Abstract
Background
The utero-placental vascular supply is a critical determinant of placental function and fetal growth. Current methods for the in vivo assessment of placental blood flow are limited.
Objective
Here we demonstrate the feasibility of utilizing contrast-enhanced ultrasound to visualize and quantify perfusion kinetics in the intervillous space of the primate placenta.
Study design
Pregnant Japanese macaques were studied at mid second trimester and in the early third trimester. Markers of injury were assessed in placenta samples from animals with or without contrast-enhanced ultrasound exposure (n=6/group). Human subjects were recruited immediately prior to scheduled first trimester pregnancy termination. All studies were performed with maternal intravenous infusion of lipid-shelled octofluoropropane microbubbles with image acquisition using a multipulse contrast-specific algorithm with destruction-replenishment analysis of signal intensity for assessment of perfusion.
Results
In macaques, rate of perfusion in the intervillous space was increased with advancing gestation. No evidence of microvascular hemorrhage or acute inflammation was found in placental villous tissue and expression levels of caspase-3, nitrotyrosine and HSP70 as markers of apoptosis, nitrative and oxidative stress respectively were unchanged by contrast-enhanced ultrasound exposure. In humans, placental perfusion was visualized at 11wks gestation and preliminary data reveal regional differences in intervillous space perfusion within an individual placenta. By electron microscopy, we demonstrate no evidence of ultrastructure damage to the microvilli on the syncytiotrophoblast following first trimester ultrasound studies.
Conclusions
Use of contrast-enhanced ultrasound did not result in placental structural damage, and was able to identify intervillous space perfusion rate differences within a placenta. Contrast-enhanced ultrasound may offer a safe clinical tool for the identification of pregnancies at-risk for vascular insufficiency; early recognition may facilitate intervention and improved pregnancy outcomes.
Keywords: Contrast-enhanced ultrasound, placenta, Japanese macaque, placental perfusion, in vivo imaging
Introduction
The blood supply to the placenta is a critical determinant of maternal-fetal nutrient exchange throughout pregnancy. In the primate placenta, establishment of utero-placental blood flow in the first trimester1 is largely dependent on trophoblast invasion of the maternal spiral arteries; inadequate remodeling of this vascular space has been demonstrated to underlie fetal growth restriction, preeclampsia and stillbirth.2,3 As gestation progresses, plasticity within the developing placenta allows for vascular adaptations to meet fetal growth demands. Unfortunately, the lack of safe, non-invasive imaging modalities that facilitate the in vivo study of both normal and abnormal placental vascular perfusion and blood volume impedes our understanding of placental vascular function.4 Specifically, currently available imaging modalities are limited in their ability to quantitatively assess utero-placental blood flow.
Contrast-enhanced ultrasound (CE-US) is a non-invasive technique that permits imaging of microvascular perfusion using acoustic detection of gas-filled, lipid-encapsulated microbubble contrast agents.5,6 This methodology has been used extensively in cardiac diagnostic imaging with microbubbles used as flow tracers.7,8 Thus safety studies have examined the rheology of microbubbles in the microcirculation and demonstrated that they are similar in size to red blood cells and do not interfere with hemodynamics.9 Microbubbles emit a high acoustic signal due to either stable or inertial cavitation.6 This established technique was originally used to highlight the ventricular endocardial borders and to assess liver tissue vasculature6 and has now advanced to be used to assess tissue perfusion as a targeted delivery system for local drug/agent distribution.10 Side effects of CE-US have been reported with severe allergic reaction occurring in approximately 1 in 10,000 patients11 and a 1 in 200 rate of flank/back pain thought to be due to complement-mediated reactions.12 No fatal events have been reported and in general, acute therapy has resolved severe allergic responses within 8 hours.11 However, the use of contrast agents during pregnancy raises appropriate safety concerns for clinical application, and consequently CE-US in pregnant women has not previously been trialed. The main concerns of CE-US use during pregnancy are 1) lodging of contrast agent in the microcirculation, 2) complement activation and 3) micro hemorrhages as a result of cavitation.
Studies performed in pregnant rats have shown that phospholipid encapsulated microbubbles containing sulfur hexafluoride do not appear to cross the feto-placental barrier at a mechanical index below 1.513 and that real-time perfusion can be measured across gestation14 with blood flow increasing between days 14 and 17.15 In addition, CE-US has been used in early pregnancy implantation studies in the Rhesus macaque16 but not later in gestation. Importantly, Keator and colleagues demonstrated no adverse effect on pregnancy outcome at full term following early CE-US use in their small sample cohort of three animals.16 Limited data are available from in vitro studies that demonstrate the bioeffects of microbubbles in combination with ultrasound on cellular properties. Primary endothelial cells in culture exposed to ultrasound with and without microbubbles exhibit short lived effects (<1 hour until restoration) on stress fibers, cytoskeletal arrangements and oxidative stress markers.17 However, studies in placental tissue have not previously been performed.
In the current study our objective was to demonstrate the feasibility of assessing perfusion kinetics of the intervillous space (IVS) at two time points in mid to later gestation in the nonhuman primate and in the late first trimester of human subjects. Although we and others have not observed microvascular damage10 associated with CE-US, there may be a subtle effect on the placental ultrastructure.13 Thus, we also sought to address whether CE-US use resulted in placental tissue injury or adversely effects pregnancy outcomes.
Methods
Animals
All protocols were approved by the Institutional Animal Care and Utilization Committee (IACUC) of the Oregon National Primate Research Center (ONPRC), and guidelines for humane animal care were followed. The ONPRC abides by the Animal Welfare Act and Regulations enforced by the USDA. Japanese macaques (Macaca fuscata) were socially housed in indoor/outdoor pens with ad libitum access to food and water. Animals were allowed to breed naturally and pregnancies were identified by routine early first trimester pregnancy 2-D ultrasound (GE Voluson 730 Expert, Kretztechnik, Austria) with fetal biometry measurements used for gestational dating.
Nonhuman primate contrast-enhanced ultrasound
Six pregnant animals underwent imaging studies at 90 days gestation (G90) and G129, where full term in the Japanese macaque is G175. Following an overnight fast, animals were initially sedated with 3-5mg/kg Telazol (Tiletamine HCl and Zolazepam HCl) IM. Once intubated and maintained under anesthesia with 1-2% isoflurane gas, Flumazenil (0.01mg/kg, IV) was administered to reverse the effects of Telazol. CE-US was performed using a multiphase amplitude-modulation and phase-inversion algorithm on a Sequoia system (Siemens Medical Systems, Mountain View, CA) equipped with a 15L8 transducer at a transmit frequency of 7MHz with a 0.18 mechanical index (MI) and a 55dB dynamic range. Lipid-shelled octofluoropropane microbubble contrast reagent (Definity®, Lantheus Medical Imaging, Billerica, MA) was prepared in 0.9% saline at a final concentration of 5%. Intravenous infusion via a cephalic catheter was initially performed at a rate of 10ml/hr to obtain baseline measurements of the maternal blood pool within the brachial artery of the catheterized arm. Digital video clips were recorded for 10 second durations to measure video intensity (VI) of the blood pool (VIpool). Three replicates of all recordings were obtained during each study. For visualization of uteroplacental blood flow, the infusion rate was increased to 60ml/hr. The acoustic beam was centered over individually identified maternal spiral artery sources and the microbubbles within the path of the beam were destroyed by a brief (2 second) increase in MI to 1.9. Microbubble re-entry in the spiral artery and the IVS was recorded at 1 frame /75 msec (rapidly re-filling vessels) or 1 frame /125 msec (slower re-filling vessels) until the area of interest reached signal saturation (VImax).
Human Contrast-enhanced ultrasound studies
This protocol was approved by the Institutional Review Board at Oregon Health and Science University (IRB#10744) with written consent obtained from all study participants. Women who were scheduled for elective termination of pregnancy were approached about participation in this study. Caucasian women (n=4) underwent ultrasound examinations at 11-13 weeks' gestation while in a reclined position with normal oxygen saturation on room air. The ultrasound protocol was the same as the animal studies with the following exceptions: A 4C1-S transducer set at a frequency of 3MHz was used to obtain all images. The resting mechanical index was 0.11. All other parameters were consistent with the nonhuman primate studies. At this early gestational age, perfusion of the entire placenta can be visualized in one field of view. In addition, acquisition parameters can be adjusted to focus in and selectively view individual maternal spiral artery sources by adjusting the depth.
Data analysis
Digital imaging data were analyzed using a custom-designed CE-US analysis program. The VIpool was analyzed using recordings obtained from the brachial artery measurements. Three overlapping regions of interest (ROI) were drawn to facilitate calculation of the VIpool. For placental assessment, ROIs were drawn over the area of each cotyledon. The data was fit to the function y=A(1-e-βt), where y is the VI at the pulsing interval t, A is the VI plateau and β is the flux rate constant. The β-value determines the rate constant of the post-destructive VI recovery and reflects the microvascular blood flux rate.18
Placental tissue collection
The animals utilized in this cohort were part of a larger study where pregnancies were delivered by cesarean section at G130. Maternal serum was collected at the time of surgery, processed and stored at -80°F for later analysis of complement activation. Following delivery, fetal and placental weights were recorded, and placental tissue was collected. Full thickness sections (maternal decidua to chorionic plate) were collected in cassettes and fixed in 10% zinc formalin for paraffin embedding. Villous tissue was dissected from each individually identified and mapped cotyledon, and flash frozen in liquid nitrogen. Tissue was collected from animals from the imaging studies described above (n=6) and a control group who were not exposed to CE-US during pregnancy (n=6).
Placental tissue analysis
Placental villous tissue was homogenized in lysis buffer containing protease inhibitors and centrifuged at 20,000×g for 5 minutes as previously described.19 Supernatant from at least three individual cotyledons per placenta was pooled in to one representative sample for protein expression analysis. Complement activation, apoptosis and nitrative stress levels were assessed using commercially available enzyme-linked immunosorbent assay kits (Monkey Terminal Complement Complex C5b-9 and Caspase 3 ELISA: NeoBioLab, Woburn, MA; Nitrotyrosine ELISA: Millipore, Billerica, MA). Assays were performed according to manufacturers' instructions and data were corrected for protein content.
Placental histology
Representative macaque placental tissue samples were fixed in formalin, paraffin-embedded and histological sections (5μm) stained for hematoxylin and eosin for evaluation by a placental pathologist (TKM). In addition, sections were stained using a TUNEL assay (APO-BRDU-IHC kit, Phoenix Flow Systems via Biorad, Hercules, CA) as a marker of apoptosis, and with anti-HSP70 (1:100, Cell Signaling, Danvers, MA) as a marker of oxidative and cellular stress.
First trimester human samples were collected and fixed for examination within one hour post-CE-US imaging. Representative first trimester chorionic villous samples from 3 subjects were sectioned and assessed by routine hematoxylin & eosin stain. In addition, 2.5% glutaraldehyde fixed samples were examined by electron microscopy. For the purposes of this pilot study, all three cases were compared to gestational age-matched negative controls (n=6) to evaluate for trophoblast surface damage20-22 by a placental pathologist (TKM) while blinded to treatment group. Notably, areas of hydropic generation related to chorionic membrane development were excluded from this analysis due to the fragmented nature of the specimens obtained post-procedure.
Statistical Analysis
Contrast ultrasound parameters were compared between the G90 and G129 time points using an unpaired t-test performed using statistical analysis software (GraphPad Prism, La Jolla, CA). Similarly, expression of markers of injury between CE-US exposed versus non-exposed maternal serum and placental tissue were assessed using an unpaired t-test. p<0.05 was considered to be significant.
Results
Contrast-enhanced ultrasound in pregnant nonhuman primates
CE-US with Definity® microbubbles was used to locate and visualize maternal spiral artery sources supplying individual placental cotyledons of the primate placenta in mid gestation. Figure 1A shows an example time series of re-filling of the placental IVS following ‘burst’ of the microbubbles until signal saturation is reached at VImax. Insert Video link. For orientation, the maternal spiral artery and a cotyledon are outlined with the yellow and red dashed lines respectively (Fig.1B). A parametric image of IVS flux rate (β) is also shown (Fig. 1C).
Figure 1. Visualization of maternal perfusion of the intervillous space.
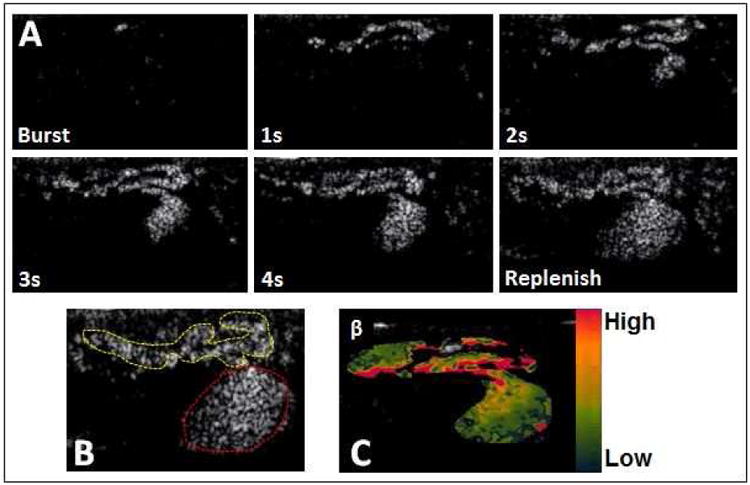
(A) Microbubbles are ‘burst’ by a 2 sec increase in mechanical index, and spiral artery filling followed by perfusion of the intervillous space in an individual cotyledon are observed in sequential frames taken at 1 second (s) intervals from a video clip obtained in an imaging study performed at G129 in a Japanese Macaque, (B) For anatomical orientation, the maternal spiral artery (yellow dashed line) and placental cotyledon (red dashed line) are identified, (C) A parametric image representing flux rate (β) in the cotyledon represented in (A). The ascending color scale bar represents high to low flow in this image.
Data were analyzed from six pregnant females at two time points in the second trimester (G90) and early third trimester (G129). Maternal blood pressure, heart rate and oxygenation were stable and unchanged over the duration of each ultrasound exam (data not shown). We demonstrate a significant increase in maternal VIpool, assessed in the brachial artery, from G90 to G129 (Fig. 2A). Flux rate (β) provides a measure of microvascular resistance with the significant increase in β at G129 vs. G90 demonstrating a decrease in resistance as gestation advances (Fig. 2B). Importantly, we observed no sequential deterioration in perfusion with replicate measurements obtained in each cotyledon (raw data not shown).
Figure 2. Contrast-enhanced ultrasound data analysis.
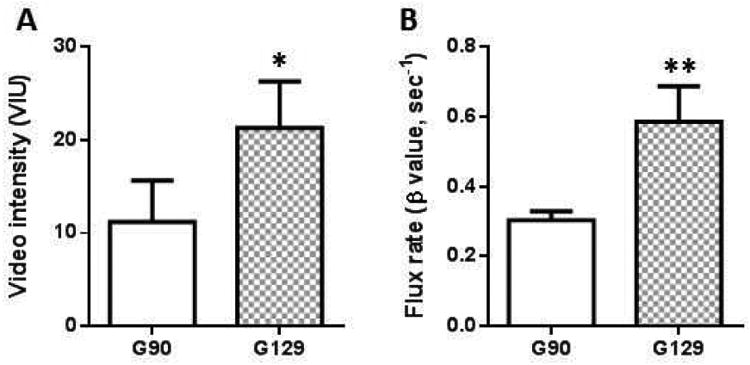
(A) Maternal blood pool video intensity calculated from gthe brachial artery and (B) flux rate (β) calculated from the slope of the curve in Japanese Macaques at G90 and G129. Data are mean±SEM from n=28 spiral artery sources from 6 animals at G90 and n=31 spiral artery sources from 6 animals at G129.
Safety of CE-US use during pregnancy
There was no significant difference in fetal or placental weights at G130 in animals with and without CE-US exposure (Table 1). Complement activation was assessed by measurement of C5b-9 in both maternal serum and placental protein. There was no significant difference in C5b-9 expression in maternal serum (Fig. 3A) and expression was below detectable levels in the placenta (data not shown). Assessment of placental tissue integrity in samples collected one day post CE-US compared to gestational age matched controls (no CE-US exposure) demonstrate no significant changes in protein expression levels of caspase-3 and nitrotyrosine, as markers of apoptosis and nitrative stress respectively (Fig. 3 B and C). Histological assessment of placental sections revealed no evidence of intervillous micro hemorrhage or acute inflammation (Fig. 3D). Additional assessment of apoptosis by TUNEL assay demonstrates minimal cell death within the placental villous tissue resulting from CE-US exposure (Fig. 3E). Expression and localization of heat shock protein 70 (HSP70, Fig. 3F), as a marker of cellular and oxidative stress, was semi-quantitatively assessed by blinded grading of staining intensity and found to be unchanged by CE-US compared to controls.
Table 1. Weight characteristics.
| No CE-US exposure (n=6) | CE-US exposure (n=6) | |
|---|---|---|
| Maternal weight (Kg) | 10.8±1.6 | 9.9±1.4 |
| Fetal weight (g) | 340±24.1 | 327±24.6 |
| Placental weight (g) | 103.0±7.1 | 106.3±17.2 |
Figure 3. Japanese macaque placental tissue integrity with and without contrast-enhanced ultrasound exposure.
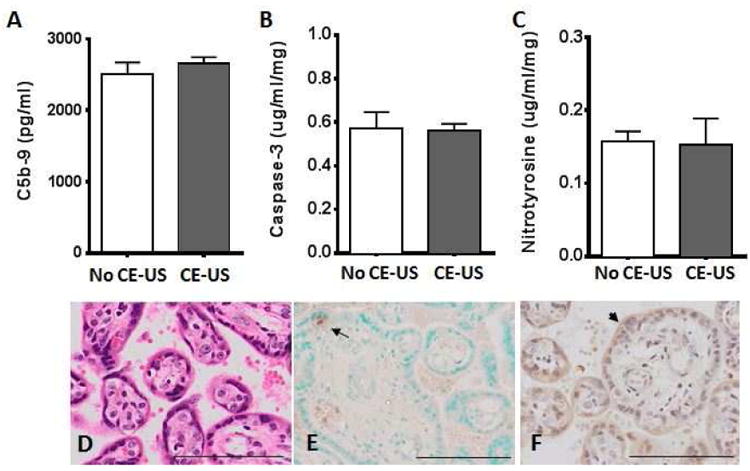
(A) Complement C5b-9 expression in maternal serum obtained at the time of G130 cesarean section delivery, (B) Caspase 3 and (C) Nitrotyrosine protein expression in placental homogenate from control animals who were not exposed to CE-US (white bar) and CE-US exposed (grey bar) animals. Data are mean±SEM for n=6 animals/group. Representative placental tissue sections from a control animal with microbubble exposure during ultrasound stained for (D) Hematoxylin and Eosin (E) TUNEL, where the arrow highlights an apoptosis-positive cell and (F) HSP70, where the arrowhead indicates positive staining on the syncytiotrophoblast. Scale bar indicates 100μm.
Contrast-enhanced ultrasound in first trimester human subjects
A representative CE-US time series from an 11 week gestation pregnancy is shown in Figure 4. Insert Video link.The earth map overlay aids visualization of placental perfusion. Preliminary analysis in this example image (Fig. 4B) reveals varying β rate in individual cotyledons reflecting regional differences in perfusion that can be detected using this technique (Fig 4C). At this early gestational age, it is possible to image the entire placenta in one field of view, something that is not possible later in gestation due to the size constraints of the transducer in proportion to the growing placenta.
Figure 4. Contrast-enhanced ultrasound in an 11 week gestation human subject.
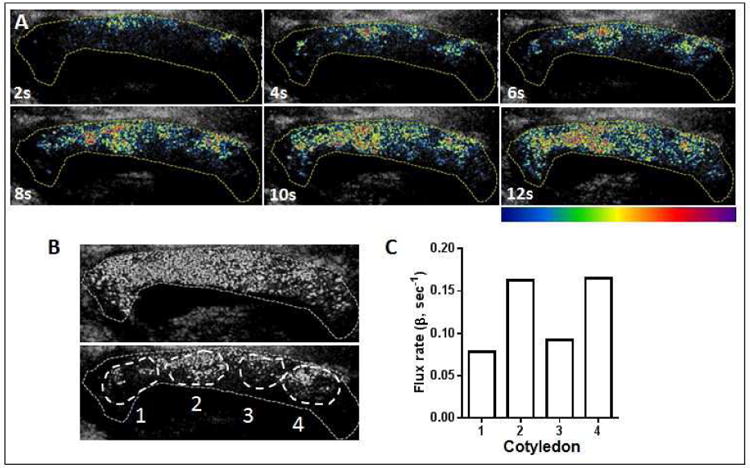
(A) Sequential frames taken at 2 second (s) intervals post ‘burst’. The yellow dotted line outlines the placenta and the earth map overlay aids visualization of placental perfusion in the late first trimester with a color scale bar indicated from low (blue) to high (red-purple) blood flow. (B) The white overlays represent four individual cotyledons within the 11 week placenta labeled 1 to 4, and (C) Flux rate in each of the four individual cotyledons demonstrating regional variance in perfusion kinetics within the placenta.
First trimester tissue histology
In first trimester chorionic villous samples collected one hour post imaging, there was no microvascular hemorrhage by H&E assessment (Fig. 5A), and the syncytiotrophoblast microvilli appeared not to be affected in cases (Fig. 5B) compared with gestational age matched negative controls (Fig. 5C).
Figure 5. Human first trimester placental tissue integrity with and without contrast-enhanced ultrasound exposure.
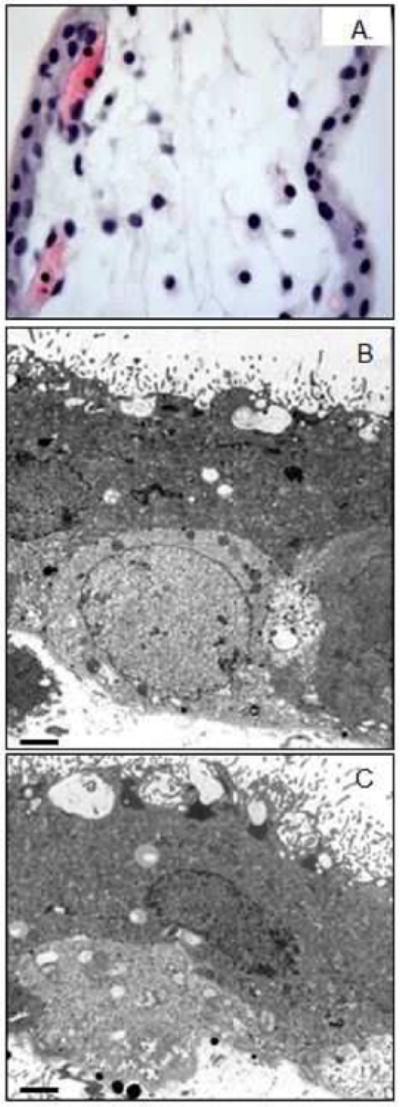
(A) Hematoxylin & eosin staining of first trimester villi, (B) electron microscopy ultrastructure in CE-US exposed and (C) non-exposed first trimester tissue. Scale bar is 2μm.
Comment
Principal findings
The current lack of imaging modalities that facilitate the in utero study of placental function impedes our ability to identify at-risk pregnancies. Here we demonstrate that CE-US provides a tool that can be used to visualize and quantify maternal perfusion of the placental intervillous space in both the macaque and human placenta. In this initial analysis, we report an increase in total maternal blood volume and an increase in flux rate within cotyledons with advancing gestational age. The human studies component of this work is in the early stages but we have been successful in demonstrating as a proof-of-concept that it is possible to visualize placental perfusion as early as 11 weeks of gestation (Range: 11 to 13 weeks). In addition, our preliminary data suggest that regional differences in perfusion kinetics can be detected using CE-US.
Our in vitro studies from the nonhuman primate provide data to support the safe use of CE-US with Definity® microbubbles during pregnancy. Firstly, we report no evidence of complement activation in the maternal circulation following CE-US use. Secondly, markers of placental apoptosis and nitrative stress were unaffected by CE-US exposure when measured one day after the second imaging study in our experimental design. In keeping with the macaque data, assessment of human first trimester placental ultrastructure by electron microscopy revealed no damage to the microvilli of the syncytiotrophoblast following CE-US examinations.
Meaning of observations as they relate to other studies
The finding of regional perfusion differences by CE-US corresponds with our recently published data where by MRI we have quantified perfusion across the placenta to demonstrate regional variance by cotyledon.23 Furthermore, we have ongoing CE-US studies in diet manipulation animal models which show promising data that suggest that abnormalities in placental perfusion with differing pregnancy phenotypes can be identified using this methodology.
The absence of placental tissue injury following CE-US is supported by in vitro data in isolated endothelial cells which showed transient changes in structural proteins and markers of oxidative stress which return to within normal parameters within one hour post exposure.17
Clinical and research implications
Based on our findings, we suggest that CE-US may provide a potential diagnostic method which will help to identify early pregnancies at-risk for placental vascular dysfunction. However, validation studies are needed in compromised pregnancies to confirm the diagnostic utility of CE-US. The correlation between flow and biomarkers is not well understood but improved methods to assess placental vascular function in vivo may facilitate the identification of biomarkers that are more predictive of adverse obstetric outcomes.
Strengths and limitations
The longitudinal study design of our nonhuman primate studies allows us to assess placental perfusion across gestation, and examine the effects of multiple exposures to CE-US. It is pertinent to note that no adverse pregnancy events occurred between the earlier imaging time point and the time of planned cesarean section delivery 40 days later. Anecdotally, this is also true in animal studies currently underway in our other cohorts where animals have delivered healthy live infants at full term following imaging studies performed at G85 and G135. Additionally, our outcomes are supported by earlier work at the primate center where three healthy live births resulted following multiple CE-US scans in the first month of pregnancy.16
A constraint of nonhuman primate studies is the relatively small sample sizes due to animal expenses. Here we present in vitro data from 6 animals with and 6 animals without CE-US exposure; with animal cohorts of this sample size we have previously demonstrated significant differences in maternal, fetal and placental parameters. 24-26
A limitation of our current capabilities with CE-US is the use of flux rate as our quantitative measure of perfusion kinetics due to the complexity of the vascular network within the placenta which results in our inability to calculate absolute blood volume values. Specifically, previously applied equations for blood flow and volume in non-tortuous vessels do not account for the complex architecture of the villous tree and the maternal blood pool in the intervillous space. Thus, we do not have the necessary image processing algorithms to determine absolute values at present. We intend to address this deficit as we refine our data analysis methods in our ongoing studies.
Conclusions
Taken together, the data presented in this study make progress towards alleviating safety concerns regarding the use of CE-US during pregnancy. Thus, although further safety studies are needed to validate these initial findings, we have described the application of CE-US for the quantification of perfusion in the primate placenta and suggest that this technology may be safely implemented in future clinical management of pregnancies.
Supplementary Material
Video 1: Maternal perfusion of the intervillous space in a Japanese macaque at gestational day 129. Microbubbles are ‘burst’ by a 2 sec increase in mechanical index, and spiral artery filling followed by perfusion of the intervillous space in an individual cotyledon are observed until signal saturation is reached.
Video 2: Maternal perfusion of an 11 week gestation human placenta. Microbubbles are ‘burst’ by a 2 sec increase in mechanical index, and perfusion of the entire placenta is observed from four maternal spiral artery sources until signal saturation is reached.
Acknowledgments
Sources of Funding: NIH grants, R21-HD-076265, R24-DK-090964, P51-OD-011092 and Seed funding from the OHSU Knight Cardiovascular Institute Center for Developmental Health and the Struble Foundation. None of the above mentioned funding sources were involved in the study design, execution or publication production.
Footnotes
Disclosure: The authors report no conflict of interest.
Paper presentation: Accepted for oral presentation at the 36th annual meeting of the Society for Maternal-Fetal Medicine, Atlanta, GA, Feb.5-8, 2016
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kliman HJ. Uteroplacental blood flow. The story of decidualization, menstruation, and trophoblast invasion. Am J Pathol. 2000;157(6):1759–1768. doi: 10.1016/S0002-9440(10)64813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13(4):591–599. doi: 10.1016/s1472-6483(10)60649-9. [DOI] [PubMed] [Google Scholar]
- 3.Silasi M, Cohen B, Karumanchi SA, Rana S. Abnormal placentation, angiogenic factors, and the pathogenesis of preeclampsia. Obstet Gynecol Clin North Am. 2010;37(2):239–253. doi: 10.1016/j.ogc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014;35(5):303–304. doi: 10.1016/j.placenta.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol. 2007;18(1):11–16. doi: 10.1016/j.copbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Curr Probl Cardiol. 2007;32(2):51–96. doi: 10.1016/j.cpcardiol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Heppner P, Lindner JR. Contrast ultrasound assessment of angiogenesis by perfusion and molecular imaging. Expert Rev Mol Diagn. 2005;5(3):447–455. doi: 10.1586/14737159.5.3.447. [DOI] [PubMed] [Google Scholar]
- 8.Lindner JR. Contrast ultrasound molecular imaging of inflammation in cardiovascular disease. Cardiovasc Res. 2009;84(2):182–189. doi: 10.1093/cvr/cvp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15(5):396–403. doi: 10.1067/mje.2002.117290. [DOI] [PubMed] [Google Scholar]
- 10.Xie A, Belcik T, Qi Y, et al. Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles. JACC Cardiovasc Imaging. 2012;5(12):1253–1262. doi: 10.1016/j.jcmg.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei K, Mulvagh SL, Carson L, et al. The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr. 2008;21(11):1202–1206. doi: 10.1016/j.echo.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Liu YN, Khangura J, Xie A, et al. Renal retention of lipid microbubbles: a potential mechanism for flank discomfort during ultrasound contrast administration. J Am Soc Echocardiogr. 2013;26(12):1474–1481. doi: 10.1016/j.echo.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua X, Zhu LP, Li R, Zhong H, Xue YF, Chen ZH. Effects of diagnostic contrast-enhanced ultrasound on permeability of placental barrier: a primary study. Placenta. 2009;30(9):780–784. doi: 10.1016/j.placenta.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Zhou YJ, Yuan ML, Li R, Zhu LP, Chen ZH. Real-time placental perfusion on contrast-enhanced ultrasound and parametric imaging analysis in rats at different gestation time and different portions of placenta. PLoS One. 2013;8(4):e58986. doi: 10.1371/journal.pone.0058986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arthuis CJ, Novell A, Escoffre JM, Patat F, Bouakaz A, Perrotin F. New insights into uteroplacental perfusion: quantitative analysis using Doppler and contrast-enhanced ultrasound imaging. Placenta. 2013;34(5):424–431. doi: 10.1016/j.placenta.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Keator CS, Lindner JR, Belcik JT, Bishop CV, Slayden OD. Contrast-enhanced ultrasound reveals real-time spatial changes in vascular perfusion during early implantation in the macaque uterus. Fertil Steril. 2011;95(4):1316–1321 e1311-1313. doi: 10.1016/j.fertnstert.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juffermans LJ, van Dijk A, Jongenelen CA, et al. Ultrasound and microbubble-induced intra- and intercellular bioeffects in primary endothelial cells. Ultrasound Med Biol. 2009;35(11):1917–1927. doi: 10.1016/j.ultrasmedbio.2009.06.1091. [DOI] [PubMed] [Google Scholar]
- 18.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97(5):473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 19.Roberts VH, Smith J, McLea SA, Heizer AB, Richardson JL, Myatt L. Effect of increasing maternal body mass index on oxidative and nitrative stress in the human placenta. Placenta. 2009;30(2):169–175. doi: 10.1016/j.placenta.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones CJ, Fox H. An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta. 1980;1(1):61–76. doi: 10.1016/s0143-4004(80)80016-6. [DOI] [PubMed] [Google Scholar]
- 21.Gould SF. The ultrastructural demonstration of placental phospholipids in preterm, term and eclamptic pregnancies. Placenta. 1983;4(3):241–254. doi: 10.1016/s0143-4004(83)80003-4. [DOI] [PubMed] [Google Scholar]
- 22.Jones CJ, Jauniaux E. Ultrastructure of the materno-embryonic interface in the first trimester of pregnancy. Micron. 1995;26(2):145–173. doi: 10.1016/0968-4328(95)00002-l. [DOI] [PubMed] [Google Scholar]
- 23.Frias AE, Schabel MC, Roberts VH, et al. Using dynamic contrast-enhanced MRI to quantitatively characterize maternal vascular organization in the primate placenta. Magn Reson Med. 2015;73(4):1570–1578. doi: 10.1002/mrm.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts VH, Pound LD, Thorn SR, et al. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014;28(6):2466–2477. doi: 10.1096/fj.13-245472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo JO, Schabel MC, Roberts VH, et al. Vitamin C supplementation ameliorates the adverse effects of nicotine on placental hemodynamics and histology in nonhuman primates. Am J Obstet Gynecol. 2015;212(3):370 e371–378. doi: 10.1016/j.ajog.2014.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009;119(2):323–335. doi: 10.1172/JCI32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1: Maternal perfusion of the intervillous space in a Japanese macaque at gestational day 129. Microbubbles are ‘burst’ by a 2 sec increase in mechanical index, and spiral artery filling followed by perfusion of the intervillous space in an individual cotyledon are observed until signal saturation is reached.
Video 2: Maternal perfusion of an 11 week gestation human placenta. Microbubbles are ‘burst’ by a 2 sec increase in mechanical index, and perfusion of the entire placenta is observed from four maternal spiral artery sources until signal saturation is reached.


