Keywords: nerve regeneration, traditional Chinese medicine, Parkinson's disease; rats, dyskinesia, tyrosine hydroxylase, neurological behavior, verbascoside, neural regeneration
Abstract
Tyrosine hydroxylase is a key enzyme in dopamine biosynthesis. Change in tyrosine hydroxylase expression in the nigrostriatal system is closely related to the occurrence and development of Parkinson's disease. Verbascoside, an extract from Radix Rehmanniae Praeparata has been shown to be clinically effective in treating Parkinson's disease. However, the underlying mechanisms remain unclear. It is hypothesized that the effects of verbascoside on Parkinson's disease are related to tyrosine hydroxylase expression change in the nigrostriatal system. Rat models of Parkinson's disease were established and verbascoside (60 mg/kg) was administered intraperitoneally once a day. After 6 weeks of verbascoside treatment, rat rotational behavior was alleviated; tyrosine hydroxylase mRNA and protein expression and the number of tyrosine hydroxylase-immunoreactive neurons in the rat right substantia nigra were significantly higher than the Parkinson's model group. These findings suggest that the mechanism by which verbascoside treats Parkinson's disease is related to the regeneration of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra.
Introduction
Parkinson's disease (PD) is a synucleinopathy characterized by degeneration of the dopaminergic nigrostriatal system, resulting in the classic motor features of the disease (Hughes et al., 2002). In advanced PD, it is well known that neuronal loss in the substantia nigra pars compacta is severe and most pronounced in the caudal and ventrolateral layer (Jellinger, 2012). Neurological behavior, often evaluated by the number of rotations per 30 minutes, is an important indicator in rats with PD, and is used to judge the success of the model and the severity of the disease. Tyrosine hydroxylase (TH) is the rate-limiting enzyme for the biosynthesis of dopamine (DA) with high specificity, which only impacts the precursor of DA synthesis, L-tyrosine. It hydroxylates the tyrosine to produce 3,4-dihydroxyphenylacetic acid (3,4-DOPAC), which is then decarboxylated by DOPA decarboxylase to dopamine (Chu et al., 2002). Thus, TH plays an important role in the pathophysiology of PD, and its level is the key to the DA level in the brain.
The neurological hallmark of PD is characterized by the degeneration of nigrostriatal dopaminergic neurons (Shastry, 2001; Lee et al., 2009). Freeman et al. (1995) found that the numbers of TH-immunoreactive neurons and TH protein expression in the substantia nigra were fewer in PD than non-PD patients when measured post mortem. Another study has shown that reductions in TH content and activity in the substantia nigra induced a lack of DA, which is the main reason for PD pathogenesis (Fujiyama et al., 2006). Systemic levodopa therapy has proved to be an effective initial treatment for this disorder. However, resistance to this therapy inevitably develops with time, necessitating other approaches including surgery. Current experimental surgical treatments for this disorder include pallidal stimulation, pallidal lesion, subthalamic stimulation, and dopaminergic cell transplants. The current limitation of these approaches is that they all treat the symptoms but not the cause, so the progressive degeneration of the substantia nigra pars compacta continues. No conventional treatment has been convincingly demonstrated to slow or stop the progression of PD (Bega et al., 2014).
In traditional Chinese medicine, PD is termed as “shaking palsy”, a syndrome characterized by tremors, numbness, limpness, and weakness of the four limbs, and the pathologic features of PD are liver-kidney Yin and Qi-blood deficiency (Li et al., 2006; Zhang et al., 2006). We analyzed the anti-parkinsonian activities of herbal medicines and herbal formulations investigated in PD models and provided future references for basic and clinical investigations as follows. All the herbal medicines and herbal formulations were tested in PD models in vitro and in vivo. The relevant compounds and herbal extracts with anti-parkinsonian activities were included and analyzed according to their general or pharmacological activities (Li et al., 2013). Morroniside and loganin can protect human dopaminergic SH-SY5Y cells against H2O2-induced apoptosis by suppressing reactive oxygen species production and apoptotic signals (Kwon et al., 2011). The neuroprotective effects of paeoniflorin and paeonol were observed in an in vitro model of PD (Tseng et al., 2012).
Verbascum thapsus commonly known as ‘mullein’ is part of a large family of scrophulariaceae consisting of more than 360 species. Verbascum thapsus has been used as a medicinal herb and it contains diverse polysaccharides, iroid glycosides, flavonoids, saponins, volatile oils, and phenylentanoids (Speranza et al., 2010). One component, verbascoside, a phenylethanoid glycoside, has been shown to possess pharmacologically beneficial activities for human health, including neuroprotective properties (Alipieva et al., 2014). Verbascoside attenuates Aβ-induced cell death via HO-1 upregulation and activation of the ERK and PI3K/Akt pathways (Wang et al., 2012). Previous studies have demonstrated that verbascoside has a strong antioxidative property (Hung et al., 2012) and radical scavenging activity (Sinico et al., 2008). There is also evidence that verbascoside may provide a useful therapeutic strategy for the treatment of oxidative stress-induced neurodegenerative disease such as PD (Sheng et al., 2002). However, the underlying mechanism remains unclear. In this study, we investigated the mechanism of verbascoside as a neuroprotective treatment against 6-hydroxydopamine (6-OHDA)-induced PD in rats.
Materials and Methods
Animals
Ninety healthy male Sprague-Dawley rats of specific pathogen-free grade, aged 5–6 weeks and weighing 180–220 g, were provided by the Animal Experimental Center of Shanghai University of Traditional Chinese Medicine, China (license No. SYXK (Hu) 2008-0016). Rats were housed in wire cages at 23 ± 2°C and 60–65% humidity, with illumination of 12-hour dark/light cycle (light 7:00–19:00, dark 19:00–7:00), with access to water and food ad libitum.
We randomly divided 30 of 90 rats into a normal control group (n = 15) and a sham-operated group (n = 15). PD was induced in the remaining 60 rats. Then 30 successful rat models of PD were randomly divided into an untreated PD group (n = 15) and a verbascoside-treated PD group (n = 15).
Drugs
Verbascoside (120 mg) was dissolved in 2 mL physiological saline and administered at a dose of 60 mg/kg body weight. It was processed by the School of Traditional Chinese Materia Medica of Shanghai University of Traditional Chinese Medicine (Shanghai, China). The molecular formula of verbascoside is shown in Figure 1.
Figure 1.
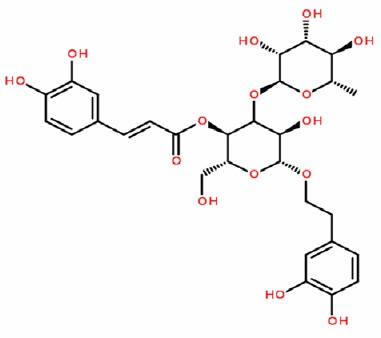
Structural formula of verbascoside.
Establishing PD rat models and interventions
6-OHDA-induced PD rat models were established according to a previously described method (Ungerstedt, 1968). In brief, after rats were confirmed to have had normal rotational behaviors before surgery, they were anesthetized by intraperitoneal injection of 3% pentobarbital sodium (50 mg/kg; Shanghai Chinese and Western Pharmaceutical Co., Ltd., Shanghai, China) and fixed in a brain stereotaxic instrument (TOW-3A; Shantou Education and Medical Instrument Factory, Shantou, Guangdong Province, China). The heads were shaved and routine disinfection with bromo geramine was performed. Under sterile conditions, an incision was made in the skin over the cranium along the midline and the periosteum was stripped to expose the bregma. The substantia nigra was located with a brain stereotaxic instrument based on the diagram of Bao et al. (1991). The two coordinates for targeting the substantia nigra were as follows: (1) 5.2 mm posterior to the bregma, 1.0 mm right lateral to the midline, 9.0 mm below the dura mater; (2) 5.2 mm posterior to the bregma, 2.5 mm right lateral to the midline, 8.5 mm below the dura mater. After locating the coordinates precisely, we carefully drilled a hole and injected 6-OHDA (diluted in normal saline containing 0.2% ascorbic acid; Sigma, St. Louis, MO, USA) into the right substantia nigra with a 5-μL micro-injector (the insertion speed was 1.0 mm/min). The volume per hole was 3 μL and injected at a speed of 1 μL/min. The retention time of the needle was 5 minutes, and the speed of needle withdrawal was 1.0 mm/min. Finally, the holes were sealed with medical gelatin sponge, and the skin incision was sutured. The rats were returned to their cages when they awoke and were given daily intramuscular injections of gentamycin for 7 days. Rats in the sham-operated group were injected with saline containing 0.2% ascorbic acid only. Rats in the normal control group were fixed in the apparatus for the same amount of time without any additional procedure. Rats in the PD groups were intraperitoneally injected with apomorphine (Sigma-Aldrich, Shanghai, China) 0.5 mg/kg to induce rotation to one side. The number of rotations was recorded over the next 30 minutes. Induction of PD in rats was considered successful if the frequency met or exceeded seven rotations per minute (Carman et al., 1991). Rats in the verbascoside-treated group were intraperitoneally administered 2 mL of verbascoside at a dose calculated according to a reported method (Sun, 1987), daily for 6 weeks. Rats in the normal control, sham-operated and PD groups were intraperitoneally administered 2 mL of physiological saline daily for 6 weeks.
Neurological behaviors in rats with PD
After 6 weeks of verbascoside injections, rotational behavior was observed in the rats after they were intraperitoneally injected with 0.5 mg/kg apomorphine to induce rotation to the left side. The time from apomorphine injection to the start of rotational behaviors and the number of rotations/30 minutes were recorded.
mRNA expression of TH in the right substantia nigra of rats as detected by real-time PCR
After neurobehavioral observation was performed at 6 weeks, six rats from each group were anesthetized with pentobarbital sodium (50 mg/kg), decapitated and their brains removed, then the right substantia nigra was dissected out and weighed. Brain tissues were crushed with liquid nitrogen in a mortar, and the pulverized powder was homogenized with 1 mL of TRIzol (Invitrogen, Carlsbad, CA, USA) in a glass homogenizer. Subsequently, the specimens were placed into a 1.5-mL centrifuge tube, thoroughly mixed and incubated on ice for 5 minutes, then centrifuged at 12,000 × g for 5 minutes at 4°C. The supernatant was then collected and transferred into another 1.5-mL centrifuge tube, gently shaken after the addition of 200 μL chloroform (Shanghai Shiyi Chemicals Reagent Co., Ltd., Shanghai, China) for 15 seconds, kept at room temperature for 15 minutes, and finally centrifuged at 12,000 × g for 15 minutes at 4°C. The supernatant was transferred to an RNAse-free 1.5-mL tube, gently mixed with 0.5 mL of isopropyl alcohol (Shanghai Shiyi Chemicals Reagent Co., Ltd.), incubated at room temperature for 10 minutes, and centrifuged at 12,000 ×g for 10 minutes at 4°C. After the supernatant was discarded, the RNA precipitate was rinsed with 1 mL of 75% ethanol and centrifuged at 7,500 × g for 5 minutes at 4°C. The centrifuge tube was inverted and dried on a clean bench, and 30 μL of DEPC-treated water was added to dissolve the RNA. The optical density of the RNA sample at 260 and 280 nm was determined with a spectrophotometer (Thermo, Boston, MA, USA) to measure the RNA concentration. The primers were designed using Primer Premier 5 (Gen-Script Real-time PCR Primer Design, https://www.genscript.com/ssl-bin/app/primer) and shown in Table 1, and DNAStar software (DNAStar company, Madison, WI, USA), and subjected to BLAST analysis (NCBI).
Table 1.
Primer sequences of genes

RT-PCR amplification protocol: 1 cycle of 94°C for 5 minutes, 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds; 30 cycles of 72°C for 7 minutes. Real-time fluorescent quantitative PCR (ABI, Foster City, CA, USA) was used to determine the expression of TH in the substantia nigra of rats. The mRNA expression was represented as 2–ΔΔCt method (Liu, 2013).
Protein expression of TH in the right substantia nigra of rats as detected by western blot analysis
Three rats from each group were used to measure TH protein expression. Protein samples of TH for western blot analysis were prepared in sodium dodecyl sulfate (SDS) sample buffer. Subsequently, the protein lysate or the immunocomplex was separated on 12% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. The membrane was then incubated at 4°C for 12 hours with rabbit anti-rat TH monoclonal antibody (1:500; Abcam, Cambridge, MA, USA). After washing, the membranes were incubated with goat anti-rabbit IgG horseradish peroxidase-linked secondary antibodies (goat anti-rabbit IgG; 1: 2,000; Abcam) for 1 hour at 37°C. The immunoreactive proteins were visualized by using an enhanced chemiluminescence kit (CST; Biological Reagents Co., Ltd., Shanghai, China). Gel bands were quantified by a densitometer (Model 375A; Molecular Dynamics, Sunnyvale, CA, USA) and normalized by reprobing the same blot for the β-actin signals. The protein expression of TH was calculated and expressed as a ratio of the integrated optical density of the TH band to β-actin. Blots were developed with chemiluminescence and the images were analyzed using Tanon Gel Image System ID 4.1.2 System (Shanghai Technology Co., Ltd., Shanghai, China).
Quantification of TH-immunoreactive neurons in the right substantia nigra of rats by immunohistochemical staining
Six rats from each group were deeply anesthetized by 3% pentobarbital sodium (50 mg/kg, i.p.) and intracardially perfused with saline followed by 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS, pH 7.4). The brains were rapidly removed from the skull, postfixed overnight in 4% paraformaldehyde and stored in 30% sucrose at 4°C. The brains were frozen and cut into 30-μm-thick coronal sections using a microtome cryostat (CUT4062, SLEE, Mainz, Germany) at −20°C. The frozen brain sections were rinsed in PBS and then incubated in 0.3% hydrogen peroxide for 15 minutes at 4°C. The sections were washed in PBS containing 0.3% Triton X-100 (PBS-T) and then incubated with rabbit anti-TH monoclonal antibody (1:500; Abcam) in PBS-T containing 10% normal goat serum for 12 hours at 4°C. After two washes with PBS-T, the sections were incubated with biotinylated secondary mouse anti-rabbit IgG (1:2,000; Abcam) in PBS-T for 1 hour at 37°C, followed by incubation in avidin-biotin complex in PBS-T for 1 hour at room temperature. Visualization of TH immunoreactivity was performed by incubating with 0.05% diaminobenzidine-HCl (DAB) for 2–5 minutes. TH-immunoreactive dopaminergic neurons were visualized and counted under a Leica DM2500 microscope equipped with a RET 2000R CCD camera (Qimaging, Surrey, BC, Canada) and with the aid of Stereo Investigator software (gd3-IMS-2000, China). Brain tissue sections of six rats were used for immunohistochemical staining.
Statistical analysis
Data are expressed as the mean ± SD and were analyzed using SPSS 16.0 Software (SPSS, Chicago, IL, USA). Intergroup differences were compared with one-way analysis of variance and pairwise comparison was performed using the least significant difference test. A P < 0.05 was considered statistically significant.
Results
Quantitative analysis of experimental animals
From the original 90 rats, 15 rats were used as the normal control group, 15 rats were injected with saline containing 0.2% ascorbic acid into the right substantia nigra to serve as the sham-operated group, and the remaining 60 rats were injected with 6-OHDA into the right substantia nigra to induce PD. Thirty rats were considered to have succeeded in PD induction and were randomly and evenly divided into a PD group and a verbascoside-treated group. Rats in the verbascoside-treated group were intraperitoneally injected with verbascoside, while those in the normal control and sham-operated groups were intraperitoneally administered an equal amount of 0.9% sodium chloride. Sixty rats were included in the final analysis, with 15 rats in each group.
Effect of verbascoside on the number of rotations in PD rats
Apomorphine-induced rotation test showed that in the verbascoside-treated group, the number of rotations decreased over the duration of treatment (P < 0.01). The difference between the PD and verbascoside-treated groups at 6 weeks was statistically significant (P < 0.01; Figure 2).
Figure 2.
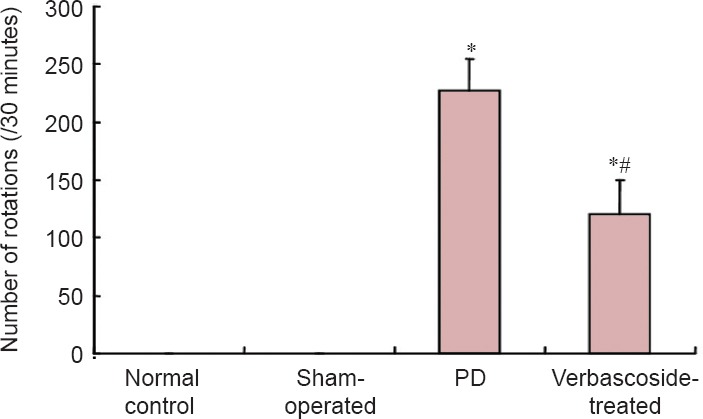
Effect of verbascoside on the rotational behavior in rats with Parkinson's disease (PD) at 6 weeks after treatment.
Verbascoside significantly reduced the numbers of rotations per 30 minutes. Data are expressed as the mean ± SD (n = 15). One-way analysis of variance was used for intergroup comparison, and pairwise comparison was performed using the least significant difference test. *P < 0.01, vs. normal control and sham-operated group; #P < 0.01, vs. PD group.
Effect of verbascoside on the mRNA expression of TH in the right substantia nigra of PD rats
Compared with the control and sham-operated groups, TH mRNA expression in the rat right substantia nigra was significantly decreased in the PD group (P < 0.01). After treatment with verbascoside, TH mRNA levels significantly increased (P < 0.01), but were still lower than those of rats in the normal control and sham-operated groups (P < 0.01; Figure 3).
Figure 3.
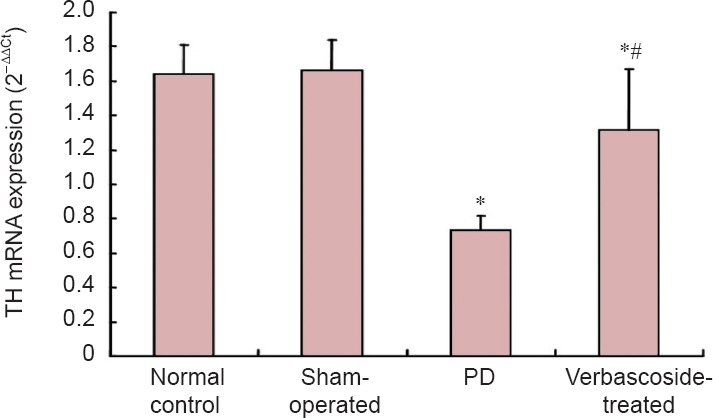
Effect of verbascoside on the mRNA expression of tyrosine hydroxylase (TH) in the right substantia nigra of rats with Parkinson's disease (PD).
The mRNA expression (mean ± SD, n = 15) was determined by real-time PCR at 6 weeks after treatment for each group. One-way analysis of variance was used for intergroup comparison, and pairwise comparison was performed using the least significant difference test. *P < 0.01, vs. normal control and sham-operated groups; #P < 0.01, vs. PD group.
Effect of verbascoside on the protein expression of TH in the right substantia nigra of PD rats
Compared with the normal control and sham-operated groups, TH protein expression in the rat right substantia nigra was significantly decreased in the PD group (P < 0.01). After treatment with verbascoside, TH protein expression was significantly better (P < 0.01), but was still lower than that in the normal control and sham-operated groups (P < 0.01; Figure 4).
Figure 4.
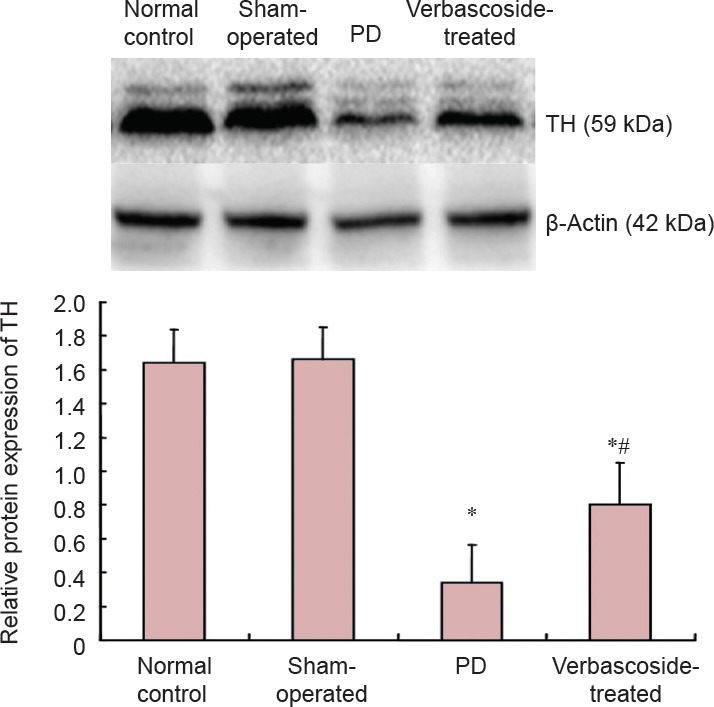
Effect of verbascoside on the protein expression of tyrosine hydroxylase (TH) in the right substantia nigra of rats with Parkinson's disease (PD) (western blot analysis).
The protein expression of TH is expressed as integrated optical density ratio of TH to β-actin, the gray value (mean ± SD, n =15) at 6 weeks after treatment for each group. One-way analysis of variance was used for intergroup comparison, and pairwise comparison was performed using the least significant difference test. *P < 0.01, vs. normal control and sham-operated groups; #P < 0.01, vs. PD group.
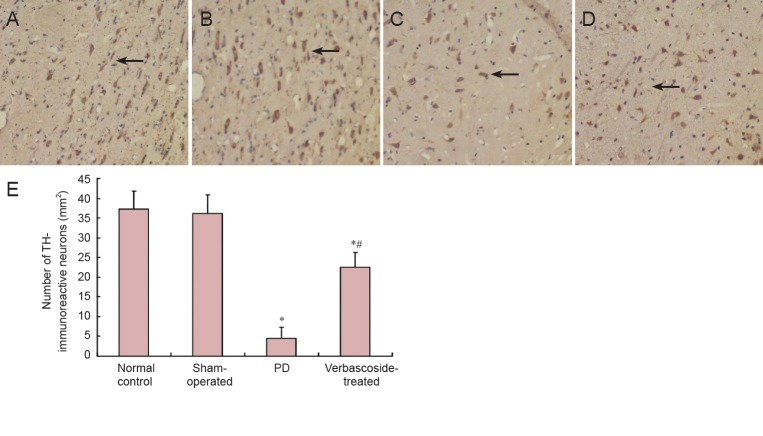
Effect of verbascoside on TH-immunoreactive neurons in the right substantia nigra of PD rats
Compared with the normal control and sham-operated groups, the number of TH-immunoreactive neurons in the rat right substantia nigra was significantly decreased in the PD group (P < 0.01). After treatment with verbascoside, the number of TH-immunoreactive neurons was significantly higher (P < 0.01), but it was still lower than that in the normal control and sham-operated groups (P < 0.01; Figure 5).
Figure 5.
Effect of verbascoside on tyrosine hydroxylase (TH) immunoreactivity in the right substantia nigra of rats with Parkinson's disease (PD) (× 400).
(A) In the normal control group, there is a high density of TH-immunoreactive neurons in the right substantia nigra, seen as brown colored round/oval (majority) or polygon-shaped (minority) appearance. (B) In the sham-operated group, TH-immunoreactive neurons are the same as the control group. (C) In the PD group, TH-immunoreactive neurons are small in number and size. (D) In the verbascoside-treated group, there are more TH-immunoreactive neurons than in the PD group. (E) TH immunoreactivity is expressed as the number of TH-immunoreactive neurons (mean ± SD, n = 15) at 6 weeks after treatment for each group. One-way analysis of variance was used for inter-group comparison, and pairwise comparison was performed using the least significant difference test. *P < 0.01, vs. normal control and sham-operated groups; #P < 0.01, vs. PD group.
Discussion
In the present study, we injected 6-OHDA into the brain of normal rats to establish a PD model. When 6-OHDA was injected to the substantia nigra, rats began to exhibit Parkinsonian signs and symptoms. After verbascoside treatment, the symptom of rotational behavior was alleviated, and TH protein and mRNA expression as well as the number of TH-immunoreactive neurons increased significantly compared to saline injected PD rats. The alterations became more striking over the course of treatment. These findings suggest that verbascoside possibly has a neuroprotective effect on the function of TH in animal experiments. The present results also provide the first direct evidence of TH changes in the lesioned substantia nigra in vivo after verbascoside treatment.
In chronic neurodegenerative disorders such as PD, the current therapies replace Levodopa but do not alter the progress of the disease. So far, there are only two Food and Drug Administration (FDA)-approved treatments, namely, administration of levodopa (L-DOPA) and deep brain stimulation of the bilateral subthalamic nuclei (Foltynie et al., 2010; Poewe et al., 2010), both of which are palliative and not disease modifying (Ping et al., 2014). Natural products could play a valuable role in the discovery of new drug candidates for the research into the treatment of neurodegenerative disorders, especially PD (Campos et al., 2011).
Several limitations and bias to this study should be noted. First, in our in vivo study, we used only one animal model, i.e., 6-OHDA-induced PD medel. This neurotoxin is a structural analog of dopamine and norepinephrine, capable of damaging catecholaminergic neurons and inducing the activation of glial cells. 6-OHDA can accumulate in the brain and, upon transport to the neurons, it inhibits mitochondrial complex I and produces superoxide and hydroxyl radicals through participation in the Fenton reaction (Schober, 2004). This causes an endogenous oxidative stress response in astrocytes (Jakel et al., 2005). Different kinds of animal models such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or a transgenic animal model should be used in further studies to confirm our conclusions. Second, in vitro studies using isolated nigral cells are needed, as we have not done in vitro experiments on the effect of verbascoside on apoptosis in the brain tissue from the 6-OHDA-induced PD rat model. Third, we studied only verbascoside extracted from Radix Rehmanniae, the therapeutic effect of the latter on Parkinson's disease needs further investigation.
Verbascoside exhibits neuroprotective effects through cholinergic, antioxidant and anti-inflammatory mechanisms and may be a promising candidate for neuroprotective applications. It has high immunoreactive activity in models of intestine inflammation and provides insight into chronic inflammatory bowel disease. This compound shows great promise for the prevention and treatment of a variety of skin disorders, from immune-mediated chronic inflammatory disease to solar UV-induced cutaneous non-melanoma tumors (Alipieva et al., 2014). Therefore, the medicinal value of verbascoside is still underutilized, and more research and more clinical trials on its efficacy and safety should be performed.
Our data suggest that verbascoside may block the effect of 6-OHDA leading to the dopaminergic neuron death in lesioned substantia nigra in vivo (Ma et al., 2005) and a reduced loss of TH expression in the substantia nigra in vivo. We believe verbascoside is a promising drug for the treatment of central nervous system-related neurodegenerative diseases such as PD. The present findings from real-time PCR, western blot analysis and immunohistochemical staining also provide evidence that verbascoside is neuroprotective against PD. However, PD involves extremely complex processes and a variety of factors, and all their underlying mechanisms need to be explored.
Acknowledgments:
We thank Associate Professor Qing-quan Li from Fudan University, China, for providing suggestions in experimental design.
Footnotes
Funding: This study was supported by a grant from Science and Technology Support Traditional Chinese Drug Research and Development Project of Shanghai of China, No. 12401900302; Traditional Chinese Medicine Research Foundation of Shanghai Municipal Health Bureau of China, No. 2012J009A; a grant from Annual Research Budget of Shanghai University of Traditional Chinese Medicine of China in 2013, No. 2013JW25; the National Natural Science Foundation of China, No. 30672684, 30973722.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Ann Dawes E, Robens J, Wang J, Li CH, Song LP, Zhao M
References
- Bao XM, Shu SY. The Stereotaxic Atlas of Rat Brain. Beijing: People's Medical Publishing House, China; 1991. [Google Scholar]
- Barreiro EJ, Viegas C., Jr The role of natural products in the discovery of new drug candidates for the treatment of neurodegenerative disorders I: Parkinson's disease. CNS Neurol Disord Drug Targets. 2011;10:239–250. doi: 10.2174/187152711794480483. [DOI] [PubMed] [Google Scholar]
- Bega D, Gonzalez-Latapi P, Zadikoff C, Simuni T. A review of the clinical evidence for complementary and alternative therapies in Parkinson's disease. Curr Treat Options Neurol. 2014;16:314. doi: 10.1007/s11940-014-0314-5. [DOI] [PubMed] [Google Scholar]
- Campos HC, da Rocha MD, Viegas FP, Nicastro PC, Fossaluzza PC, Fraga CA, Carman LS, Gage FH, Shults CW. Partial lesion of the substantia nigra: relation between extent of lesion and rotational behavior. Brain Res. 1991;553:275–283. doi: 10.1016/0006-8993(91)90835-j. [DOI] [PubMed] [Google Scholar]
- Chu Y, Kompoliti K, Cochran EJ, Mufson EJ, Kordower JH. Age-related decreases in Nurrl immunoreactivity in the human substantia nigra. J Comparative Neurol. 2002;450:203–214. doi: 10.1002/cne.10261. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Hariz MI. Surgical management of Parkinson's disease. Expert Rev Neurotherapeut. 2010;10:903–914. doi: 10.1586/ern.10.68. [DOI] [PubMed] [Google Scholar]
- Freeman TB, Olanow CW, Hauser RA, Nauert GM, Smith DA, Borlongan CV, Sanberg PR, Holt DA, Kordower JH, Vingerhoets FJ. Bilateral fetal nigral transplantation into the post commissural putamen in Parkinson's disease. Ann Neurol. 1995;38:379–388. doi: 10.1002/ana.410380307. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Unzai T, Nakamura K, Nomura S, Kaneko T. Difference in organization of corticostriatal and thalamostriatal synapses between patch and matrix compartments of rat neostriatum. Eur J Neurosci. 2006;24:2813–2824. doi: 10.1111/j.1460-9568.2006.05177.x. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- Hung CY, Tsai YC, Li KY. Phenolic antioxidants isolated from the flowers of osmanthus fragrans. Molecules. 2012;17:10724–10737. doi: 10.3390/molecules170910724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel RJ, Kern JT, Johnson DA, Johnson JA. Induction of the protective antioxidant response element pathway by 6-hydroxydopamine in vivo and in vitro. Toxicol Sci. 2005;87:176–186. doi: 10.1093/toxsci/kfi241. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathology of sporadic Parkinson's disease: evaluation and changes of concepts. Mov Disord. 2012;27:8–30. doi: 10.1002/mds.23795. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Kim JA, Hong SI, Jung YH, Kim HC, Lee SY, Jang CG. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 2011;58:533–541. doi: 10.1016/j.neuint.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhao D, Bezard E. Traditional Chinese medicine for Parkinson's disease: a review of Chinese literature. Behav Pharmacol. 2006;17:403–410. doi: 10.1097/00008877-200609000-00006. [DOI] [PubMed] [Google Scholar]
- Li XZ, Zhang SN, Liu SM, Lu F. Recent advances in herbal medicines treating Parkinson's disease. Fitoterapia. 2013;84:273–285. doi: 10.1016/j.fitote.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Liu Q, Gu LG, Lu NN, Zhou XP, Wu J, Qiu ZJ, Zhang HC, Chao EX. The regulatory effects of Shufeng Xuanfei and Jiebiao Qingli Chinese medicine formulae on helper T cell 1/2 cytokines of mice with influenza viral pneumonia. Zhongguo Zhongxiyi Jiehe Jijiu Zazhi. 2013;20:1–2. [PubMed] [Google Scholar]
- Ma JH, Hu GH, Dong LH, Xiong Y, Wang W, Song YP, Li SJ. Role of 6-hydroxydopamine in pathogenesis of Parkinson's disease. Jilin Daxue Xuebao: Yixue Ban. 2005;31:355–356. [Google Scholar]
- Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F. Levodopa in the treatment of Parkinson's disease: an old drug still going strong. Clin Interv Aging. 2010;5:229–238. doi: 10.2147/cia.s6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Shastry BS. Parkinson disease: etiology, pathogenesis and future of gene, therapy. Neurosci Res. 2001;41:5–12. doi: 10.1016/s0168-0102(01)00254-1. [DOI] [PubMed] [Google Scholar]
- Sheng GQ, Zhang JR, Pu XP, Ma J, Li CL. Protective effect of verbascoside on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in PC12 cells. Eur J Pharmacol. 2002;451:119–124. doi: 10.1016/s0014-2999(02)02240-9. [DOI] [PubMed] [Google Scholar]
- Sinico C, Caddeo C, Valenti D, Fadda AM, Bilia AR, Vincieri FF. Liposomes as carriers for verbascoside: stability and skin permeation studies. J Liposome Res. 2008;18:83–90. doi: 10.1080/08982100801894067. [DOI] [PubMed] [Google Scholar]
- Speranza L, Franceschelli S, Pesce M, Reale M, Menghini L, Vinciguerra I, De Lutiis MA, Felaco M, Grilli A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother Res. 2010;24:1398–1404. doi: 10.1002/ptr.3173. [DOI] [PubMed] [Google Scholar]
- Sun RY. Quantitative Pharmacology. Beijing: People's Medical Publishing House, China; 1987. [Google Scholar]
- Tseng YT, Hsu YY, Shih YT, Lo YC. Paeonol attenuates microglia-mediated inflammation and oxidative stress-induced neurotoxicity in ratprimary microglia and cortical neurons. Shock. 2012;37:312–318. doi: 10.1097/SHK.0b013e31823fe939. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Xu YX, Zhu CQ. Upregulation of heme oxygenase-1 byacteoside through ERK and PI3K/Akt pathway confer neuroprotection againstbeta-amyloid-induced neurotoxicity. Neurotox Res. 2012;21:368–378. doi: 10.1007/s12640-011-9292-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu SM. Recent developments of traditional Chinese medicine in treatment of Parkinson disease. Zhongguo Linchuang Kangfu. 2006;10:152–154. [Google Scholar]
- Zhao P, Luo Z, Tian W, Yang J, Ibáñez DP, Huang Z, Tortorella MD, Esteban MA, Fan W. Solving the puzzle of Parkinson's disease using induced pluripotent stem cells. Exp Biol Med. 2014;239:1421–1432. doi: 10.1177/1535370214538588. [DOI] [PubMed] [Google Scholar]