol. 2014;85:131–141. doi: 10.1016/j.neuropharm.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Sufka KJ, Schomburg FM, Giordano J. Receptor mediation of 5-HT-induced inflammation and nociception in rats. Pharmacol. Biochem. Behav. 1992;41:53–56. doi: 10.1016/0091-3057(92)90058-n. [DOI] [PubMed] [Google Scholar]
- 23.Santos CMF, Francischi JN, Lima-Paiva P, Sluka KA, Resende MA. Effect of transcutaneous electrical stimulation on nociception and edema induced by peripheral serotonin. International J. Neurosci. 2013;123:507–515. doi: 10.3109/00207454.2013.768244. [DOI] [PubMed] [Google Scholar]
- 24.Pierce PA, Xie GX, Peroutka SJ, Green PG, Levine JD. 5-hydroxytryptamine-induced synovial plasma extravasation is mediated via 5-hydroxytryptamine2a receptors on sympathetic efferent terminals. J. Pharmacol. Exp. Ther. 1995;275:502–508. [PubMed] [Google Scholar]
- 25.Tokunaga A, Saika M, Senba E. 5-HT2A receptor subtype is involved in the thermal hyperalgesic mechanism of serotonin in the periphery. Pain. 1998;76:349–55. doi: 10.1016/S0304-3959(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 26.Sommer C. Serotonin in pain and analgesia: actions in the periphery. Mol. Neurobiol. 2004;30:117–125. doi: 10.1385/MN:30:2:117. [DOI] [PubMed] [Google Scholar]
- 27.Wu JC, Ziea ET, Lao L, Lam EF, Chan CS, Liang AY, Chu SL, Yew DT, Berman BM, Sung JJ. Effect of electroacupuncture on visceral hyperalgesia, serotonin and fos expression in an animal model of irritable bowel syndrome. J. Neurogastroenterol. Motil. 2010;16:306–314. doi: 10.5056/jnm.2010.16.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meller ST, Lewis SJ, Brody MJ, Gebhart GF. Age, strain and anesthetic dependent differences in the nociceptive responses produced by i.v. 5-HT in the rat. Brain Res. 1992;587:88–94. doi: 10.1016/0006-8993(92)91431-d. [DOI] [PubMed] [Google Scholar]
- 29.Coelho AM, Fioramonti J, Bueno L. Mast cell degranulation induces delayed rectal allodynia in rats: role of histamine and 5-HT. Dig. Dis. Sci. 1998;43:727–737. doi: 10.1023/a:1018853728251. [DOI] [PubMed] [Google Scholar]
- 30.Giordano J, Rogers LV. Peripherally administered serotonin 5-HT3 receptor antagonists reduce inflammatory pain in rats. Eur. J. Pharmacol. 1989;170:83–86. doi: 10.1016/0014-2999(89)90137-4. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto K, Imbe H, Tashiro A, Kumabe S, Senba E. Blockade of peripheral 5HT3 receptor attenuates the formalin-induced nocifensive behavior in persistent temporomandibular joint inflammation of rat. Neurosci. Lett. 2004;367:259–263. doi: 10.1016/j.neulet.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Giordano J, Daleo C, Sacks SM. Topical ondansetron attenuates nociceptive and inflammatory effects of intradermal capsaicin in humans. Eur. J. Pharmacol. 1998;354:13–14. doi: 10.1016/s0014-2999(98)00492-0. [DOI] [PubMed] [Google Scholar]
- 33.Gu M, Miyoshi K, Dubner R, Guo W, Zou S, Ren K, Noguchi K, Wei F. Spinal 5-HT(3) receptor activation induces behavioral hypersensitivity via a neuronal-glial-neuronal signaling cascade. J. Neurosci. 2011;31:12823–12836. doi: 10.1523/JNEUROSCI.1564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Dogrul A, Ossipov MH, Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009;1280:52–59. doi: 10.1016/j.brainres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Oatway MA, Weaver LC. Blockade of the 5-HT3 receptor for days causes sustained relief from mechanical allodynia following spinal cord injury. J. Neurosci. Res. 2009;87:418–424. doi: 10.1002/jnr.21860. [DOI] [PubMed] [Google Scholar]
- 36.Rahman W, Bauer CS, Bannister K, Vonsy JL, Dolphin AC, Dickenson AH. Descending serotonergic facilitation and the antinociceptive effects of pregabalin in a rat model of osteoarthritic pain. Mol. Pain. 2009;5:45–61. doi: 10.1186/1744-8069-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donovan-Rodriguez T, Urch CE, Dickenson AH. Evidence of a role for descending serotonergic facilitation in a rat model of cancer-induced bone pain. Neurosci. Lett. 2006;393:237–242. doi: 10.1016/j.neulet.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 38.Lagraize SC, Guo W, Yang K, Wei F, Ren K, Dubner R. Spinal cord mechanisms mediating behavioral hyperalgesia induced by neurokinin-1 tachykinin receptor activation in the rostral ventromedial medulla. Neurosci. 2010;171:1341–1356. doi: 10.1016/j.neuroscience.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kayser V, Elfassi IE, Aubel B, Melfort M, Julius D, Gingrich JA, Hamon M, Bourgoin S. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A−/−, 5-HT1B−/−, 5-HT2A−/−, 5-HT3A−/− and 5-HTT−/− knock-out male mice. Pain. 2007;130:235–248. doi: 10.1016/j.pain.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Meller ST, Lewis SJ, Ness TJ, Brody MJ, Gebhart GF. Vagal afferent-mediated inhibition of a nociceptive reflex by intravenous serotonin in the rat. I. Characterization. Brain Res. 1990;524:90–100. doi: 10.1016/0006-8993(90)90496-x. [DOI] [PubMed] [Google Scholar]