Abstract
Curcumin is the active component of turmeric extract derived from the Curcuma longa plant. In the last decade, curcumin has raised a considerable interest in medicine owing to its negligible toxicity and multiple therapeutic actions including anti-cancer, anti-inflammatory and anti-microbial activities. Among the various molecular targets of curcumin, some are involved in bone remodeling, which strongly suggests that curcumin can affect the skeletal system. The review sheds light on the current and potential applications of curcumin to treat bone disorders characterized by an excessive resorption activity. Within the scope of this review, the novel formulations of curcumin to overcome its physico-chemical and pharmacokinetic constraints are also discussed.
Introduction
Curcumin is a polyphenolic compound derived from the Indian spice, turmeric (Curcumin longa). Turmeric contains three principal curcuminoids (Figure 1) in which curcumin is the most abundant (77%) and biologically active, followed by demethoxycurcumin (17%) and bisdemethoxycurcumin (3%).1 Curcumin has been demonstrated to possess multi-faced therapeutic actions and biological activities, including anti-infectious, anti-oxidant, anti-inflammatory, thrombosuppressive, anti-arthritic, chemopreventive and anti-carcinogenic properties.2,3,4 For example, curcumin has undergone more than 40 clinical trials for the treatment of inflammatory diseases and various human cancers.5 In addition to its numerous biological activities, curcumin is well-tolerated by organism even after high peros dose administration.
Figure 1.
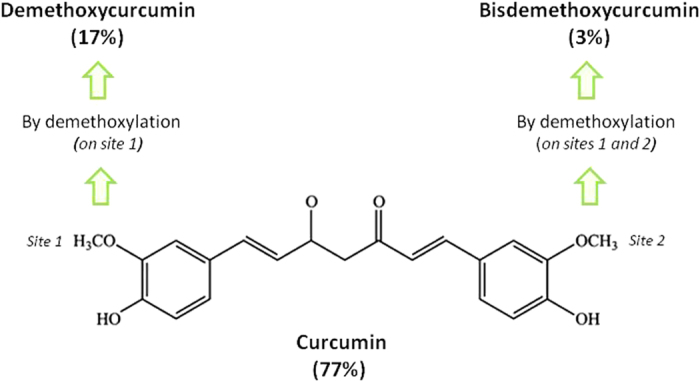
Chemical structure of curcumin. Turmeric contains three principal curcuminoids in which curcumin is the most abundant (77%) and biologically active, followed by demethoxycurcumin (17%) and bisdemethoxycurcumin (3%). These analogs were obtained by demethoxylation of curcumin on site 1 and on both sites, respectively.
In spite of the enormous potential of curcumin in medicine, its clinical application is hampered by its low water solubility and physico-chemical stability, resulting in poor absorption and weak bioavailability on oral administration.6 Rapid systemic clearance and low cellular uptake considerably compromise its potential therapeutic action in human. For instance, it was shown that the plasmatic concentration of curcumin remained low (nanomolar range) even after high oral doses (that is, 10–12 g per day) were administered.7 Approximately 65% of the orally administered curcumin undergoes rapid clearance and is eliminated from body mainly in the feces.
After reporting generalities on pharmacological mechanisms of curcumin, this review details deeper the in vitro and in vivo studies on curcumin that focus on the treatment of bone disorders including osteoporosis and tumor. The review will also cover novel strategies developed to overcome the above-mentioned limitations with curcumin's physico-chemical and pharmacokinetic characteristics.
General pharmacological mechanisms of curcumin
Curcumin modulates important molecular targets in a wide variety of cells including transcription factors such as activating protein-1, β-catenin, peroxisome proliferator-activated receptor-γ and nuclear factor-κB (NF-κB).8 Decreasing NF-κB activity is one of the major biological effects of curcumin in which NF-κB tends to maintain bonding with IκB (inhibitor of NF-κB), as curcumin hinders the phosphorylation and the degradation of IκBα. The inactivated NF-κB/IκB complex is kept in the cytoplasm and therefore not able to enter into the nucleus. Inactivation of the NF-κB signaling pathway is particularly important in curcumin's anti-cancer activities, as in tumor cells activated NF-κB is relatively higher than normal cells, and this results in the development of carcinogenesis, such as antiapoptotic genes, metastasis, tumor promotion and malignancy. As a result of the inactivation of NF-κB, curcumin downregulated the carcinogenesis-related expression of genetic products of NF-κB, including cell cycle proteins (cyclin D1 and p21), inducible cyclooxygenase (COX-2) and Bcl-2. Moreover, curcumin disturbs activity of enzymes by suppressing their expression such as lipoxygenase and inducible nitric oxide (NO) synthase.9 Finally, curcumin also alters the expression of numerous cytokines (tumor necrosis factor-α, interleukin-1 (IL-1), IL-6 and chemokines), receptors (epidermal growth factor receptor, low-density lipoprotein receptor and estrogen receptor-α) and cell surface adhesion molecules.1,5,6,7
Consequently, curcumin displays both anti-oxidant and anti-inflammatory properties and found to suppress tumor initiation, promotion and metastasis.10 For example, curcumin was shown to induce apoptosis in MCF-7 breast cancer cells (25 μM for 24 h),11 human leukemia HL 60 cells (10–40 μM for 16–24 h)12 and human melanoma cells (30–60 μM for 24 h).13 In contrast, curcumin treatment (10 μM for 12 h) inhibited dexamethane-induced apoptosis in rat thymocytes and chemotherapy-induced apoptosis in breast cancer cells.14 Figure 2 recapitulates the main molecular targets of curcumin responsible for its therapeutic activities.
Figure 2.
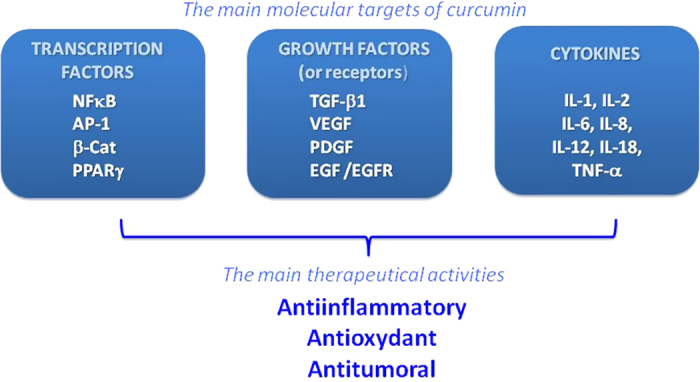
Main molecular targets regulated by curcumin involved in its therapeutical activities. Curcumin modulates (i) various transcription factors such as NF-κB (nuclear factor κB), AP-1 (activating protein-1), β-cat (β-catenin) and PPARγ (peroxisome proliferator-associated receptor gamma), (ii) growth factors including TGF-β1 (tumor growth factor -β1), VEGF (vascular endothelial growth factor), PDGF (platelet-derived growth factor), EGF (epidermal growth factor) and its receptor EGFR and (iii) cytokines such as IL (interleukin) and TNF-α (tumor necrosis factor α). Regulation of these molecular targets contributes to its therapeutical interest as anti-inflammatory, antioxydant and anti-cancer agent.
As many of the curcumin targets mentioned above including NF-κB participate in the regulation of bone remodeling, curcumin can potentially affect the skeletal system. The specific effects of curcumin on bone cells/tissue will be discussed in the following sections.
Effects of curcumin on bone
Physiological bone environment
Osteoblasts
Using 5–10 μM treatment, Notoya et al.15 demonstrated the inhibitory effects of curcumin on proliferation of osteoblasts derived from calvaria of rats (ROB) without inducing apoptosis.This effect might result from the arrest of cell cycle progression via expression of p21 protein. In contrast, Chan et al.16 reported that curcumin induced two distinct cell death programs in osteoblasts according to its concentration. In fact, curcumin decreased dose dependently the intracellular ATP levels in osteoblasts and reactive oxygen species (ROS) generation, which could switch curcumin-induced apoptosis to necrosis. Concerning the oxidative stress, they found that 12.5–25 μM curcumin treatment increased intracellular ROS levels by approximately sixfold, whereas 50–200 μM curcumin had far less effect. The authors supported theirs results on the hypothesis that ATP can act as a switch to determine curcumin-induced apoptosis versus necrosis. They concluded that curcumin treatment induces osteoblast apoptosis at the doses of 12.5–25 μM and causes necrosis at the doses greater than 50 μM.
Concerning the activity of osteoblasts, it appears that the stimulated-expression of p21 by curcumin might also be implied in the inhibition of mineralization by ROB cells.15 By contrast, it has recently been investigated that a curcumin analog, UBS109, has a positive role in the differentiation of mouse preosteoblastic MC3T3 cell line by stimulating Smad signaling involved in the activation of bone morphogenetic protein-2.17 In addition, UBS109 tested in vitro at 10–200 nM stimulates osteoblastic mineralization from bone marrow mesenchymal stem cells, whereas it suppresses the process of adipogenesis of these stem cells.
Given the few studies performed on osteoblasts and their contradictory results, further experiments are required to determine the impact of curcumin on their differentiation process and their activity.
Osteoclasts
Ozaki et al.18 observed that curcumin stimulates in a dose-dependent manner apoptosis of osteoclasts derived from long bone of rabbit. As a consequence, curcumin inhibits osteoclastic bone resorption in a dose-dependent manner. For example, the treatment with 10 μM curcumin for 24 h decreases approximatively by 80% the number of bone resorption pits as compared with the untreated cells. Even if they did not address in their study the mechanism whereby curcumin stimulates apoptosis in mature rabbit osteoclasts, they postulated on the inhibitory effect of curcumin on two factors implied in the survival of osteoclasts, AP-1 and NF-k B. In this regard, Bharti et al.19 demonstrated that curcumin suppressed RANKL signaling and osteoclastogenesis by interfering with the NF-κB pathway. Treatment of RAW 264.7 cells, a murine monocytic cell line, with 50 μM curcumin for 2 h blocked phosphorylation of IκB, which is a prerequisite to the activation of NF-κB.
In addition, owing to its anti-oxidant properties, curcumin suppresses osteoclast differentiation by scavenging the generated intracellular ROS, which acts as a secondary messenger in the RANKL-induced osteoclast differentiation signaling pathway.20 This effect attenuates ROS-induced NF-κB signaling pathways for osteoclastogenesis and then causes a decrease in NFATc1 gene expression, which is the master gene involved in the osteoclastic differentiation.
Other bone cells
Considering the key role of osteocytes as bone sensor, it could be interesting in deciphering the impact of curcumin on their activity. Unfortunately, the authors could not find any studies in the literature on this subject.
Pathological bone environment
Osteoporosis
Despite the few biological data, curcumin might be a promising candidate for preventing and/or treating the development of the osteoporotic fractures. Hie et al.21 reported the inhibitory effects of curcumin on the stimulated osteoclastic activity in insulin-dependent diabetes mellitus using rats with streptozotocin-induced diabetes. In their diabetic rat model, the elevated levels of TRAP (1.5-fold) and cathepsin K (2.4-fold) activities strongly induced degradation of bone matrix with consequently decreased the level of hydroxyproline in the distal femur and increased the urinary excretion of deoxypyridinoline (1.6 fold), which is a product of the degradation of collagen. Interestingly, curcumin oral administration (120 mg per day for 14 days) in diabetic rats restored the levels of mRNA and activity of cathepsin K and TRAP to the control value. The urinary deoxypyridinoline level was also decreased by curcumin. Further, the histochemical analysis showed that the increased number of osteoclasts in the distal femur of the diabetic rats was recovered to the control level in the curcumin-supplemented rats. However, the decreased levels of expression of ALP and osteocalcin in diabetic rats were not affected by the curcumin supplement.
Although the inhibition of mineralization by curcumin was observed in the cultured osteoblasts,15 the calcium content of the distal femur was not decreased by the curcumin supplement in the diabetic rats. This observation probably indicated that curcumin affected the activity and number of osteoclasts rather than osteoblasts. They also showed that curcumin reversed the increased number of osteoclast progenitors in response to M-CSF and RANKL observed in diabetic rats. They reported that this alteration of osteoclastic formation in the bone marrow cells was probably due to the inhibition of c-fos and c-jun expression and consequently of the AP-1 complex, which is essential for osteoclastogenesis. Interestingly, curcumin appeared to suppress osteoclastogenesis by inhibiting the expression of c-fos and c-jun but not the expression of c-fms or RANK in the diabetic rats. As mentioned previously, curcumin also inhibited osteoclastogenesis through inhibition of NF-κB in RAW 264.7 cells.19
Folwarczna et al.22 have investigated the effects of curcumin administrated orally on bone in a postmenopausal osteoporosis model induced by ovariectomizing female rat (OVX). They examined serum total cholesterol and estradiol levels, bone mass, bone mineral and calcium content, mechanical properties, and macrometric and histomorphometric parameters of the bone. In rats with normal estrogen levels, curcumin decreased serum estradiol level and slightly increased cancellous bone formation, along with decreased mineralization. In OVX rats, oral administration of curcumin (10 mg kg−1 per day for 4 weeks) (i) decreased serum total cholesterol level and body mass gain and and (ii) slightly improved some bone histomorphometric parameters including width of endosteal osteoid in the tibia impaired by estrogen deficiency. The decrease in the width of endosteal osteoid in the tibia of the treated OVX rats could be due to the inhibition of bone matrix formation, resulting in the decrease in transverse growth. Moreover, curcumin did neither improve bone mineralization nor the mechanical properties in treated OVX rats compared with the non-OVX animals. In fact, the strength of the femoral neck in OVX rats treated with curcumin was significantly lower as compared with non-OVX control rats.
French et al.23 have also explored the in vivo effects of curcumin on OVX female rats. They quantified bone mineral density at the spine, femur before and at 2, 4 and 6 months after ovariectomy in each of the 40 mature rats. They performed dosages of serum osteocalcin and C-telopeptide as indicators of bone formation and resorption rates. At the highest dose of curcumin (15 mg per day), they observed a significant improvement in the size of the femur after 6 months of administration. In addition, curcumin administration increased the energy to fracture of the femur in a dose-dependent manner.
Osteosarcoma
Considering that uptake of curcumin by malignant cells seems to be higher than by normal cells, a preferential action of curcumin toward tumor than healthy tissue might be expected.24 Chang et al.25 compared the in vitro cytotoxicity of curcumin in MG-63 osteosarcoma and healthy human osteoblasts treated at different concentrations (5, 10, 25, 50, 75 and 100 μM). Treatment with 10 μM curcumin reduced viability of MG-63 osteosarcoma cells by 50% as compared with the untreated control. By contrast, healthy osteoblast cells had at least 80% viability throughout all the tested concentrations. The results demonstrated that MG-63 osteosarcoma cells were much more sensitive in terms of cytotoxicity to curcumin, whereas the healthy human osteoblasts exhibited a higher viability after 24 h of curcumin treatment. On the basis of the protective effect of NO in bone tissue, Moran et al.26 elucidated that curcumin might affect growth and mineralization of MG-63 by a mechanism partially regulated by NO. Even if the mechanism underlying the protective effect of NO against bone damage has not been fully elucidated, it seems that the effect of NO on osteoblast activity is bi-directional depending on its concentration. Under physiological conditions, the low level of NO is an important second messenger for systemic hormones involved in the regulation of bone metabolism such as calcitonin gene-related peptide, parathyroid hormone and sex steroids, particularly estrogen.27 It has been shown that the constitutively NO produced by osteoblasts stimulates both osteoblastic growth and differentiation.28 By contrast, excess local production of NO induces cytotoxicity in osteoblastic cells,27 which is probably mediated in part by cGMP.28 Interestingly, curcumin has been widely described to inhibit inducible NO synthase expression and NO production, at least in part via direct interference in NF-κB activation.
Similar result was reported in a study that tested the cytotoxicity of curcumin on U2OS osteosarcoma cells at different concentrations (5, 10, 25, 50, 75 and 100 μM) for different time points (6, 12, 24 and 36 h).29 They showed that curcumin stimulated apoptosis of U2OS cells in a time- and a concentration-dependent manner. In addition, the curcumin-treated cancer cells displayed higher expression of apoptosis-related proteins including Bax and Bak, as well as a lower expression of anti-apoptosis proteins such as Bcl-2. Furthermore, one consequence of loss of mitochondrial membrane potential induced by curcumin is probably the release of the mitochondrial cytochrome c and therefore its accumulation into cytosol. Consequently, caspase-3 is activated leading to the clivage of specific substrates and finally to block cells successively in G1/S and G2/M phases. According to Li et al.,30 curcumin's effect on osteosarcoma cells is mediated through a novel mechanism involving the inactivation of the Notch-1 signaling pathway including its downstream target gene matrix metalloproteinase (MMP). It is an interesting therapeutical target considering that Notch-1 has a pivotal role in the process of cell survival, invasion and metastasis and therefore largely contributing to the pathogenesis of human osteosarcoma.31
A particular attention should be payed on multidrug resistance (MDR). Interestingly, curcumin displays reversion effects on MDR through downregulation of P-glycoprotein (P-gp) and inhibition of the function of P-gp efflux pump.32 In addition to the downregulation of P-gp, curcumin can also affect the expression of breast cancer resistance protein (ABCG2) and MDR protein (MRP-1) expression. These alterations contribute to in vivo inhibition of the proliferation, invasion and metastasis of osteosarcoma MDR cells.32
Bone metastases
Bone tissue is one of the most favored sites for metastasis of various solid tumors including thyroid, breast, prostate and kidney cancers. For example, up to 70% of all patients diagnosed with breast cancer will develop bone metastases.33 This is potentially due to the high vascularity of the bone environment and the abundance of a variety of ions, cytokines and growth factors that serve as a ‘fertile soil' in which cancer cells can grow.34 Bone lesions are preferentially localized in the spine and pelvic bone, resulting in severe pain and serious neurological complications.33 Development of bone metastasis is a complex, multistep process that implied a cascade of interrelated, sequential steps including invasion, migration, adhesion, infiltration, colonization at distant tissue and the subsequent formation of new capillaries.35 During the process of invasion, the adhesion of cancer cells to the blood vessels and then spilling out and then to the bone tissue interstitium is the key step of bone metastasis.
Bone metastasis from thyroid cancer
Thyroid cancer is one of the aggressive cancers resulting in the development of severe osteolytic bone lesions (bone destruction). Zhang et al.36 examined the antimetastatic effects of curcumin on K1 papillary thyroid cancer cells by focusing on the cancer cell attachment, spreading, migration and invasion abilities, as well as the activity and expression levels of MMP-9. In summary, they demonstrated that curcumin significantly altered the main steps involved in metastasis. For example, curcumin used at 12.5, 25 and 50 μM promoted mesenchymal–epithelial transition and decreased the migration rate of K1 papillary thyroid cancer cells by 24–87%. The anti-metastatic mechanisms of curcumin may involve (i) the upregulation of E-cadherin expression levels and (ii) the downregulation of the activity and expression of MMP-9.
Bone metastasis from breast cancer
By using the well-known estrogen-independent human breast cancer cell line MDA-MB-231 with enhanced bone metastatic ability, Ganguly et al.37 reported that curcumin markedly inhibited cell adhesion ability through the regulation of FAK and thereby MMP-9 expression and activity. In addition, curcumin decreased MDA-MB-231 viability dose dependently (IC50=82 μM).38 Recently, Yamaguchi et al.39 have designed an in vitro model of bone metastases from breast cancer. Co-culture with MDA-MB-231 bone metastatic cells suppressed osteoblastic mineralization and stimulated osteoclastogenesis in bone marrow culture. These effects were reversed by treating cells with 50–200 nM curcumin analog (USB109). Interestingly, they confirmed in vivo the efficacy of curcumin to reduce bone loss in a breast cancer bone metastasis mouse model.40 Nude mice were inoculated with breast cancer MDA-MB-231 bone metastastic cells into the tibia. Trabecular bone tissues in the femur and tibia of mice were profoundly damaged, demonstrating defective and fragmentary bone. This bone destruction was prevented by curcumin peros administration (50 or 150 mg kg−1 bodyweight) or i.p. administration (10 or 20 mg kg−1 bodyweight) once daily for 5 days per week during 7 weeks. Similarly, Wright et al.38 demonstrated that administration of curcumin (25 or 50 mg kg−1) for 21 days prevented the development of severe osteolytic bone lesions in mice with breast cancer bone metastases. Consistently, the number of osteoclasts at the bone–tumor interface was reduced up to 53%, whereas the tumor area within the bone was unaltered. Interestingly, they showed that this effect is related to the inhibition of the osteolytic peptide parathyroid hormone-related protein (PTHrP) secretion (IC50=24 μM). This inhibitory effect of curcumin is independent of its effects on the tumor cell growth and is mediated by the downregulation of phospho-Smad 2/3 and Ets-1 protein.38
From these promising results on preventing breast cancer osteolytic bone metastasis using curcumin trearment, a clinical trial has been conducted to investigate the feasibility and tolerability of the combination of docetaxel and curcumin in breast cancer patients. This open-label phase I trial included 14 patients with advanced or metastatic breast cancer.41 The recommended peros dose of curcumin is 6000, mg per day for 7 consecutive days every 3 weeks in combination with a standard dose of docetaxel. According to the authors, it could be expected that a response rate up to 50% in a population of all evaluable patients treated with the combination of docetaxel/curcumin. On the basis of this beneficial effect, a comparative phase II trial of this regimen plus docetaxel versus docetaxel alone is ongoing in advanced and metastatic breast cancer patients.
Bone metastasis from prostate cancer
By disturbing the protein kinase C (PKC) signaling pathway, curcumin decreased the expression of CCL2 CC motif ligand 2 (CCL2; aka monocyte chemoattractant protein-1 or MCP-1) in a human bone-derived androgen-independent PCa cell line named PC-3 cells.42 Consequently, curcumin blocked adhesion, invasion and motility of PC-3 cells. For example, treatment with 30 μM curcumin for 18 h blocked 23% the invasive characteristics of PC-3 cells as compared with the control cells. Dorai et al.43,44 demonstrated the anti-osteomimetic properties of the castration-resistant prostate cells by blocking signaling pathways mediated by colony stimulation factor receptor, epidermal growth factor receptor, Cbfa1 (an osteoblast transcription factor) and NFkB.Their preliminary results strongly suggested that curcumin might severely alter the establishment of bone metastases. By using an adapted in vivo model of C4-2B prostate carcinoma bone metastasis progression, they have recently confirmed that curcumin inhibits prostate cancer bone metastasis by upregulating the expression of bone morphogenic protein-7.45
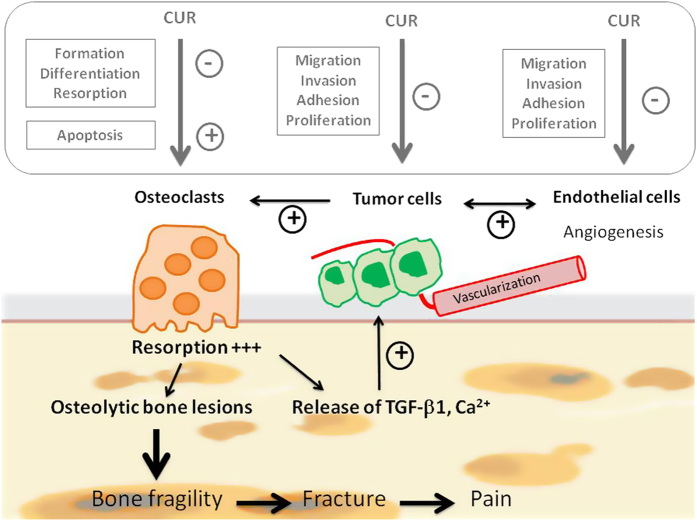
Althought further in vivo experiments are required to confirm its beneficial effects, curcumin might be a promising candidate for preventing and/or treating the development of bone metastases regardless of the origin of the primary tumor (Figure 3).
Figure 3.
Effects of curcumin (CUR) on bone metastases. Summary of the physiopathology of bone metastases (in black). Excessive osteoclastic resorption activity is related to the presence of tumor cells secreting various osteolytic factors. This leads progressively in the development of severe bone lesions. Among the growth factors released from bone matrix, TGF-β1 is the most implied in the tumor growth and invasion. Effects of curcumin (in the gray box) on osteoclasts, tumor cells and endothelial cells. In addition to the decrease in the proliferation of endothelial cells and the formation of cell colonies,67,68 curcumin inhibits TNF-induced expression of adhesion molecules on endothelial cells,69 such as intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1 and endothelial leukocyte adhesion molecule (ELAM)-1 on endothelial cells, which are critical for tumor cell invasion and metastases. The anti-angiogenic properties of curcumin may be mediated in part by the inhibition ofcyclic nucleotide phosphodiesterases, which are strongly involved in the regulation of the VEGF-induced endothelial cell activity.
Osteoarthritis
Osteoarthritis has been defined as a progressive, degenerative disease of joints involving the articular cartilage, subchondral bone and synovium; joints in the fingers, hips, knees, and spine are most frequently affected.46 In vitro studies revealed that curcumin decreases the expression of inflammatory markers such as IL-6 and IL-8, 5-lipooxygenase and COX-2.47 Interestingly, by selectively inhibiting COX-2 instead of COX-1, curcumin improves gastrointestinal tolerance for patients used to be treated by classical anti-inflammatory drugs. Moreover, curcumin displays anti-catabolic properties by suppressing gene expression of several MMPs largely involved in the degradation of the joint matrix.47,48 In addition, by altering the proliferation of synoviocytes, curcumin limits the excessive inflammatory process so prejudiciable for the integrity of the joint matrix.49 Finally, it has been demonstrated that curcumin can reduce the formation of reactive species (oxygen and nitrogen ones) responsible for the degradation of the cartilage.50
Recently, a randomized clinical trial strongly showed the efficiency of curcumin to reduce pain and improve physical outcome of patients.51 Despite these positive results, further clinical studies should be designed to evaluate whether curcumin could prevent the development of OA or limit its progression.
Novel strategies to improve biological activities of curcumin
Even if curcumin effects on the skeletal system require further pre-clinical studies, the in vivo therapeutic activities of curcumin does not seem reflecting the positive benefitial effects that were largely observed in vitro. One of the reasons explaining the discrepancy between the in vitro and the in vivo outcomes may be due to the low bioavalability of curcumin in tissues, including bone. As previously mentioned, curcumin has poor water solubility, stability and in general short elimination half-life from body.7 To overcome these pharmacokinetic limits, smart approches have been developed to improve curcumin delivery into the body. As it has been demonstrated with various therapeutical agents, delivering drugs directly in situ considerably improves both efficiency and tolerance of treatment.52,53,54 These strategies include implantable curcumin carrier,55 curcumin microparticles56 and nanoparticles.57 Pharmacokinetic studies reported that curcumin nano-formulation displayed a larger area under curve (AUC)–time curve as compared with free curcumin.57,58,59 For example, encapsulation of curcumin by poly(lactic-co-glycolide) (PLGA) nanoparticles provided a significant higher maximal concentration (1000-fold increase) as compared with free curcumin following 60 min of i.v. administration in mice.57
Curcumin nano-formulation has been explored especially for cancer therapy.60,61,62 In this attempt, modulation of key properties of nanoparticles such as their size, surface charge and chemistry has been reported to favor their accumulation into the tumor tissue and optimize biological interactions with tumor cells. For example, it has been suggested that particles with size ranging between 100 and 150 nm with outer positive charges may penetrate easily into the tumor cells. According to Lee et al.5, cellular uptake and anti-cancer potency of curcumin nanoparticles are directly correlated with their size where the smallest size (28 nm) was the most potent.
As compared with free curcumin, nano-encapsulation of curcumin with polymers not only improved considerably its bioavailability but also protected curcumin from various physiological degradation. Consequently, nano-formulation of curcumin displayed higher therapeutic activities. For example, curcumin was loaded into micelles based on MPEG-P(CL-co-PDO) (methoxypoly(ethylene glycol)-b-poly(ɛ-caprolactone-co-p-dioxanone)) copolymers by a solid dispersion method.63 Interestingly, the release of curcumin from these nano-sized polymeric micelles (∼30 nm) was slow without any burst effect. After 48 h of incubation, curcumin-loaded micelles markedly inhibited the human bone-derived androgen-independent PCa cell growth in a dose-dependent manner. Yalaapu et al.64 have encapsulated curcumin in PLGA (biodegradable polymer) nanoparticles, in the presence of poly(vinyl alcohol) and poly(L-lysine) stabilizers, using a nano-precipitation technique.They observed a sixfold increase in the cellular uptake of these nanoparticles into metastatic MDA-MB-231 breast cancer cells as compared with free curcumin. As expected, they demonstrated that these nano-formulations enhanced the anti-proliferative effect of curcumin.
Human serum albumin is a very attractive curcumin carrier as it is well-tolerated by organism, non-immunogenic and stable without requiring surfactants or polymers.59,65 In addition, because of its various drug-binding sites, albumin enhances considerably the amount of curcumin within particle matrices. By modulating the curcumin polymer ratio, Jithan et al.59 obtained a final entrapment efficiency of 86.42% and a percent yield of 85.2%. Interestingly, the curcumin released from albumin nanoparticles dissolved in phosphate buffer pH 7.4 was almost fourfold higher than that of free curcumin, strongly suggesting that solubility of curcumin with curcumin nanoparticles can be enhanced in vivo. This will lead to higher plasma levels, as well as higher intracellular levels of curcumin. Consequently, this enhancement leads to a significant anti-proliferative effect (IC50=70 μM) in MDA-MB-231 cells as compared with free curcumin (IC50=90 μM at 72 h).59 In consistence with that data, Wartlick et al.66 previously reported that the presence of albumin in nano-formulation increased the endocytic uptake and the accumulation of the loaded drug within tumor site.
To summarize, nanotechnology requires a technological expertise to obtain in a reproducibly manner nanoparticles homogeneous in terms of size and composition, well-dispersed and stable as it conditions their biological efficiency.
Conclusion
To our knowledge, this is the first time that a review shed light on the potential of curcumin in the treatment of these bone disorders. Even if these preliminary data on bone tissue are very convincing and encouraging, further in vivo experiments are required to explore deeper its bone effects before envisaging human clinical studies. Unfortunately, its clinical use for bone pathologies may be limited by its instability in water and its low bioavailability. To overcome these constraints, smart strategies including nanotechnology already developed for cancer indication may also be adapted for bone application. Obviously, these development processes must be simple, efficient, safe, reproducible and suitable for large-scale production.
Acknowledgments
We acknowledge the ‘Fondation ARC' (dossier SAE 20140600962), the Faculty of Pharmacy of Nantes in France, the University of Nantes in France, the ‘Fondation de France' in France and the Faculty of Pharmacy of Sydney in Australia for their financial and technical supports.
Footnotes
The authors declare no conflict of interest.
References
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the indian solid gold. Adv Exp Med Biol 2007; 595: 1–75. [DOI] [PubMed] [Google Scholar]
- Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME et al. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015; 20: 2728–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad A, Rehman G, Lee YS. Curcumin in inflammatory diseases. Biofactors 2013; 39: 69–77. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 2013; 15: 195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Loo CY, Young PM, Traini D, Mason RS, Rohanizadeh R. Recent advances in curcumin nanoformulation for cancer therapy. Exp Opin Drug Deliv 2014; 11: 1183–1201. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm 2007; 4: 807–818. [DOI] [PubMed] [Google Scholar]
- Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 2008; 17: 1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood 2003; 101: 1053–1062. [DOI] [PubMed] [Google Scholar]
- Ye MX, Li Y, Yin H, Zhang J. Curcumin: updated molecular mechanisms and intervention targets in human lung cancer. Int J Mol Sci 2012; 13: 3959–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 2003; 23: 363–398. [PubMed] [Google Scholar]
- Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett 2002; 512: 334–340. [DOI] [PubMed] [Google Scholar]
- Anto RJ, Mukhopadhyay A, Denning K, Aggarwal BB. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis 2002; 23: 143–150. [DOI] [PubMed] [Google Scholar]
- Bush JA, Cheung KJ Jr, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res 2001; 271: 305–314. [DOI] [PubMed] [Google Scholar]
- Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res 2002; 62: 3868–3875. [PubMed] [Google Scholar]
- Notoya M, Nishimura H, Woo JT, Nagai K, Ishihara Y, Hagiwara H. Curcumin inhibits the proliferation and mineralization of cultured osteoblasts. Eur J Pharmacol 2006; 534: 55–62. [DOI] [PubMed] [Google Scholar]
- Chan WH, Wu HY, Chang WH. Dosage effects of curcumin on cell death types in a human osteoblast cell line. Food Chem Toxicol 2006; 44: 1362–1371. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Moore TW, Sun A, Snyder JP, Shoji M. Novel curcumin analogue UBS109 potently stimulates osteoblastogenesis and suppresses osteoclastogenesis: involvement in Smad activation and NF-kappaB inhibition. Integr Biol (Camb) 2012; 4: 905–913. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Kawata Y, Amano S, Hanazawa S. Stimulatory effect of curcumin on osteoclast apoptosis. Biochem Pharmacol 2000; 59: 1577–1581. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Takada Y, Aggarwal BB. Curcumin (diferuloylmethane) inhibits receptor activator of NF-kappa B ligand-induced NF-kappa B activation in osteoclast precursors and suppresses osteoclastogenesis. J Immunol 2004; 172: 5940–5947. [DOI] [PubMed] [Google Scholar]
- Moon HJ, Ko WK, Han SW, Kim DS, Hwang YS, Park HK et al. Antioxidants, like coenzyme Q10, selenite, and curcumin, inhibited osteoclast differentiation by suppressing reactive oxygen species generation. Biochem Biophys Res Commun 2012; 418: 247–253. [DOI] [PubMed] [Google Scholar]
- Hie M, Yamazaki M, Tsukamoto I. Curcumin suppresses increased bone resorption by inhibiting osteoclastogenesis in rats with streptozotocin-induced diabetes. Eur J Pharmacol 2009; 621: 1–9. [DOI] [PubMed] [Google Scholar]
- Folwarczna J, Zych M, Trzeciak HI. Effects of curcumin on the skeletal system in rats. Pharmacol Rep 2010; 62: 900–909. [DOI] [PubMed] [Google Scholar]
- French DL, Muir JM, Webber CE. The ovariectomized, mature rat model of postmenopausal osteoporosis: an assessment of the bone sparing effects of curcumin. Phytomedicine 2008; 15: 1069–1078. [DOI] [PubMed] [Google Scholar]
- Kunwar A, Barik A, Mishra B, Rathinasamy K, Pandey R, Priyadarsini KI. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim Biophys Acta 2008; 1780: 673–679. [DOI] [PubMed] [Google Scholar]
- Chang R, Sun L, Webster TJ. Short communication: selective cytotoxicity of curcumin on osteosarcoma cells compared to healthy osteoblasts. Int J Nanomed 2014; 9: 461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Roncero-Martin R, Rodriguez-Velasco FJ, Calderon-Garcia JF, Rey-Sanchez P, Vera V et al. Effects of curcumin on the proliferation and mineralization of human osteoblast-like cells: implications of nitric oxide. Int J Mol Sci 2012; 13: 16104–16118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Yinghui T, Bo Y, Gang Z, Xian X, Lu Z. Effect of calcitonin gene-related peptide on nitric oxide production in osteoblasts: an experimental study. Cell Biol Int 2011; 35: 757–765. [DOI] [PubMed] [Google Scholar]
- Son MJ, Lee SB, Byun YJ, Lee HO, Kim HS, Kwon OJ et al. Sodium nitroprusside induces autophagic cell death in glutathione-depleted osteoblasts. J Biochem Mol Toxicol 2010; 24: 313–322. [DOI] [PubMed] [Google Scholar]
- Jin S, Xu HG, Shen JN, Chen XW, Wang H, Zhou JG. Apoptotic effects of curcumin on human osteosarcoma U2OS cells. Orthopaed Surg 2009; 1: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang J, Ma D, Zhang L, Si M, Yin H et al. Curcumin inhibits proliferation and invasion of osteosarcoma cells through inactivation of Notch-1 signaling. FEBS J 2012; 279: 2247–2259. [DOI] [PubMed] [Google Scholar]
- Engin F, Bertin T, Ma O, Jiang MM, Wang L, Sutton RE et al. Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum Mol Genet 2009; 18: 1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si M, Zhao J, Li X, Tian JG, Li YG, Li JM. Reversion effects of curcumin on multidrug resistance of MNNG/HOS human osteosarcoma cells in vitro and in vivo through regulation of P-glycoprotein. Chin Med J 2013; 126: 4116–4123. [PubMed] [Google Scholar]
- Verron E, Schmid-Antomarchi H, Pascal-Mousselard H, Schmid-Alliana A, Scimeca JC, Bouler JM. Therapeutic strategies for treating osteolytic bone metastases. Drug Discov Today 2014; 19: 1419–1426. [DOI] [PubMed] [Google Scholar]
- Chen YC, Sosnoski DM, Mastro AM. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res 2010; 12: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004; 350: 1655–1664. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Zhang L, Yu HX, Bao JD, Sun Z, Lu RR. Curcumin inhibits invasion and metastasis in K1 papillary thyroid cancer cells. Food Chem 2013; 139: 1021–1028. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Sen T, Pal S, Biswas J, Chatterjee A. Studies on focal adhesion kinase in human breast cancer cell MDA-MB-231. Adv Biol Chem 2012; 2: 29–42. [Google Scholar]
- Wright LE, Frye JB, Gorti B, Timmermann BN, Funk JL. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Curr Pharm Des 2013; 19: 6218–6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Zhu S, Weitzmann MN, Snyder JP, Shoji M. Curcumin analog UBS109 prevents bone marrow osteoblastogenesis and osteoclastogenesis disordered by coculture with breast cancer MDA-MB-231 bone metastatic cells in vitro. Mol Cell Biochem 2015; 401: 1–10. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Zhu S, Zhang S, Wu D, Moore TM, Snyder JP et al. Curcumin analogue UBS109 prevents bone loss in breast cancer bone metastasis mouse model: involvement in osteoblastogenesis and osteoclastogenesis. Cell Tissue Res 2014; 357: 245–252. [DOI] [PubMed] [Google Scholar]
- Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther 2010; 9: 8–14. [DOI] [PubMed] [Google Scholar]
- Herman JG, Stadelman HL, Roselli CE. Curcumin blocks CCL2-induced adhesion, motility and invasion, in part, through down-regulation of CCL2 expression and proteolytic activity. Int J Oncol 2009; 34: 1319–1327. [PMC free article] [PubMed] [Google Scholar]
- Dorai T, Dutcher JP, Dempster DW, Wiernik PH. Therapeutic potential of curcumin in prostate cancer--V: Interference with the osteomimetic properties of hormone refractory C4-2B prostate cancer cells. Prostate 2004; 60: 1–17. [DOI] [PubMed] [Google Scholar]
- Dorai T, Cao YC, Dorai B, Buttyan R, Katz AE. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 2001; 47: 293–303. [DOI] [PubMed] [Google Scholar]
- Dorai T, Diouri J, O'Shea O, Doty SB. Curcumin inhibits prostate cancer bone metastasis by up-regulating bone morphogenic protein-7. J Cancer Ther 2014; 5: 369–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Saase JL, van Romunde LK, Cats A, Vandenbroucke JP, Valkenburg HA. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis 1989; 48: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res 2009; 58: 899–908. [DOI] [PubMed] [Google Scholar]
- Moon DO, Kim MO, Choi YH, Park YM, Kim GY. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol 2010; 10: 605–610. [DOI] [PubMed] [Google Scholar]
- Jackson JK, Higo T, Hunter WL, Burt HM. The antioxidants curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflamm Res 2006; 55: 168–175. [DOI] [PubMed] [Google Scholar]
- Jancinova V, Perecko T, Nosal R, Kostalova D, Bauerova K, Drabikova K. Decreased activity of neutrophils in the presence of diferuloylmethane (curcumin) involves protein kinase C inhibition. Eur J Pharmacol 2009; 612: 161–166. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Rahimnia AR, Sharafi M, Alishiri G, Saburi A, Sahebkar A. Curcuminoid treatment for knee osteoarthritis: a randomized double-blind placebo-controlled trial. Phytother Res 2014; 28: 1625–1631. [DOI] [PubMed] [Google Scholar]
- Verron E, Khairoun I, Guicheux J, Bouler JM. Calcium phosphate biomaterials as bone drug delivery systems: a review. Drug Discov Today 2010; 15: 547–552. [DOI] [PubMed] [Google Scholar]
- Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements: competitive drug carriers for the musculoskeletal system? Biomaterials 2006; 27: 2171–2177. [DOI] [PubMed] [Google Scholar]
- Verron E, Bouler JM, Guicheux J. Controlling the biological function of calcium phosphate bone substitutes with drugs. Acta Biomater 2012; 8: 3541–3551. [DOI] [PubMed] [Google Scholar]
- Shehzad A, Khan S, Sup Lee Y. Curcumin molecular targets in obesity and obesity-related cancers. Future Oncol 2012; 8: 179–190. [DOI] [PubMed] [Google Scholar]
- Pandey A, Pandey KB, Gupta RK, Rizvi SI. Ferric reducing, antiradical and beta-carotene bleaching activities of nicotinic acid and picolinic acid bioconjugates of curcumin. Nat Prod Commun 2011; 6: 1877–1880. [PubMed] [Google Scholar]
- Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010; 31: 6597–6611. [DOI] [PubMed] [Google Scholar]
- Duan J, Zhang Y, Han S, Chen Y, Li B, Liao M et al. Synthesis and in vitro/in vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int J Pharm 2010; 400: 211–220. [DOI] [PubMed] [Google Scholar]
- Jithan A, Madhavi K, Madhavi M, Prabhakar K. Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int J Pharm Invest 2011; 1: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu MM, Dobberpuhl MR, Maher DM, Jaggi M, Chauhan SC. Design of curcumin loaded cellulose nanoparticles for prostate cancer. Curr Drug Metab 2012; 13: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today 2012; 17: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu MM, Othman SF, Curtis ET, Bauer NA, Chauhan N, Kumar D et al. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int J Nanomed 2012; 7: 1761–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Feng R, Sun M, Guo C, Gao Y, Li L et al. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J Colloid Interface Sci 2011; 354: 116–123. [DOI] [PubMed] [Google Scholar]
- Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci 2010; 351: 19–29. [DOI] [PubMed] [Google Scholar]
- Bourassa P, Kanakis CD, Tarantilis P, Pollissiou MG, Tajmir-Riahi HA. Resveratrol, genistein, and curcumin bind bovine serum albumin. J Phys Chem B 2010; 114: 3348–3354. [DOI] [PubMed] [Google Scholar]
- Wartlick H, Spankuch-Schmitt B, Strebhardt K, Kreuter J, Langer K. Tumour cell delivery of antisense oligonuclceotides by human serum albumin nanoparticles. J Control Release 2004; 96: 483–495. [DOI] [PubMed] [Google Scholar]
- Vyas D, Gupt S, Dixit V, Anita K, Kaur S. To study the effect of curcumin on the growth properties of circulating endothelial progenitor cells. In Vitro Cell Dev Biol Anim 2015; 51: 488–494. [DOI] [PubMed] [Google Scholar]
- Abusnina A, Keravis T, Zhou Q, Justiniano H, Lobstein A, Lugnier C. Tumour growth inhibition and anti-angiogenic effects using curcumin correspond to combined PDE2 and PDE4 inhibition. Thromb Haemost 2015; 113: 319–328. [DOI] [PubMed] [Google Scholar]
- Gupta B, Ghosh B. Curcuma longa inhibits TNF-alpha induced expression of adhesion molecules on human umbilical vein endothelial cells. Int J Immunopharmacol 1999; 21: 745–757. [DOI] [PubMed] [Google Scholar]