Keywords: nerve regeneration, cerebral ischemia/reperfusion, Shenqi Fuzheng injection, aged rats, neurological function, Ca2+, oxygen free radicals, NSFC grant, neural regeneration
Abstract
Shenqi Fuzheng injection is extracted from the Chinese herbs Radix Astragali and Radix Codonopsis. The aim of the present study was to investigate the neuroprotective effects of Shenqi Fuzheng injection in cerebral ischemia and reperfusion. Aged rats (20–22 months) were divided into three groups: sham, model, and treatment. Shenqi Fuzheng injection or saline (40 mL/kg) was injected into the tail vein daily for 1 week, after which a cerebral ischemia/reperfusion injury model was established. Compared with model rats that received saline, rats in the treatment group had smaller infarct volumes, lower brain water and malondialdehyde content, lower brain Ca2+ levels, lower activities of serum lactate dehydrogenase and creatine kinase, and higher superoxide dismutase activity. In addition, the treatment group showed less damage to the brain tissue ultrastructure and better neurological function. Our findings indicate that Shenqi Fuzheng injection exerts neuroprotective effects in aged rats with cerebral ischemia/reperfusion injury, and that the underlying mechanism relies on oxygen free radical scavenging and inhibition of brain Ca2+ accumulation.
Introduction
Severe cerebral ischemia/reperfusion injury is the leading cause of cerebral resuscitation failure (Vaahersalo et al., 2014). Hemodynamic disorders may induce chronic brain ischemia, decrease cortical cell number, trigger massive neurofibrillary and nerve cell degeneration, and cause diffuse demyelination of white matter, ultimately leading to deterioration in brain function, and vascular dementia (Lin et al., 2002). These changes cause severe impairments in cognition and memory (Hailer, 2008; Dirnagl et al., 2009; Nakano et al., 2014; Chen et al., 2015; Wang et al., 2015), and older people are particularly at risk. Existing preventive and therapeutic drugs focus on the immediate effects of cerebral ischemia/reperfusion damage (Yuan et al., 2011; Li et al., 2012; Huang et al., 2014). However, the pathogenesis of this type of injury is very complex (Benderro and LaManna, 2013; Patel et al., 2014), and a single medication is unlikely to produce a satisfactory outcome. Shenqi Fuzheng injection (SFI) is an extract from Radix Astragali and Radix Codonopsis, two Chinese herbs known for strengthening the body's resistance. Indeed, the majority of studies on SFI emphasize its beneficial effects on immunity in cancer patients (Dai et al., 2008). Radix Codonopsis invigorates the spleen, improves vital energy, produces blood fluid, and nourishes the blood. We therefore hypothesized that this Chinese drug preparation will protect the brain and body from cerebral ischemia/reperfusion damage. Here, we used older rats, aged ≥ 20 months (Shi, 1999), to model cerebral ischemia/reperfusion injury in older people, and demonstrated the therapeutic efficacy of SFI in these models by improvements in functional, morphological and biochemical indicators.
Materials and Methods
Animals
One hundred and forty male Sprague-Dawley rats, aged 20–22 months and weighing 200–300 g, were provided by the Laboratory Animal Center of Xi’an Jiaotong University Health Science Center (Xi’an, Shaanxi Province, China; license No. b08005). All rats were housed in a room at 24°C and 50% humidity. The experimental protocols were approved by the Animal Ethics Committee of Xi’an Jiaotong University Health Science Center in China. The rats were randomly allocated to a sham group (n = 40), a model group (n = 50) and an SFI group (n = 50).
SFI administration
Rats in the SFI group received 40 mL/kg SFI (containing 10-g Radix Astragali and 10-g Radix Codonopsis per 250 mL; Livzon Pharmaceutical Group Co., Ltd., Zhuhai, Guangdong Province, China; batch No. Z19990065) (Xu et al., 2001) into the tail vein, once daily for 7 days. Rats in the model and sham groups were injected with an equal volume of saline.
Establishment of cerebral ischemia/reperfusion injury models
A model of cerebral ischemia/reperfusion injury was established using a modification of the method described by Longa et al. (1989). In brief, rats were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg; Shanghai First Pharmaceutical Factory, Shanghai, China) and fixed in the supine position on an operating table. The left common carotid artery was exposed through a midline neck incision, and the external carotid artery and branches were carefully separated from the adjacent tissue by electrocoagulation. A 5-0 silk suture was tied loosely around the mobilized external carotid artery stump. A 5-cm length of 4-0 monofilament nylon suture was then inserted through the incision in the external carotid artery into the internal carotid artery, slowly advanced to the anterior cerebral artery, and tightened to prevent bleeding. The suture was positioned 20–21 mm from the origin of the internal carotid artery, occluding blood flow from the middle cerebral artery and collateral blood flow from the internal carotid, anterior cerebral, and posterior cerebral arteries. This resulted in focal ischemia in the middle cerebral artery. The incisions were then closed, with the nylon suture raised to the level of the incision, and rats were returned to their home cages. After 3 hours of middle cerebral artery occlusion, and without additional anesthesia, the nylon suture was withdrawn until resistance was felt. The suture tip was returned to the stump of the external carotid artery and reperfusion then started in the ischemic brain area for a further 3 hours. Sham-operated rats underwent identical surgical procedures but without carotid occlusion. Respiratory rhythm was monitored during surgery and rectal temperature was maintained at 37 ± 0.5°C under a heat lamp.
Neurological evaluation
Three hours after reperfusion, rats were scored using a 5-point evaluation method (Longa et al., 1989): 0, no neurological symptoms; 1, inability to extend the forepaw fully; 2, circling to the right when walking; 3, falling towards the right side; 4, inability to walk spontaneously, or loss of consciousness.
Determination of infarct volume
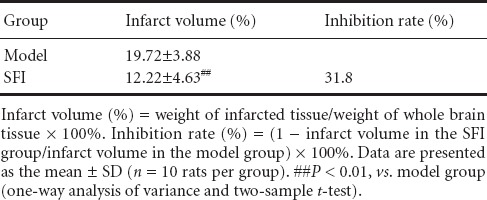
Three hours after reperfusion, rats in the model and SFI groups were killed by decapitation, and the olfactory bulb, cerebellum and lower brainstem were removed. The remaining brain tissue was sliced into six coronal sections of equal thickness using a double-sided blade. The sections were stained with 1% TTC solution (Shanghai Chemical Reagent Factory, Shanghai, China), incubated in a water bath at 37°C for 30 minutes, and fixed in 10% formaldehyde. Whole and infarcted brain tissue were weighed using a TG3238 photoelectric analytical balance (Shanghai Balance Instrument Factory, Shanghai, China), and infarct volume and inhibition rate were calculated according to the following formulae: infarct volume (%) = weight of infarcted tissue/weight of whole brain × 100%; inhibition rate (%) = (1 − infarct volume in intervention group/infarct volume in model group) × 100%.
Determination of brain water content and Ca2+ level
Brain water content and Ca2+ level were measured using the method described by Gotoh et al. (1985). Briefly, 3 hours after the start of reperfusion, rat brains were quickly harvested, and the anterior hemisphere was obtained from the infarcted side. The wet tissue was weighed, then dried in an oven for 48 hours at 105°C, and weighed again. Brain water content (%) = [(wet weight − dry weight)/wet weight] × 100%.
Brain tissue was digested in concentrated sulfuric acid and mixed with a deionized solution of 1% lauramidopropylamine oxide and 0.5 M HCl. Brain Ca2+ level was detected using a 721-UV-visible spectral luminance meter (Shanghai Third Analytical Instrument Factory, Shanghai, China) according to the atomic absorption spectrometry method (Wu, 2009).
Determination of serum lactate dehydrogenase (LDH) and creatine kinase (CK) activities
Six hours after the start of reperfusion, orbital blood samples were harvested and the serum was separated. Serum LDH activity at λ440 nm was detected using an AU640 biochemical analyzer (Olympus, Shizuoka, Japan) according to a colorimetry method described previously (Gui and Zhou, 2003). CK activity at λ520 nm was measured by creatine chromogenic assay (Ren et al., 2009).
Determination of superoxide dismutase (SOD) activity and malondialdehyde (MDA) content in the rat brain
Three hours after the start of reperfusion, the posterior hemisphere on the infarction side was harvested, and the tissue was rinsed with ice-cold saline, patted dry, and weighed. The tissue was then added to nine volumes of ice-cold saline and centrifuged at 1,500 r/min for 20 minutes. The supernatant was collected and MDA content was measured at λ532 nm by TBA assay (Wei, 1984). SOD activity was determined by the pyrogallol autoxidation method (Xu et al., 2006).
Ultrastructural observation
One rat from each group was used to observe the tissue ultrastructure at the infarct site. The animals were perfused intracardially with cold saline followed by 4% paraformaldehyde, and the brain was dissected out and cut into 2 mm thick sections. The fourth section at the infarct site, containing part of the parietal and frontal cortices, was collected and cut into ultrathin (50 nm) sections. The sections were prepared as previously described (Wu et al., 2011) and observed under an electron microscope (Shanghai Optical Instrument Factory, Shanghai, China).
Statistical analysis
Data are presented as the mean ± SD. Measurement data between groups were compared using one-way analysis of variance and the two-sample t-test. Statistical analysis was performed using SAS 9.1.3 software (SAS Institute, Inc., Cary, NC, USA). P < 0.05 was considered statistically significant.
Results
SFI pretreatment improved movement behaviors in aged rats with cerebral ischemia/reperfusion injury
Sham-operated rats had normal neurological function. In the model group, rats had dyskinesias such as right forelimb adduction, internal rotation contracture of the shoulder, and right forelimb adhering to the chest when lifting the tail. However, rats in the SFI group showed significantly better movement and had lower Longa behavior scores than those in the model group (P < 0.01; Figure 1).
Figure 1.
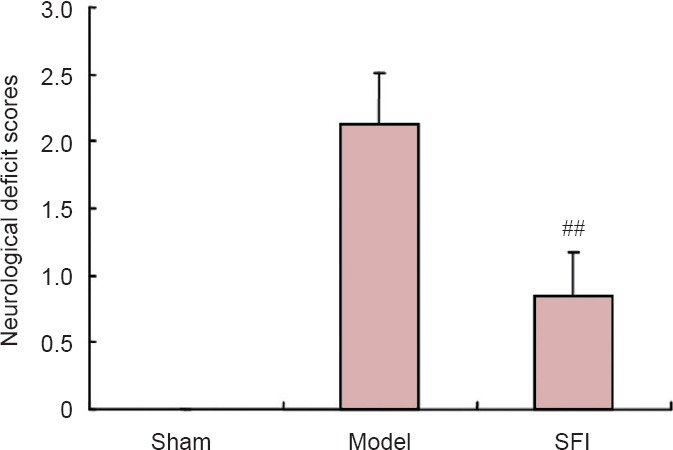
Effect of Shenqi Fuzheng injection (SFI) on behavioral deficits in aged rats after cerebral ischemia/reperfusion injury.
Higher Longa scores indicate greater impairment. Data are expressed as the mean ± SD (n = 10 per group). ##P < 0.01, vs. model group (one-way analysis of variance and two-sample t-test).
SFI pretreatment reduced infarct volume in aged rats with cerebral ischemia/reperfusion injury
Compared with the model group, infarct volume was significantly smaller when SFI was administered before ischemia and reperfusion (P < 0.01; Figure 2, Table 1).
Figure 2.
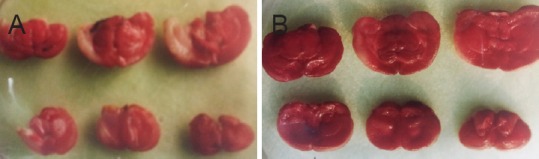
Infarct area shown by TTC staining of coronal sections.
(A) Model group; (B) Shenqi Fuzheng injection group. Red staining, normal tissue; white (unstained) areas, infarcted tissue.
Table 1.
Effect of Shenqi Fuzheng injection (SFI) on infarct volume in aged rats with cerebral ischemia/reperfusion injury
SFI pretreatment reduced brain water content in aged rats with cerebral ischemia/reperfusion injury
Brain water content was significantly greater in the model group than in the sham group (P < 0.01). However, in rats that received SFI pretreatment, water content was lower than in model rats (P < 0.05; Figure 3).
Figure 3.
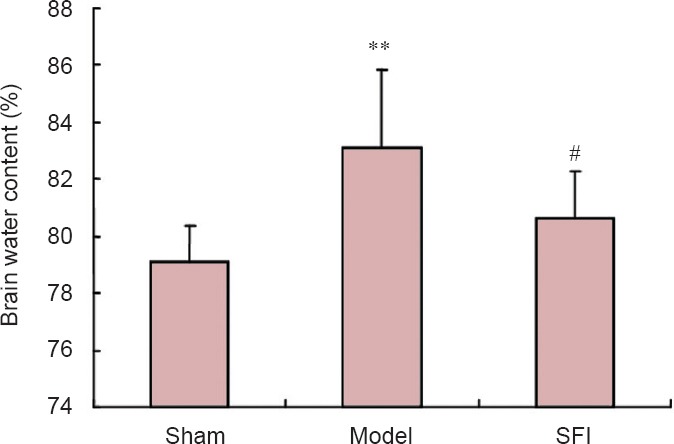
Effect of Shenqi Fuzheng injection (SFI) on brain water content in aged rats with cerebral ischemia/reperfusion injury.
Brain water content (%) = [(wet weight − dry weight)/wet weight] × 100%. Data are presented as the mean ± SD (n = 10 rats per group). Measurement data were compared between groups using one-way analysis of variance and two-sample t-test. **P < 0.01, vs. sham group; #P < 0.05, vs. model group.
SFI pretreatment decreased brain Ca2+ level in aged rats with cerebral ischemia/reperfusion injury
Levels of brain Ca2+ were significantly greater in the model group than in the sham group (P < 0.01). However, rats that underwent SFI pretreatment had significantly less brain Ca2+ than those in the model group (P < 0.01), with levels very similar to those measured in the sham group (Figure 4).
Figure 4.
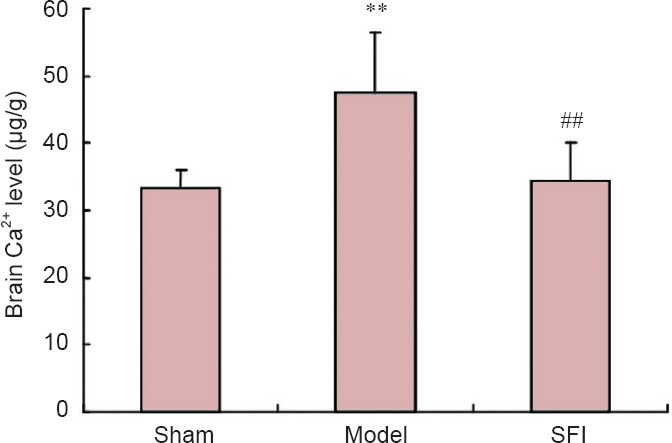
Effect of Shenqi Fuzheng injection (SFI) on brain Ca2+ level in aged rats with cerebral ischemia/reperfusion injury.
Data are presented as the mean ± SD (n = 10 rats per group). **P < 0.01, vs. sham group; ##P < 0.01, vs. model group (one-way analysis of variance and two-sample t-test).
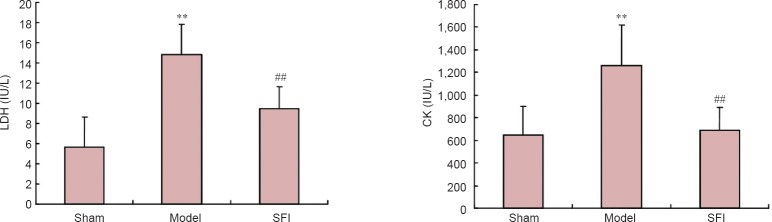
SFI pretreatment decreased serum LDH and CK activities in aged rats with cerebral ischemia/reperfusion injury
Serum LDH and CK activities were significantly greater in the model group than in the sham group (P < 0.01), but significantly lower after SFI pretreatment compared with levels observed in the model group (P < 0.01; Figure 5).
Figure 5.
Effect of Shenqi Fuzheng injection (SFI) on serum lactate dehydrogenase (LDH; left) and creatine kinase (CK; right) activities in aged rats with cerebral ischemia/reperfusion injury.
Data are presented as the mean ± SD (n = 10 rats per group). **P < 0.01, vs. sham group; ##P < 0.01, vs. model group (one-way analysis of variance and two-sample t-test).
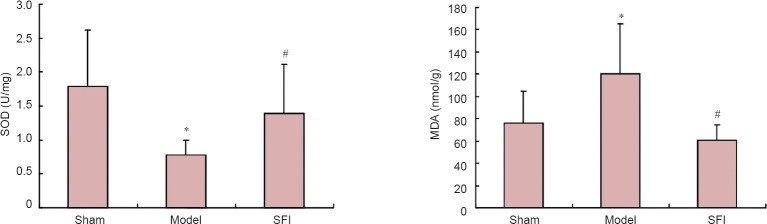
Effect of SFI pretreatment on brain SOD activity and MDA content in aged rats with cerebral ischemia/reperfusion injury
SOD activity in rat brain was significantly lower, and MDA content significantly greater, in the model group than in the sham group (P < 0.01). However, SFI pretreatment prevented these changes, resulting in significantly greater SOD activity and lower MDA content in brain tissue in the intervention group than in the model group (P < 0.05; Figure 6).
Figure 6.
Effect of Shenqi Fuzheng injection (SFI) on brain superoxide dismutase (SOD) activity (left) and malondialdehyde (MDA) content (right) in aged rats with cerebral ischemia/reperfusion injury.
Data are presented as the mean ± SD (n = 10 rats per group). *P < 0.01, vs. sham group; #P < 0.05, vs. model group (one-way analysis of variance and two-sample t-test).
SFI pretreatment improved the ultrastructure of brain tissue in aged rats with cerebral ischemia/reperfusion injury
The sham-operated rat brains showed normal neurons with mild condensation of neuronal nuclear chromatin. Organelles were abundant, with mitochondria, rough endoplasmic reticulum and ribosomes clearly visible. Mitochondrial morphology was normal, well encapsulated and with dense cristae. In the model group, there was considerable neuronal injury. Organelles were damaged and dissolved, and a large degree of cell swelling was observed. Mitochondria were also swollen, cristae had disappeared, and vacuoles, peripheral nucleoplasm and dilated endoplasmic reticulum were evident. However, in the SFI group, the damage was noticeably attenuated. Neuronal membranes were intact and tortuous, chromatin aggregation was visible, mitochondria were mildly swollen with partial cristae, and the nuclear membrane was intact. Glial cells in the sham-operated rats were normal, whereas in the model group they were shrunken and showed nuclear chromatin aggregation, homogeneous cytoplasmic changes, and noticeably fewer organelles. In the SFI group, however, glial cells were normal, without any visible damage. Furthermore, capillaries in the model group were thinner than in the sham-operated group, and the vascular wall was thickened, with an unclear boundary; after SFI intervention, capillary damage was noticeably prevented (Figure 7).
Figure 7.

Effect of Shenqi Fuzheng injection (SFI) on the ultrastructure of brain tissue in aged rats with cerebral ischemia/ reperfusion injury (transmission electron microscopy, × 6,000).
Sham group: Normal morphology of neurons (red arrows), glial cells (yellow arrows), blood capillaries (green arrows) and neuronal organelles. Model group: Neurons (red arrows) were visibly damaged, glial cells (yellow arrows) condensed, and organelles had disappeared; capillaries (green arrows) were thinner and the vascular wall was thickened. SFI group: Structure and morphology of neurons (red arrows), glial cells (yellow arrows), and capillaries (green arrows) were noticeably more normal than in the model group.
Discussion
Rat models are widely used in brain research owing to the similarity between rat and human brain (Bernstein et al., 2011). Blood flow and metabolism in the human brain slow down with age, meaning that older people are more susceptible to cerebral ischemia/reperfusion injury than younger people (Henning et al., 2010). It is therefore necessary to model such conditions in aged rats.
SFI is a Chinese materia medica preparation of Radix Astragali and Radix Codonopsis. Radix Codonopsis is known to invigorate the spleen, improve vital energy, nourish the blood and produce blood fluid; furthermore, it improves memory in mice (Wang et al., 2013). Radix Astragali also functions to invigorate the spleen and improve vital energy. Modern pharmacological studies have found that both Radix Astragali and Radix Codonopsis dilate blood vessels, lower blood pressure, and enhance body immunity, in addition to activating and restoring erythrocyte deformability and improving the microcirculation (Gu et al., 2009).
To identify the mechanism underlying the protective effects of SFI intervention, we measured various cerebral ischemia/reperfusion injury-related indicators. The first was brain water content, as brain edema is an inevitable consequence of cerebral ischemia/reperfusion (Pu et al., 1999; Zhao et al., 2013; Rakhunde et al., 2014; Saad et al., 2015) and significantly affects morbidity and mortality. After cerebral ischemia and hypoxia, brain cells show energy metabolism disorders which result in noticeable retention of intracellular Na+ and water, damage to the cell membrane, and abnormally high intracellular fluids (Zhao et al., 2014). At the same time, free radicals are generated after hypoxia, leading to vascular epithelium lipid peroxidation and blood-brain barrier damage, increasing vascular permeability, and inducing plasma leakage into the extracellular space, ultimately causing cerebral edema (Liu et al., 2014). The results from the present study indicate that SFI inhibits swelling and water accumulation in the infarcted hemisphere.
After cerebral ischemia/reperfusion injury, hypoxia causes lactic acidosis, and the permeability of the cell membrane and capillaries is increased, leading to membrane damage and massive protease release (Bederson et al., 1986; Deng et al., 2002; Liu et al., 2013; Xia et al., 2013). Intracellular LDH and brain-type CK are released into the extracellular fluid, then enter the cerebrospinal fluid and blood. As a result, the activities of LDH and CK become reduced in the brain, and increased in the serum. Increased serum LDH and CK activities correlate with the severity and extent of damage (Zhang et al., 2008; Zheng et al., 2010; Austin et al., 2011; Maslov and Lishmanov, 2012; Joo et al., 2013). Previous studies revealed that SFI inhibited the decline of LDH and CK activities in aged rats after cerebral ischemia and reperfusion, indicating that SFI can stabilize the cell membrane. Generation of oxygen free radicals and lipid peroxidation are the important mechanisms in ischemia/reperfusion injury (Wang et al., 2008; Park et al., 2009; Sitailo et al., 2009). Following cerebral ischemia and reperfusion, an overload of intracellular calcium occurs in addition to a large increase in free fatty acids and excitatory amino acids. This activates a chain reaction producing a large number of free radicals. MDA is the end product of lipid peroxidation by free radicals, principally affecting unsaturated fatty acids, and as such it is an indirect indicator of oxygen radicals in tissue (Maksimovich et al., 2014; Song et al., 2014; Wan et al., 2014). In the present study, 3 hours after reperfusion, SOD activity in the brain tissue was significantly decreased while MDA content was significantly elevated. This indirectly confirmed the generation of oxygen free radicals and lipid peroxidation upon ischemia/reperfusion. Our results indicate that, when administered prior to an ischemic event, SFI increases SOD activity in brain tissue, scavenges free radicals, attenuates lipid peroxidation, and reduces MDA content. Our finding supports previous studies, which found that Radix Astragali increases SOD activity and attenuates free radical damage (Wang et al., 2004; Sun et al., 2014, 2015; Kim et al., 2015; Li et al., 2015; Mohammadi and Dehghani, 2015; Yue et al., 2015).
In summary, SFI pretreatment is neuroprotective in rats with focal cerebral ischemia/reperfusion injury, and the protective mechanism may be related to the inhibition of brain Ca2+ accumulation and prevention of lipid peroxidation. Our results open up a new avenue for the application of SFI in the treatment of cerebrovascular disease.
Footnotes
Funding: This study was supported by a grant from Key Technology Research and Development Program of Shaanxi Province of China, No. 2003K10-G86; the National Natural Science Foundation of China, No. 30070731.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Slone-Murphy, Raye W, Yu J, Yang Y, Song LP, Zhao M
References
- Austin EW, Yousif NG, Li J, Ao L, Reece T, Weyant MJ, Cleveland JC, Fullerton DA, Meng X. Ghrelin reduces myocardial injury following global hypothermic ischemia/reperfusion via suppression of the myocardial inflammatory response. J Surg Res. 2011;165:192. [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Benderro GF, LaManna JC. Kidney EPO expression during chronic hypoxia in aged mice. Adv Exp Med Biol. 2013;765:9–14. doi: 10.1007/978-1-4614-4989-8_2. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Derst C, Stich C, Prüss H, Peters D, Krauss M, Bogerts B, Veh RW, Laube G. The agmatine-degrading enzyme agmatinase: a key to agmatine signaling in rat and human brain. Amino Acids. 2011;40:453–65. doi: 10.1007/s00726-010-0657-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Tian M, Jin L, Jia H, Jin Y. PUMA is invovled in ischemia/reperfusion-induced apoptosis of mouse cerebral astrocytes. Neuroscience. 2015;284:824–832. doi: 10.1016/j.neuroscience.2014.10.059. [DOI] [PubMed] [Google Scholar]
- Dai Z, Wan X, Kang H, Ji Z, Liu L, Liu X, Song L, Min W, Ma X. Clinical effects of shenqi fuzheng injection in the neoadjuvant chemotherapy for local advanced breast cancer and the effects on T-lymphocyte subsets. J Tradit Chin Med. 2008;28:34–38. doi: 10.1016/s0254-6272(08)60010-2. [DOI] [PubMed] [Google Scholar]
- Deng J, Zhou HD, Zhang LL, Shen C. The effect of Bcl-2 protection on neuronal DNA damage after cerebral ischemia and reperfusion. Zhongguo Linchuang Kangfu. 2002;6:2216–2217. [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh O, Asano T, Koide T, Takakura K. Ischemic brain edema following occlusion of the middle cerebral artery in the rat. I: The time courses of the brain water, sodium and potassium contents and blood-brain barrier permeability to 125I-albumin. Stroke. 1985;16:101–109. doi: 10.1161/01.str.16.1.101. [DOI] [PubMed] [Google Scholar]
- Gu N, Sang HF, Sun YY, Xiong LZ. Shenqi Fuzheng injection delay the neuropathic pain in CCI rats. Disi Junyi Daxue Xuebao. 2009;30:865–868. [Google Scholar]
- Gui SY, Zhou YQ. Determination of Ginsenosides in Panax Ginseng by colorimetric method. Anhui Zhongyi Xueyuan Xuebao. 2003;22:51–54. [Google Scholar]
- Hailer NP. Immunosuppression after traumatic or ischemic CNS damage: It is neuroprotective and illuminates the role of microglial cells. Prog Neurobiol. 2008;84:211–233. doi: 10.1016/j.pneurobio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Henning EC, Warach S, Spatz M. Hypertension-induced vascular remodeling contributes to reduced cerebral perfusion and the development of spontaneous stroke in aged SHRSP rats. J Cereb Blood Flow Metab. 2010;30(4):827–36. doi: 10.1038/jcbfm.2009.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XP, Qiu YY, Wang B, Ding H, Tang YH, Zeng R, Deng CQ. Effects of Astragaloside IV combined with the active components of Panax notoginseng on oxidative stress injury and nuclear factor-erythroid 2-related factor 2/heme oxygenase-1 signaling pathway after cerebral ischemia-reperfusion in mice. Pharmacogn Mag. 2014;10:402–409. doi: 10.4103/0973-1296.141765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo SP, Xie W, Xiong X, Xu B, Zhao H. Ischemic postconditioning protects against focal cerebral ischemia by inhibiting brain inflammation while attenuating peripheral lymphopenia in mice. Neuroscience. 2013;243:149–157. doi: 10.1016/j.neuroscience.2013.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Cho JH, Cho JH, Park JH, Ahn JH, Tae HJ, Cho GS, Yan BC, Hwang IK, Lee CH, Bae EJ, Won MH, Lee JC. Impact of hyperthermia before and during ischemia-reperfusion on neuronal damage and gliosis in the gerbil hippocampus induced by transient cerebral ischemia. J Neurol Sci. 2015;348:101–110. doi: 10.1016/j.jns.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Li L, Zhu K, Liu Y, Wu X, Wu J, Zhao Y, Zhao J. Targeting thioredoxin-1 with siRNA exacerbates oxidative stress injury after cerebral ischemia/reperfusion in rats. Neuroscience. 2015;284:815–823. doi: 10.1016/j.neuroscience.2014.10.066. [DOI] [PubMed] [Google Scholar]
- Li ZY, Liu B, Yu J, Yang FW, Luo YN, Ge PF. Ischaemic postconditioning rescues brain injury caused by focal ischaemia/reperfusion via attenuation of protein oxidization. J Int Med Res. 2012;40:954–966. doi: 10.1177/147323001204000314. [DOI] [PubMed] [Google Scholar]
- Lin XJ, Hu YX, Hu CL. Progress of vascular dementia. Zhongguo Linchuang Kangfu. 2002;6:978–979. [Google Scholar]
- Liu G, Song J, Guo Y, Wang T, Zhou Z. Astragalus injection protects cerebral ischemic injury by inhibiting neuronal apoptosis and the expression of JNK3 after cerebral ischemia reperfusion in rats. Behav Brain Funct. 2013;9:36. doi: 10.1186/1744-9081-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang X, Zhang J, Kang N, Zhang N, Wang H, Xue J, Yu J, Yang Y, Cui H, Cui L, Wang L, Wang X. Diosmin protects against cerebral ischemia/reperfusion injury through activating JAK2/STAT3 signal pathway in mice. Neuroscience. 2014;268:318–327. doi: 10.1016/j.neuroscience.2014.03.032. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Maksimovich NY, Dremza IK, Troyan EI, Maksimovich YN, Borodinskii AN. The correcting effects of dihydroquercetin in cerebral ischemia-reperfusion injury. Biomed Khim. 2014;60:643–650. doi: 10.18097/pbmc20146006643. [DOI] [PubMed] [Google Scholar]
- Maslov LN, Lishmanov IuB. Neuroprotective effect of ischemic postconditioning and remote preconditioning. Prospective of clinical use. Angiol Sosud Khir. 2012;18:27–34. [PubMed] [Google Scholar]
- Mohammadi MT, Dehghani GA. Nitric oxide as a regulatory factor for aquaporin-1 and 4 gene expression following brain ischemia/reperfusion injury in rat. Pathol Res Pract. 2015;211:43–49. doi: 10.1016/j.prp.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Suzuki Y, Takagi T, Kitashoji A, Ono Y, Tsuruma K, Yoshimura S, Shimazawa M, Iwama T, Hara H. Glycoprotein nonmetastatic melanoma protein B (GPNMB) as a novel neuroprotective factor in cerebral ischemia-reperfusion injury. Neuroscience. 2014;277:123–131. doi: 10.1016/j.neuroscience.2014.06.065. [DOI] [PubMed] [Google Scholar]
- Park SE, Park C, Kim SH, Hossain MA, Kim MY, Chung HY, Son WS, Kim G-Y, Choi YH, Kim ND. Korean red ginseng extract induces apoptosis and decreases telomerase activity in human leukemia cells. J Ethnopharmacol. 2009;121:304–312. doi: 10.1016/j.jep.2008.10.038. [DOI] [PubMed] [Google Scholar]
- Patel FJ, Volkmann DT, Taylor GW, Hansson MA, Anderson JF, Zhou Y, Scoazec LM, Hartford CV, Hainz DL. IL-37 reduces inflammatory response after cerebral ischemia and reperfusion injury through down-regulation of pro-inflammatory cytokines. Cytokine. 2014;69:234–239. doi: 10.1016/j.cyto.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Pu CQ, Lang SY, Wu WP. Cerebral Vascular Disease. Beijing: People's Military Medcial Press; 1999. [Google Scholar]
- Rakhunde PB, Saher S, Ali SA. Neuroprotective effect of Feronia limonia on ischemia reperfusion induced brain injury in rats. Indian J Pharmacol. 2014;46:617–621. doi: 10.4103/0253-7613.144920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ji XJ, Sun SW, Nie ZK, Du J, Huang H. Rapid determination of acetoin level in fermentation broth by creatine colorimetric method. Shipin Keji. 2009;34:260–262. [Google Scholar]
- Saad MA, Abdel Salam RM, Kenawy SA, Attia AS. Pinocembrin attenuates hippocampal inflammation, oxidative perturbations and apoptosis in a rat model of global cerebral ischemia, reperfusion. Pharmacol Rep. 2015;67:115–122. doi: 10.1016/j.pharep.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Shi XY. Medical Experimental Zoology. Beijing: People's Military Medical Press; 1999. [Google Scholar]
- Sherif C, Wambacher B, Loyoddin M, Karaic R, Krafft P, Valentin A, Tscholakoff D, Kleinpeter G. Repeated combined endovascular therapy with milrinone and nimodipine for the treatment of severe vasospasm: preliminary results. Acta Neurochir Suppl. 2015;120:203–207. doi: 10.1007/978-3-319-04981-6_35. [DOI] [PubMed] [Google Scholar]
- Sitailo LA, Jerome-Morais A, Denning MF. Mcl-1 functions as major epidermal survival protein required for proper keratinocyte differentiation. J Invest Dermatol. 2009;129:1351–1360. doi: 10.1038/jid.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T, Liu J, Tao X, Deng JG. Protection effect of atorvastatin in cerebral ischemia-reperfusion injury rats by blocking the mitochondrial permeability transition pore. Genet Mol Res. 2014;13:10632–10642. doi: 10.4238/2014.December.18.5. [DOI] [PubMed] [Google Scholar]
- Sun C, Lai X, Huang X, Zeng Y. Protective effects of ginsenoside Rg1 on astrocytes and cerebral ischemic-reperfusion mice. Biol Pharm Bull. 2014;37:1891–1898. doi: 10.1248/bpb.b14-00394. [DOI] [PubMed] [Google Scholar]
- Sun M, Li M, Huang Q, Han F, Gu JH, Xie J, Han R, Qin ZH, Zhou Z. Ischemia/reperfusion-induced upregulation of TIGAR in brain is mediated by SP1 and modulated by ROS and hormones involved in glucose metabolism. Neurochem Int. 2015;80:99–109. doi: 10.1016/j.neuint.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Vaahersalo J, Skrifvars MB, Pulkki K, Stridsberg M, Røsjø H, Hovilehto S, Tiainen M, Varpula T, Pettilä V, Ruokonen E FINNRESUSCI Laboratory Study Group. Admission interleukin-6 is associated with post resuscitation organ dysfunction and predicts long-term neurological outcome after out-of-hospital ventricular fibrillation. Resuscitation. 2014;85:1573–1579. doi: 10.1016/j.resuscitation.2014.08.036. [DOI] [PubMed] [Google Scholar]
- Wan Q, Pan P, Xu C, Li W. Effects of electroacupuncture preconditioning on jugular vein glucose level and cerebral edema in rats undergoing cerebral ischemia reperfusion that induced injury. Int J Clin Exp Med. 2014;7:4384–4388. [PMC free article] [PubMed] [Google Scholar]
- Wang FW, Jiang N, Tong ZQ, Wang ZK, Tian JH, Liu Y, Yin DY. Clinical study on shenlong tang for improving neurological impairment in patients with vascular dementia. Zhongguo Linchuang Kangfu. 2004;8:679–681. [Google Scholar]
- Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, Liang HW, Bruce IC, Luo BY, Xia Q. Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke. 2008;39:983–990. doi: 10.1161/STROKEAHA.107.499079. [DOI] [PubMed] [Google Scholar]
- Wang W, Tang L, Li Y, Wang Y. Biochanin A protects against focal cerebral ischemia/reperfusion in rats via inhibition of p38-mediated inflammatory responses. J Neurol Sci. 2015;348:121–125. doi: 10.1016/j.jns.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Zhong L, Wen DJ. The protection of shenqi fuzheng injection for the lesion of myocardial ischemia/reperfusion. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2013;29:409–410. 415. [PubMed] [Google Scholar]
- Wei MJ. Determination of peroxide expression in plasma lipid and its clinical significance. Guowai Yixue: Linchuang Shengwu Huaxue yu Jianyan Xue Fence; 1984. pp. 6–8. [Google Scholar]
- Wu HJ, Zhang SM, Zhuang BX. Key process of preparing ultrathin sections. Jiepou Xue Zazhi. 2011:133–134. [Google Scholar]
- Wu YH. Application of environmental analysis of atomic absorotion spectrometry in environmental analysis. Neimenggu Huanjing Kexue. 2009;21:94–95. [Google Scholar]
- Xia DY, Li W, Qian HR, Yao S, Liu JG, Qi XK. Ischemia preconditioning is neuroprotective in a rat cerebral ischemic injury model through autophagy activation and apoptosis inhibition. Braz J Med Biol Res. 2013;46:580–588. doi: 10.1590/1414-431X20133161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SY, Chen X, Bian RL. Methodology of Pharmacological Experiment. Beijing: People's Medical Publishing House; 2001. [Google Scholar]
- Xu YJ, Zhao YJ, Hu H. Research on the measurement of the SOD activity via pyrogallol auto-oxidation. Xinan Minzu Daxue Xuebao: Ziran Kexue Ban. 2006;32:1207–1209. [Google Scholar]
- Yuan Y, Guo Q, Ye Z, Pingping X, Wang N, Song Z. Ischemic postconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PI3K-Akt pathway. Brain Res. 2011;1367:85–93. doi: 10.1016/j.brainres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Yue ZY, Dong H, Wang YF, Liu Y, Song CY, Yang WC, Qian H, Lu SJ, Chang FF. Propofol prevents neuronal mtDNA deletion and cerebral damage due to ischemia/reperfusion injury in rats. Brain Res. 2015;1594:108–114. doi: 10.1016/j.brainres.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Fan XJ, Li X, Peng LL, Wang GH, Ke KF, Jiang ZL. Ginsenoside Rg1 protects neurons from hypoxic–ischemic injury possibly by inhibiting Ca 2+ influx through NMDA receptors and L-type voltage-dependent Ca 2+ channels. Eur J Pharmacol. 2008;586:90–99. doi: 10.1016/j.ejphar.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Zhao H, Luo Y, Liu X, Wang R, Yan F, Liu X, Li S, Leak RK, Ji X. Ischemic post-conditioning partially reverses cell cycle reactivity following ischemia/reperfusion injury: a genome-wide survey. CNS Neurol Disord Drug Targets. 2013;12:350–359. doi: 10.2174/1871527311312030008. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wang R, Tao Z, Gao L, Yan F, Gao Z, Liu X, Ji X, Luo Y. Ischemic postconditioning relieves cerebral ischemia and reperfusion injury through activating T-LAK cell-originated protein kinase/protein kinase B pathway in rats. Stroke. 2014;45:2417–2424. doi: 10.1161/STROKEAHA.114.006135. [DOI] [PubMed] [Google Scholar]
- Zheng GQ, Wang XM, Wang Y, Wang XT. Tau as a potential novel therapeutic target in ischemic stroke. J Cell Biochem. 2010;109:26–29. doi: 10.1002/jcb.22408. [DOI] [PubMed] [Google Scholar]