After spinal cord injury (SCI), a cascade of events begins. At first, there is physical damage with disruption of the blood-brain barrier (BBB) and the integrity of the nervous tissue. The disruption of central nervous system (CNS) BBB alters the endothelial permeability, the protein and chemokines expression and the propensity to release in situ inflammatory cytokines, overcoming anti-inflammatory signals, facilitating the attraction and entry of immune system cells into the injured spinal cord parenchyma (Gaudet et al., 2011). As a result, there is a neuroinflammatory response with changes in blood flow, edema, cell infiltration, apoptosis and release of axonal growth inhibitory factors. Nerve function loss occurs when the nerve impulse propagation is interrupted and do not reach its target. This disorder encompasses neuron and glia apoptosis, accompanied by Wallerian degeneration of disconnected axons, and CNS cells exposure to a hostile microenvironment that hampers axon regeneration (Mautes et al., 2000; Harkey et al., 2003). Additionally, the damage spreads further in a phenomenon called progressive hemorrhagic necrosis – PHN, with the appearance of petechial hemorrhagic foci and deterioration in areas outside the lesion epicenter during the next 2 – 24 hours after the trauma (Simard et al., 2007). Our laboratory investigates the role of galectin-3, a protein linked to mechanisms of inflammation, behind the cellular mechanisms of neural degeneration/regeneration with perspectives of a novel treatment (Mostacada et al., 2015).
After CNS trauma there is a cellular response that leads to neuroinflammation. Neutrophils are the first cells to infiltrate the injured site in a response to the exposure of damage-associated molecule patterns (DAMP) and to alterations of the microenvironment. They appear in the first 24 hours, reach a peak, and start to decline around 48 hours after the injury. Even though neutrophils have an immunological role against infections, in the injured CNS they can be harmful due to its degranulation and release of proteolytic and hazardous enzymes such as elastase, matrix metalloproteinases (MMPs) which contribute to tearing down the vascular and nervous tissue architecture. Neutrophils also release inflammatory cytokines (interleukins-1β, -6, tumor necrosis factor alpha [TNF-α]), that amplify inflammation and tissue injury, and chemokines that attract macrophages (such as macrophage inflammatory protein 1 – MIP-1, macrophage chemoattractant protein 1 – MCP-1/CCL2, interleukin-8). In the subacute phase, monocytes start to arrive (they reach a peak in 72 hours). At the same time, there is a more prominent response from the resident microglia, which changes its morphology to a macrophage-like form (Willians et al., 2011). Monocytes assume two phenotypes according to what is expressed in the surrounding tissue: Ly6chi/Cx3CR1lo (inflammatory type responsive to MCP-1/CCL2 and MCP-3/CCL7) and Ly6cLo/Cx3CR1hi (anti-inflammatory type responsive to CX3CL1). This polarization also occurs when monocytes assume macrophage morphology, becoming M1 or M2. M1 is an inflammatory macrophage lineage activated by T helper 1 (Th1) intercession and exposure to inflammatory cytokines such as interferon gamma (IFN-γ), which starts to produce inflammatory cytokines (IL-12, IL-23, IL-1β, TNF-α), cytotoxic mediators and harmful factors (proteases, reactive oxygen species, nitrogen metabolites). A predominant M1 activation, therefore, may cause more damage to the tissue, hindering the recovery. M2 is an anti-inflammatory macrophage lineage activated by T helper 2 (Th2) and exposure to anti-inflammatory cytokines such as interleukin-4 (IL-4), which starts to drive to an anti-inflammatory state, inhibiting the production of inflammatory cytokines and harmful factors, rising the expression of major histocompatibility complex II (MHC-II). Besides the fact that macrophages have a cleaning role in phagocyting dead cells and stromal debris, it is already known that they have also a major role in neuroregeneration since they secrete neurotrophic factors such as nerve growth factor (NGF), transforming growth factor beta (TGF-β), basic fibroblast growth factor (bFGF) and others. However, if there is a M1 overall activation, the inflammatory/detrimental state will prevail. Between 3 – 7 days after SCI both M1 and M2 populations are found in the lesion site, but the stimuli that maintain M2 population start to decrease, resulting in a predominant M1 activity. Some M1 cells can reside in the lesion site for months, perpetuating its neurotoxic effect (Kigerl et al., 2009; Xiang et al, 2014).
Galectins are members of 14 lectins family proteins phylogenetically preserved, 12 of them are present in the human species. One of this members, galectin-3, our research focus, is expressed by neoplasic cells, macrophages, epithelial cells, fibroblasts, and activated T cells, having a pleiotropic action. It is involved in cellular activation, chemoattraction, cellular growth and differentiation, cellular cycle, apoptosis, and cell adhesion. Together with other molecules and factors, galectin-3 shows an important role as an inflammation regulatory factor. They are involved in the expression of L-selectin and IL-8, being associated with neutrophil migration, and the promotion of superoxide anion production. They facilitate the phagocytic role of macrophages and monocytes, as well as the polarization of macrophages to M1 or M2 phenotypes (MacKinnon et al., 2008), thereafter, exerting a role in degeneration or regeneration of the injured nervous system. Previous works of our group (Narciso et al., 2009; Mietto et al., 2013) showed that C57BL/6 mice knockout for galectin-3 (Gal3–/–) have a better functional score in the recovery period compared to wild type C57BL/6, after a peripheral nerve injury. Both works showed a prominent neuroinflammatory and degenerative state in the early stages after the injury, accompanied by an efficient phagocytic and clearance role by macrophages in the lesion site, which is important to neural regeneration. Some researchers showed a controversial association of the rise of TNF- α and other interleukins with neural regeneration, even in Gal3–/– mice subjected to sciatic nerve compression. The rise of these interleukins and other chemoattractants are apparently necessary to drive inflammatory cells to the site of injury and begin the process of cleaning the area to allow the axonal growth cone elongation (Mietto et al., 2013).
Our latest research elucidated the effects of galetin-3 absence in the motor function recovery, correlating it with morphological and cellular alterations in Gal3–/– C57BL/6 mice compared to wild type C57BL/6 after a spinal cord compression. The lesion was performed in the spinal cord with a 30 g vascular clip during one minute at T8-10 vertebral level, inducing hind limb paralysis. We evaluated the motor function using the Basso Mouse Scale (BMS) at 1, 3, 7, 28, and 42 days after SCI. All 39 animals started with a 9 score (highest score), and after the compression they scored 0, showing that the compression injury was successful (Figure 1). After 42 days after lesion, sham animals (those just underwent a laminectomy surgery with no spinal cord compression) had the same 9 score. On the other hand, wild type animals achieved a weak recovery (score ~2) compared to Gal3–/– (score ~4) after 42 days (P < 0.001 – P < 0.0001 in Mostacada et al, 2015, Figure 1). These results demonstrate that the absence of galectin-3 had a favorable effect in the neurological function recovery. We evaluated the spinal cord lesion extension and the preserved white matter to measure the magnitude of the lesion by histological analysis with Luxol Fast Blue plus Hematoxylin and Eosin, and compared Gal3–/– and wild type mice at 1, 3, 7 and 42 days after SCI. We observed a smaller extension of the lesion in Gal3–/–, after 7 days, with a decrease of the lesion extension in the subsequent days, compared to wild type (P = 0.0003 at 7 days and 42 days after injury according to Mostacada et al., 2015, Figure 2). In Gal3–/– the lesion was concentrated near the compression epicenter. The average preserved white matter area was higher in Gal3–/– compared to wild type (P < 0.009 at 7 days, P < 0.05 at 42 days and P < 0.009 comparing the averages between 7 and 42 days). In the morphologic analysis of semi-thin sections we observed that 28 days after lesion, even with both Gal3–/– and wild type passing through an intense degeneration and tissue disorganization, there were more spared fibers in Gal3–/– animals (P < 0.05).
Figure 1.
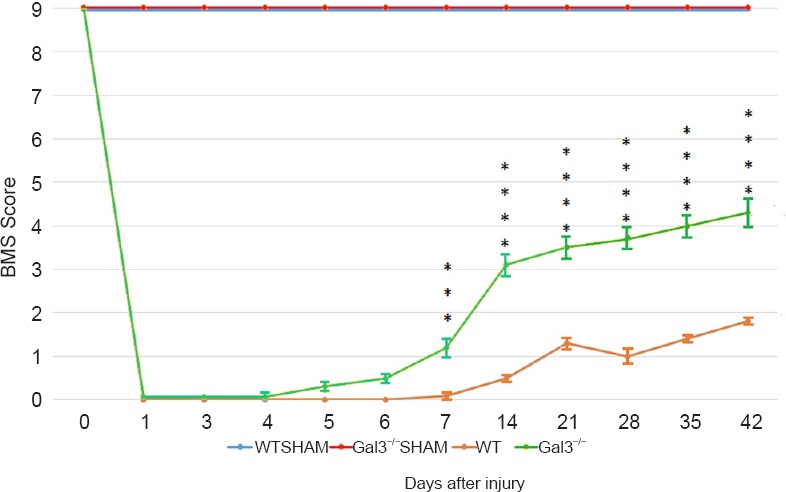
Basso Mouse Scale evolution on groups of mice with spinal cord compression injury.
Since SHAM group (WT SHAM, Gal3–/– SHAM) did not go through a compression lesion, the score of this group was always the highest (Xiang et al., 2014). Forty-two days after lesion l Gal3–/– mouse had a BMS score of 4 with a substantial motor function recovery. At the same time, wild type animals did not reach that score and stopped in BMS score 2, representing a weaker motor function recovery. ***P< 0.001, ****P < 0.0001. Adapted from Mostacada et al., 2015.
Figure 2.
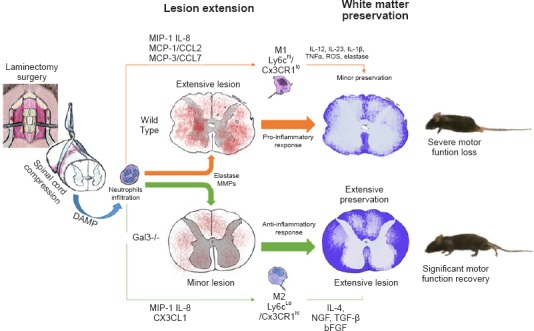
Evolution of the damage to the spinal cord in C57BL/6 wild type mouse (upper orange row) in comparison to galectin-3 knockout (Gal3–/–) mice (bottom green row).
The image shows the laminectomy surgery to access the spinal cord and the compression with vascular clips. The sequence shows the neutrophils orchestrating a pro-inflammatory response intermediated by M1 (macrophage) and Ly6chi/Cx3CR1lo (monocytes) or an anti-inflammatory response by M2 (macrophage) and Ly6cLo/Cx3CR1hi. The thin arrows indicate the interleukins, chemokines and factors released in each situation. The column “Lesion extension” shows the extension of hemorrhage characterized by red dots. In the column “White matter preservation”, representing a Luxol Fast Blue staining, the preserved white matter corresponds to the blue area, and the bluish pale area corresponds to either gray matter or damaged white matter. The last column shows the outcome in each lineage, with a paraplegic wild type C57BL/6 mouse and a partial motor function recovery in a non-paraplegic Gal3–/– C57BL/6 mouse. Adapted from Mostacada et al., 2015.
By immunohistochemistry, we observed a higher proportion of neutrophils reaching the parenchyma at 3 days after lesion in Gal3–/– as compared to wild type animals. We evaluated the number of microglia and macrophages of both M1 and M2 lineages at 3 and 7 days after lesion. All these cells were marked with anti-CD11b, and just M2 lineage cells were also labeled with Arginase-1 once M1 can not be marked with Arginase-1. At 3 days after lesion both group of animals expressed anti-CD11b and Arginase-1. These markers were found in outlying areas away from the lesion epicenter, near sites of damaged tissue, and were found all over the spinal cord parenchyma at the epicenter level. There was no significant difference between Gal3–/– and wild type groups comparing the number of cells labeled with anti-CD11b. However, in the subacute phase (7 days after lesion) there was a higher proportion of spinal cord area labeled with anti-CD11b cell in wild type compared to Gal3–/– (P = 0.0008). These anti-CD11b cells were found at all spinal cord extension, but were concentrated near the lesion epicenter. Unlike anti-CD11b, Arginase-1 labeled cells (M2 cells) were found in higher proportion in Gal3–/– phenotype (P < 0.0009). In order to know the proportion of Arginase-1positive cells (M2) compared to the totality of Anti-CD11b cells in Gal3–/– phenotype, we performed a double labeling and co-localization of positive cells for these two markers. Gal3–/– animals presented a high proportion of double positive cells for Arginase-1 and anti-CD11b, which presupposes that the of absence of Gal3 led to either less inflammation or a more anti-inflammatory response after 7 days, since there was a decreased cellularity and at that time resident cells exhibited an M2 phenotype (Figure 2).
Altogether, our results and those from other groups (MacKinnon et al., 2008; Kigerl et al., 2009) suggest that galectin-3 has a role in inducing inflammation, sustaining an M1 phenotype over M2, which hampers neural regeneration, diminishing functional recovery. Therefore, treatments that suppress galectin-3 could lead to better results after SCI, giving a chance of better life quality for the patients. Our group is now searching for more information on galectin-3 role in neural regeneration and neuroinflammatory responses, such as the boundaries between neurodegenerative diseases and traumatic injury to nervous system, and how the adaptive immune system intermediates the cellular response in these situations.
References
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;9:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkey HL, 3rd, White EA, 4th, Tibbs RE, Jr, Haines DE. A clinician's view of spinal cord injury. Anat Rec B New Anat. 2003;271:41–48. doi: 10.1002/ar.b.10012. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma VY, Chan L, Carruters BS. Incidence, prevalence, costs and impact on disability of common conditions requiring reheabilitation in the United States: stroke, spinals cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss and back pain. Arch Phys Med Rehabil. 2014;95:986–995. doi: 10.1016/j.apmr.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- Mautes A, Weinzierl MR, Donovan F, Noble LJ. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80:673–687. [PubMed] [Google Scholar]
- Mietto BS, Jurgensen S, Alves L, Pecli C, Narciso MS, Assunção-Miranda I, Villa-Verde DM, de Souza Lima FR, de Menezes JR, Benjamim CF, Bozza MT, Martinez AM. Lack of galectin-3 speeds Wallerian degeneration by altering TLR and pro-inflammatory cytokines expression in the injured sciatic nerve. Eur J Neurosci. 2013;37:1682–1690. doi: 10.1111/ejn.12161. [DOI] [PubMed] [Google Scholar]
- Mostacada K, Oliveira FL, Villa-Verde DM, Martinez AM. Lack of galectin-3 improves the functional outcome and tissue sparing by modulating inflammatory response after a compressive spinal cord injury. Exp Neurol. 2015;271:390–400. doi: 10.1016/j.expneurol.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Narciso MS, Mietto Bde S, Marques SA, Soares CP, Mermelstein Cdos S, El-Cheikh MC, Martinez AM. Sciatic nerve regeneration is accelerated in galectin-3 knockout mice. Exp Neurol. 2009;217:7–15. doi: 10.1016/j.expneurol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Batha S, Geng Z, Woo SK, Gerzanich V. Endothelial sulfonylurea receptor1-regulated NCCa-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117:2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willians MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–469. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, He X, Ren Y. Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen Res. 2014;9:1787–1795. doi: 10.4103/1673-5374.143423. [DOI] [PMC free article] [PubMed] [Google Scholar]


