Abstract
In response to peripheral nerve injury, the inflammatory response is almost entirely comprised of infiltrating macrophages. Macrophages are a highly plastic, heterogenic immune cell, playing an indispensable role in peripheral nerve injury, clearing debris and regulating the microenvironment to allow for efficient regeneration. There are several cells within the microenvironment that likely interact with macrophages to support their function – most notably the Schwann cell, the glial cell of the peripheral nervous system. Schwann cells express several ligands that are known to interact with receptors expressed by macrophages, yet the effects of Schwann cells in regulating macrophage phenotype remains largely unexplored. This review discusses macrophages in peripheral nerve injury and how Schwann cells may regulate their behavior.
Keywords: nerve, macrophage, traumatic injury, Schwann cells, polarization
Introduction
It has long been accepted that Schwann cells are a primary force behind the efficient regenerative capacity of the peripheral nervous system (PNS) following injury (Gaudet et al., 2011; Zochodne, 2012). It comes as no surprise that Schwann cells have this reputation, given that they have been shown time and time again to act directly to aid axonal outgrowth and remyelinate the regenerating axon in vivo and in vitro (Gaudet et al., 2011; Zochodne, 2012). What is less often discussed is the complex role that Schwann cells play in regulating the immune response (Stratton et al., 2015). Here we review previously published studies, in addition to previously published microarray datasets (Painter et al., 2014; Jha et al., 2015), to describe the inflammatory gene expression profile following peripheral nerve injury (PNI) and hypothesize potential mechanisms of macrophage polarization by Schwann cells.
In the healthy adult nerve, the inflammatory response following injury is, aside from an early wave of neutrophils, almost entirely comprised of infiltrating macrophages (Gaudet et al., 2011; Nadeau et al., 2011). Like most other inflamed injury sites, this process is initiated by chemoattractants, such as monocyte chemoattractant protein 1 (MCP-1), being released from local cells, including Schwann cells (Tofaris et al., 2002). Thereafter, circulating immune cells are attracted to the injury site, whereby they play a well-established and indispensable role in engulfing debris, including inhibitory myelin debris (Kang and Lichtman, 2013), a clearing process that is important for efficient axonal regeneration (Barrette et al., 2008; Gaudet et al., 2011). In addition, however, the macrophage's pro-regenerative role involves direct regulation of several other cellular events, including but not limited to the regulation of neurotrophin production and angiogenesis (Barrette et al., 2008; Gaudet et al., 2011; Bastien and Lacroix, 2014; Cattin et al., 2015).
It is clear that macrophages are highly plastic and exist in a spectrum of activation states as dictated by their microenvironment. In recent years, the complexities of mammalian macrophage activation have been elegantly described in vitro (Xue et al., 2014). Using a genomics approach to study macrophage-activation gene signatures in response to 29 stimulation conditions, Xue et al. (2014) demonstrated that there are at least 9 subtypes of macrophage-activation patterns, and at least 5 distinct clusters of biological processes that lead to various macrophage activation states. Such datasets nicely illustrate the shift away from traditional linear polarization models of macrophage activation to one that appreciates the true spectrum of macrophage activation. Nevertheless, and at least in the context of the nerve, macrophages are generally, and usefully, conceptualized into two opposing but complementary activation states: either proinflammatory (often referred to as M1) – involved in free radical production and pathogen protection; or anti-inflammatory (often referred to as M2) – considered to play reparative roles in inducing wound healing and mediating the resolution of inflammation (Xue et al., 2014; Franco and Fernández-Suárez, 2015; Gensel and Zhang, 2015).
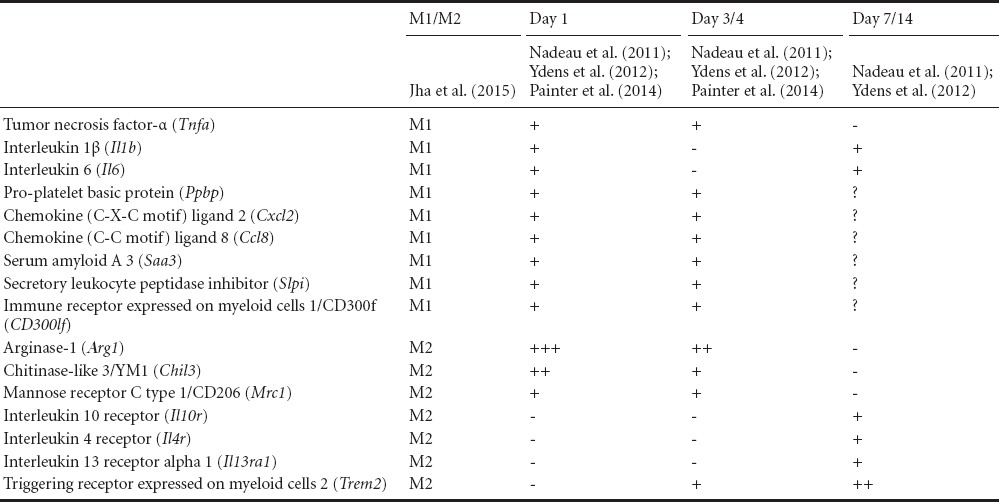
Macrophage Polarization in PNI
In response to PNI, neutrophils and monocytes infiltrate the injury site within 6–12 hours post-injury (Figure 1) (Nadeau et al., 2011; Bastien and Lacroix, 2014). Proinflammatory macrophages derived from infiltrating monocytes, characterized by Ly6Chi/Ly6G–, peak at 1–2 days post-injury (Nadeau et al., 2011; Bastien and Lacroix, 2014). This is accompanied by increased expression of proinflammatory-associated factors, including tumor necrosis factor-α (Tnfa), interleukin 1β (Il1b), interleukin 6 (Il6), pro-platelet basic protein (Ppbp), chemokine (C-X-C motif) ligand 2 (Cxcl2), chemokine (C-C motif) ligand 8 (Ccl8), serum amyloid A 3 (Saa3), secretory leukocyte peptidase inhibitor (Slpi) and immune receptor expressed on myeloid cells 1/CD300f (CD300lf) at 1 day post-injury (Table 1) (Nadeau et al., 2011; Ydens et al., 2012; Painter et al., 2014; Jha et al., 2015). This closely parallels the proinflammatory profile of macrophages analyzed acutely at the injury site, after skeletal muscle injury (Arnold et al., 2007; Cheng et al., 2008) and central nervous system (CNS) injury (Kroner et al., 2014; Gensel and Zhang, 2015). Interestingly, prototypical proinflammatory-associated genes such as interferon-γ receptor (Ifngr), and in some cases inducible nitric oxide synthase/iNOS (Nos2), are not routinely detected at early or late-stages following PNI (Ydens et al., 2012; Painter et al., 2014; Peluffo et al., 2015). This is in contrast to traumatic injury in the CNS, where there is delayed but persistent expression of Ifngr and other proinflammatory proteins including iNOS, CD16/32, and CD86 (Kigerl et al., 2009; Kroner et al., 2014).
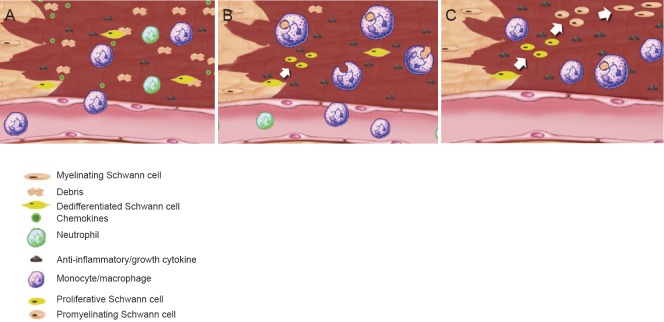
Figure 1.
Schematic of the macrophage and Schwann cell response in peripheral nerve injury.
(A) Neutrophils and monocytes infiltrate the injury site within hours post-injury. This process is initiated by chemokines being released from local cells, including dedifferentiating Schwann cells. (B) Recruited monocytes differentiate into phagocytic macrophages whereby, among other things, they play a well-established and indispensable role in engulfing debris. We hypothesize that the dedifferentiated Schwann cell plays a substantial role in regulating macrophage function, via the secretion of cytokines/factors, although the mechanisms that underlie this regulation remain undetermined. (C) The macrophage's pro-regenerative role involves direct regulation of several cellular events via the secretion of anti-inflammatory/growth supportive cytokines. We hypothesize this includes the regulation of Schwann cell differentiation, the promotion of Schwann cell remyelination, axonal support and angiogenesis.
Table 1.
Temporal profile of the expression of M1-and M2-associated genes as defined by Jha et al. (2015) in peripheral nerve injury
Although proinflammatory factors are often referred to as detrimental (Arnold et al., 2007; Aurora and Olson, 2014; Kroner et al., 2014; Gensel and Zhang, 2015), it is clear that certain proinflammatory cytokines and chemokines, such as MCP-1, macrophage inflammatory protein-α (MIP-α), TNF and IL1-β, are pivotal for efficient regeneration in PNI, with multiple studies suggesting these cytokines play important roles in the recruitment/activation of immune cells to clear myelin debris and the regulation of Schwann cells (Perrin et al., 2005; Lu and Zhu, 2011). More generally, it seems the role of proinflammatory macrophages, with their complex cytokine profile, in the context of nerve injury is less clear. In vitro, it appears that the exposure of Schwann cells and neurons to M1-primed macrophage conditioned media enhances Schwann cell proliferation, reduces axonal outgrowth and compromises neuronal survival (Kigerl et al., 2009; Mokarram et al., 2012). In vivo, however, there is a suggestion that M1 macrophages are necessary for efficient regeneration, given a recent study which interfered with the function of proinflammatory macrophages in PNI, found that recovery was compromised (Peluffo et al., 2015). By interfering with the CD300f receptor – highly expressed by M1-associated macrophages and thought to play a role in regulating inflammation and debris clearance – the authors demonstrated a shift in macrophage phenotypes towards an anti-inflammatory profile with an increase in propensity to clear debris (Peluffo et al., 2015). Interestingly, although this macrophage profile would usually suggest a pro-growth supportive phenotype, the authors described a reduction in axonal regrowth and compromised behavioral outcomes compared to controls. Such findings demonstrate the complex orchestration of the proinflammatory response in PNI (Bastien and Lacroix, 2014), and the contextual effects that influence overall tissue repair.
Following the initial proinflammatory response in PNI, there is a transition to an anti-inflammatory macrophage phenotype, characterized by Ly6Clo/Ly6G– cells that begin to prevail the nerve by 3 days post-injury (Nadeau et al., 2011). At the same time, anti-inflammatory genes, such as arginase-1 (Arg1), chitinase-like 3/YM1 (Chil3) and mannose receptor C type 1/CD206 (Mrc1), are expressed (Painter et al., 2014; Jha et al., 2015). Interestingly, as described above, the M1-associated macrophage is the predominant macrophage phenotype present at 1 day post-injury (Bastien and Lacroix, 2014), yet it seems some of the most highly upregulated genes at 1 day after PNI are, in fact, those that encode for enzymes, such as Arg1 and Chil3, associated with an anti-inflammatory response (Painter et al., 2014). This observation has also been made in other tissues (Novak and Koh, 2013a, b; Gensel and Zhang, 2015). Thereafter, it seems that between 7–14 days post PNI, a different anti-inflammatory macrophage subtype wave dominates the nerve, characterized by the expression of interleukin 10 (Il10) and receptors such as interleukin 10 receptor (Il10r), interleukin 4 receptor (Il4r), interleukin 13 receptor alpha 1 (Il13ra1) and triggering receptor expressed on myeloid cells 2 (Trem2) (Be’eri et al., 1998; Ydens et al., 2012; Bastien and Lacroix, 2014). These kinetics of macrophage polarization at later stages of nerve injury are similar to what is observed at sites of injury in tissues such as skin and skeletal muscle (Arnold et al., 2007; Daley et al., 2010; Novak and Koh, 2013b) but intriguingly, different from what occurs in spinal cord injury where it is believed the expression of M2-associated genes and proteins are only transient (Kigerl et al., 2009; Bastien and Lacroix, 2014). Taken together, in recent years, the spectrums of genetic profiles regulating the inflammatory response elicited by PNI are becoming better characterized, but the outcome or role of this genetic profile on cellular function, for the most part (Chen et al., 2015), still remains poorly understood.
At least in vitro, it seems that the myelin-ingested, phagocytic macrophage shows a strikingly enhanced M2 phenotype, where these cells are not only growth-promoting, but also incapable of producing a proinflammatory macrophage profile when stimulated with prototypical proinflammatory stimuli (Kroner et al., 2014). Anti-inflammatory macrophages, and associated M2-cytokines, are clearly crucial in several injury environments given that this phenotype promotes muscle and myelin cell maturation and differentiation, and, in vitro at least, appears to provide trophic support to enhance axonal outgrowth in neurons (Kigerl et al., 2009; Miron et al., 2013; Novak and Koh, 2013b; Aurora and Olson, 2014; Kroner et al., 2014). Interestingly, given that Lazarov-spiegler et al. (1998) demonstrated that macrophages stimulated with sciatic nerve explants are far more phagocytic than those left unstimulated or stimulated by optic nerve explants, it seems likely that macrophages subjected to the PNS microenvironment have a particularly robust capacity to be M2-like. Indeed, Lazarov-spiegler et al. (1998) demonstrated that the transplant of nerve-stimulated macrophages into CNS injury accelerated axonal regeneration compared to unprimed macrophage transplants.
Macrophage-Schwann Cell interactions
What could be disparate about the immune response in injuries of the peripheral nerve compared with other systems, such as the CNS, is the key involvement of Schwann cells, a tissue-specific cell that is known to express particular growth factors at extremely high levels post-injury (Painter et al., 2014). When assessing PNI over time, the expression of growth factors such as glial cell line-derived neurotrophic factor (Gdnf), fibroblast growth factor-5 (Fgf5) and sonic hedgehog (Shh), but not classic M2-inducing cytokines (Il4, Il10, Il13), seem to dominate the nerve during peak stages of debris clearance (Painter et al., 2014). With this in mind, given that we have recently shown in vitro that Schwann cells do not secrete high levels of classic M2-associated cytokines, but are potent inducers of M2-phenotypes in macrophages (as evidenced by a >1,000-fold increase in Arg1 expression) and that these macrophages promote axonal outgrowth (Stratton et al., 2015), the question arises as to what drives macrophages to be M2-like in the PNS. Interestingly, following Schwann cell-targeted knockout of c-Jun expression – a transcription factor that regulates the transition of myelinating Schwann cells into a dedifferentiated state, and regulates the expression of neurotrophic factors such as Gdnf and brain-derived neurotrophic factor (Bdnf) – there is a decrease in the clearance of myelin debris after nerve injury (Arthur-Farraj et al., 2012). The authors attributed this to a malfunction in the myelinophagic capacity of the c-Jun-null Schwann cell to degrade myelin (Gomez-Sanchez et al., 2015). However, and to their surprise, they also made the observation that macrophages, although genetically normal, contained 7 times more lipid droplets per macrophage and were substantially larger compared to wild-type controls. It was not determined whether this observation was simply a compensatory mechanism (i.e., the macrophage had more debris to clear given the impaired capacity of the Schwann cell to clear debris) or whether a given macrophage could no longer efficiently digest debris. It is possible that these findings indicate that c-Jun, in Schwann cells, regulates the expression, and subsequent secretion, of yet-to-be identified ligand(s) that interact with macrophages to modulate their function. Given GDNF is one such factor which is strikingly reduced following c-Jun knockout, and given that macrophages have the capacity to express the GDNF receptor, Gfra2 (Jha et al., 2015), it would be interesting to assess whether this ligand enhances the phagocytic capacity of macrophages.
Relevance
Following severe nerve injury, delayed surgical repair or when patients are either aged or diseased (Zochodne, 2012; Painter et al., 2014), regeneration is often incomplete and permanent disability can occur. Usually it is unclear as to what underlying mechanisms are responsible for this lack of regeneration, but some suggest it may be 1) a diminished capacity of Schwann cells to promote regeneration over time; 2) axonal misdirection; 3) the vast distances an axon must grow to reinnervate target organs/tissues (Sulaiman and Gordon, 2000; Gordon et al., 2011; Zochodne, 2012). Another possible contributing mechanism is that the typical macrophage response is compromised. Indeed, in other tissues, including, for example the aged CNS, it seems that improper macrophage responses following injury might be a key factor contributing to inefficient regeneration (Ruckh et al., 2012; Natrajan et al., 2015). Moreover, dysregulated macrophage responses are also believed to contribute to the pathology of PNS and CNS autoimmune diseases (Shin et al., 2012, 2013a, b). With this in mind, it is possible that, in certain circumstances where regeneration is inadequate in PNI, there is potential to therapeutically modulate the immune response to be more growth supportive, ultimately improving regenerative outcomes. Indeed, it has been shown that macrophages become more M2-like following IL-4 recombinant therapy delivered at the injury/repair site in PNI (increased ratio of CD206+:CD68+ cells) and that this results in enhanced axonal regeneration (Kigerl et al., 2009; Mokarram et al., 2012). It has also been shown, recently, using Schwann cell transplant in PNI, that macrophage modulation may be one of the contributing mechanisms leading to enhanced regeneration following Schwann cell transplant therapy (Stratton et al., 2015). Together, although only a few studies to-date have demonstrated the potential of macrophage-directed therapies to improve regenerative outcomes in PNI, we believe that such an approach holds potential for patients who suffer from chronic disability following PNI.
Conclusion
The immune response in PNI has several features common to any injury site in the body - a rapid influx of immune cells, an early proinflammatory response followed by an anti-inflammatory response. Even so, there appears to be distinct features of macrophages in PNS microenvironments suggesting that this microenvironment may induce a unique, and as-of-yet largely uncharacterized macrophage phenotype, with a particular propensity to clear debris and contribute to regenerative cascades. Although largely unexplored, several studies hint that one of the underlying cellular interactions, which have the potential to regulate this unique macrophage response in PNI, involves the Schwann cell. Further research is required to fully unravel the potential contribution of macrophages in efficient PNI recovery. Gaining such insight could lead to more targeted immunomodulatory treatments not only for patients who suffer from chronic disability following PNI, but also for patients suffering from other diseases and injury states where the macrophage-response is suboptimal.
References
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurora AB, Olson EN. Immune modulation of stem cells and regeneration. Cell Stem Cell. 2014;15:14–25. doi: 10.1016/j.stem.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette B, Hébert MA, Filali M, Lafortune K, Vallières N, Gowing G, Julien JP, Lacroix S. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien D, Lacroix S. Cytokine pathways regulating glial and leukocyte function after spinal cord and peripheral nerve injury. Exp Neurol. 2014;258:62–77. doi: 10.1016/j.expneurol.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Be’eri H, Reichert F, Saada A, Rotshenker S. The cytokine network of Wallerian degeneration: IL-10 and GM-CSF. Eur J Neurosci. 1998;10:2707–2713. [PubMed] [Google Scholar]
- Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJA, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Cescon M, Zuccolotto G, Nobbio L, Colombelli C, Filaferro M, Vitale G, Feltri ML, Bonaldo P. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta neuropathologica. 2015;129:97–113. doi: 10.1007/s00401-014-1369-9. [DOI] [PubMed] [Google Scholar]
- Cheng M, Nguyen MH, Fantuzzi G, Koh TJ. Endogenous interferon-ã is required for efficient skeletal muscle regeneration. AJP Cell Physiol. 2008;294:C1183–1191. doi: 10.1152/ajpcell.00568.2007. [DOI] [PubMed] [Google Scholar]
- Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Fernández-Suárez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HB, Aspichueta P, Elortza F, Aransay AM, Martínez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, et al. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J Cell Biol. 2015;210:153–168. doi: 10.1083/jcb.201503019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011;31:5325–5334. doi: 10.1523/JNEUROSCI.6156-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Huang SCC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B, Pearce EJ, Driggers EM, Artyomov MN. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Kang H, Lichtman JW. Motor axon regeneration and muscle reinnervation in young adult and aged animals. J Neurosci. 2013;33:19480–19491. doi: 10.1523/JNEUROSCI.4067-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuong HT, Kumar R, Senjaya F, Grochmal J, Ivanovic A, Shakhbazau A, Forden J, Webb A, Biernaskie J, Midha R. Skin derived precursor Schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp Neurol. 2014;254:168–179. doi: 10.1016/j.expneurol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Lazarov-Spiegler O, Solomon AS, Schwartz M. Peripheral nerve-stimulated macrophages simulate a peripheral nerve-like regenerative response in rat transected optic nerve. Glia. 1998;24:329–337. [PubMed] [Google Scholar]
- Lu MO, Zhu J. The role of cytokines in Guillain-Barré syndrome. J Neurol. 2011;258:533–548. doi: 10.1007/s00415-010-5836-5. [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao J, Yuen TJ, Ruckh JM, Shadrach JL, Wijngaarden P Van, Wagers AJ, Williams A, Franklin RJM. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33:8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, Iwakura Y, Pablo J, Vaccari DR, Keane RW, Lacroix S. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. J Neurosci. 2011;31:12533–12542. doi: 10.1523/JNEUROSCI.2840-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natrajan MS, de la Fuente AG, Crawford AH, Linehan E, Nuñez V, Johnson KR, Wu T, Fitzgerald DC, Ricote M, Bielekova B, Franklin RJM. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain. 2015;138:3581–3597. doi: 10.1093/brain/awv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol. 2013a;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013b;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter MW, Brosius Lutz A, Cheng YC, Latremoliere A, Duong K, Miller CM, Posada S, Cobos EJ, Zhang AX, Wagers AJ, Havton LA, Barres B, Omura T, Woolf CJ. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron. 2014;83:331–343. doi: 10.1016/j.neuron.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo H, Solari-Saquieres P, Negro-Demontel ML, Francos-Quijorna I, Navarro X, López-Vales R, Sayós J, Lago N. CD300f immunoreceptor contributes to peripheral nerve regeneration by the modulation of macrophage inflammatory phenotype. J Neuroinflammation. 2015;12:145. doi: 10.1186/s12974-015-0364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin FE, Lacroix S, Avilés-Trigueros M, David S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian, degeneration. Brain. 2005;128:854–866. doi: 10.1093/brain/awh407. [DOI] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, Van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJM. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Ahn M, Matsumoto Y. Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: recent insights from macrophages. Anat Cell Biol. 2012;45:141. doi: 10.5115/acb.2012.45.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Ahn M, Matsumoto Y, Moon C. Mechanism of experimental autoimmune neuritis in Lewis rats: the dual role of macrophages. Histol Histopathol. 2013a;28:679–684. doi: 10.14670/HH-28.679. [DOI] [PubMed] [Google Scholar]
- Shin T, Ahn M, Moon C, Kim S, Sim KB. Alternatively activated macrophages in spinal cord injury and remission: another mechanism for repair. Mol Neurobiol. 2013b;47:1011–1019. doi: 10.1007/s12035-013-8398-6. [DOI] [PubMed] [Google Scholar]
- Stratton JA, Shah PT, Kumar R, Stykel MG, Shapira Y, Grochmal J, Guo GF, Biernaskie J, Midha R. The immunomodulatory properties of adult skin-derived precursor Schwann cells: implications for peripheral nerve injury therapy. Eur J Neurosci. 2015 doi: 10.1111/ejn.13006. doi: 10.1111/ejn.13006. [DOI] [PubMed] [Google Scholar]
- Sulaiman OA, Gordon T. Effects of short-and long-term Schwann cell denervation on peripheral nerve regeneration, myelination and size. Glia. 2000;32:234–246. doi: 10.1002/1098-1136(200012)32:3<234::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S, Biernaskie J, Kemp SWP, Midha R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience. 2009;164:1097–1107. doi: 10.1016/j.neuroscience.2009.08.072. [DOI] [PubMed] [Google Scholar]
- Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ydens E, Cauwels A, Asselbergh B, Goethals S, Peeraer L, Lornet G, Almeida-Souza L, Van Ginderachter JA, Timmerman V, Janssens S. Acute injury in the peripheral nervous system triggers an alternative macrophage response. J Neuroinflammation. 2012;9:176. doi: 10.1186/1742-2094-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW. The challenges and beauty of peripheral nerve regrowth. J Peripher Nerv Syst. 2012;17:1–18. doi: 10.1111/j.1529-8027.2012.00378.x. [DOI] [PubMed] [Google Scholar]