Abstract
Objective
Blood‐based biomarkers for neurodegenerative conditions could improve diagnosis and treatment development. Neurofilament light chain (NfL), a marker of axonal injury, is elevated in cerebrospinal fluid (CSF) of patients with progressive supranuclear palsy (PSP). The goal of this study was to determine the diagnostic and prognostic value of plasma NfL in patients with PSP.
Methods
Plasma NfL was measured with ultrasensitive digital immunoassay‐based technology at baseline and 1‐year follow‐up in a pilot cohort of 15 PSP patients and 12 healthy controls, and a validation cohort of 147 PSP patients. Mixed linear models tested the ability of plasma NfL to predict neurological, cognitive and functional decline, and brain atrophy.
Results
Baseline mean plasma NfL levels were elevated in PSP patients (31 ± 4 pg/mL, vs. control, 17.5 ± 1 pg/mL, P < 0.05) and this difference persisted at follow‐up. A cutoff value of 20 pg/mL related to the diagnosis of PSP with a sensitivity of 0.80 and specificity of 0.83 (positive likelihood ratio = 4.7 and a negative likelihood radio of 0.24). Patients with higher NfL levels had more severe neurological (PSPRS, −36.9% vs. −28.9%, P = 0.04), functional (SEADL, −38.2% vs. −20%, P = 0.03), and neuropsychological (RBANS, −23.9% vs. −12.3%, P = 001) deterioration over 1 year. Higher baseline NfL predicted greater whole‐brain and superior cerebellar peduncle volume loss. Plasma and CSF NfL were significantly correlated (r = 0.74, P = 0.002).
Interpretation
Plasma NfL is elevated in PSP and could be of value as a biomarker both to assist clinical diagnosis and to monitor pharmacodynamic effects on the neurodegenerative process in clinical trials.
Introduction
Progressive supranuclear palsy (PSP) is a cause of atypical parkinsonism associated with deposition of primarily 4 microtubule binding domain repeat (4R) tau and neuronal loss, most prominently in the brainstem, basal ganglia, and cerebellum, with variable cortical involvement.1 PSP has an estimated prevalence of 5.8–6.5 cases per 100,000 individuals, and most commonly presents after age 40 with gait instability, recurrent falls, and eye movement abnormalities.2, 3 No effective treatments are available for PSP and mortality is usually associated with falls and bronchoaspiration, with a median survival of 7 years.4 Development of effective therapies has been hindered by the lack of sensitive tools to diagnose the disease and quantify disease activity and progression in clinical trials. Magnetic resonance imaging (MRI) and positron emission tomography have been used in the diagnosis of PSP, but these have suboptimal sensitivity and specificity, limited availability, and high cost.4 Development of sensitive fluid biomarkers could advance early detection and therapeutic development for PSP.
Neurofilament light chain (NfL) has shown consistent cerebrospinal fluid (CSF) elevations in PSP and other disorders associated with atypical parkinsonism, compared to healthy controls and patients with idiopathic Parkinson's disease (PD).5, 6, 7 In a recent PSP clinical trial, CSF NfL increased over 1 year and changes correlated with progression of ocular motor deficits and superior cerebellar peduncle (SCP) atrophy.8 NfL is the smallest intermediate filament protein of neurons, and it contributes axonal structure, mechanoresistance, and scaffolding for organelle organization.9 Elevated NfL concentrations in CSF are believed to reflect neurodegeneration‐associated axonal injury, and have been documented in demyelinating disease and brain trauma.9, 10, 11 CSF NfL is believed to reflect extracellular brain concentrations, but blood‐based quantification of NfL is highly desirable, since sample collection is comparatively more accessible and inexpensive. Recent studies have demonstrated the feasibility of blood NfL quantification in neurological conditions such as multiple sclerosis, amyotrophic lateral sclerosis (ALS), and Alzheimer's disease (AD).12, 13, 14 Such studies, however, have been based on standard immunoassays, which lack the analytical sensitivity to quantify NfL except in samples with very high concentrations. For this reason, we set out to develop an NfL assay based on the ultrasensitive Single Molecule Array (Simoa) technique.15 Further, no prior attempts have been made for assessing the clinical value of blood NfL concentrations in PSP.
The goal of this study was to measure plasma NfL concentrations using an ultrasensitive digital array‐based technology to determine the diagnostic and prognostic value of plasma NfL in PSP. An original cohort was used to determine the differences in plasma NfL concentrations between PSP patients and healthy controls and their ability to predict dementia severity upon follow‐up after 1 year. A separate validation cohort of PSP patients from a large, international clinical trial8 further examined the ability of plasma NfL levels to predict clinical decline and MRI‐based measures of brain atrophy over 1 year.
Subjects and Methods
Participants
This retrospective study was conducted in an original cohort of volunteers evaluated at the University of California, San Francisco Memory and Aging center (UCSF cohort, n = 27). Analyses were also performed in a validation cohort consisting of patients with PSP recruited for a previously published international multicenter clinical trial8 (Allon cohort, n = 147). Patients with PSP met NINDS‐Society for Progressive Supranuclear Palsy diagnostic criteria for probable or possible PSP.16 PSP patients and controls were included if two plasma samples were available with a resampling interval of 365 ± 90 days. CSF values for beta amyloid, total tau, and NfL were available only in seven subjects in the UCSF cohort and in 22 subjects in the Allon cohort. Plasma and CSF samples were collected on the same day. Neuropathological diagnosis was available in six of the 15 cases at the UCSF cohort. All six cases had PSP pathology. Volumetric MRI data8 were available from 124 Allon cohort patients. All patients provided informed consent at the time of recruitment and procedures were approved by the UCSF Institutional Review Board.
Plasma and CSF analyses
Blood samples were collected by venipuncture in ethylenediaminetetraacetic acid (EDTA) tubes for plasma. After centrifugation, plasma samples were aliquoted and stored at −80°C. Plasma NfL levels were determined using the NF‐Light kit from UmanDiagnostics (UmanDiagnostics, Umeå, Sweden), transferred onto the Simoa platform using a homebrew kit (Quanterix Corp, Boston, MA). The lower limit of quantification (LLOQ), determined by the blank mean signal + 10 SD, was 1.95 pg/mL. All samples measured well above the LLOQ. The analyses were performed by a board‐certified laboratory technician using one batch of reagents with intra‐ and interassay coefficients of variation below 10% and 15%, respectively. Samples from both cohorts were analyzed using the same NfL‐Light kit batch. In the Allon cohort, lumbar punctures were performed for CSF collection and NfL measurement using an enzyme‐linked immunosorbent assay (ELISA) as described previously.7
Clinical measures
Neurological assessments were based on the Progressive Supranuclear Palsy Rating Scale (PSPRS).8, 17 The Mini‐Mental State Examination (MMSE)18 was obtained at baseline in all subjects. In the Allon cohort, the Repeatable Battery for the Assessment of Neuropsychological Disease Severity (RBANS) was used to measure neuropsychological status.19 Assessment for overall disability was completed with the Clinical Dementia Rating Sum of Boxes scale (CDR‐sb)20 in the UCSF cohort and with the Schwab and England Activities of Daily Living (SEADL) scale21 in the Allon cohort. The CDR‐sb was measured at baseline and 52 weeks. The PSPRS and SEADL scales were obtained at baseline and at 6, 13, 26, 39, and 52 weeks visits. The RBANS was obtained at baseline and at 26 and 52 weeks visits.
Volumetric MRI data
Whole‐brain, ventricular, midbrain, and SCP volumetric measures obtained from 1.5 or 3 T structural MRI scans were available in the Allon group. MRI scans met standards established by the Mayo Clinic's Aging and Dementia Imaging Research laboratory and volumetric data were generated using the Boundary Shift Integral technique or label propagation in voxel‐based morphometry (SPM5) as described previously.8
Statistics
Tests for normality were conducted with the Shapiro–Wilk test. Means were compared with independent samples t‐test and expressed with their associated standard error of the mean. If data were not normally distributed, they were analyzed with the Mann–Whitney U test or chi‐square test when appropriate and expressed with their associated confidence interval. Contingency matrices used data from the UCSF cohort to determine the sensitivity and specificity of the plasma NfL in the diagnosis of PSP. Classification of controls and patients based on plasma NfL levels was tested by means of principal component analysis with inclusion of baseline characteristics (i.e., age, gender, education level, MMSE, CDR‐sb, and plasma NfL). Baseline plasma NfL levels were dichotomized using a median split for evaluation of the relationship with changes in clinical and imaging variables.
In the validation Allon cohort, the ability of baseline plasma NfL to predict a change in clinical measures at 6, 13, 26, 39, and 52 weeks was assessed with age, gender, and baseline MMSE‐adjusted mixed linear models with PSPRS, SEADL, and RBANS scores as dependent variables. In these analyses, baseline plasma NfL was introduced as a categorical measure (dichotomized at the 50th percentile value). In a second series of analyses, baseline plasma NfL was also introduced as a continuous variable. Age‐ and gender‐controlled Pearson's partial correlations were determined between baseline plasma NfL levels and changes in regional and whole‐brain volume. Pairwise correlations were used to assess the relationship between plasma and CSF NfL levels. For all analyses, a P < 0.05 was considered significant. Data were analyzed using SPSS (version 23; SPSS/IBM, Chicago, IL).
Results
Plasma NfL levels differentiate PSP patients from controls
PSP patients had baseline mean plasma NfL levels (31 ± 4 pg/mL) that were nearly twice as high as those of controls (17.5 ± 1 pg/mL, P < 0.05). This difference between controls and PSP patients persisted at 1‐year follow‐up (Fig. 1A). Principal component analysis revealed that classification of baseline plasma NfL values into low and high medians was the most important clinical variable contributing to the prediction of diagnosis (score coefficient for Component 1: baseline NfL 0.41 and baseline CDR‐sb 0.36) (Fig. 1B). The cutoff value of 20 pg/mL related to the diagnosis of PSP with a sensitivity of 0.80 and specificity of 0.83. At this cutoff value, elevated plasma NfL had a positive likelihood ratio of 4.7 and a negative likelihood radio of 0.24. Tables 1, 2 summarize baseline demographic, clinical, imaging, and biomarker characteristics for participants in the UCSF and Allon cohorts, respectively. Certain baseline demographic features such as age and gender influenced baseline plasma NfL levels. There were more female participants in the UCSF cohort, but the between‐group differences in plasma NfL were not confounded by gender effects. The proportion of male‐to‐female participants was similar in control and PSP groups, and the gender differences in baseline plasma NfL levels were not significant in either group (control, P = 0.25) or PSP (PSP, P = 0.25). In addition, gender did not influence baseline or follow‐up plasma NfL levels in the Allon cohort. Gender was also used as a covariate in mixed linear analyses.
Figure 1.
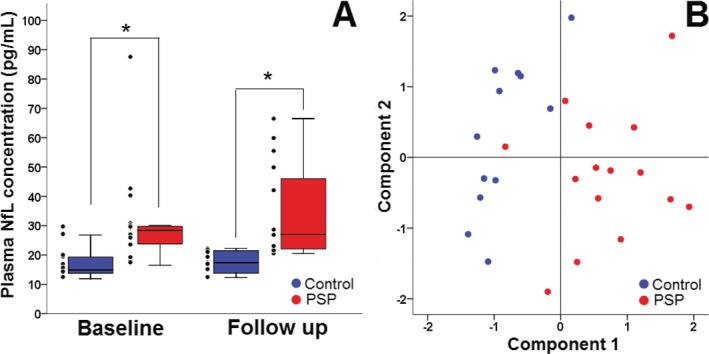
Plasma NfL is elevated in PSP patients, UCSF cohort. (A) Plasma NfL concentrations in control (blue) and PSP (red) patients at baseline and follow‐up. In both groups, NfL levels remained constant overtime, but they were comparatively elevated and showed wider variability in PSP patients. Thick horizontal bars represent the median. Upper and lower box limits represent the 75th and 25th percentiles. Error bars represents the minimum and maximum values. Individual data points, including extreme values, appear to the left of each box. (B) Principal component analysis showing classification of PSP patients (red circles) from healthy controls (blue circles) with high accuracy. Clustering was observed on “Component 1” axis which contained baseline NfL level as the major contributing factor. Major contributing variables on “Component 2” were age and education level. *Significant within‐group difference, P < 0.05. NfL, neurofilament light chain; PSP, progressive supranuclear palsy.
Table 1.
Demographic, clinical, and plasma neurofilament light‐chain characteristics of study participants, UCSF cohort
| Control (n = 12) | PSP (n = 15) | P‐value | |
|---|---|---|---|
| Demographics | |||
| Age at sampling, year, mean (SD) | 70 (8) | 66.4 (6) | 0.20 |
| Gender, N, male/female | 2/10 | 5/10 | 0.40 |
| Education, year, mean (SD) | 18 (2) | 15 (2) | 0.008 |
| Baseline MMSE, mean (SD) | 29.4 (0.7) | 27.3 (1) | 0.002 |
| Baseline CDR‐sb, mean, (SD) | 0 (0.1) | 3.7 (2) | <0.001 |
| Levodopa dose equivalent, mg/day, mean (SD) | 0 | 282 (301) | <0.001 |
| Interval between plasma samples, day, mean (SD) | 348 (40) | 369 (30) | 0.13 |
| Baseline CSF total tau, pg/mL, mean (SD)a | N/A | 69.9 (9) | N/A |
| Baseline CSF NfL, pg/mL, mean (SD)a | N/A | 2789 (1206) | N/A |
| Mean baseline plasma NfL levels, pg/mL (SD) | |||
| Total | 17.5 (5) | 31 (17) | 0.001 |
| Age at sampling, year | |||
| 56–67 | 15.2 (2) | 25.6 (6) | 0.004 |
| 68–82 | 19.1 (6) | 39.2 (24) | 0.02 |
| Gender | |||
| Male | 13.1 (1) | 23 (5) | 0.09 |
| Female | 18.3 (5) | 34 (20) | 0.003 |
| MMSE | |||
| 25–28 | 13.8 (2) | 32.1 (19) | 0.02 |
| >28 | 18.2 (5) | 28 (8) | 0.07 |
| Mean year‐1 plasma NfL levels, pg/mL (SD) | |||
| Total | 17.5 (3) | 34.2 (16) | <0.001 |
| Age at sampling, year | |||
| 56–67 | 15.2 (3) | 28.3 (12) | 0.001 |
| 68–82 | 19.2 (3) | 43.1 (17) | 0.001 |
| Gender | |||
| Male | 12.4 (0.1) | 28.4 (12) | 0.09 |
| Female | 18.5 (3) | 37.1 (17) | <0.001 |
| MMSE | |||
| ≤28 | 13.8 (1) | 36.4 (17.6) | 0.02 |
| >28 | 18.3 (3) | 28.1 (9) | 0.02 |
PSP, progressive supranuclear palsy; MMSE, Mini‐Mental State Examination; CDR‐sb, Clinical Dementia Rating Sum of Boxes; CSF, cerebrospinal fluid; NfL, neurofilament.; SD, standard deviation.
Samples available for seven patients.
Table 2.
Demographic, clinical, and plasma neurofilament light‐chain characteristics of patients with progressive supranuclear palsy, Allon cohort
| Low baseline NfL (n = 73) | High baseline NfL (n = 74) | P‐valuea | Total (n = 147) | |
|---|---|---|---|---|
| Age at sampling, year, mean (SD) | 66.6 (6) | 67.7 (7) | 0.35 | 67.1 (6) |
| Gender, N, male/female | 34/39 | 44/30 | 0.13 | 78/69 |
| Baseline MMSE, mean (SD) | 26.5 (3) | 25.7 (3) | 0.27 | 26.1 (3) |
| Baseline PSPRS, mean (SD) | 38.1 (13) | 40 (11) | 0.15 | 39.6 (12) |
| Baseline SEADL, mean (SD) | 0.54 (0.2) | 0.50 (0.2) | 0.37 | 0.52 (0.24) |
| Baseline RBANS, mean (SD) | 147 (32) | 138 (35) | 0.34 | 144.1 (34) |
| Baseline CSF total tau, pg/mL, mean (SD)b | 55.3 (31) | 55.2 (16) | 0.99 | 55.2 (26) |
| Baseline CSF NfL, pg/mL, mean (SD)c | 4036.5 (3074) | 6272.8 (1995) | 0.08 | 4887.3 (2962) |
| Whole‐brain volume, mm3 ×106, mean (SD) | 1.29 (0.1) | 1.25 (0.1) | 0.07 | 1.2x106 (1.3x105) |
| Ventricles volume, mm3 ×104, mean (SD) | 4.58 (2.4) | 4.81 (2) | 0.20 | 4.6x104 (2.3x104) |
| Midbrain volume, mm3 ×103, mean (SD) | 6.99 (0.9) | 6.61 (0.7) | 0.01 | 6.8x103 (863) |
| SCP volume, mm3, mean (SD) | 404.3 (111) | 344.2 (94) | 0.002 | 378.4 (108) |
| Volume change at 1 year, % median (CI) | ||||
| Whole brain | −0.6 (−0.9 to −0.3) | −1.0 (−1.3 to −0.8) | 0.02 | −0.8 (−1.0 to −0.6) |
| Ventricles | 8.2 (6.8–9.6) | 11.5 (9.2–13.8) | 0.05 | 9.7 (8.4–11) |
| Midbrain | −3.0 (−3.8 to −2.2) | −3.7 (−4.5 to −2.8) | 0.28 | −3.3 (−3.9 to −2.7) |
| SCP | −4.5 (−6.8 to −2.1) | −9.1 (−11.8 to −6.5) | 0.005 | −6.6 (−8.3 to −4.8) |
| Baseline CSF NfL level, pg/mL ×103, mean (SD) | 4.03 (3) | 6.27 (1.9) | 0.08 | 4.8 (2.8) |
| Mean baseline plasma NfL levels, pg/mL (SD) | ||||
| Total | 26 (6) | 61.2 (36) | 43.7 (31) | |
| Age at sampling, year | ||||
| 45–67 | 23.9 (6) | 54.3 (19) | 37.6 (20) | |
| >67–84 | 28.5 (6) | 66.8 (44) | 49.7 (38) | |
| P‐valued | 0.004 | 0.07 | 0.007 | |
| Gender | ||||
| Male | 27 (6) | 55.8 (16) | 43.3 (19) | |
| Female | 25 (7) | 69.1 (52) | 44.2 (33) | |
| P‐valued | 0.24 | 0.60 | 0.11 | |
| MMSE | ||||
| ≤27 | 26.6 (7) | 65.7 (44) | 47.1 (38) | |
| >27 | 25.3 (6) | 55.3 (18) | 39.6 (20) | |
| P‐valued | 0.37 | 0.28 | 0.23 | |
| Mean year 1 plasma NfL levels, pg/mL (SD) | ||||
| Total | 35.3 (14) | 69.3 (45) | 52.4 (38) | |
| Age at sampling, year | ||||
| 45–67 | 32 (11) | 58.6 (24) | 44 (22) | |
| >67–84 | 39.3 (16) | 77.9 (56) | 60 (47) | |
| P‐valued | 0.03 | 0.09 | 0.006 | |
| Gender | ||||
| Male | 34.2 (10) | 64.1 (30) | 51.1 (28) | |
| Female | 36.2 (16) | 76.9 (62) | 53.9 (47) | |
| P‐valued | 0.98 | 0.40 | 0.84 | |
| MMSE | ||||
| ≤27 | 36.7 (13) | 77 (55) | 58.3 (46) | |
| >27 | 33.8 (15) | 58.1 (24) | 45.4 (23) | |
| P‐valued | 0.17 | 0.09 | 0.05 | |
NfL, neurofilament; SD, standard deviation; MMSE, Mini‐Mental State Examination; PSPRS, Progressive Pupranuclear Palsy Rating Scale; SEADL, Schwab and England Activities of Daily Living scale; RBANS, Repeatable Battery for the Assessment of Neuropsychological Disease severity; CSF, cerebrospinal fluid; SCP, superior cerebellar peduncle.
Low baseline versus high baseline NfL between‐group comparison.
Fourteen samples available.
Eight samples available.
Within‐group comparison.
High baseline plasma NfL levels in PSP predict worse functional status at 1‐year follow‐up
A median split of baseline plasma NfL values from the UCSF cohort PSP group was used to define PSP patients with low (<28.4 pg/mL) and high (≥28.4 pg/mL) baseline levels. This median split within the PSP group was implemented because a median split was highly sensitive to classify controls and patients. PSP patients with baseline plasma NfL levels in the upper median for the PSP group had a higher CDR‐sb score at 1‐year follow‐up compared to baseline (PSP patients 6.7 [CI: 3.5–9.6] vs. controls 4.1 [CI: 2.5–5.6] 1‐year follow‐up, P = 0.04) (Fig. 2). There was no significant 1‐year interval change in the CDR‐sb score in PSP patients with baseline plasma NfL levels in the lower median (3.3 [CI: 0.82–5.8] baseline NfL vs. 3 [CI: 0.6–5.3] 1‐year follow‐up, P = 0.33).
Figure 2.
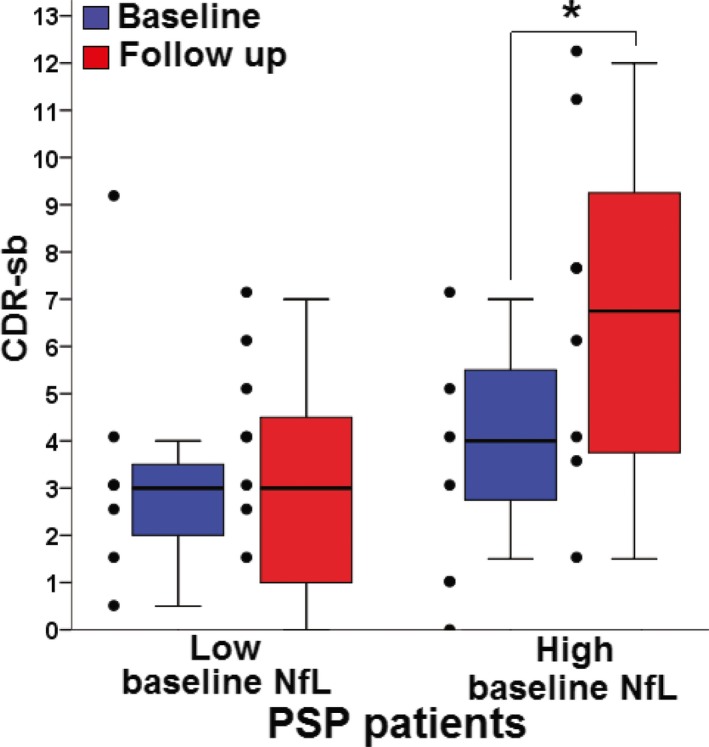
Plasma NfL levels predict cognitive function at 1‐year follow‐up in PSP, UCSF cohort. CDR‐sb scores at baseline (blue) and follow‐up (red) in patients in the low and high median baseline plasma NfL. No change over time is seen in the low baseline group, whereas a significant CDR‐sb score worsening is observed in PSP patients in high baseline plasma NfL levels. Thick horizontal bars represent the median. Upper and lower box limits represent the 75th and 25th percentiles. Error bars represents the minimum and maximum values. Individual data points appear to the left of each box. *Significant within‐group difference, P < 0.05. NfL, neurofilament light chain; PSP, progressive supranuclear palsy; CDR‐sb, clinical dementia rating sum of boxes.
In a validation cohort, high baseline plasma NfL levels in PSP patients predict more severe neurologic, cognitive, and functional decline at 1‐year follow‐up
Mean baseline plasma NfL levels in PSP patients were higher in the Allon cohort than in the UCSF cohort (43.7 ± 2 pg/mL vs. 31 ± 4 pg/mL, respectively, P = 0.01). Therefore, we derived new low and high baseline plasma NfL levels based on a median split (<36.7 pg/mL vs. ≥36.7 pg/mL) of values from this group. Similar to the original cohort, PSP patients with high baseline plasma NfL levels had higher levels at 1‐year follow‐up compared to those with low baseline levels (Table 2). Plasma NfL levels positively correlated with CSF NfL levels at baseline (r = 0.70, P = 0.001) and at year‐1 follow‐up (r = 0.74, P = 0.002) (Fig. 3). Mixed linear models assessed the ability of baseline plasma NfL levels, to predict the annual change in neurologic, cognitive, and functional status, as measured with PSPRS, RBANS, and SEADL, respectively (Fig. 4). Overall, PSPRS scores worsened as a function of time, and baseline NfL plasma concentrations higher than the median predicted a more severe worsening over time, compared to those with plasma NfL lower than the median (P = 0.02). A time‐by‐group interaction supported significant worsening by the end of the year in patients in the high baseline plasma NfL level group (P = 0.04): the percent change difference in the PSPRS score between high and low baseline plasma NfL groups was 5.5 points at week 13 (−7.3% [CI: −11.8 to −2.8] vs. −1.7% [CI: −6.2 to 2.6], respectively), and by week 52, it increased to 7.6 points (−36.5% [CI: −44.3 to −28.8] vs. −28.9% [CI: −35.9 to −22], respectively). Similarly, compared to patients in the lower median, patients with higher baseline plasma NfL had a more rapid decline in functional status, as measured by the SEADL scale (P = 0.03). The mean percent change in the SEADL score at week 52 was −38% (CI: −45.5 to −30.6) in the high NfL level group, but only −20.2% (CI: −31.8 to −8.6) in the lower median group. High baseline plasma NfL also predicted a faster decline in neuropsychological performance at 1 year (P = 0.007). A significant time‐by‐group interaction was observed for the decline in the RBANS score (P = 0.001). Upon 1‐year follow‐up, the decline in the RBANS score in patients with high NfL levels was 23.9% (CI: 18.4–29.3), whereas it was only 11.8% (CI: 7.9–15.7) in those with low baseline NfL levels. When treated as a continuous variable, baseline NfL predicted SEADL (P = 0.003) and RBANS scores (P = 0.002), and terms for time by group interactions were significant for the prediction of PSPRS (P = 0.03) and RBANS scores (P = 0.001).
Figure 3.
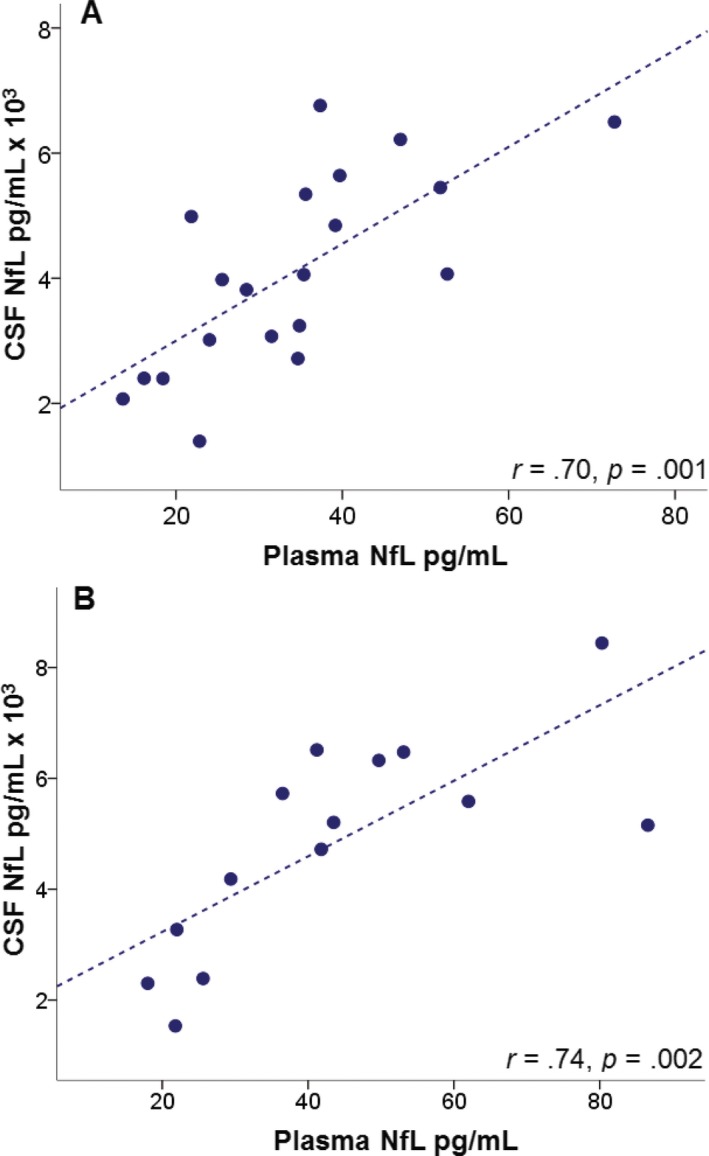
Plasma NfL levels correlate with CSF NfL levels in PSP, Allon cohort. The graph shows plasma and their correlations with CSF NfL values at (A) baseline (n = 20) and (B) 1 year follow‐up (n = 14). NfL, neurofilament light chain; CSF, cerebrospinal fluid; PSP, progressive supranuclear palsy.
Figure 4.
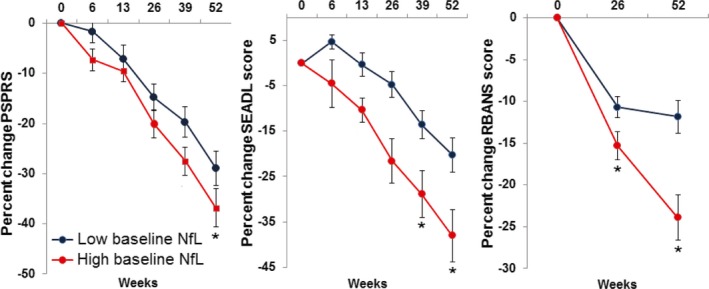
High baseline plasma NfL levels in PSP are associated with more severe neurologic, functional and cognitive decline at 1 year follow‐up, Allon cohort. Graphs represent percent change in clinical scale scores in PSP patients with low (blue) and high (red) median plasma NfL levels at baseline. *Fixed effects of baseline NfL level at the specified time point, P < 0.05. NfL, neurofilament light chain; PSP, progressive supranuclear palsy; PSPRS, progressive supranuclear palsy rating scale score; SEADL, Schwab and England activities of daily living score; RBANS, repeatable battery for the assessment of neuropsychological disease severity score.
Mean baseline plasma NfL values for PSP patients in the Allon cohort were 29.1% higher than in the UCSF cohort. This difference was likely explained by differences in the clinical features of patients in the two cohorts. For example, comparison of available baseline CSF total tau levels showed that PSP patients in the Allon cohort had levels 21% lower (55.2 ± 5 pg/mL vs. 69.9 ± 3 pg/mL P = 0.01) and CSF NfL levels 42% higher (4849 ± 617 vs. 2789 ± 455 P = 0.04) than those in the UCSF cohort. We have previously shown that CSF tau levels are lower in PSP than controls, and relatively lower CSF tau concentrations would be consistent with greater severity.7 In addition, mean baseline PSPRS scores in the UCSF cohort (36 [CI: 27.7–44.2]) were numerically higher (P = 0.39) than the Allon cohort (39.4 [CI: 37.4–41.4]). After log transformation and normalization of plasma values from both cohorts, baseline NfL values in PSP patients in the Allon cohort also differed from control values in the UCSF cohort. These transformed values also significantly predicted changes in clinical variables at 1‐year follow‐up (not shown).
Baseline plasma NfL levels predict changes in whole‐brain and PSP‐related regional brain volumes
Patients with high baseline NfL had greater magnitudes of reduction in whole‐brain volume compared to those with lower baseline NfL levels. A higher degree of volume change at 1‐year was also observed in the SCP of patients with high baseline NfL compared to those with low baseline NfL (Fig. 5).
Figure 5.
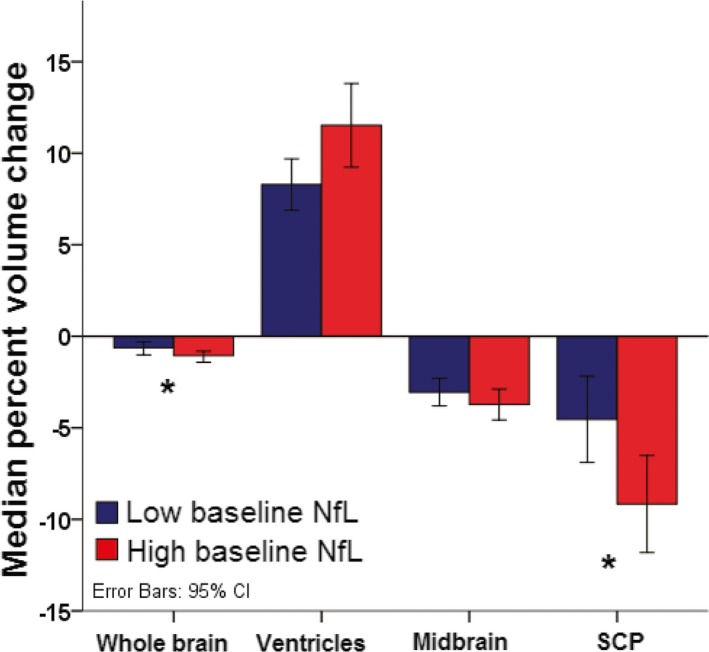
Baseline plasma NfL levels predict higher brain atrophy at 1 year follow‐up, Allon cohort. Median percent decrease in regional volume in PSP patients with low (blue, <36.7 pg/mL) and high (red, ≥36.7 pg/mL) baseline NfL concentrations. Error bars represent 95% confidence intervals. *P < 0.05. NfL, neurofilament light chain; PSP, progressive supranuclear palsy; SCP, superior cerebellar peduncle.
Discussion
This report presents proof of concept that plasma NfL concentrations are quantifiable and elevated in PSP patients compared to age‐matched healthy individuals. NfL concentrations in PSP patients were twice as high as those in controls, and a median cutoff value of 20 pg/mL allowed differentiation of patients and controls with a high level of accuracy. Plasma NfL levels in PSP patients consistently correlated with CSF NfL levels. NfL levels were also useful in predicting clinical progression of PSP. PSP patients with higher baseline NfL levels had a more rapid functional decline compared to patients with low baseline NfL levels. Prediction of functional decline over 1 year was observed in both the original and validation cohorts. NfL levels were sensitive for detection of patients with worse neurological progression, functional decline, and brain atrophy. Differences in the PSPRS scores between PSP patients with high and low baseline NfL levels were observed as early as at 6‐weeks follow‐up. Higher baseline plasma NfL levels also predicted more severe whole‐brain and SCP atrophy at 1‐year follow‐up.
The findings suggest that plasma NfL could be a useful biomarker for assessing disease severity and predicting disease progression in PSP. High baseline plasma NfL levels were associated with a more severe neurological, functional, and cognitive decline at 1 year. The clinical predictive value of NfL is consistent with findings from the davunetide in PSP trial, in which a post hoc analysis in a subset of patients (n = 19) revealed that longitudinal CSF NfL increases correlated with worsening of clinical ocular motor ratings on the PSPRS.8 Similarly, CSF NfL levels have been observed to correlate with more severe parkinsonian symptoms, as measured with the Hoehn and Yahr staging scale, but not with disease duration in patients with PSP and PD.5 We have previously observed elevations of CSF NfL in PSP patients compared to controls.7 Although CSF NfL levels did not correlate with clinical or neuropsychological ratings in PSP, high CSF NfL levels were associated with higher CDR‐sb and lower MMSE scores in behavioral variant frontotemporal dementia, semantic dementia, and nonfluent primary progressive aphasia.7 Multiple sclerosis patients with increased CSF NfL levels also show significantly higher clinical severity scores and elevated CSF lymphocyte cell counts and oligoclonal bands.22 In contrast to the longitudinal elevations observed in CSF NfL in the davutenide trial,8 no significant within‐group changes in plasma NfL concentrations were observed with time in this study. This was observed in the control and PSP groups in the UCSF cohort and in the PSP group in the Allon cohort (Tables 1, 2). Our findings are consistent with a recent study in ALS patients, in which blood NfL levels did not increase as a function of time, but maintained a relatively constant expression.14 Nevertheless, Lu and colleagues also observed that plasma NfL distinguished ALS patients from healthy controls with sensitivity of 0.90 and specificity of 0.71, and ALS patients with higher baseline blood NfL had an increased mortality hazard ratio.14 Together, these findings support that plasma NfL may be of value in monitoring disease progression, although whether levels change over time within subjects will require further study. The findings also suggest that plasma NfL could be a useful theragnostic biomarker in PSP clinical trials of drug candidates with disease‐modifying potential.
In this study, plasma NfL levels at baseline also predicted changes in global and regional brain volume detected by MRI. Greater rates of volume loss were observed at 1 year in the SCP in patients with high baseline plasma NfL. The SCP is mainly formed by the dentatorubrothalamic tract, a white matter structure that has been shown to undergo severe and selective neurodegenerative changes in PSP, compared not only to normal controls, but also to patients with other forms of atypical parkinsonism.23 In addition, partial correlation analyses revealed a biologically relevant relationship between plasma NfL levels and the amount of brain atrophy at 1 year (Fig. 5). In agreement with these data, changes in CSF NfL showed a negative correlation with changes in SCP volumes over 1 year in a subset of patients in the recent davunetide trial.8 CSF NfL levels have also accompanied neuroimaging changes in other neurological conditions. For example, CSF NfL levels showed negative correlations with frontal, temporal, parietal, occipital, and cingulate gray matter density in frontotemporal dementia.7 CSF NfL correlated with the number of T1, T2, and enhancing lesions seen on MRI in a cohort of patients with untreated multiple sclerosis.22 In addition, higher baseline CSF NfL levels in multiple sclerosis patients have been observe to correlate with lower brain volumes at baseline and with higher T2 lesions counts after 1 year.24
Plasma NfL levels in PSP patients correlated with CSF NfL levels at baseline and at 1‐year follow‐up. These data are in agreement with previous observations of a strong correlation between serum and CSF NfL levels in patients with neurodegenerative conditions such as ALS and AD, as well as in multiple sclerosis and Guillain–Barré syndrome, but not in normal controls.13, 14 Detection of NfL in blood likely reflects rising CSF NfL concentrations secondary to neuronal injury and blood–brain barrier disruption.9 Accordingly, NfL has been observed to be an extremely sensitive marker of axonal injury, with elevated concentrations measured after neuronal insults even in the absence of clinical symptoms.10 The data presented here add to the body of evidence supporting that NfL is a biomarker of pathophysiologic relevance in PSP and may be of value to track disease progression and outcomes.
There are several limitations in this study. The sample size in the original cohort was limited and the diagnostic sensitivity of plasma NfL could not be confirmed in the validation cohort, since it did not include control subjects. In addition, although patients and controls were matched for age in the UCSF cohort, disease duration is difficult to accurately determine in PSP participants due to the retrospective nature of the research diagnostic criteria. This raises the possibility that plasma NfL measurement may have limited diagnostic sensitivity for patients in early stages of the disease. Most PSP subjects lacked neuropathological diagnostic confirmation. It is therefore possible that a small number of subjects may not have underlying PSP pathology. Nevertheless, a strength of the diagnostic criteria for PSP is that they are highly predictive of underlying PSP or 4R tau pathology.25 Although PSP pathology was confirmed in all cases for which autopsy was available, additional studies in controls, atypical parkinsonism, and PD will be necessary to fully establish the diagnostic sensitivity of plasma NfL for PSP. Mean baseline plasma NfL levels were 29.1% higher in the Allon cohort than in the UCSF cohort. It is suspected that biological differences contributed significantly to this discrepancy. The mean interval between symptom onset and baseline sample collection was 5.3 years in the UCSF cohort, but this interval was not determined in the Allon cohort, making it possible that samples in the Allon cohort were collected at a different point in the natural history of the disease. This is supported by differences between cohorts observed in CSF total tau and NfL. This limitation is important because NfL was only measured at two time points per subject over 1 year. Control for interval of disease onset to sampling, and more frequent plasma sampling and prolonged follow‐up periods will be needed to determine the utility of plasma NfL as a marker of disease progression. Variability was likely not due to immunoassay‐related variables as all samples were run using the same ELISA kit batch. Nevertheless, measurements could have been affected by variations in sample handling conditions that were not controlled, such as time of sample collection, length of exposure to room temperature, or length of frozen storage. Further testing that accounts for these variables should also be conducted to assess the external validity of plasma NfL measurements for diagnostic or prognostic purposes. Although the available number of CSF samples to correlate with plasma NfL levels was only a fraction of the Allon cohort, CSF and plasma NfL values showed robust correlations at baseline and follow‐up, and they were consistent with previous reports.13, 14
This study suggests that measurement of plasma NfL concentrations is feasible and may be of value for monitoring PSP disease activity and determining prognosis in clinically diagnosed PSP patients. A strength of this study was that our validation cohort was derived from a large, multicenter clinical trial, suggesting that plasma NfL concentration may be useful for monitoring the effects of therapeutic interventions in PSP. This biomarker is being increasingly employed in clinical trials in other neurological diseases. For example, CSF NfL levels in patients with multiple sclerosis receiving treatment were unchanged at 1 year follow‐up, while patients receiving placebo showed significant increases.24 Similarly, CSF NfL levels have been shown to be sensitive to therapeutic interventions in paraneoplastic disease26 and central nervous system HIV infection.27 Clinical trials of new tau directed agents are increasingly being pursued in PSP.8, 28 Since PSP is a rare disease in which CSF and MRI data can be difficult to collect due to patient factors (e.g., axial rigidity) and geographic dispersion, the availability of a peripheral biomarker of disease activity, such as plasma NfL, could greatly accelerate therapeutic development.
Authors Contribution
J. C. R. analyzed the data, contributed to clinical data gathering and prepared the manuscript; A. K., J. B. and R. T. participated in clinical data gathering; K. B., V. L. and H. Z. performed sample analysis and provided critical revision of data; J. H. K., H. R. and B. L. M. contributed patient recruitment and provided critical revision of data; A. L. B. contributed conception, design, study coordination and critical revision of data. All authors reviewed the manuscript.
Conflict of Interest
None declared.
Acknowledgments
This study was supported by the National Institutes of Health, 4‐Repeat Tauopathy Neuroimaging Initiative (4RTNI, AG03879); the National Center for Advancing Translational Science (NCATS) and National Institute of Neurological Disorders and Stroke (NINDS), Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL, NS092089); the National Institute on Aging, Frontotemporal Dementia: Genes, Images and Emotions (AG019724); the National Research Service Award, Institutional Research Training Grant (AG23481‐11); the Tau Consortium; the Hillblom Foundation, Hillblom Aging Network (P0502788) the Swedish Research Council; the Torsten Söderberg Foundation; the Knut and Alice Wallenberg Foundation; the Swedish State Support for Clinical Research; and VINNOVA.
References
- 1. Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Arch Neurol 1964;10:333–359. [DOI] [PubMed] [Google Scholar]
- 2. Kawashima M, Miyake M, Kusumi M, et al. Prevalence of progressive supranuclear palsy in Yonago, Japan. Mov Disord 2004;19:1239–1240. [DOI] [PubMed] [Google Scholar]
- 3. Nath U, Ben‐Shlomo Y, Thomson RG, et al. The prevalence of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome) in the UK. Brain 2001;124:1438–1449. [DOI] [PubMed] [Google Scholar]
- 4. Golbe LI. Progressive supranuclear palsy. Semin Neurol 2014;34:151–159. [DOI] [PubMed] [Google Scholar]
- 5. Hall S, Ohrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 2012;69:1445–1452. [DOI] [PubMed] [Google Scholar]
- 6. Holmberg B, Rosengren L, Karlsson JE, Johnels B. Increased cerebrospinal fluid levels of neurofilament protein in progressive supranuclear palsy and multiple‐system atrophy compared with Parkinson's disease. Mov Disord 1998;13:70–77. [DOI] [PubMed] [Google Scholar]
- 7. Scherling CS, Hall T, Berisha F, et al. Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol 2014;75:116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boxer AL, Lang AE, Grossman M, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double‐blind, placebo‐controlled phase 2/3 trial. Lancet Neurol 2014;13:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gentil BJ, Tibshirani M, Durham HD. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res 2015;360:609–620. [DOI] [PubMed] [Google Scholar]
- 10. Neselius S, Brisby H, Marcusson J, et al. Neurological assessment and its relationship to CSF biomarkers in amateur boxers. PLoS One 2014;9:e99870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martinez MA, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler 2015;21:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Limberg M, Disanto G, Barro C, Kuhle J. Neurofilament light chain determination from peripheral blood samples. Methods Mol Biol 2016;1304:93–98. [DOI] [PubMed] [Google Scholar]
- 13. Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 2013;8:e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu CH, Macdonald‐Wallis C, Gray E, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015;84:2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rissin DM, Kan CW, Campbell TG, et al. Single‐molecule enzyme‐linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 2010;28:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome): report of the NINDS‐SPSP international workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 17. Golbe LI, Ohman‐Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain 2007;130:1552–1565. [DOI] [PubMed] [Google Scholar]
- 18. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 19. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 20. O'Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol 2008;65:1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwab R, England A. Projecton technique for evaluating surgery in Parkinson's disease In: Gillinham F, Donaldson M, eds. Third symposium on Parkinson's disease research. Edinburgh, Scotland: ES Livingston, 1969. p. 152–157. [Google Scholar]
- 22. Villar LM, Picon C, Costa‐Frossard L, et al. Cerebrospinal fluid immunological biomarkers associated with axonal damage in multiple sclerosis. Eur J Neurol 2015;22:1169–1175. [DOI] [PubMed] [Google Scholar]
- 23. Surova Y, Nilsson M, Latt J, et al. Disease‐specific structural changes in thalamus and dentatorubrothalamic tract in progressive supranuclear palsy. Neuroradiology 2015;57:1079–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuhle J, Disanto G, Lorscheider J, et al. Fingolimod and CSF neurofilament light chain levels in relapsing‐remitting multiple sclerosis. Neurology 2015;84:1639–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Respondek G, Stamelou M, Kurz C, et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord 2014;29:1758–1766. [DOI] [PubMed] [Google Scholar]
- 26. Pranzatelli MR, Tate ED, McGee NR, Verhulst SJ. CSF neurofilament light chain is elevated in OMS (decreasing with immunotherapy) and other pediatric neuroinflammatory disorders. J Neuroimmunol 2014;266:75–81. [DOI] [PubMed] [Google Scholar]
- 27. Meulendyke KA, Queen SE, Engle EL, et al. Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. J Neurovirol 2014;20:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsai RM, Boxer AL. Clinical trials: past, current, and future for atypical Parkinsonian syndromes. Semin Neurol 2014;34:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]


