Keywords: nerve regeneration, traumatic brain injury, umbilical cord mesenchymal stem cells, transplantation, hyperbaric oxygen, rats, craniocerebral trauma, neurological function, neural regeneration
Abstract
Transplantation of umbilical cord-derived mesenchymal stem cells (UC-MSCs) for repair of traumatic brain injury has been used in the clinic. Hyperbaric oxygen (HBO) treatment has long been widely used as an adjunctive therapy for treating traumatic brain injury. UC-MSC transplantation combined with HBO treatment is expected to yield better therapeutic effects on traumatic brain injury. In this study, we established rat models of severe traumatic brain injury by pressurized fluid (2.5–3.0 atm impact force). The injured rats were then administered UC-MSC transplantation via the tail vein in combination with HBO treatment. Compared with monotherapy, aquaporin 4 expression decreased in the injured rat brain, but growth-associated protein-43 expression, calaxon-like structures, and CM-Dil-positive cell number increased. Following combination therapy, however, rat cognitive and neurological function significantly improved. UC-MSC transplantation combined with HBO therapyfor repair of traumatic brain injury shows better therapeutic effects than monotherapy and significantly promotes recovery of neurological functions.
Introduction
Traumatic brain injury (TBI) is often followed by cerebral hypoxia and ischemia, alterations in biochemical indices, and severe brain function disorders, making clinical treatment very difficult. Animal experiments (Guo et al., 2013; Jiao et al., 2013) have demonstrated that transplantation of human umbilical cord-derived mesenchymal stem cells (UC-MSCs) is an effective treatment method for neurological function impairment. The UC-MSCs transplanted into the injured brain tissue can differentiate into new neural cells that promote repair and regeneration of injured brain tissue and functional recovery.
UC-MSCs exhibit strong proliferative ability, low immunogenicity, and multi-potential differentiation, all of which contribute to the success of cell transplantation (Kunke et al., 2009). In the case of TBI, these transplanted cells undergo cell division, proliferation, differentiation, integration, and migration, thereby exhibiting repairing effects (Yu et al., 2014). However, the number of surviving UC-MSCs following transplantation into the brain remains limited (Xiong et al., 2014). UC-MSCs can be transplanted to substitute or repair injured nerve cells with the hopes of establishing neural pathways and recovery of neuronal functions, although the local microenvironment determines the differentiation and survival rate of the transplanted UC-MSCs (Guo et al., 2009). Although UC-MSC transplantation has been used in the clinic to treat traumatic brain injury, only a small number of transplanted UC-MSCs differentiate into mature neurons, resulting in limited therapeutic effects (Chang et al., 2011).
Hyperbaric oxygen (HBO) therapy has been widely used in the clinic to effectively treat TBI (Chuang et al., 2011). There is evidence that HBO therapy can increase the arterial partial pressure of oxygen, enhance blood oxygen content, and promote aerobic metabolism in central nervous system tissue (Ding et al., 2012; Jasaitis, et al., 2012).
Recent studies have demonstrated that effective and successful TBI treatment involves the local hypoxic/ischemic brain microenvironment, as well as cell proliferation and tissue repair/regeneration in the injured brain region (Aries et al., 2012; Bor-Seng-Shu et al., 2012). HBO promotes proliferation and differentiation of exogenous UC-MSCs and their migration toward injured brain tissue, protects injured brain cells, and reduces disabilities and mortality rates (Tong et al., 2010). However, the majority of existing studies have used only HBO therapy, with sub-par therapeutic effects.
The present study hypothesized that UC-MSC transplantation in combination with HBO therapy has greater synergistic effects on the repair of TBI compared with monotherapy.
Materials and Methods
Umbilical cord source
Umbilical cords were harvested from healthy fetuses delivered through uterine incision after 9 months of pregnancy in the Department of Gynecology and Obstetrics, Renmin Hospital of Wuhan University, China. Written informed consent was obtained from each included woman regarding use of umbilical cord and experimental purpose. The experimental protocol was approved by the Ethics Committee, First Hospital of Wuhan University, China.
Animals
A total of 160 adult, specific pathogen-free, Sprague-Dawley (SD), 80 males and 80 females, weighing 300–350 g, were purchased from the Animal Center, Peking Union Medical College, China (license No. SCXK (Jing) 20070001).
All rats were maintained and housed under controlled conditions (22°C), with a 12-hour reversed light/dark cycle and access to food and water ad libitum. All operations were performed under anesthesia, and all efforts were made to minimize pain and distress of the experiment animals. All animal experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986).
Primary culture and identification of UC-MSCs
The umbilical cords were thoroughly washed with phosphate-buffered saline (PBS). After removal of the umbilical cord vessel, the left tissue was chopped into 1-mm3 blocks, and digested with 0.25% trypsin and 0.1% collagenase II (Shanghai Alading Biochemistry Science & Technology, Shanghai, China) at 37°C. After centrifugation, the supernatant was removed, the cells were re-suspended with a sufficient amount of culture medium, and cultured with DMEM containing 5% fetal bovine serum (Shanghai Alading Biochemistry Science & Technology). After the first passage, the cells were sub-cultured once every 3 days at a 1:3 ratio. Passage 3 cells were digested and then treated with CD34, CD45, CD29, CD 90, CD105 and CD49 antibodies which were labeled by PE FITC. Flow cytometry was performed using a Flow Cytometer System (Becton Dickinson, San Jose, CA, USA).
Chloromethyl-1,1-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (CM-Dil) labeling of UC-MSCs
A total of 5 μL CM-Dil solution and 1 mL of complete culture medium was added to a 1.5-mL Eppendorf tube and kept in the dark. The UC-MSCs that fully adhered to the culture flask were harvested. After removal of culture medium, the cells were washed three times with PBS and then incubated with the above-prepared CM-Dil labeling solution at 40 μL/cm2 for 20 minutes in an incubator of saturated humidity at 37°C and 5% CO2 environment. After removal of the CM-Dil labeling solution, 5 mL of 37°C complete culture medium was added. After a 10-minute incubation, the complete culture medium was discarded. Culture medium was added again and cells were washed three times with PBS. After 24 hours of culture, CM-Dil labeling and UC-MSC morphology were observed under an inverted fluorescence microscope (Olympus, Japan, USA). During sub-culture, sufficient amounts of UC-MSCs were used for flow cytometry (Becton Dickinson) to detect the CM-Dil labeling rate.
Establishment of TBI rat models and group management
Rat models of severe TBI induced by pressurized fluid were used (Wang et al., 2000). In brief, after anesthesia by intraperitoneal injection of 20% urethane (1.2 g/kg; Shanghai Maikelin Biochemical Reagent Co., Ltd., Shanghai, China), a cranial window was opened with the dura mater intact, which was then impacted once by pressurized fluid [2.5–3.0 atm (253.3–304.0 kPa) impact force] using an internationally standard lateral fluid percussion Injury device (CIC, USA). If lack of spontaneous breathing occurred after injury, an oxygen mask was provided. After another 24 hours, the rats were anesthetized again and the original bone window was opened for stereotaxic injection (ALC-IP600 animal brain stereotactic apparatus, Beijing, China).
A total of 160 rat models of TBI were randomly assigned to four groups, with 40 rats per group. In the TBI group, rats were injected with 1 mL DMEM/F12 culture medium via the tail vein. In the UC-MSC group, an equal amount of UC-MSCs suspension (1 × 1010/L) was injected via the tail vein. In the UC-MSCs + HBO group, an equal amount of UC-MSCs suspension (1 × 1010/L) was injected via the tail vein and then the rats were placed in an HBO chamber (Weifang Huaxin Company, Weifang, Shandong Province, China). The HBO chamber was washed with pure oxygen for 10 minutes and pressurized at a speed of 0.01 MPa/min until 0.2 MPa was reached. After pressure stabilization for 30 minutes, the HBO chamber was intermittently pressurized to > 96.5% and then decompressed at a constant speed for 10 minutes until normal pressure was reached. Then rats were routinely housed outside the HBO chamber. HBO therapy was performed 4 times a day with 6-hour intervals for four successive days. In the HBO group, HBO treatment was administered as described above.
Six rats per group were selected for reverse transcription-polymerase chain reaction (RT-PCR) and western blot analysis; 10 rats per group for neurological function evaluation; 10 rats per group for Morris water maze test; 6 rats per group for hematoxylin-eosin staining; and 6 rats per group for immunohistochemical staining, resulting in 38 rats per group and 2 extra rats.
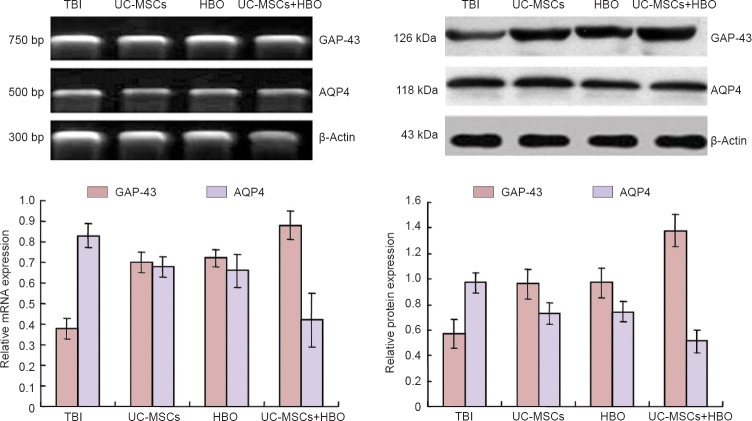
Detection of aquaporin 4 (AQP4) and growth-associated protein-43 (GAP-43) mRNA expression
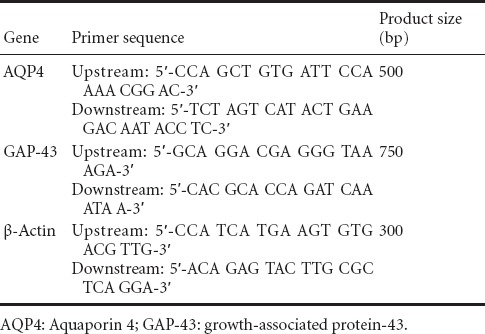
At 4 days after injury and following anesthesia, the rats were decapitated and 50 g brain tissue was harvested and homogenized. According to Trizol reagent instruction, total RNA was extracted from brain tissue and RNA levels were measured using an ultraviolet spectrophotometer (Shimadzu-3550, Tokyo, Japan). Using the two-step RT-PCR kit, mRNA was reverse-transcribed into cDNA and then PCR was performed. The primers used are shown in Table 1. PCR products were electrophoresed. The optical density of each band was analyzed using a gel image analysis system (Tanon-3500, Tokyo, Japan). The optical density ratio of GAP-43 and AQP4 to β-actin was calculated and used as the relative mRNA expression of GAP-43 and AQP4.
Table 1.
Primer sequence and product size
Western blot analysis of AQP4 and GAP-43 protein expression
At 3–5 days after injury, the cell lysate remaining after RT-PCR was centrifuged at 1,500 r/min for 30 minutes and the supernatant was collected for protein concentration measurements using the Bradford method. The sample was added to a 5% stacking gel and electrophoresed at a constant voltage of 40 V for 1 hour, followed by a 10% separating gel at a constant voltage of 60 V for 3.5 hours, and then subsequently transferred to a membrane using a wet transfer method at a constant voltage of 14 V for 14 hours. The membranes were then blocked at 37 °C for 2 hours while shaking, followed by three wash steps for 10 minutes each. Rabbit anti-rat AQP4/GAP-43 monoclonal antibodies (1:1,000; Sigma, St. Louis, MO, USA) that were diluted to 1:200 were dissolved in TBST and incubated at room temperature for 60 minutes. The membranes were then washed with above-prepared TBST for 10 minutes, three times/minute, treated with horseradish peroxidase-labeled goat anti-rabbit IgG diluted at 1:500 (Tiangen Biotech (Beijing) Co., Ltd., Beijing, China) at room temperature for 60 minutes, and then washed three times for 10 minutes each. Subsequently, the membranes were washed with Tris-buffered saline for 10 minutes and developed with 3,3’-diaminobenzidine (DAB). The optical density of AQP4 and GAP-43 products were measured by Quantity One analysis software (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China). The optical density ratios of AQP4 and GAP-43 expression to β-actin expression were used to determine relative AQP4 and GAP-43 protein expressions.
Neurological function assessment
Rat neurological function was assessed at 24 hours, 3 days, and 1, 2, 3, and 4 weeks after traumatic brain injury using the neurological examination grading system of Bederson et al. (1986): grade 0, no observable deficit; 1, contralateral forelimb flexion; 2, decreased gripping ability of contralateral forelimb when held by the tail; 3, automatic action, left circling when held by the tail; 4, spontaneous left circling. Lower grades indicate better recovery of neurological function.
Morris water maze test
Within 1–5 days after UC-MSC transplantation, the Morris water maze test was performed to analyze learning and memory abilities (Li et al., 2011). The rats were allowed to acclimate to the environment by placing them for 10 seconds on the platform in quadrant 3 (Q3). The rats were the randomly placed facing the wall in different quadrants, and the time spent from entry into the water to 5 seconds after climbing on the platform was recorded, with the longest time spent of 120 seconds. If the rats did not climb onto the platform within 120 seconds, they were guided to climb onto the platform and were allowed to remain on the platform for 10 seconds, after which they were returned to their cages. The rats were placed into the pool as mentioned above four times per day with 1-hour intervals. The time taken from entry into water to climbing onto the platform (i.e., escape latency) was recorded. The rats were allowed to freely swim in the same water maze without a platform for 60 seconds, and the percentage of time spent in Q3 (where platform was originally positioned) compared with total time (60 seconds) was calculated as an index to evaluate memory (i.e., percentage of swimming time spent in target quadrant).
Hematoxylin-eosin staining and immunohistochemical staining
Four weeks after injury, the brain tissues were embedded in paraffin and sectioned for histological examination. Five minutes after hematoxylin-eosin staining, brain tissue slices were washed with tap water, differentiated in hydrochloric acid/ethanol for 10 seconds, washed with tap water, stained with eosin for 7 minutes, washed with tap water, dehydrated with ethanol gradients, cleared with xylene, and mounted in neutral gum. In the CM-Dil immunohistochemical staining protocols, the primary antibody was rabbit anti-rat IgG (1:1,000; Sigma), and the secondary antibody was goat anti-rabbit IgG (1:1,000; Sigma). A 2-hour incubation was performed at 25°C once after addition of primary antibody and again after addition of secondary antibody. Ten non-overlapping visual fields were selected from each slice under 200-fold magnification. CM-Dil-positive cells (i.e., UC-MSCs) were quantified in the visual field, and the mean value across 10 visual fields was calculated as the number of CD-Dil-positive cells per group. Image Pro-Plus 6.0 Software (Media Cybernetics, Silver Spring, MD, USA) was used to analyze the results.
Statistical analysis
All measurement data are expressed as the mean ± SD and were statistically analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance and the least significance difference were used for analysis of differences of the means among groups. A level of P < 0.05 was considered statistically significant.
Results
UC-MSC morphology
After 4–5 days in culture, there was an increased number of UC-MSCs that adhered to the flask wall. They appeared as long shuttle-shaped or polygonal cells. Some cell colonies grew in clusters with expanded somas. After 8–9 days in culture, the UC-MSCs were confluent, appeared to grow in a whirlpool-like manner, and the majority of UC-MSCs in the periphery appeared as radiating or whirlpool migrating shapes (Figure 1A). Flow cytometry showed that these cells were positive for CD34, CD45, CD29, CD90, CD105, and CD49, with good homogenicity and a > 97% purity. After 24 hours in culture, CM-Dil-labeled UC-MSCs appeared as red fluorescence under a fluorescence microscope (Figure 1B). Flow cytometry showed that cell-labeling rate was 100%.
Figure 1.
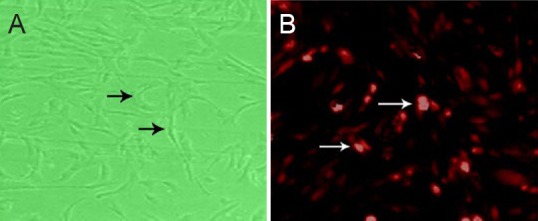
Morphology of passage 3 UC-MSCs
(A, B) After proliferation and purification (A, optical microscope, × 200) and CM-Dil-labeled UC-MSCs after 24 hours of culture (B, fluorescence microscope, × 200). (A) Cells are long shuttle-shaped or polygonal (arrows) and mitotic, and some cell colonies grow in clusters with enlarged somas. (B) CM-Dil-stained UC-MSCs exhibited red fluorescence under a fluorescence microscope (arrows). UC-MSCs: Human umbilical cord-derived mesenchymal stem cells; CM-Dil: chloromethyl-1,1-dioctadecyl-3,3,3’,3’-tetramethyl-indocarbocyanine perchlorate.
AQP4 and AQP-43 mRNA and protein expression in injured rat brain tissue after UC-MSC transplantation in combination with HBO therapy
RT-PCR and western blot assay showed that 4 days after UC-MSCs transplantation, AQP4 mRNA and protein expression in the injured rat brain tissue was greatest in the TBI group, followed by the UC-MSCs or HBO groups, and lowest in the UC-MSCs + HBO group (P < 0.05). GAP-43 mRNA and protein expression in injured rat brain tissue was lowest in the TBI group, followed by the UC-MSCs or HBO group, and highest in the UC-MSCs + HBO group (P < 0.05) (Figure 2).
Figure 2.
GAP-43 and AQP4 mRNA and protein expression in injured rat brain tissue after UC-MSC transplantation in combination with HBO therapy.
AQP4 mRNA and protein expression (optical density value relative to β-actin) was greatest in the TBI group, followed by the UC-MSCs or HBO group, and lowest in the UC-MSCs + HBO group (P < 0.05). GAP-43 mRNA and protein expression (optical density value relative to β-actin) was lowest in the TBI group, followed by the UC-MSCs or HBO group, and highest in the UC-MSCs + HBO group (P < 0.05). All data are expressed as the mean ± SD (n = 6). One-way analysis of variance and the least significance difference test were used. UC-MSCs: Human umbilical cord-derived mesenchymal stem cells; HBO: hyperbaric oxygen; TBI: traumatic brain injury; GAP-43: growth associated protein-43; AQP4: aquaporin-4.
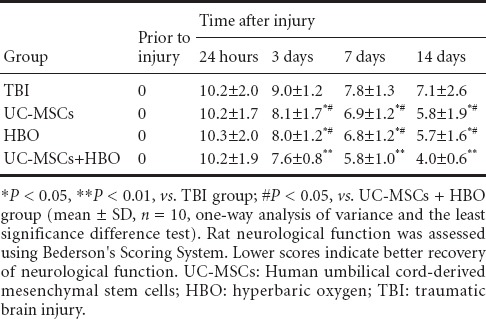
TBI rat neurological function changes after UC-MSC transplantation in combination with HBO therapy
At 3–14 days after UC-MSC transplantation, scores revealing neurological deficits were greatest in the TBI group, followed by the UC-MSC group or HBO group, and lowest in the UC-MSCs + HBO group (P < 0.05). These results suggested that UC-MSC transplantation in combination with HBO therapy resulted in the best outcome as assessed by neurological functions (Table 2).
Table 2.
Changes in TBI neurological function (score) after UC-MSCs transplantation in combination with HBO therapy
Changes in learning and memory abilities in TBI rats after UC-MSCs transplantation in combination with HBO therapy
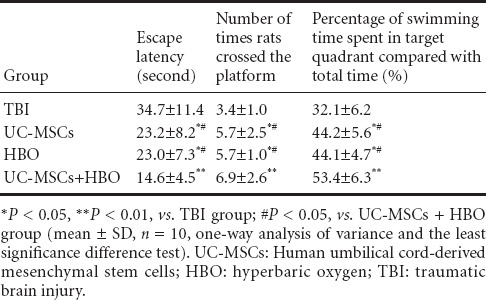
Morris water maze test results showed that mean escape latency gradually decreased in each group. There was no significant difference in mean latency between groups at 1 day after UC-MSC transplantation. Mean escape latency was significantly decreased in the UC-MSCs + HBO group compared with the TBI group at 2 days. Mean escape latency in the UC-MSCs + HBO group was significantly less than in the HBO group (P < 0.05), UC-MSC group (P < 0.05), or the TBI group (P < 0.01) at 3–5 days. The number of times that the rats crossed the platform and the percentage of swimming time spent in the target quadrant compared with total time were significantly greater in the UC-MSCs + HBO group than in the HBO group (P < 0.05), UC-MSC group (P < 0.05), or the TBI group (P < 0.01). These results showed that UC-MSCs + HBO treatment resulted in the best learning and memory scores, followed by UC-MSCs or HBO group, and worst in the TBI group (all P < 0.05) (Table 3).
Table 3.
Changes in learning and memory abilities in TBI rats at 3–5 days after UC-MSC transplantation in combination with HBO therapy
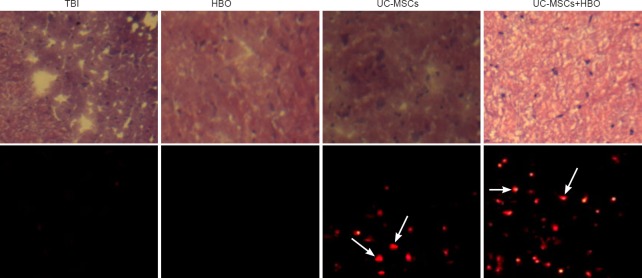
Morphological changes in TBI brain tissue after UC-MSC transplantation in combination with HBO therapy
Four weeks after transplantation, hematoxylin-eosin staining results in the TBI group revealed glial scarring, with some atrophy and softening foci. In the UC-MSCs and HBO groups, the injured brain tissue was filled with glial cells and glial fibers, and the sizes of scar and softening foci were smaller than in the TBI group, but larger than in the UC-MSCs + HBO group. In the UC-MSCs + HBO group, the injured brain tissue was filled with glial cells and glial fibers, increased number of neuraxon-like structures, lack of softening foci, and a small amount of scar tissue. Immunohistochemical staining results showed no CM-Dil-positive cells in the injured brain tissue in the TBI and HBO groups; the number of CM-Dil-positive cells was 30.53 ± 9.43/200-fold visual field in the UC-MSC group and 54.21 ± 14.52/200-fold visual field in the UC-MSCs + HBO group, with significantly less CM-Dil-positive cells in the UC-MSC group compared with the UC-MSCs + HBO group (P < 0.01) (Figure 3).
Figure 3.
TBI rat brain tissue morphology after UC-MSC transplantation in combination with HBO therapy (× 200).
Upper panels: Hematoxylin-eosin staining results under optical microscope: in the TBI group, brain tissue exhibits a disordered structure, i.e., scar tissue with obvious cavitations; in the UC-MSC group and HBO group, sizes of scarring and softening foci are less than in the TBI group, but greater than in the UC-MSCs + HBO group. In the UC-MSCs + HBO group, cavities are obviously reduced. Lower panels: Immunohistochemical staining results under fluorescence microscope: The number of CM-Dil-positive cells (arrows; i.e., surviving UC-MSCs) is less in the UC-MSC group than in the UC-MSCs + HBO group. UC-MSCs: Umbilical cord-derived mesenchymal stem cells; HBO: hyperbaric oxygen; TBI: traumatic brain injury; CM-Dil: chloromethyl-1,1-dioctadecyl-3,3,3’,3’-tetramethyl-indocarbocyanine perchlorate.
Discussion
HBO therapy alone does not result in ideal therapeutic effects on TBI. In the present study, we investigated the therapeutic effects of UC-MSC transplantation in combination with HBO therapy on TBI. Our results showed that this combined therapy resulted in better therapeutic effects on TBI than monotherapy. There is also evidence that HBO therapy can decrease malonaldehyde and calcium ion levels (Fuentes-Raspall et al., 2011), inhibit lipid peroxidation, increase the antioxidant activity of cell membranes, reduce intracellular calcium ion inflow, protect neurons, and promote neural regeneration. HBO therapy given in the early stage of UC-MSC transplantation promotes UC-MSC proliferation and induces migration toward the injured area, reduces cell apoptosis, promotes cell survival, alleviates swelling in the injured region, and improves microcirculation (Lin et al., 2011). These findings suggest that HBO therapy for the treatment of TBI can increase UC-MSC survival, promote endogenous and exogenous neural cells to proliferate and differentiate into neurons, improve microcirculation of brain tissue cells, alleviate cerebral edema, and reduce injury. Future studies are needed to determine the involved cellular and molecular mechanisms of UC-MSC transplantation used in combination with HBO therapy.
Results from the present study showed that HBO therapy promoted proliferation and differentiation of UC-MSCs and significantly improved neurological functions in a rat TBI model. There were, however, several limitations to this study. First, the sample size was small, and larger sample sizes should be used in future studies to increase result reliability. Second, we only transplanted human UC-MSCs into a rat model of injury, but did not perform human experiments. Therefore, different biological models should be used to validate the generalization of these experimental results. Third, we did not investigate the molecular mechanisms involved in UC-MSC transplantation combined with HBO therapy.
Taken together, HBO therapy promoted proliferation and differentiation of transplanted UC-MSCs and improved the local microenvironment. UC-MSC transplantation used in combination with HBO therapy to treat a rat TBI model exhibits synergistic effects in terms of the promotion of neurological function, the reconstruction of a local microenvironment for injured brain tissue. Therefore, this combined therapy holds great potential for clinical application.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Cooper C, Robens J, Wang J, Li CH, Song LP, Zhao M
References
- Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, Hutchinson PJ, Brady KM, Menon DK, Pickard JD, Smielewski P. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Critical Care Med. 2012;40:2456–2463. doi: 10.1097/CCM.0b013e3182514eb6. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Bor-Seng-Shu E, Figueiredo EG, Amorim RL, Teixeira MJ, Valbuza JS, de Oliveira MM, Panerai RB. Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury: a review. J Neurosurg. 2012;117:589–596. doi: 10.3171/2012.6.JNS101400. [DOI] [PubMed] [Google Scholar]
- Chang D, Jeong M, Song J. Discovery of small molecules that enhance astrocyte differentiation in rat fetal neural stem cells. ACS Med Chem Lett. 2011;21:7050–7053. doi: 10.1016/j.bmcl.2011.09.099. [DOI] [PubMed] [Google Scholar]
- Chuang S. Limited evidence to demonstrate that the use of hyperbaric oxygen (HBO) therapy reduces the incidence of osteoradionecrosis in irradiated patients requiring yooth extraction. J Evid Based Dent Pract. 2011;11:129–131. doi: 10.1016/j.jebdp.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Fuentes-Raspall R, Inoriza JM, Martí-Utzet MJ, Auñón-Sanz C, Garcia-Martin P, Oliu-Isern G. Hyperbaric oxygen therapy for late rectal and bladder toxicity after radiation in prostate cancer patients. A symptom control and quality-of-life study. Clin Oncol (R Coll Radiol) 2012;24:e126. doi: 10.1016/j.clon.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Guo B, Dong M. Application of neural stem cells in tissue-engineered artificial nerve original. Otolaryngol Head Neck Surg. 2009;140:159–164. doi: 10.1016/j.otohns.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Guo J, Wang J, Liang C. proNGF inhibits proliferation and oligodendrogenesis of postnatal hippocampal neural stem/progenitor cells through p75NTR in vitro. Stem Cell Res. 2013;11:874–887. doi: 10.1016/j.scr.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Jasaitis A, Ouellet H, Lambry J. Ultrafast hemeligand recombination in truncated hemoglobin HbO from Mycobacterium tuberculosis: a ligand cage. J Chem Phys. 2012;396:10–16. [Google Scholar]
- Kunke D, Bryja V, Mygland L. Inhibition of canonical Wnt signaling promotes gliogenesis in P0-UC-MSCs. Biochem Biophys Res Commun. 2009;386:628–633. doi: 10.1016/j.bbrc.2009.06.084. [DOI] [PubMed] [Google Scholar]
- Li L, Ding J, Marshall C, Gao J, Hu G, Xiao M. Pretraining affects Morris water maze performance with different patterns between control and ovariectomized plus D-galactose-injected mice. Behav Brain Res. 2011;217:244–247. doi: 10.1016/j.bbr.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Lin SS, Yuan LJ, Niu CC. HBO suppressed nitric oxide and apoptosis in articular cartilage defect via up-regulation of hsp 70 expression-In vitro and in vivo study. Bone. 2011;48:143. [Google Scholar]
- Qian J, Xie W, Wang Y. Spatial relationship between UC-MSCs/NPCs and microvessels in rat brain along prenatal and postnatal development. Int J Dev Neurosci. 2013;31:280–285. doi: 10.1016/j.ijdevneu.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Rocha FS, Gomes Moura CC, Rocha Rodrigues DB, Zanetta-Barbosa D, Nakamura Hiraki KR, Dechichi P. Influence of hyperbaric oxygen on the initial stages of bone healing. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:581–587. doi: 10.1016/j.oooo.2015.06.039. [DOI] [PubMed] [Google Scholar]
- Tong L, Ji L, Zhenyu Wang Z. Differentiation of neural stem cells into Schwann-like cells in vitro. Biochem Biophys Res Commun. 2010;401:592–597. doi: 10.1016/j.bbrc.2010.09.107. [DOI] [PubMed] [Google Scholar]
- Wang QH, Xu RX, Li LP. Models of graded brain trauma in rats. Chuangshang Waike Zazhi. 2000;2:42–44. [Google Scholar]
- Xiong Z, Zhao S, Mao X. Selective neuronal differentiation of neural stem cells induced by nanosecond microplasma agitation. Stem Cell Res. 2014;12:387–399. doi: 10.1016/j.scr.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Wang XL, Yu XH, Wang X, Xie M, Liu CT. Hyperbaric oxygen induces endogenous neural stem cells to proliferate and differentiate in hypoxic-ischemic brain damage in neonatal rats. Undersea Hyperb Med. 2008;35:113–129. [PubMed] [Google Scholar]
- Yu M, Jiang M, Yang C. Maternal high-fat diet affects Msi/Notch/Hes signaling in neural stem cells of offspring mice. J Nutr Biochem. 2014;25:227–231. doi: 10.1016/j.jnutbio.2013.10.011. [DOI] [PubMed] [Google Scholar]