Abstract
A replicated chromosome possesses two discrete, complex, dynamic, macromolecular assemblies, known as kinetochores, that are positioned on opposite sides of the primary constriction of the chromosome. Here, the authors review how kinetochores control chromosome segregation during mitosis in vertebrates. They attach the chromosome to the opposing spindle poles by trapping the dynamic plus-ends of microtubules growing from the poles. They then produce much of the force for chromosome poleward motion, regulate when this force is applied, and act as a site for microtubule assembly and disassembly. Finally, they control the metaphase–anaphase transition by inhibiting chromatid separation until the chromatids are properly attached.
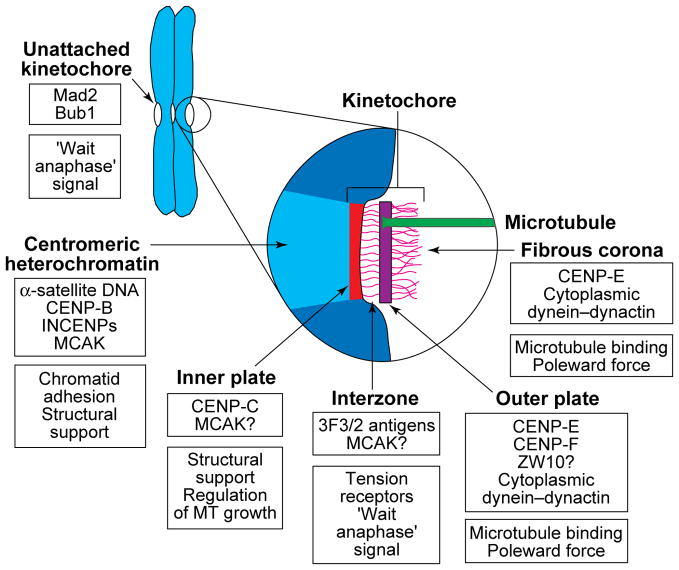
In electron micrographs of sections cut from conventionally fixed cells, the vertebrate kinetochore appears as a disk-shaped structure that can be differentiated into four domains (Fig. 1). An outer plate of thickness 35–40 nm is separated by an electron-lucent central zone, 15–35 nm thick, from a less conspicuous inner plate associated with the surface of the centromeric heterochromatin (reviewed in Ref. 1). The outer plate comprises a dense but loosely organized meshwork of 10–20 nm fibres, and a filamentous ‘corona’ material extends ~0.1–0.3 μm away from this structure. A subset of dynamic spindle microtubule (MT) plus-ends, known as kinetochore MTs (K-MTs), terminate on or in this plate. Kinetochores contain MT motor proteins, including CENP-E and cytoplasmic dynein, that are involved in their attachment and motility. They also contain non-motor proteins, at least some of which (e.g. Mad2 and Bub1) are involved in regulating the metaphase–anaphase (M–A) transition (Fig. 1).
FIGURE 1.
A diagram depicting the putative location and possible function of reported kinetochore proteins. Sister kinetochores are located on opposite sides of the centromere region of replicated chromosomes, and the centromeric heterochromatin between them is rich in α-satellite DNA, its binding protein CENP-B62, and an inner centromere protein (INCENP)63 that might be involved in maintaining sister-chromatid cohesion. This region also appears to contain mitotic centromere-associated kinesin (MCAK)17, which has been shown in Xenopus extracts to be required for spindle formation and maintenance64. In electron micrographs of sections cut from conventionally fixed and stained preparations, the kinetochore region itself appears to consist of four structurally differentiated domains. The inner plate is closely associated with the centromeric heterochromatin and contains CENP-C, the presence of which is required for the maintenance of a functional kinetochore65, and perhaps MCAK. The zone between the inner and outer plates (the interzone) appears to contain the 3F3/2 phosphoepitope, which has been proposed to control the metaphase–anaphase (M–A) transition by sensing tension47, and MCAK might also be located in this region. Kinetochore microtubule (MT) plus-ends attach to and terminate at various levels within the outer plate, which has been reported to contain CENP-F22 (also called mitosin), CENP-E18–20, ZW1023 and possibly cytoplasmic dynein and its associated dynactin complex16. The fibrous corona extends from the outer plate and is apparent only on unattached kinetochores. It contains CENP-E20, ZW1023 and cytoplasmic dynein (reviewed in Ref. 16), the latter possibly being involved in MT attachment and poleward force production. Unattached (but not fully attached) kinetochores also contain Mad224 and Bub125 proteins, which are known to play important roles in regulating the M–A transition. A question mark indicates that the location of a protein is inferred from immunofluorescence microscopy rather than determined by immunoelectron microscopy.
Each vertebrate kinetochore contains multiple functional subunits, which have the complete molecular machinery for kinetochore function (reviewed in Ref. 2). Although the structural basis of these units is unknown, each might define a MT-binding site. Budding yeast kinetochores bind to a single MT yet, with the exception that the sister kinetochores do not become aligned on the spindle equator prior to anaphase, they exhibit motile behaviour largely similar to that of vertebrate kinetochores3. In vertebrates, the MT-binding capacity of sister kinetochores is similar and related to their diameter, which, although it can vary between chromosomes of a genome, correlates only weakly with chromosome size4. In most organisms, kinetochores range between 0.1–0.5 μm in diameter and bind 10–45 MTs1.
This article focuses on the roles kinetochores play during mitosis in vertebrate cells. We discuss how they attach chromosomes to the spindle and how they control chromosome positioning and the onset of anaphase. We also review new information on these topics, including how their structure and composition change with K-MT formation, and highlight areas requiring additional work.
Kinetochores attach sister chromatids to the opposing poles of the spindle
During spindle formation, one kinetochore on a chromosome becomes attached to and oriented towards (i.e. faces) one spindle pole, while the other becomes attached to and oriented towards the opposing pole (Fig. 2). This ‘bipolar’ attachment and orientation is crucial for the two chromatids to be segregated faithfully during the ensuing anaphase.
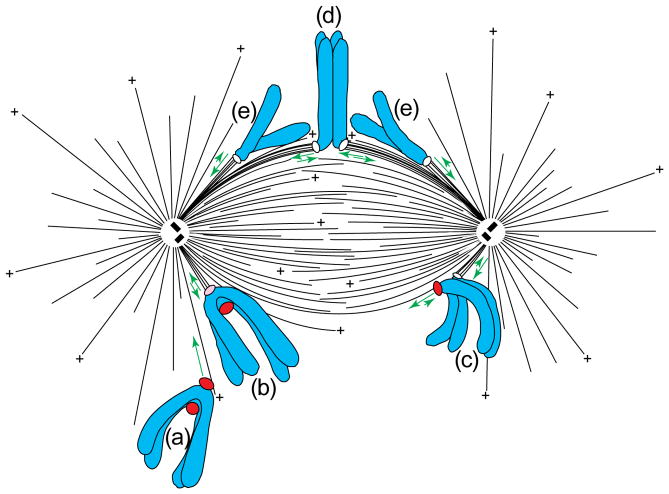
FIGURE 2.
Spindle structure and chromosome behaviour. The mitotic spindle in vertebrate cells comprises two overlapping arrays of polar microtubules (MTs), some of which have been released from the centrosome, and all of which are oriented with their ‘plus’ (+) ends distal and their minus-ends proximal to their poles. This diagram illustrates the typical sequence of events that chromosomes in vertebrate cells experience during mitosis. Initially, one kinetochore on the chromosome becomes attached to, and glides rapidly poleward (long arrow) along the surface of a single MT (a). During this poleward motion, the attaching kinetochore on the now mono-oriented chromosome acquires additional MTs that terminate in its outer plate (b). It then begins to oscillate between poleward and away from the pole states of motion (short double arrows) around a position between the pole and the spindle equator. When the unattached kinetochore on this chromosome encounters a MT growing from the distal pole, the chromosome moves to the spindle equator in a process known as congression (c). As a result of congression, the chromosome adopts an average position near the spindle equator around which it oscillates, and, over time, the sister kinetochores acquire similar numbers of MTs (d). During anaphase, the chromatids separate (e), and, although the single kinetochore on each still exhibits a modified form of directionally unstable behaviour, there is net movement of each towards their respective spindle poles. Unattached kinetochores (red label) stain strongly for proteins such as CENP-E and cytoplasmic dynein/dynactin, which are involved in attachment and movement, and Mad2, Bub1 and the 3F3/2 epitope, which are involved in the checkpoint controlling the metaphase–anaphase transition. Modified, with permission, from Ref. 5.
The steps by which a chromosome acquires a bipolar attachment and orientation are known (Fig. 2; reviewed in Ref. 5). As the two radial MT arrays defining the spindle poles separate, their MT plus-ends, which are directed away from the poles, grow and shorten in a dynamically unstable fashion. This provides an efficient ‘search and capture’ mechanism for kinetochore attachment6, which occurs when the wall or growing end of a MT contacts the kinetochore7 (Fig. 2a). However, because of the random nature of this mechanism, sister kinetochores rarely attach simultaneously. Instead, the one closest to and facing a pole during nuclear envelope breakdown usually attaches first, and, as it does, the chromosome initiates poleward motion towards the minus-end of the contacting MT. This motion orients the kinetochore towards the pole, and its velocity (25–55 μm min−1; Ref. 7) can approach that produced in vitro by the MT minus-end-directed motor cytoplasmic dynein. As the now mono-oriented chromosome moves poleward, its attaching kinetochore encounters and binds to more MT plus-ends, which it bundles into a kinetochore fibre (K-fibre; Fig. 2b). During this process, the poleward velocity of the chromosome slows to 1.5–2.5 μm min−1. Serial-section analyses reveal that the attached kinetochore on a mono-oriented chromosome does not acquire its full complement of K-MTs until its sister attaches and the chromosome moves to the spindle equator8. The reason for this remains unclear, but it does not appear to be related to tension9, which stabilizes K-MT attachment in insect spermatocytes10. It might, however, be due to the fact that, although the density of MTs is highest near poles, most of the MT plus-ends are located away from the kinetochore near the forming spindle equator (Fig. 2)11.
Ultimately, a MT growing from the distal pole contacts the unattached kinetochore on the mono-oriented chromosome and initiates formation of its K-fibre (Fig. 2c). This bi-orients the chromosome and leads to a process, termed congression, that aligns it on the spindle equator (Fig. 2d). During the early stages of spindle formation, sister kinetochores sometimes become attached to the same pole, or one kinetochore can become attached to both poles12. These mal-orientations are unstable and are usually corrected by an unknown mechanism before anaphase. One hypothesis is that the MTs on mal-oriented kinetochores are unstable because their ends experience abnormal tension or mechanical stress10,12.
Remarkably, K-MTs shorten and grow, as the kinetochore moves towards and away from the pole by MT subunit deletion and addition within the kinetochore outer plate (Fig. 3), and the K-MTs do so without frequently detaching13. Although the minus-ends of many MTs are released from the centrosome11, those polar MTs that become associated with a kinetochore appear to remain more stably attached to the pole14. As a result, the half-life of a K-MT (5–9 min) is much greater than that of spindle MTs with free plus-ends (0.3–1 min)15.
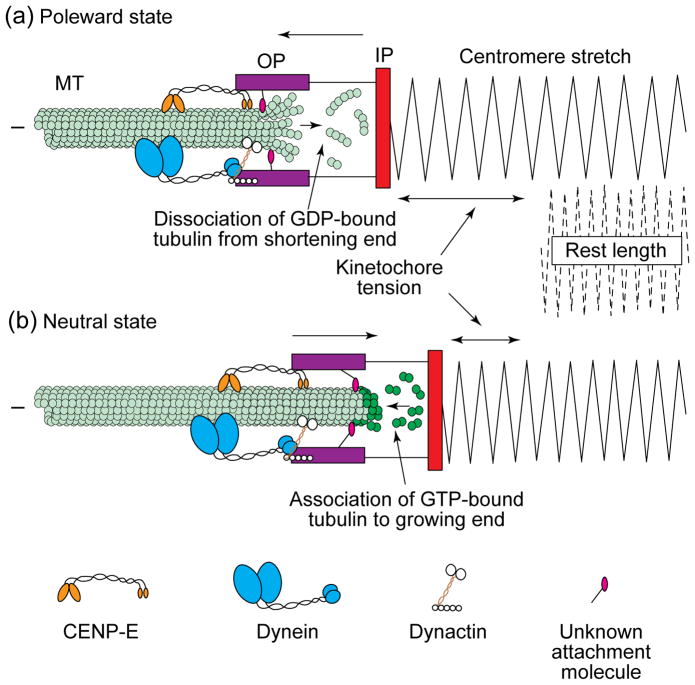
FIGURE 3.
Attached kinetochores switch abruptly between a state of constant-velocity poleward motion and a neutral state, which allows motion away from the pole also at a constant velocity. Both of these motility or activity states occur in association with the plus-ends of relatively stationary kinetochore microtubules (K-MTs). An attached kinetochore moves poleward at 1.5–2.5 μm min−1, and, during this time, it pulls on its associated MTs, which stretches the centromere region from its rest length (a). The force for poleward motion probably involves MT motors (e.g. dynein) located in the kinetochore corona or outer plate (see Fig. 1) and the dissociation of the terminal tubulin subunits on the MT plus-ends, which might splay during this process. These motors might also regulate velocity and maintain the MT–kinetochore attachment as the K-MT plus-ends shorten. Once the tension (stretch) on the kinetochore reaches a critical level, and/or when the kinetochore runs out of a crucial component involved in poleward force production, it switches into a neutral state (b) that allows it to be transported away from the pole. This motion away from the pole is associated with, and allowed by, the elongation of K-MTs. On a mono-oriented chromosome, it is powered probably by ejection forces, produced by MTs associated with the proximal spindle pole, that push the chromosome and its arms away from the pole. On a bi-oriented chromosome, motion away from the pole is thought to be powered by the proximal ejection force and also the poleward movement of the opposing sister kinetochore. During motion away from the pole, K-MTs elongate as GTP–tubulin subunits add to a stabilizing cap of GTP–tubulins at their growing plus-ends within the kinetochore. Beneath the cap, the GTP is hydrolysed to GDP in the MT lattice. Movement away from the pole reduces tension (stretch) on the centromere. A combination of active MT plus-end-directed motors, and/or an unknown attachment molecule, might maintain attachment to the growing ends of MTs and also regulate the velocity of motion away from the pole. Abbreviations: OP, kinetochore outer plate; IP, kinetochore inner plate.
We are beginning to understand how kinetochores bind and remain attached in a dynamic fashion to MT plus-ends. These processes probably involve several MT motor and accessory proteins*, including cytoplasmic dynein and its dynactin binding complex (reviewed in Ref. 16), as well as the kinesin-related proteins MCAK17 and CENP-E18–20. Two of these (the dynein and CENP-E) are located in the corona and/or outer plate (Fig. 1) and appear to form an ‘antenna complex’ of corona motor and non-motor proteins for capturing polar MT plus-ends and inserting them into binding sites in the outer plate. Overexpressing the 50-kDa subunit of dynactin16, or microinjecting antibodies against dynein into vertebrate cells21, inhibits chromosome congression but not necessarily kinetochore attachment. However, since a normal bipolar spindle fails to form under these conditions, and because chromosome behaviour has not been followed in vivo, the role of dynein in vertebrate kinetochore function remains ambiguous. To date, the best evidence that this motor is involved in kinetochore attachment comes from Tetrahymena (S. Lee, J. C. Wisniewski and D. J. Asai, pers. commun.). When the gene encoding cytoplasmic dynein is knocked out of this organism, chromosomes in the micronucleus fail to attach to the intranuclear mitotic apparatus and, as a result, become spread randomly along the spindle as it elongates during anaphase B. When CENP-E function is compromised in vertebrate cells by antibody injection or overexpressing a mutant protein, chromosomes form at least a monopolar attachment to the spindle18. However, even though the spindle appears to form normally, bi-orientation, and thus congression, is often inhibited. The roles of MCAK17 and the known kinetochore-associated nonmotor proteins (e.g. CENP-F22, ZW1023) are not clear, but they might serve important structural or regulatory functions to ensure proper chromosome bi-orientation.
Elucidating the molecular changes that occur during kinetochore attachment will be important for understanding how these organelles achieve their various functions. We know that the diameter of the outer plate decreases as the kinetochore binds to MTs and that its corona becomes less distinct1. During this time, the inner plate appears to form (or become more conspicuous) on the surface of the centromeric heterochromatin. While the attaching kinetochore acquires more MTs, the concentrations of corona-associated dynein–dynactin, CENP-E and ZW10 appear to decrease and become redistributed into the K-fibre16,20,23. As discussed below, these changes in motor concentration with K-MT formation might bias bi-oriented chromosome congression to the spindle equator. Also, the concentration of other kinetochore proteins involved in regulating the M–A transition decreases with attachment24,25, and it remains to be determined how the activity and concentration of these proteins relate to those proteins responsible for attachment and motion.
Kinetochores generate most of the force for poleward chromosome movement
There is now little doubt that kinetochores produce most of the force for chromosome poleward motion. The high (25–55 μm min−1) poleward velocity of attaching kinetochores7 implicates a fast kinetochore-associated MT minus-end-directed motor in this motion, and such a motor, cytoplasmic dynein, is present on kinetochores throughout mitosis. CENP-E has also been reported to be a fast MT minus-end-directed motor26, but, when expressed in bacteria, the motor domains of this molecule exhibit a slow (2–4 μm min−1) MT plus-end-directed motility19. Thus, although CENP-E is clearly necessary for normal kinetochore function18, in view of the conflicting data its role remains unclear.
After the initial attachment, kinetochore poleward velocity slows to 1.5–2.5 μm min−1. Photoactivation studies reveal that tubulin subunits are added continuously to K-MT plus-ends within the kinetochore and simultaneously removed from their minus-ends anchored in the poles13. Although this poleward MT ‘flux’ along the K-fibre exerts a continuous poleward force on the kinetochore in vertebrate somatic cells27, its rate accounts for only 25% of that exhibited by poleward-moving kinetochores. The other 75% of the force for poleward motion is produced at the kinetochore, which ‘pulls’ the chromosome towards the minus-ends of its K-MTs. During this poleward motion, K-MTs shorten by disassembly at the kinetochore (Fig. 3a; reviewed in Ref. 28). As the kinetochore moves poleward, the pulling force transmitted to the chromosome depends on how much the kinetochore stretches its underlying centromere (Fig. 3a). However, regardless of the magnitude of the poleward force, or the resistance of the centromere to stretching, the poleward velocity of the kinetochore remains constant (reviewed in Ref. 29).
We still do not fully understand how kinetochores generate their share of the poleward force or how their velocity of motion is governed. Although cytoplasmic dynein plays an important role in the attachment and poleward motion of Tetrahymena chromosomes (S. Lee, J. C. Wisniewski and D. J. Asai, pers. commun.), it is not required for chromosome segregation in yeast (which occurs predominantly by elongation of the central spindle)3,30. Currently, there is also no direct evidence that cytoplasmic dynein is essential in producing the force for kinetochore poleward motion in vertebrates. The observation that disrupting CENP-E function does not affect kinetochore motion, or the velocity of movements towards and away from the pole18, suggests that this molecule is also not essential in poleward force generation. However, the role of any kinetochore-associated MT motor in powering chromosome motion might be difficult to establish with certainty if it is masked by the presence of other redundant force-producing mechanisms. Potential candidates here include unknown kinetochore-associated proteins and/or other mechanisms such as K-MT disassembly28 or elastic components within the K-fibre31.
In contrast to the poleward pulling forces produced by kinetochores, kinetochores moving away from their pole do not exert a pushing force on the chromosomes in PtK1 cells32 and rarely in newt cells9. Instead, when not in a poleward state of motion, kinetochores exist in a neutral state (Fig. 3b) that allows them to be dragged away from their attached pole on the ends of their elongating K-MTs32. Kinetochores that are moving away from the pole do so with a constant velocity (again, 1.5–2.5 μm min−1) even if their associated chromosome arms are highly stretched away from the pole with a microneedle29. Thus, as for poleward motion, the velocity of motion away from the pole is regulated and independent of the force acting on the kinetochore. The molecules that regulate the rate of motion and hold the K-MT plus-ends as they elongate to allow for motion away from the pole are unknown. CENP-E might play one or both of these roles especially if ultimately it is established to be a MT plus-end-directed motor in vivo (Fig. 3b).
Kinetochores switch between poleward-moving and neutral states
When viewed in time-lapse movies, many of the mono-oriented chromosomes seen on a developing vertebrate spindle exhibit oscillatory motions towards and away from their associated pole. During this behaviour, the only attached kinetochore on these chromosomes switches constantly and autonomously between periods of movement poleward and away from the pole32,33,35 (Figs 2 and 3), and, during these motions, the K-MTs remain relatively stationary13. Since kinetochores do not generate a force for motion away from the pole (see above), this component of an oscillation is thought to be due to ejection forces associated with the closest pole that push the whole chromosome away from the pole (see below). Although not seen in some insect spermatocytes, this oscillatory behaviour is found in other spermatocytes from flatworms to newts (reviewed in Ref. 5). One striking feature of this ‘kinetochore directional instability’ is that the motions poleward and away from the pole are similar in velocity and, except during congression, in duration32,33. Switching often occurs abruptly (within 6 sec), implying that the mechanism induces all K-MT plus-ends to change rapidly between shortening (poleward) or growing (away from the pole) phases. How this switching is coordinated between the multiple subunits of vertebrate kinetochores, so that the kinetochore moves poleward and away from the pole as a single unit, is an important unresolved issue.
After bi-orientation, sister kinetochores can continue to exhibit directionally unstable behaviour throughout congression, metaphase and even during the initial stages of anaphase (reviewed in Ref. 5). As occurs for mono-oriented chromosomes, the kinetochore that is moving away from its pole on a bi-oriented chromosome appears to be pushed away from the pole by the proximal polar-ejection forces. However, on a bi-oriented chromosome, it can also be pulled away from its associated pole by the poleward motion of its sister kinetochore.
An important question for the future is how switching is controlled. Time-lapse video33 and laser-microsurgery34 studies reveal that attached kinetochores tend to exist in a poleward-moving state when under low tension levels and then switch into neutral (and move away from the pole) when experiencing high tension levels. This has led to the idea that kinetochore switching is mediated simply by tension on the kinetochore (or on the kinetochore–chromosome junction)33,34. However, the evidence that kinetochore switching is controlled by tension is circumstantial, and other switching mechanisms are possible. When the poleward-moving kinetochore on a congressing chromosome is destroyed suddenly by laser microsurgery, the chromosome stops moving immediately32. The kinetochore that was originally moving away from its pole then remains motionless (i.e. in neutral) for up to 50 s before switching into a poleward state of motion. Thus, if tension alone controls kinetochore switching, there might be a significant amount of hysteresis in the mechanism. Alternatively, the finding that an attached kinetochore can remain motionless for prolonged periods when tension on it is suddenly relieved could mean that it must spend a defined time in a neutral phase before it can reinitiate poleward motion32. In this model, a kinetochore switches into neutral when an associated component needed for poleward motion is depleted below a threshold, and it is then free to be displaced away from the pole until it becomes fully ‘recharged’, at which time it switches back into poleward motion. The depletion rate could also be related to the level of tension experienced by the poleward-moving kinetochore. Although speculative, this idea is consistent with the observation that, in most cases, the centromere region undergoes at least one, and sometimes several, oscillations as a bi-orienting and congressing chromosome moves from a pole to the spindle equator32. These oscillations would not be expected if switching were mediated simply by an increased tension on the poleward-moving kinetochore because this kinetochore should not experience additional tension until it reaches the spindle equator. The idea that switching is controlled by the depletion/regeneration of a kinetochore component needed for poleward motion can also explain why the sister kinetochores on many bi-oriented chromosomes do not exhibit a high degree of coordinated behaviour32.
Kinetochores help position chromosomes on the spindle equator
Given that each kinetochore on a bi-oriented chromosome can exist either in a poleward moving state, or a neutral state that allows it to be displaced away from the pole, how does a bi-oriented chromosome end up at the spindle equator? The answer to this age-old question remains unknown. We envision that forces are generated within each half-spindle that progressively resist chromosome pole-ward motion but not motion away from the pole (reviewed in Ref. 5). The idea behind this model is that the poleward motion of a chromosome is resisted because it is moving into an increasingly dense array of MTs that exert on it a progressively stronger pushing or resistance force away from the pole (envision moving into the end of a broom!). Indeed, although the number of MTs and free-MT plus-ends is highest near the equator, the density of spindle MTs is clearly highest near the poles (Fig. 2)11,35. The other tenet is that switching is induced by tension and/or that a crucial component needed for poleward motion is depleted more rapidly when the kinetochore is required to work against high tension levels. Under these conditions, the length of time that a newly attaching kinetochore spends in poleward motion would be exaggerated because it initiates this motion next to the opposite pole, in a region of high MT density. During the initial motion, the tension level on it remains less than that on the sister kinetochore because it is moving into a region of progressively lower MT density.
During bi-orientation, the attaching kinetochore would also undergo an exaggerated duration of poleward motion if its poleward force-producing potential, or the concentration of a factor required for poleward movement, is initially greater than that of its attached sister. There are reliable data that attaching kinetochores do possess a higher concentration of MT motor proteins relative to fully attached kinetochores (see above). It is easy to envisage how such a property could work in concert with the spindle-resistance/kinetochore-tension model described above to bias the duration of sister kinetochore motilities during congression.
Although forces are clearly associated with the polar regions of vertebrate spindles that ‘push’ chromosomes away from the pole and/or impede poleward motion (reviewed in Refs 5 and 35), their magnitude and how they are generated remain mysterious. This force appears to have two components: a steric resistance to chromosome penetration based on MT density and the tendency of dense arrays of dynamic spindle MTs to exclude large objects (reviewed in Refs 32 and 36), and an active pushing force on the chromosome arm (reviewed in Refs 5 and 35). This latter component might be generated by the impact of growing MT ends on the chromosome or by kinesin-like MT plus-end-directed motor proteins (e.g. chromokinesins37) on the chromosome surface. However, there is no experimental evidence that such proteins are involved in the spindle-ejection forces. Moreover, at least in vertebrates, there are no electron microscopy data to suggest that chromatin is noticeably stretched away from the pole along polar MTs that penetrate through or laterally contact a prometaphase chromosome.
Kinetochores control the M–A transition
There are two ‘points of no return’ during mitosis (reviewed in Ref. 38). One is in late prophase – passage through this point leads to nuclear envelope breakdown and a commitment to enter mitosis. The other is at the M–A transition – when pathways are activated that trigger chromatid separation (i.e. anaphase) and exit from mitosis (i.e. telophase). The presence of just one unattached or weakly attached kinetochore delays the M–A transition in many cells (reviewed in Ref. 39). Laser-micro-surgery40 and micromanipulation41 experiments have shown that this delay is mediated by a negative-feedback pathway – that is, that unattached or weakly attached kinetochores produce a signal that inhibits anaphase onset. The duration of spindle formation is variable, and kinetochores start mitosis unattached. Therefore, this cell-cycle checkpoint is not ‘activated’ suddenly in response to an experimental intervention. Instead, it appears to be a constitutive mechanism, present from after the onset of spindle formation, that inhibits the pathway(s) that trigger anaphase.
We refer to the checkpoint controlling the M–A transition as the kinetochore-attachment checkpoint42, but it has also been termed the ‘spindle assembly’39, ‘chromosome distribution’14, ‘mitotic’43 or just ‘spindle’44 checkpoint. We currently favour the term kinetochore-attachment checkpoint for vertebrate somatic cells because, once a commitment to enter mitosis is made, some aspect of kinetochore attachment appears to be the only event monitored before initiating anaphase. Although inhibiting spindle pole replication/separation, spindle formation or the normal behaviour of spindle MTs does inevitably induce a mitotic delay, all of these conditions affect some state of kinetochore attachment. Mitosis is not prolonged in cells containing tri- or tetrapolar spindles in which all kinetochores are properly attached, implying that mechanisms are not in place for detecting excess numbers of spindle poles or the absence of bipolar spindle symmetry45.
The kinetochore-attachment checkpoint monitors microtubule accumulation and/or tension
What is monitored by the kinetochore-attachment checkpoint remains unclear, but possibilities include the accumulation of K-MTs42 or the generation of tension between the kinetochore and its K-MTs and/or chromosome41. These alternatives are difficult to distinguish because the acquisition of MTs produces tension on the kinetochore, which promotes MT accumulation10. Micromanipulation experiments on how non-natural univalent chromosomes delay progression through meiosis in insect spermatocytes demonstrate clearly that the inhibitory signal is abrogated in some systems when kinetochores are placed under tension41. However, it is not clear whether this tension relieves the checkpoint by distorting stretch- or tension-sensing enzymes in the kinetochore/centromere complex or by promoting the acquisition of K-MTs. When the unattached kinetochore on the last mono-oriented chromosome is destroyed in PtK1 cells by laser microsurgery, the cell enters anaphase40 (Fig. 4a), even though the remaining attached kinetochore on the chromosome lacks a full complement of MTs8,40 but is (on average) stretched9. Anaphase is also inhibited in taxol-treated metaphase PtK1 cells under conditions in which the kinetochores are, on average, saturated with MTs but not under tension (reviewed in Ref. 44). However, conclusions based on averages might mask the presence of one or more kinetochores that are not saturated with MTs. Thus, whether the kinetochore-attachment checkpoint monitors tension, MT numbers or both remains to be resolved.
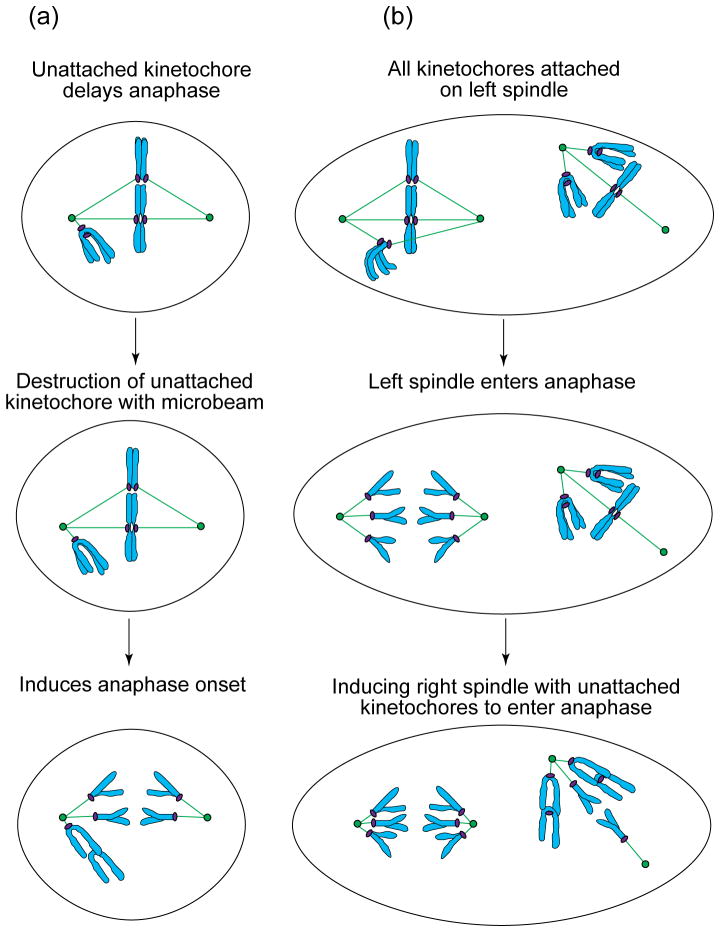
FIGURE 4.
(a) In PtK1 cells, the unattached kinetochore on a single mono-oriented chromosome is sufficient to inhibit the onset of anaphase for many hours. However, anaphase occurs shortly after this unattached kinetochore is destroyed by laser microsurgery40. Thus, unattached kinetochores produce a ‘wait-anaphase’ signal. (b) PtK1 cells containing two independent spindles can be created by fusing neighbouring cells53. In these cells, even multiple unattached kinetochores on the mono-oriented chromosomes on one spindle do not prevent anaphase onset in an adjacent spindle when all of its kinetochores become attached. In addition, after a variable delay, anaphase in this spindle induces anaphase in the spindle with mono-oriented chromosomes and unattached kinetochores.
Although it is unclear how kinetochores convert lack of tension or unfilled MT-attachment sites into a signal inhibiting anaphase, several kinetochore proteins have been implicated in this pathway. In PtK1 cells and grasshopper spermatocytes, the 3F3/2 antibody, which recognizes a phosphorylated epitope common to many proteins, labels unattached but not attached kinetochores (reviewed in Ref. 10). Injecting this antibody into prometaphase PtK1 cells does not inhibit metaphase alignment of chromosomes but does inhibit dephosphorylation of the phosphoepitope and prolongs the duration of metaphase. This suggests that the kinetochore protein possessing the 3F3/2 epitope is involved in regulating the M–A transition47. However, the role of the 3F3/2 epitope in the kinetochore-attachment checkpoint remains unclear in view of the report that, in newt cells, the 3F3/2 antibody labels all kinetochores with a similar intensity throughout spindle formation9.
In most cells, the M–A transition is delayed when the spindle poles fail to separate or when the behaviour of spindle MTs is disturbed. Yeast proteins involved in this checkpoint include those encoded by the MAD, BUB and MPS1 genes (reviewed in Ref. 39). Evidence is accumulating that Mad and Bub protein homologues in vertebrates are involved in the kinetochore-attachment checkpoint24,43,44,46, which, with few exceptions48, remains active when the spindle poles fail to separate or when MT behaviour is perturbed. For example, antibodies to Xenopus (XMAD2)24 and human (hsMAD2)43 homologues of the yeast Mad2 protein stain unattached but not fully attached kinetochores in vertebrates. Furthermore, when added to Xenopus extracts25 or electroporated into HeLa cells43, antibodies to Mad2 prevent the mitotic arrest induced by inhibiting MT assembly. Similarly, microinjecting antibodies to MAD2 into prometaphase PtK1 cells, or metaphase-arrested taxol-treated cells, rapidly induces precocious anaphase44,46. The murine homologue of Bub1p is also a component of unattached but not attached kinetochores and is required for delaying the M–A transition in response to spindle disassembly25.
The checkpoint mechanism
What event does the ‘wait-anaphase’ signal produced by unattached kinetochores inhibit? Entry into mitosis correlates with the activation of the CDK1 (cdc2) mitotic kinase. The subsequent inactivation of CDK1, through ubiquitination and destruction of its associated cyclin B, similarly correlates with exit from mitosis. Originally, both chromatid separation and exit from mitosis were thought to be triggered simultaneously by CDK1 inactivation. However, we now know that these events are regulated by different but coordinated pathways49. One leads to chromatid separation, which, in turn, allows the chromosomes to move towards their respective poles. In yeast, this pathway involves the ubiquitin-mediated degradation of one or more proteins, not complexed with cyclin, that maintain sister-chromatid cohesion (e.g. see review in Ref. 50). By contrast, the ‘exit mitosis’ pathway is triggered by the inactivation of CDK1 and leads to the hallmark features of telophase, including spindle disassembly, chromosome decondensation, nuclear envelope reformation and cytokinesis. In yeast, the separation of sister chromatids precedes CDK1 inactivation51. Similarly, in vertebrates, expression of nondegradable cyclin B inhibits exit from mitosis but not chromatid disjunction, suggesting that the latter pathway is initiated upstream from the former49,52. Thus, the wait-anaphase signal produced by unattached kinetochores probably works by inhibiting the chromatid-separation pathway.
Recent strides have also been made in determining where the target for the wait-anaphase signal resides. In fused PtK1 cells, multiple unattached kinetochores on one spindle do not inhibit anaphase onset in a neighbouring spindle that lacks unattached kinetochores (Fig. 4b)53. Thus, the functional target of the wait-anaphase signal produced by an unattached or weakly attached kinetochore is associated with the spindle. What is this target? Currently, the most attractive candidates are large (20S) assemblies, known as cyclosomes or anaphase-promoting complexes (APCs), that appear by immunofluorescent microscopy to be a component of the spindle (reviewed in Ref. 54). APCs are ubiquitin ligases that function to target proteins selectively for destruction. Studies in fission yeast reveal that chromatid disjunction requires the APC-mediated degradation of cut2 (and its budding yeast homologue Pds1p – reviewed in Ref. 50) and that this protein, like the APCs, also appears associated with the spindle. Clearly, determining how APC activity is regulated will be important for elucidating the molecular events underlying the kinetochore-attachment checkpoint, and work on this topic is accelerating. Recent data from yeast (reviewed in Ref. 55) as well as mammalian tissue-culture cells56 suggest that APC activity is mediated by a complex of Mad proteins and Cdc20p (fission yeast homologue is slp1; mammalian homologue is P55cdc56).
In the cell-fusion experiment described above, anaphase in the mature spindle initiated anaphase in an adjacent spindle containing unattached kinetochores (Fig. 4b)53. Thus, anaphase is the ‘dominant’ mitotic state since, when triggered locally within a spindle, it spreads globally and overrides the wait-anaphase signal associated with a neighbouring spindle. This conclusion leads to several testable predictions. One is that a chromosome that is too far away to attach to a spindle will not inhibit anaphase. Another is that anaphase cytoplasm will quickly and prematurely trigger the M–A transition when injected into prometaphase cells. Finally, it suggests that the local activation of APCs within a spindle lacking unattached kinetochores starts a chain reaction that spreads globally by targeting for destruction a soluble factor that itself inhibits APC activity.
Some apparently conflicting results regarding the kinetochore-attachment checkpoint can be ascribed to the fact that some embryos are checkpoint challenged. Drosophila embryos, for example, lack checkpoints until cellularization during the 14th mitotic cycle57. Similarly, the M–A transition in sea urchin zygotes is not delayed by the presence of numerous unattached kinetochores58. However, in sea urchins (as in Xenopus), the M–A transition is delayed if the zygote nucleus, and thus all the kinetochores, are removed (reviewed in Ref. 58). In sea urchins, the enucleation-induced cell-cycle delay appears to occur during the ‘mitosis’ portion because similar delays are produced in controls when spindle formation is inhibited or when the spindle is cut into two monopolar spindles (reviewed in Ref. 58). Together, these data suggest that spindle formation, which does not occur in sea urchin zygotes in the absence of chromosomes59, is crucial for proper timing of the M–A transition. Unattached kinetochores in Xenopus embryos might also fail to produce an operational wait-anaphase signal. The term ‘operational’ is used because the presence of sufficiently high densities of sperm nuclei (i.e. chromosomes and/or unattached kinetochores) does arrest nocodazole-treated and activated Xenopus oocyte extracts in a meiotic state60. Clearly, the rules that control the M–A transition in some zygotes differ from those in somatic cells, and this is a crucial area for future research.
Concluding remarks
Kinetochores are dynamic complexes containing MT motor and cell-cycle regulatory proteins, which serve three functions during cell division. They attach each replicated chromosome to the opposing poles of the mitotic spindle, help position the chromosome on the spindle and then inhibit chromatid separation (and anaphase onset) until all of the chromosomes are properly attached and positioned. The failure of a kinetochore to execute these functions properly leads to the formation of aneuploid cells that contain abnormal numbers of chromosomes. When this occurs during development, organisms are formed that are mosaic for birth-defect syndromes characterized by missing or additional chromosomes. In adults, the genesis of aneuploid cells is thought to be a major mechanism behind cell transformation. Indeed, the advances discussed above in understanding how the kinetochore-attachment checkpoint works have already implicated mutations in the BUB1 gene with the genesis of colorectal tumours61. It is likely that other types of cancers or birth defects will be linked in the future to additional defects in kinetochore function.
Acknowledgments
The authors thank K. Bloom, C. Waterman-Storer, A. Khodjakov, B. F. McEwen, J. Waters, J. Canman and S. Nowogrodzki for their thoughtful comments on the manuscript. Work discussed from the authors’ laboratories was supported by NIH grants GMS 40198 (to C. L. R.), GMS 24364 (to E. D. S.) and NCRR P41-01219 (C. L. R.).
Footnotes
Note added in proof: Dujardin, D. et al. [(1998) J. Cell Biol. 141, 849–862] have shown that CLIP170, a protein needed for in vitro binding of endocytic transport vesicles to MTs, localizes strongly to unattached but not to fully attached kinetochores.
Contributor Information
Conly L. Rieder, Email: Rieder@Wadsworth.Org, Division of Molecular Medicine, Wadsworth Center, New York State Dept of Health, PO Box 509, Albany, NY 12201-0509, USA
E. D. Salmon, Dept of Biology, CB 3280-607 Fordham Hall, University of North Carolina, Chapel Hill, NC 27599-3280, USA
References
- 1.Rieder CL. Internat Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- 2.Khodjakov A, et al. J Cell Biol. 1997;136:229–241. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straight AF, et al. Science. 1997;277:574–578. doi: 10.1126/science.277.5325.574. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BF, Ding Y, Heagle AB. Chromosome Res. 1997;6:123–132. doi: 10.1023/a:1009239013215. [DOI] [PubMed] [Google Scholar]
- 5.Rieder CL, Salmon ED. J Cell Biol. 1994;124:223–233. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirschner M, Mitchison TJ. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 7.Rieder CL, Alexander SP. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEwen BF, et al. J Cell Biol. 1997;137:1567–1580. doi: 10.1083/jcb.137.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waters JC, Skibbens RV, Salmon ED. J Cell Sci. 1996;109:2823–2831. doi: 10.1242/jcs.109.12.2823. [DOI] [PubMed] [Google Scholar]
- 10.Nicklas RB. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 11.McIntosh JR, Landis SC. J Cell Biol. 1971;49:468–497. doi: 10.1083/jcb.49.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ault J, Rieder CL. Cell Motil Cytoskeleton. 1992;22:155–159. doi: 10.1002/cm.970220302. [DOI] [PubMed] [Google Scholar]
- 13.Mitchison TJ, Salmon ED. J Cell Biol. 1992;119:569–582. doi: 10.1083/jcb.119.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastronarde DN, et al. J Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhai Y, Kronebusch PJ, Borisy GG. J Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Echeverri CJ, et al. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wordeman L, Mitchison TJ. J Cell Biol. 1995;128:95–105. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaar BT, et al. J Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood K, et al. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 20.Cooke CA, et al. Chromosoma. 1997;106:446–455. doi: 10.1007/s004120050266. [DOI] [PubMed] [Google Scholar]
- 21.Vaisberg EA, Koonce MP, McIntosh JR. J Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao H, et al. J Cell Biol. 1995;130:507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams BC, Gatti M, Goldberg ML. J Cell Biol. 1996;134:1127–1140. doi: 10.1083/jcb.134.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen RH, et al. Science. 1996;274:242–246. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- 25.Taylor SS, McKeon F. Cell. 1997;89:727–735. doi: 10.1016/s0092-8674(00)80255-x. [DOI] [PubMed] [Google Scholar]
- 26.Thrower DA, et al. EMBO J. 1995;14:918–926. doi: 10.1002/j.1460-2075.1995.tb07073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waters JC, et al. Mol Biol Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inouè S, Salmon ED. Mol Biol Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skibbens RV, Salmon ED. Exp Cell Res. 1997;235:314–324. doi: 10.1006/excr.1997.3691. [DOI] [PubMed] [Google Scholar]
- 30.Yeh E, et al. J Cell Biol. 1995;130:687–700. doi: 10.1083/jcb.130.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickett-Heaps JE, Forer A, Spurck T. Protoplasma. 1996;192:1–10. doi: 10.1007/s00709-007-0265-8. [DOI] [PubMed] [Google Scholar]
- 32.Khodjakov A, Rieder CL. J Cell Biol. 1996;135:315–328. doi: 10.1083/jcb.135.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skibbens RV, Skeen VP, Salmon ED. J Cell Biol. 1993;122:859–875. doi: 10.1083/jcb.122.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skibbens RV, Rieder CL, Salmon ED. J Cell Sci. 1995;108:2537–2548. doi: 10.1242/jcs.108.7.2537. [DOI] [PubMed] [Google Scholar]
- 35.Cassimeris L, Rieder CL, Salmon ED. J Cell Sci. 1994;107:285–297. doi: 10.1242/jcs.107.1.285. [DOI] [PubMed] [Google Scholar]
- 36.Bajer AS, Mole-Bajer J. Int Rev Cytol (Suppl) 1972;3:1–271. [Google Scholar]
- 37.Molina I, et al. J Cell Biol. 1997;139:1361–1371. doi: 10.1083/jcb.139.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rieder CL, Cole RW. J Cell Biol. in press. [Google Scholar]
- 39.Wells WAE. Trends Cell Biol. 1996;6:228–234. doi: 10.1016/0962-8924(96)10018-0. [DOI] [PubMed] [Google Scholar]
- 40.Rieder CL, et al. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Nicklas RB. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- 42.Rieder CL, et al. J Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Benezra R. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 44.Waters JC, et al. J Cell Biol. 1998;141:1181–1191. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sluder G, et al. J Cell Sci. 1997;110:421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- 46.Gorbsky GJ, Chen RH, Murray AW. J Cell Biol. 1998;141:1193–1205. doi: 10.1083/jcb.141.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell MS, Gorbsky GJ. J Cell Biol. 1995;129:1195–1204. doi: 10.1083/jcb.129.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss E, Winey M. J Cell Biol. 1996;132:111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holloway SL. Curr Opin Genet Dev. 1995;5:243–248. doi: 10.1016/0959-437x(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 50.Cohen-Fix O, et al. Genes Dev. 1996;10:3081–3093. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 51.Straight AF, et al. Curr Biol. 1996;6:1599–1608. doi: 10.1016/s0960-9822(02)70783-5. [DOI] [PubMed] [Google Scholar]
- 52.Wheatley SP, et al. J Cell Biol. 1997;138:385–393. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rieder CL, et al. Proc Natl Acad Sci U S A. 1997;94:5107–5112. doi: 10.1073/pnas.94.10.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Page AM, Hieter P. Cancer Surv. 1997;29:133–150. [PubMed] [Google Scholar]
- 55.Kim SH, et al. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- 56.Kallio M, et al. J Cell Biol. 1998;141:1393–1406. doi: 10.1083/jcb.141.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glover DM. Trends Genet. 1991;7:125–132. doi: 10.1016/0168-9525(91)90457-2. [DOI] [PubMed] [Google Scholar]
- 58.Sluder G, et al. J Cell Biol. 1994;126:189–198. doi: 10.1083/jcb.126.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sluder G, et al. J Cell Biol. 1986;103:1873–1881. doi: 10.1083/jcb.103.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clute P, Masui Y. Dev Biol. 1995;171:273–285. doi: 10.1006/dbio.1995.1280. [DOI] [PubMed] [Google Scholar]
- 61.Cahill DP, et al. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 62.Cooke CA, Bernat RL, Earnshaw WC. J Cell Biol. 1990;110:1475–1488. doi: 10.1083/jcb.110.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mackay AM, et al. J Cell Biol. 1998;140:991–1002. doi: 10.1083/jcb.140.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walczak CE, Mitchison TJ, Desai A. Cell. 1996;84:37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- 65.Tomkiel J, et al. J Cell Biol. 1994;125:531–545. doi: 10.1083/jcb.125.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]