Abstract
Human neural stem cells (hNSCs) derived from the ventral mesencephalon are powerful research tools and candidates for cell therapies in Parkinson's disease. However, their clinical translation has not been fully realized due, in part, to the limited ability to track stem cell regional localization and survival over long periods of time after in vivo transplantation. Magnetic resonance imaging provides an excellent non-invasive method to study the fate of transplanted cells in vivo. For magnetic resonance imaging cell tracking, cells need to be labeled with a contrast agent, such as magnetic nanoparticles, at a concentration high enough to be easily detected by magnetic resonance imaging. Grafting of human neural stem cells labeled with magnetic nanoparticles allows cell tracking by magnetic resonance imaging without impairment of cell survival, proliferation, self-renewal, and multipotency. However, the results reviewed here suggest that in long term grafting, activated microglia and macrophages could contribute to magnetic resonance imaging signal by engulfing dead labeled cells or iron nanoparticles dispersed freely in the brain parenchyma over time.
Keywords: human neural stem cells, Parkinson's disease, magnetic resonance imaging, magnetic nanoparticles, stem cell transplantation
In Vivo Tracking of Human Neural Stem Cells
One of the goals of stem cell research is the in vitro and in vivo generation of neurons which could replace the neurons lost in certain acute conditions and chronic diseases of the nervous system. Stem cell therapies are promising strategies for the treatment of several neurodegenerative diseases, such as Parkinson's disease (PD) characterized by a progressive loss of integrity, function and number of dopaminergic neurons (DAn) present in the substantia nigra pars compacta (SNpc). Stem cells, and more specifically neural stem cells (NSCs), constitute a viable option for regenerative medicine in PD, in particular for cell replacement therapies (CRT) both in the pre-clinical and clinical experimental settings (Lindvall et al., 2004).
Previous clinical studies of cell replacement in PD were based on the transplantation of fresh human fetal ventral mesencephalic (VM) tissue into the caudate and putamen of PD patients. Even when these studies provided clear-cut proof-of-concept type of evidence of CRT efficacy, fetal tissue transplants will not reach the status of a routine clinical practice due to practical and ethical issues. Indeed, some of those clinical experiments provided mixed outcomes, an aspect that still needs to be clarified. Among the practical issues, CRT for PD using fresh fetal tissue requires from six to seven human fetuses to provide enough cells for transplantation of a single patient, there is an elevated cell death rate of the transplanted cells, a lack of reproducibility between centers and the appearance of serious adverse side effects (dyskinesias) in some patients.
A tremendous progress has been achieved in recent years in our understanding of stem cell biology. Stem cells, in particular human induced pluripotent stem cells (hi-PSCs) and human NSCs (hNSCs) are today considered as of potential help to circumvent the mentioned problems, and to constitute the best alternative source of DAn (Lindvall et al., 2004). Stem cell therapies could overcome many of the issues associated with primary fetal tissue, thus helping to bring forward CRT to become a reliable and effective treatment for neurodegenerative disorders (Politis and Lindvall, 2012).
Recent in vitro and in vivo work has thus aimed at obtaining suitable sources of human NSCs with the capacity to differentiate into DAn, in turn endowed with the required genuine properties of SNpc neurons lost in PD. Previous studies with rodent and human DAn precursors indicated poor growth potential and unstable phenotypical properties. In this regard, we have established a new immortalized cell line obtained from human fetal ventral midbrain (VM) tissue and modified to express Bcl-XL, (hVM1-Bcl-XL or hVM, in short) which shows a great potential for the generation of SNpc DAn in vitro (Courtois et al., 2010). Bcl-XL allows for human fetal VM stem cells to stably generate mature SNpc DAn both in vitro and in vivo. Mechanistically, Bcl-XL not only exerts the expected anti-apoptotic effect but also induces pro-neural and dopamine-related transcription factors, resulting in a high yield of DAn with the correct phenotype for the development of cell therapies for PD (Courtois et al., 2010).
Since functional maturation of human DAn in vivo requires long survival times, the behavioral amelioration induced by the transplantation of hVM cells in the striatum of parkinsonian rats has been analyzed for up to 5 months, demonstrating an amelioration of spontaneous motor behavior and cognitive performance in parkinsonian animals after receiving human VM neural stem cell grafts (Ramos-Moreno et al., 2012). VM-derived cell lines did survive and integrated well from the second month after transplantation and mature neurons were generated, but the morphology of many DAn in hVM cell transplants was still immature. Electrophysiological maturation and integration has also been demonstrated in more recent short- to mid-term transplantation experiments (Ramos-Moreno T. and Martinez-Serrano A., unpublished results). Thus, future studies will be focused on determining the safety and therapeutic effect on biochemical, electrophysiological and behavioral grounds of hVM cells at longer time points (up to 12 months).
Human NSCs derived from the VM region are thus powerful research tools and candidates for cell therapies in PD. However, their clinical translation has not been fully realized due, in part, to the limited ability to track stem cell regional localization and survival over long periods of time after in vivo transplantation. Until now, behavioural tests and the subsequent histological analysis have been the preferred methods used to evaluate graft viability, differentiation and effects of transplanted cells. Research in CRT requires non-invasive and sensitive imaging techniques to unequivocally track the localization of transplanted cells. Tracking noninvasively the long-term spatial destination and final residence of transplanted cells in vivo, to monitor their survival, migration, differentiation and regenerative impact, has become a critical methodology in evaluating the efficacy of stem cell therapy procedures.
Magnetic resonance imaging (MRI), due to its inherent high spatial resolution, provides an excellent non-invasive method to study the fate of transplanted cells in vivo. To detect the transplanted cells by MRI with high sensitivity, it is necessary to label them with magnetic nanoparticles (MNPs) prior to their implantation. Labeling cells with MNPs has been shown to induce sufficient contrast for MRI visualization without impairment of their functional properties, allowing the detection of even ~500 labeled cells (Kallur et al., 2011). Therefore, MRI, in combination with other in vivo molecular imaging techniques, like positron emission tomography (PET) or bioluminescence live imaging (BLI) can provide insight into important grafting parameters such as optimal cell “dose”, localization and migration of the cells, cell survival and proliferation kinetics, graft volume, and cell differentiation or de-differentiation patterns, which can aid clinical implementation of cell therapy (Swijnenburg et al., 2007). To determine some of these parameters in a reliable manner, for instance graft volume or cell proliferation, very precise titration controls are needed to determine the detection limit of the MRI equipment.
For MRI cell tracking, cells need to be labeled with the contrast agent, such as MNPs, at a concentration high enough to be easily detected by MRI. Most labeling techniques currently use one of the following approaches: attaching magnetic nanoparticles to the stem cell surface or internalizing biocompatible magnetic nanoparticles by endocytosis. Surface labeling tends to result in lower iron content per cell and promotes a rapid reticuloendothelial recognition and clearance of labeled cells (Syková and Jendelová, 2005). Therefore, endocytosis of MNPs during in vitro cell cultivation is the preferred method to label cells for in vivo tracking by MRI.
Since MNPs do not efficiently label either non-phagocytic or non-rapidly dividing mammalian cells in vitro, in most studies, cells are co-incubated with MNPs in the presence of transfection agents (TAs), such us poly-L-lysine or protamine sulfate, to improve their uptake by the cells. However, the use of TAs to label cells might have harmful side effects. Since most TAs are toxic to cells producing a slight increase in apoptosis shortly after labeling (Thu et al., 2009), they may reduce cell viability. This toxic effect is probably due to the use of TAs and not to the MNPs themselves, since it disappeared at later stages of NSC culture, while iron nanoparticles still remained inside the cells. Moreover, it has also been reported that MNPs complexed with poly-L-lysine blocked the differentiation of human mesenchymal stem cells into chondrocytes (Kostura et al., 2004). Therefore, the use of TAs remains problematic, although recent evidence indicates that incubation of hNSCs with MNPs in the absence of TAs is not always ineffective, rather the contrary (Ramos-Gómez et al., 2015). The labeling efficiency can be improved by prolonged incubation times and elevated iron doses, resulting in a high number of internalized MNPs per cell (Figure 1). However, the use of relative high concentrations of MNPs to label hNSCs might be toxic or affect some of their functional properties, causing alterations in their differentiation processes. Therefore, a careful titration of MNP concentration is required to set optimal conditions resulting in efficient cell labeling without impairment of cell survival, proliferation, self-renewal, and multipotency (Kallur et al., 2011; Eamegdool et al., 2014; Ramos-Gómez et al., 2015). Thus, an extensive study of the properties of NSCs labeled with MNPs must be carried out to identify the effects of MNPs on hNSCs biology. Upon loading, cellular viability and differentiation, average distance of migration and changes in intracellular calcium ion concentration have been analyzed in many studies to determine optimal loading conditions and the impact of iron on cellular metabolism (Kallur et al., 2011; Eamegdool et al., 2014; Ramos-Gómez et al., 2015). These studies have confirmed that MNPs-labeling of human NSCs showed no adverse effects on cell biology when used at an optimized dosage. MNPs did not affect short-term survival, proliferation rates, multipotency or differentiation capacity of NSCs. However, after long-term in vitro differentiation, some changes appeared in both the proportion of neural phenotypes generated, as well as in the number of proliferating cells in some of the labeled cultures when compared with non-labeled control cell cultures. MNP-labeled cell cultures contained significantly less nestin- and GFAP-positive cells than the unlabeled controls. These effects have been shown in hNSCs after 18 days in culture and could be caused by a delayed toxicity due to iron overloading, which leads to cellular stress responses that may have effects on cell properties, manifested only after longer experimental times (Kallur et al., 2011). Moreover, with a prolonged exposure, MNPs translocated from the cytoplasm into the peri-nuclear region, and the migration capacity of hNSCs may be affected (Eamegdool et al., 2014).
Figure 1.
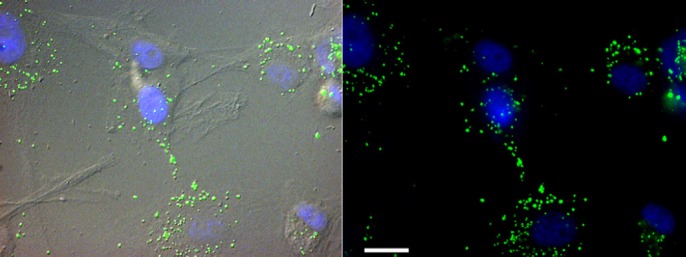
hVM-MNPs labeled cells.
Fluorescence micrographs showing human ventral midbrain (hVM) cells labeled with magnetic nanoparticles (MNPs) marked in green. Nuclei are shown in blue stained with Hoechst. Scale bar: 20 μm.
In spite of the effects described when cells are cultured in the presence of MNPs for extended periods of time, properly labeled hVM cells maintain their stemness and differentiation potential. Labeling hVM cells with MNPs did not affect the percentage of tyroxyne hydroxilase positive cells, a typical dopaminergic marker, obtained during the differentiation process. No significant changes in the percentage of nestin, GFAP or β-III-tubulin hVM cells compared to MNPs-hVM cells were observed after 7 days of in vitro differentiation (Ramos-Gómez et al., 2015). Similar findings were also obtained with the neuronal transcription factor doublecortin, a marker of immature neurons. In addition, it was found that once MNP-labeled progenitor cells differentiated into astrocytes or neurons, they did not lose their MNP label (Shen et al., 2013). Therefore, after optimization of the appropriate dose and incubation times, MNP-labeling of human NSCs left the quantitative profile of the in vitro proliferation and differentiation capacity of the cells unaltered. According to the literature, no differences in MNP-labeling have been described for different sources of stem cells. Efficient labeling of hi-PSCs (Ruan et al., 2011) and embryonic stem cells (Parsa et al., 2015) can be achieved with no negative effects on growth, morphology, viability, proliferation and differentiation allowing noninvasive in vivo MRI tracking of the transplanted cells. In addition, in vivo MR tracking of MNP-labeled bone marrow-derived NSCs after autologous transplantation can be accomplished (Ke et al., 2009).
MRI can be used for tracking of MNP-labeled neural stem cells for studying the in vivo localization and migration of grafted stem cells in longitudinal studies. It has been shown that labeling of hVM cells with MNPs does not affect their overall biology and survival rate. No significant changes have been detected in the phenotypes generated in vivo after transplantation in response to cell labeling (Eamegdool et al., 2014; Ramos-Gómez et al., 2015). The MNPs-labeled hNSCs grafted into hemiparkinsonian rats can be successfully visualized using MRI (Figure 2) at different time points, up to 5 months after transplantation, supporting the use of commercial MNPs to track cells for short- and mid-term periods in vivo (Ramos-Gómez et al., 2015). MNPs are detected by MRI as a hypointense signal which persisted for at least 5 months after transplantation. This hypointense signal, corresponding to the presence of MNPs, showed no significant changes in both the location and volume over time (Kallur et al., 2011). However, of great concern is the notion that activated microglia and macrophages will engulf dead labeled cells or iron nanoparticles dispersed freely in the brain parenchyma, contributing thus to the MRI signal and probably inducing a significant microglia proliferation (Cupaioli et al., 2014). Dead NSCs and the iron nanoparticles expelled from them may be phagocytosed by activated microglia and macrophages that are present at the NSC injection site over time (Ramos-Gómez et al., 2015). Thus, long-term MR images should be interpreted with caution due to the possibility that some MNPs may be expelled from the transplanted cells and internalized by host microglia cells. Among complementary aiding tools, the simultaneous use of BLI in the same animals seems to be accurate enough (although resolution is lower) to confirm that the MRI signal is coming from alive transplanted cells. Although several clinical trials using MNP-labeled cells have already been performed, clinical application of MNP-cell tracking by MRI is still many years away (Bernsen et al., 2015). However, much can already be learned from animal models which may help to elucidate the mechanisms underlying the success of cell-based therapies.
Figure 2.
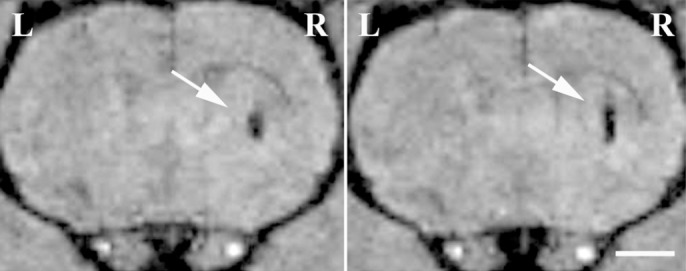
MRI of hVM-MNP labeled cells transplanted into rat brain.
Two consecutive in vivo magnetic resonance images of hVM cell suspensions (3 × 105 cells) transplanted into the left (hVM cells, L) and right (hVM-MNP labeled cells, R) striata. MRI was performed eight weeks after cell transplantation. hVM-MNP labeled cells can be easily detected in coronal T2*-weighted images as dark hypointense signals in the area where the cells have been injected (arrows). No MRI signal was detected when unlabeled hVM cells were transplanted (left striatum, L). Scale bar: 2 mm. hVM: Human ventral midbrain; MNP: magnetic nanoparticle; MRI: magnetic resonance imaging; R: right stratum.
Conclusions
To sum up, the ability to monitor the time course of survival, migration, and distribution of stem cells following transplantation by MRI will provide critical information for optimizing future therapies based on cell transplantation for several neurodegenerative diseases, such as Parkinson's disease. It has been demonstrated that labeling hNSCs with MNPs, using optimal doses of iron, does not affect their overall biology, survival rate and in vitro and in vivo differentiation patterns. Grafting of hNSC labeled with MNPs allows cell tracking by MRI for long periods of time without showing significant neuronal or systemic toxicities or behavioral changes. However, the results reviewed here suggest that in long term grafting, activated microglia and macrophages could contribute to the MRI signal by engulfing dead labeled cells or iron nanoparticles dispersed freely in the brain parenchyma over time. Thus, long-term MR images should be interpreted with caution, and interpreted in combination with other information like that offered by in vivo PET and BLI.
Footnotes
Funding: Work at the author's laboratories was supported by: To AMS: Instituto de Salud Carlos-III (RETICS TerCel RD12/0019/0013), Comunidad Autónoma de Madrid (S2010-BMD-2336), MINECO (SAF2010-17167) and the institutional grant of the Fundación Ramón Areces to the CBMSO. To MRG: Reina Sofia Foundation and Comunidad Autónoma Madrid (S2010-BMD-2460).
References
- Bernsen MR, Guenoun J, van Tiel ST, Krestin GP. Nanoparticles and clinically applicable cell tracking. Br J Radiol. 2015;88:20150375. doi: 10.1259/bjr.20150375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois ET, Castillo CG, Seiz EG, Ramos M, Bueno C, Liste I, Martínez-Serrano A. In vitro and in vivo enhanced generation of human A9 dopamine neurons from neural stem cells by Bcl-XL. J Biol Chem. 2010;285:9881–9897. doi: 10.1074/jbc.M109.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupaioli FA, Zucca FA, Boraschi D, Zecca L. Engineered nanoparticles. How brain friendly is this new guest? Prog Neurobiol. 2014;119-20:20–38. doi: 10.1016/j.pneurobio.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Eamegdool SS, Weible MW, Pham BT, Hawkett BS, Grieve SM, Chan-ling T. Ultrasmall superparamagnetic iron oxide nanoparticle prelabeling of human neural precursor cells. Biomaterials. 2014;35:5549–5564. doi: 10.1016/j.biomaterials.2014.03.061. [DOI] [PubMed] [Google Scholar]
- Kallur T, Farr TD, Böhm-Sturm P, Kokaia Z, Hoehn M. Spatio-temporal dynamics, differentiation and viability of human neural stem cells after implantation into neonatal rat, brain. Eur J Neurosci. 2011;34:382–393. doi: 10.1111/j.1460-9568.2011.07759.x. [DOI] [PubMed] [Google Scholar]
- Ke YQ, Hu CC, Jiang XD, Yang ZJ, Zhang HW, Ji HM, Zhou LY, Cai YQ, Qin LS, Xu RX. In vivo magnetic resonance tracking of Feridex-labeled bone marrow-derived neural stem cells after autologous transplantation in rhesus monkey. J Neurosci Methods. 2009;179:45–50. doi: 10.1016/j.jneumeth.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17:513–517. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10(Suppl):S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- Parsa H, Shamsasenjan K, Movassaghpour A, Akbarzadeh P, Amoghli Tabrizi B, Dehdilani N, Lotfinegad P, Soleimanloo F. Effect of superparamagnetic iron oxide nanoparticles-labeling on mouse embryonic stem cells. Cell J. 2015;17:221–230. doi: 10.22074/cellj.2016.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis M, Lindvall O. Clinical application of stem cell therapy in Parkinson's disease. BMC Med. 2012;10:1. doi: 10.1186/1741-7015-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Gómez M, Seiz EG, Martínez-Serrano A. Optimization of the magnetic labeling of human neural stem cells and MRI visualization in the hemiparkinsonian rat brain. J Nanobiotechnology. 2015;13:20. doi: 10.1186/s12951-015-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Moreno T, Castillo CG, Martínez-Serrano A. Long term behavioral effects of functional dopaminergic neurons generated from human neural stem cells in the rat 6-OH-DA Parkinson's disease model. Effects of the forced expression of BCL-X(L) Behav Brain Res. 2012;232:225–232. doi: 10.1016/j.bbr.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Ruan J, Shen J, Wang Z, Ji J, Song H, Wang K, Liu B, Li J, Cui D. Efficient preparation and labeling of human induced pluripotent stem cells by nanotechnology. Int J Nanomedicine. 2011;6:425–435. doi: 10.2147/IJN.S16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WB, Plachez C, Chan A, Yarnell D, Puche AC, Fishman PS, Yarowsky P. Human neural progenitor cells retain viability, phenotype proliferation, and lineage differentiation when labeled with a novel iron oxide nanoparticle, Molday ION Rhodamine B. Int J Nanomedicine. 2013;8:4593–4600. doi: 10.2147/IJN.S53012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swijnenburg RJ, van der Bogt KE, Sheikh AY, Cao F, Wu JC. Clinical hurdles for the transplantation of cardiomyocytes derived from human embryonic stem cells: role of molecular imaging. Curr Opin Biotechnol. 2007;18:38–45. doi: 10.1016/j.copbio.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Syková E, Jendelová P. Magnetic resonance tracking of implanted adult and embryonic stem cells in injured brain and spinal cord. Ann N Y Acad Sci. 2005;1049:146–160. doi: 10.1196/annals.1334.014. [DOI] [PubMed] [Google Scholar]
- Thu MS, Najbauer J, Kendall SE, Harutyunyan I, Sangalang N, Gutova M, Metz MZ, Garcia E, Frank RT, Kim SU, Moats RA, Aboody KS. Iron labeling and pre-clinical MRI visualization of therapeutic human neural stem cells in a murine glioma model. PLoS One. 2009;4:e7218. doi: 10.1371/journal.pone.0007218. [DOI] [PMC free article] [PubMed] [Google Scholar]


