Abstract
Background:
Intracranial tumors with heterogeneous histopathology are a well-described pathologic entity. Pathologically, distinct tumors in direct contact with one another, also known as collision tumors are exceptionally rare, and collision between meningioma subtypes has not been previously described in the literature.
Case Description:
A 79-year-old female with a history of breast carcinoma presenting with visual and motor deficits and imaging/intraoperative findings consistent with separate, distinct lesions. Histopathologic findings provided evidence for a collision between World Health Organization Grade III anaplastic and papillary meningioma.
Conclusion:
We report a possible collision tumor between two separate meningioma subtypes based on the unique radiologic, intraoperative, and histopathologic findings. Submission of multiple pathologic specimens during surgical resection is key for accurate histopathologic diagnosis.
Key Words: Anaplastic, collision tumor, meningioma, papillary
INTRODUCTION
Solitary intracranial tumors with intratumoral heterogeneity consisting of varying meningioma subtypes are a known reported pathologic entity.[12,17] Collision tumors are rarer entities that consist of well-demarcated, discrete tumors of differing histopathology that occur in direct contact within the same anatomic location. These tumors are rare, especially in the brain, with less 30 cases of collision tumors between intrinsic brain tumors reported in the literature since 1970.[1,3,6,9,11,13,14] A collision between two distinct meningioma subtypes has not been reported in the literature. Here, we provide possible evidence for a unique case of collision tumor between World Health Organization (WHO) III papillary and WHO III anaplastic meningiomas and describe the radiologic, intraoperative, and histopathologic features of this rare diagnosis.
CLINICALLY SUMMARY
A 79-year-old right-handed female with a history of recently diagnosed Stage III, Grade II invasive ductal breast carcinoma presented with a 2-week history of progressive left-sided weakness, visual loss, gait imbalance, and headache. Her neurological examination was remarkable for a left homonymous hemianopia as well as mild left-sided hemiparesis.
Contrast-enhanced head computed tomography scan and subsequent brain magnetic resonance imaging with gadolinium demonstrated a large, multilobulated, contrast enhancing, dural-based, and predominantly extra-axial mass in the right parietooccipital region with apparent invasion of the underlying right parietal brain parenchyma [Figure 1]. Two radiologically distinct components of this tumor were apparent, each with differing enhancement patterns with the anterior component showing greater enhancement and central necrosis. This was suggestive of two pathologically distinct components with associated moderate vasogenic edema in the surrounding brain parenchyma with mass effect on the right lateral ventricle and mild midline shift to the left.
Figure 1.
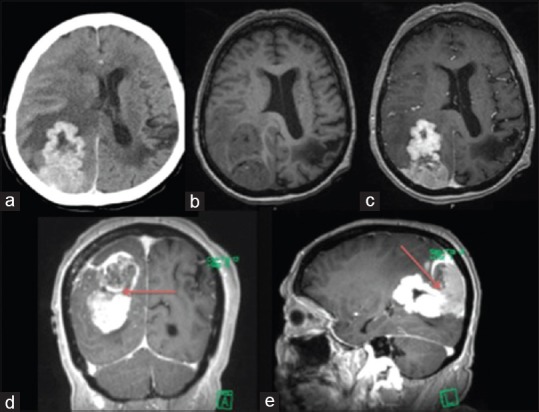
Axial contrast-enhanced computed tomography (a), T1-weighted noncontrast (b) and T1-weighted contrasted (c) magnetic resonance imaging showing contrast-enhancing predominantly extra-axial dural-based lesion with invasion into underlying brain parenchyma. Two radiologic distinct components are delineated by the red arrow in the T1-weighted contrast-enhanced coronal and sagittal images (d and e)
The patient was taken to the operating room where a right parasagittal craniotomy was performed for gross total resection of the lesion. Intraoperative frozen section was consistent with meningioma. This diagnosis was consistent with the initially encountered vascular dural-based lesion. The deeper portion of the tumor was noticeably less vascular, lobular, and largely intraventricular. This portion was adherent to the choroid plexus of the atrium. Because of concerns of the differing appearance of this portion of the tumor, a second permanent pathology specimen was submitted.
The patient had an uneventful postoperative course with no new deficits. She was discharged to a skilled nursing facility.
Histologic examination revealed two distinct malignant neoplasms, which intermingled in areas. The sections that were submitted from the superficial dural-based lesion showed a predominantly solid growth pattern forming focal papillary structures [Figure 2]. Mitotic activity was high with more than 20 mitotic figures per 10 high-power fields [Figure 2]. The meningioma cells showed prominent nucleoli with high nuclear to cytoplasmic ratios. Necrosis was focally present [Figure 2]. The morphologic features were consistent with papillary meningioma, WHO Grade III. The sections that were submitted from the deeper ventricular lesion showed a different morphology with features consistent with a transitional-type meningioma with identifiable whorl formations and psammoma bodies [Figure 3]. In addition, it showed areas of hypercellularity with small cell change, macronucleoli, and tumor cell necrosis. This lesion also showed more than 20 mitotic figures per 10 high-power fields [Figure 3], thus meeting the histologic features of anaplastic meningioma, WHO Grade III. Both tumors invaded underlying brain parenchyma.
Figure 2.
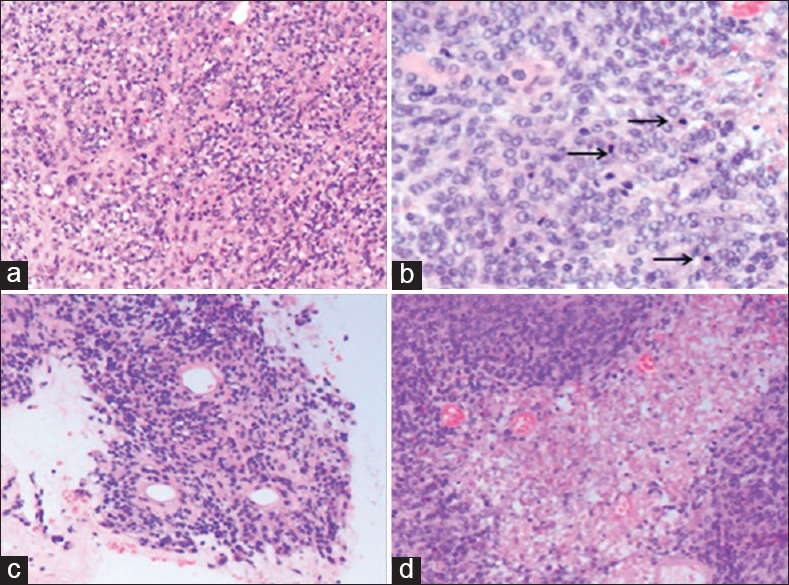
Papillary meningioma (H and E, ×40). This tumor was characterized by sheet-like growth pattern (a) high mitotic activity with more than 20 mitotic figures per 10 high-power fields (arrows) (b) forming focal papillary structures (c) and area of necrosis (d)
Figure 3.
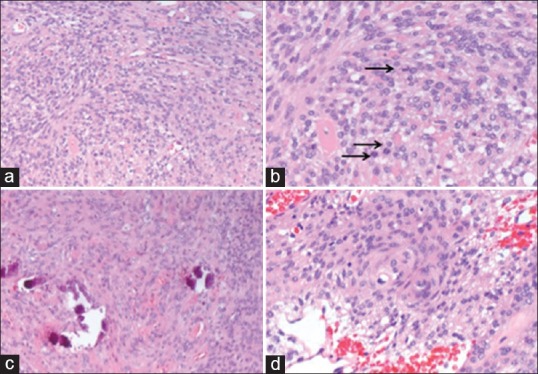
Anaplastic meningioma (H and E ×40). This tumor was characterized by sheet-like growth pattern (a) high mitotic activity with more than 20 mitotic figures per 10 high-power fields (arrows) (b) better-differentiated area showed morphologic features of a transitional meningioma with psammoma bodies (c) and identifiable whorl formations (d)
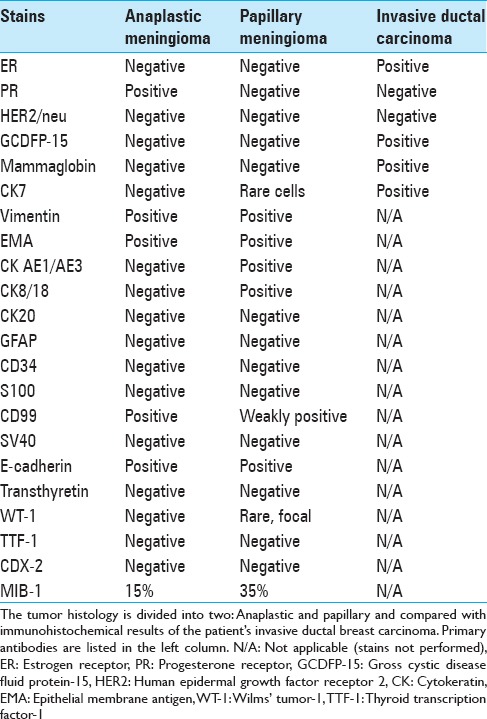
By immunohistochemistry, both tumors showed an immunophenotype consistent with meningioma with strong positivity for epithelial membrane antigen [Figure 4] and vimentin. The most striking difference, however, was the very strong immunoreactivity for cytokeratin (CK) AE1/AE3 [Figure 5] and CK8/18 that was seen only in the papillary meningioma and not in the anaplastic meningioma. The CK positivity was seen in virtually every cell in the papillary meningioma and was completely negative in the anaplastic meningioma, and it clearly delineated the two tumors.
Figure 4.
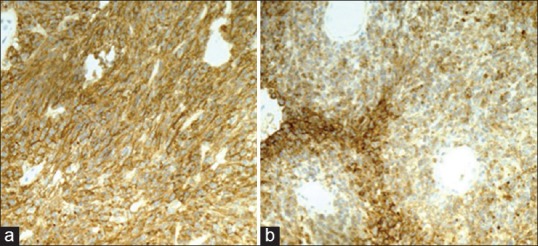
Strong epithelial membrane antigen positivity in both the anaplastic (a) and papillary (b) meningiomas (a and b: EMA IHC, ×20)
Figure 5.
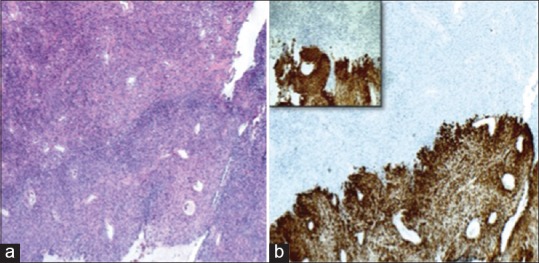
Collision between the anaplastic (above) and papillary (below) meningiomas (a) H and E, ×4. Cytokeratin AE1/AE3 is positive in the papillary meningioma and completely negative in the anaplastic meningioma; clearly delineating the two tumors (b) CK AE1/AE3 IHC ×4, higher power ×10 of the same area (inset)
Review of the patient's breast and axillary lymph node biopsy results showed histopathology consistent with Grade II invasive ductal breast carcinoma. Immunohistochemical analysis showed strong estrogen receptor (ER), CK7, mammaglobin, and gross cystic disease fluid protein 15 (GCDFP-15) positivity. Immunohistochemistry was negative for progesterone receptor and human epidermal growth factor receptor 2-neu.
A detailed description and comparison of the morphologic features and the immunohistochemistry can be found in Tables 1 and 2.
Table 1.
Detailed description of the morphologic features of the anaplastic meningioma and papillary meningioma
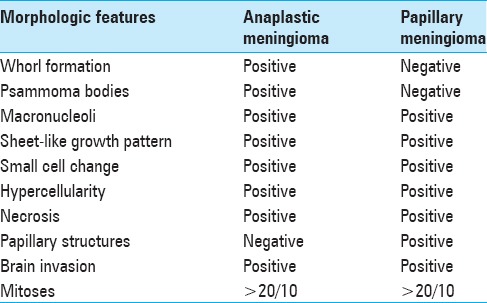
Table 2.
Immunohistochemical results
DISCUSSION
Here, we present a unique case of an intracranial tumor consisting of two differing meningioma subtypes in a patient with recently diagnosed invasive ductal breast carcinoma. In terms of entertaining the diagnosis of collision tumor, meningioma is the second most common intracranial tumor to collide with another, but all other reported cases have been in conjunction with an entirely different tumor (astrocytoma, metastatic tumor).[3,4,5,8,11,14,19] There have been six reported cases of intracranial meningioma in a collision with astrocytoma, but no reported cases of intracranial collision tumors between meningioma of varying subtypes.[6,9] The pathogenesis of collision tumors has been hypothesized to be secondary to simple chance alone or by one tumor acting as an irritating agent for the local proliferation and growth of the other and factors such as surgical trauma, ionizing radiation, and genetic predisposition have been associated with these tumors.[6,16,23] In our case, the patient had no prior history of cranial surgery or radiotherapy making the hypothesis of local proliferative factors of each tumor stimulating the growth of the other or genetic predisposition the most likely causes.
Interestingly, our patient had been recently diagnosed with Stage III, Grade II invasive ductal breast carcinoma. Cases of intracranial collision tumor between metastatic carcinoma/sarcoma and meningioma have been previously described.[1,4,15,18,22] An association between both solitary intracranial meningioma and breast carcinoma has also been reported as well as rare cases of metastatic breast carcinoma to meningioma (tumor-to-tumor metastasis).[7,10,20] The papillary meningioma component did show areas of carcinoma-like pathology, raising the question of possible intracranial meningioma with breast metastasis; however, in our case, the patient's breast cancer was ER-positive while both the papillary and anaplastic portions were not. In addition, immunohistochemical markers that are commonly positive in and highly sensitive and specific for breast carcinoma (mammaglobin and GCDFP-15) were negative in both tumors.[2,21] Furthermore, in most reported cases of carcinoma metastatic to meningioma, the host tumor typically appears benign.[12] Furthermore, the patient did not have any other evidence of metastatic disease on staging imaging. This constellation of findings makes metastatic carcinoma to meningioma less likely.
Intracranial meningiomas with intratumoral heterogeneity (adenocarcinoma-like metaplasia) have also been reported in the literature.[12,17] These cases have shown a WHO Grade I–III histology intermingled heterogeneously within the same mass lesion.[12,17] In these reported cases, the radiologic appearance of the tumors differed significantly from our case in that they were heterogeneous lesions without a clearly demarcated interface. In our case, an interface between what appeared to be two discrete lesions was evident and was reported by the radiologist. Moreover, during surgical resection, each lesion had a unique consistency and vascularity, which ultimately led to our suspicion of the possibility of two discrete lesions in a collision with one another. These above observations taken in conjunction with immunohistochemistry, in particular CK staining that showed a sharp boundary of demarcation between the papillary and anaplastic meningioma subtypes at the gross interface between the lesions, seems to favor collision tumor over a single tumor with intratumoral heterogeneity.
Despite imaging findings suggestive of two separate compartments, the provisional diagnosis preoperatively in our case was that of one tumor type; however, a demarcation between the two differing meningioma subtypes during gross resection as evidenced by the differing consistency and vascularity of the intraventricular lesion led to our suspicion of two distinct tumors and the submission of multiple specimens. The submission of multiple specimens is imperative when concern for multiple distinct lesions arises during surgical resection. Without this suspicion, this unique histopathologic diagnosis would not be reported.
CONCLUSION
We report a rare case of two distinct intracranial lesions with unique radiologic, histopathologic, and gross appearing characteristics. These characteristics provide evidence for a possible collision tumor between meningioma subtypes. During surgical resection, multiple pathologic specimens should be submitted if there is a concern for differing pathologic diagnoses or subtypes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Ryan B. Kochanski, Email: ryan_b_kochanski@rush.edu.
Nika Byrne, Email: rbyrne137@gmail.com.
Leonidas Arvanitis, Email: leonidas_arvanitis@rush.edu.
Sudeep Bhabad, Email: sudeep_bhabad@rush.edu.
Richard W. Byrne, Email: richard_w_byrne@rush.edu.
REFERENCES
- 1.Anlyan FH, Heinzen BR, Carras R. Metastasis of tumor to second different tumor: Collision tumors. JAMA. 1970;212:2124. [PubMed] [Google Scholar]
- 2.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: An immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–13. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 3.Binello E, Bederson JB, Kleinman GM. Hemangiopericytoma: Collision with meningioma and recurrence. Neurol Sci. 2010;31:625–30. doi: 10.1007/s10072-010-0227-3. [DOI] [PubMed] [Google Scholar]
- 4.Chahlavi A, Staugaitis SM, Yahya R, Vogelbaum MA. Intracranial collision tumor mimicking an octreotide-SPECT positive and FDG-PET negative meningioma. J Clin Neurosci. 2005;12:720–3. doi: 10.1016/j.jocn.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Drlicek M, Aichholzer M, Wurm G, Bodenteich A, Fischer J. Collisiontumour composed of glioblastoma and meningioma-a case report. Pathologe. 2004;25:402–5. doi: 10.1007/s00292-004-0696-3. [DOI] [PubMed] [Google Scholar]
- 6.Hakan T. A case of sphenoid wing meningioma and glioblastoma multiforme in collision. Neurol Neurochir Pol. 2010;44:102. [PubMed] [Google Scholar]
- 7.Jun P, Garcia J, Tihan T, McDermott MW, Cha S. Perfusion MR imaging of an intracranial collision tumor confirmed by image-guided biopsy. AJNR Am J Neuroradiol. 2006;27:94–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Khalatbari M, Borghei-Razavi H, Shayanfar N, Behzadi AH, Sepehrnia A. Collision tumor of meningioma and malignant astrocytoma. Pediatr Neurosurg. 2010;46:357–61. doi: 10.1159/000321596. [DOI] [PubMed] [Google Scholar]
- 9.Kurdi M, Al-Ardati H, Baeesa SS. Malignant trigeminal nerve sheath tumor and anaplastic astrocytoma collision tumor with high proliferative activity and tumor suppressor p53 expression. Case Rep Pathol 2014. 2014:153197. doi: 10.1155/2014/153197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieu AS, Hwang SL, Howng SL. Intracranial meningioma and breast cancer. J Clin Neurosci. 2003;10:553–6. doi: 10.1016/s0967-5868(02)00305-3. [DOI] [PubMed] [Google Scholar]
- 11.Mitsos AP, Konstantinou EA, Fotis TG, Lafazanos SA, Kontogeorgos G, Georgakoulias NV. Sphenoid wing meningioma and glioblastoma multiforme in collision – Case report and review of the literature. Neurol Neurochir Pol. 2009;43:479–83. [PubMed] [Google Scholar]
- 12.Patil S, Scheithauer BW, Strom RG, Mafra M, Chicoine MR, Perry A. Malignant meningiomas with epithelial (adenocarcinoma-like) metaplasia: A study of 3 cases. Neurosurgery. 2011;69:884–92. doi: 10.1227/NEU.0b013e318222dc6f. [DOI] [PubMed] [Google Scholar]
- 13.Perry A, Scheithauer BW, Szczesniak DM, Atkinson JL, Wald JT, Hammak JE. Combined oligodendroglioma/pleomorphic xanthoastrocytoma: A probable collision tumor: Case report. Neurosurgery. 2001;48:1358–61. doi: 10.1097/00006123-200106000-00038. [DOI] [PubMed] [Google Scholar]
- 14.Prayson RA, Chowdhary S, Woodhouse S, Hanson M, Nair S. Collision of a syncytial meningioma and malignant astrocytoma. Ann Diagn Pathol. 2002;6:44–8. doi: 10.1053/adpa.2002.30612. [DOI] [PubMed] [Google Scholar]
- 15.Shah A, Goel A, Goel N. A case of cerebellopontine angle epidermoid tumor and brainstem squamous cell carcinoma presenting as collision tumor. Acta Neurochir (Wien) 2010;152:1087–8. doi: 10.1007/s00701-010-0606-9. [DOI] [PubMed] [Google Scholar]
- 16.Tajika Y, Kubo O, Takeshita M, Tajika T, Shimizu T, Kitamura K. An intracranial collision tumor composed of intrasellar gangliocytoma and pituitary adenoma. No Shinkei Geka. 1989;17:1181–6. [PubMed] [Google Scholar]
- 17.Takayama Y, Nobusawa S, Ochiai I, Watanabe H, Ishigame H, Ikota H, et al. Malignant meningioma with adenocarcinoma-like metaplasia: Demonstration of intestinal phenotype. Neuropathology. 2015;35:158–64. doi: 10.1111/neup.12155. [DOI] [PubMed] [Google Scholar]
- 18.Tang GC, Piao YS, Zhao L, Lu DH. Lung adenocarcinoma metastasizing to cerebellopontine angle schwannoma (collision tumor) Acta Neurochir (Wien) 2007;149:87–90. doi: 10.1007/s00701-006-1049-1. [DOI] [PubMed] [Google Scholar]
- 19.Wanifuchi H, Kadowaki H, Kubo O, Kitamura K. Intracranial collision tumor composed of ganglioglioma and meningioma. Case report. Neurol Med Chir (Tokyo) 1988;28:195–9. doi: 10.2176/nmc.28.195. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Fujisawa H, Hasegawa M, Arakawa Y, Yamashita J, Ueda F, et al. Metastasis of breast cancer to intracranial meningioma: Case report. Am J Clin Oncol. 2002;25:414–7. doi: 10.1097/00000421-200208000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, Kiang DT. Gross cystic disease fluid protein-15 as a marker for breast cancer: Immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol. 1989;20:281–7. doi: 10.1016/0046-8177(89)90137-8. [DOI] [PubMed] [Google Scholar]
- 22.Wu WQ, Hiszczynskyj R. Metastasis of carcinoma of cervix uteri to convexity meningioma. Surg Neurol. 1977;8:327–9. [PubMed] [Google Scholar]
- 23.Zhang D, Yu J, Guo Y, Zhao S, Shao G, Huang H. An intraventricular meningioma and recurrent astrocytoma collision tumor: A case report and literature review. World J Surg Oncol. 2015;13:37. doi: 10.1186/s12957-015-0436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


