Abstract
Structural connectivity analyses by means of diffusion‐weighted imaging have substantially advanced the understanding of stroke‐related network alterations and their implications for motor recovery processes and residual motor function. Analyses of the corticospinal tract, alternate corticofugal pathways as well as intrahemispheric and interhemispheric corticocortical connections have not only been related to residual motor function in cross‐sectional studies, but have also been evaluated to predict functional recovery after stroke in longitudinal studies. This review will consist of an update on the available literature about structural connectivity analyses after ischemic motor stroke, followed by an outlook of possible future directions of research and applications.
Introduction
During the past decades, various imaging studies have substantially contributed to the understanding of motor recovery after stroke. Dynamic alterations of regional brain activity, neuronal excitability and interregional interactions both at the cortical and subcortical level have been related to residual motor functioning and recovery processes after stroke.1, 2, 3 Aside from its importance for acute stroke diagnosis and management, magnetic‐resonance‐based structural imaging has provided pivotal insights into the functional role of stroke‐related changes in the underlying structural networks (Table 1). In this article, we will review the available literature on stroke‐related structural connectivity changes and their implications for both residual motor functioning and motor recovery.
Table 1.
Selection of important studies for structural connectivity analyses in motor recovery research after stroke
| Study (Title, Author, Journal, Year) | Comment |
|---|---|
| Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke, Lindenberg et al., Neurology, 201016 | This is one of the first studies which nicely demonstrates that alternate corticofugal fibers, such as of the cortico‐rubro‐spinal tract, might play a role in motor recovery after stroke, in addition to the contribution of the corticospinal tract |
| Network analysis detects changes in the contralesional hemisphere following stroke, Crofts et al., Neuroimage, 201124 | This graph‐theoretical whole‐brain network analysis shows that alterations in “communicability,” a measure of information flow through neuronal networks, can separate chronic stroke patients and controls and adds to the understanding of large‐scale network alterations after stroke |
| The PREP algorithm predicts potential for upper limb recovery after stroke, Stinear et al., Brain, 201255 | This study proposes a simple algorithm including clinical scores, measures of corticospinal excitability and structural integrity of the corticospinal tract for the prognosis of upper limb recovery after stroke in individual patients. |
| A new early and automated MRI‐based predictor of motor improvement after stroke, Granziera et al., Neurology, 201230 | This longitudinal study applies modern diffusion spectrum imaging to explore time‐dependent changes in white matter integrity of intra and interhemispheric corticocortical motor connections between primary and secondary motor areas and to relate them to motor recovery after stroke |
| Parietofrontal motor pathways and their association with motor function after stroke, Schulz et al., Brain, 201522 | This study evaluates the functional importance of the structural integrity of ipsilesional parietofrontal motor pathways for recovered hand function while specifically considering the impact of the damage to the corticospinal tract |
Diffusion‐Weighted Imaging as a Tool for Structural Connectivity Analyses
The motor system of the human brain comprises a complex, distributed and bilateral network including multiple cortical and subcortical brain regions. Proper neuronal information throughput within this network requires intact nodes and interconnecting neuronal pathways. Acute ischemic strokes can disrupt these nodes and edges and lead to time‐ and recovery‐dependent changes in the structural network characteristics. Diffusion‐weighted magnetic resonance imaging is one modern technique for the analysis of such structural network alterations after stroke. It is based on measuring the diffusion of water molecules within brain tissue. The most commonly used application to describe its characteristics voxel‐by‐voxel is diffusion tensor imaging (DTI). This applies a one‐tensor model, which is a geometric object in the three‐dimensional space. In white matter tissue with coherent white matter fibers, the free water diffusion is restricted, primarily due to the lipophilic characteristics of myelin sheaths, axonal membranes, and glial cells.4 Second, it is further restricted due to fiber bundle orientation on a macroscopic scale. This leads to a fast diffusion of water molecules along the main fiber direction and a slowed perpendicular diffusion.4, 5, 6 In DTI, three eigenvalues describe the ellipsoid shape of the tensor and allow the estimation of different diffusivity parameters7: The mean diffusivity is thought to describe the average magnitude of molecular water translation in all directions. The radial (RD) and axial diffusivities (AD) primarily represent the directional diffusions in or orthogonal to the direction of the fiber bundles and have been associated with the constitution of myelin sheaths and axons, respectively. Most commonly used in the literature is the fractional anisotropy (FA), a measure of directionality of the diffusion tensor with values between one (intact white matter) and zero (disrupted white matter), serving as a surrogate parameter for the microstructural white matter integrity.8 Given that ischemic strokes lead to dramatic microstructural white matter changes and the principal assumption that proper neuronal information flow critically depends on structural integrity, FA and the other diffusion metrics can thus serve as sensitive tools to (1) noninvasively monitor both time‐dependent changes in structural network properties and (2) to make inferences about functional aspects of the underlying pathways as well. For FA, for instance, both recovery‐dependent decreases and increases are reported: While the primary lesion and also secondary neurodegenerative processes, for example, Wallerian degeneration and gliosis in regions remote to the primary stroke lesion, can result in FA decreases, increases in FA have also been reported and related to reorganizational white matter processes and restoration of neuronal connectivity during recovery after stroke.9, 10, 11, 12, 13
Aside from voxel‐wise inferences about the integrity suitable for whole‐brain analyses,11, 12, 14 DTI also allows the noninvasive reconstruction of white matter tracts. Having estimated one or multiple fiber directions15 in each voxel, deterministic or probabilistic diffusion tensor tractography algorithms can be applied to estimate probable trajectories between selected seed and target regions that can be further analyzed in various ways. For instance, the absolute fiber count between two areas of interest,16, 17, 18 the lesion load to the reconstructed tracts,19, 20, 21 or the white matter integrity (absolute or proportional FA values/FA asymmetry) along the whole tracts or at predefined regions within the tracts22, 23 are some approaches. On a whole‐brain and large‐scale network perspective, fiber counts between numerous cortical and subcortical brain regions can be analyzed by means of graph‐theoretical measures.24 In fact, while fiber counts are thought to better represent connectivity than regional diffusion metrics, we will summarize both approaches as measures of connectivity in this review.
For DTI and tract reconstructions, a notable limitation is its inability to resolve intravoxel fiber orientation heterogeneity. Despite that multiple tensor fitting algorithms have been developed,15 DTI has been found to perform poorly in regions of crossing or kissing fibers. Therefore more powerful, model‐free approaches have been developed such as high angular resolution diffusion,25 and q‐ball imaging,26 diffusion spectrum imaging,27 or constrained spherical deconvolution imaging.28 However, so far only a few studies have applied these techniques in stroke patients.29, 30, 31
Network Concepts for Structural Connectivity Analyses After Stroke
Figure 1 gives an introductory overview about the concept of different, distributed motor networks at the corticofugal and corticocortical level which have been investigated by means of structural connectivity analysis after stroke. The corticospinal tract (CST) originating from the primary motor cortex is considered the most crucial outflow tract of the motor system in the human brain.32, 33 It has been hypothesized that its structural properties and recovery‐related changes after stroke would critically relate to residual motor functioning and recovery processes which has been studied in detail in the past decades (Fig. 1‐1). In fact, as indicated by Figure 2 which gives a synopsis of the publication history of structural connectivity analyses in motor stroke recovery research, early work goes back to the year 2000. Over time, this tract has clearly been the most extensively addressed pathway in structural analyses related to motor recovery after stroke. Considering the motor system as a bilateral network involving multiple cortical and subcortical brain regions and neuronal circuits, subsequent research has hypothesized that not only the primary CST but other motor networks may contribute to motor recovery after stroke as well. For example, based on animal data, alternate corticofugal fibers, such as corticospinal pathways from secondary motor areas or cortico‐subcortical circuits have gained particular interest in 2010 and later (Fig. 1‐2). For a long time, corticocortical networks have been largely neglected and brain imaging has focused on pathways at the corticofugal level. However, more recent studies on intra (Fig. 1‐3) and interhemispheric (Fig. 1‐4) circuits between primary and secondary motor and nonmotor areas at the corticocortical level have significantly extended the concept of interacting networks for recovery processes after motor stroke (Fig. 2). For example, functional imaging and electrophysiological studies applying transcranial magnetic stimulation (TMS) have suggested a critical importance of interhemispheric interactions for stroke recovery.34 Subsequent brain imaging has sought to investigate these relationships from the structural point of view. Taken together, there is converging evidence that the structural status of each of these important networks can be related to recovery processes and residual motor functioning after stroke. However, future structural connectivity analyses will have to explore how the properties of these networks might interact and add to better understanding recovery‐related brain plasticity. Notably, in order to present a rather illustrative and instructive than exhaustive overview, the selection of studies included in this review was guided by subjective criteria such as novelty, quality of the study and impact to the field. Hence, objective criteria of a systematic search of the literature cannot be given.
Figure 1.
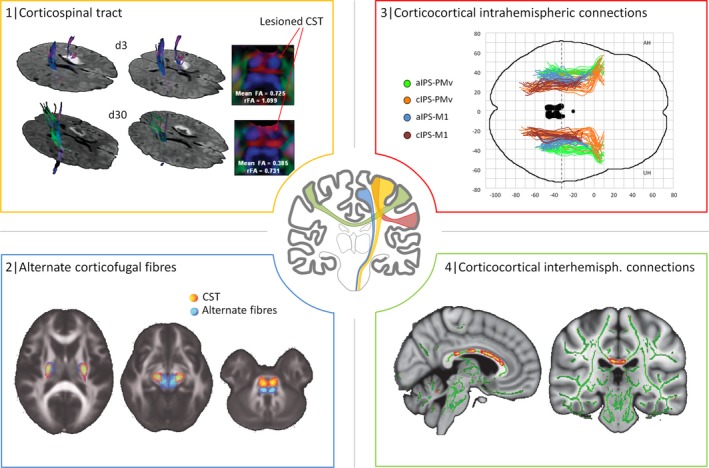
Networks of interest in structural connectivity analyses after stroke. This figure is to illustrate how structural imaging can be used to study stroke‐related changes in structural connectivity in different networks. The CST originating from the primary motor cortex has been studied by numerous studies (represented in yellow): For example, diffusion tensor imaging has been used to reconstruct the CST in a 47‐year‐old man 3 (d3) and 30 days (d30) after left‐sided stroke. Reduction in fractional anisotropy in the lesioned left CST at pons level at d30 (FA, rFA values compared to the contralateral side) was regarded as Wallerian degeneration (1, adapted with permission).45 Recent imaging data have suggested that not only the CST but also alternate motor fibers might contribute to motor functioning and recovery after stroke (represented in blue): For instance, tractography was used to reconstruct such alternate fibers (in blue), probably paralleling the cortico‐rubro‐spinal system, in addition to the CST (2, adapted with permission).38 Intrahemispheric corticocortical connections (in red) have been addressed by more recent analyses, for instance, between frontal and parietal motor areas. It could be shown that aside from the CST, also parietofrontal structural connectivity relates to residual motor function after stroke. M1 primary motor cortex, PMv ventral premotor cortex, aIPS anterior/cIPS caudal intraparietal sulcus (3, adapted with permission from Robert Schulz et al. Parieto frontal motor pathways and their association with motor function after stroke. Brain (2015) 138 (7): 1949–1960. (Fig. 1.) By permission of Oxford University Press on behalf of The Guarantors of Brain. This image is not covered by the terms of the Creative Commons/Open Access license of this publication. For permission to reuse, please contact the rights holder).22 Ultimately, also structural connections between both hemispheres have been evaluated in regard of their contribution for motor functioning and recovery after stroke. For example, regional FA values along the corpus callosum were related to residual motor function in the chronic stage of recovery (4, adapted with permission).72 Notably, applications of large‐scale network analyses were not included in this synopsis for the sake of clarity. CST, corticospinal tract; FA, fractional anisotropy; rFA, relative FA.
Figure 2.
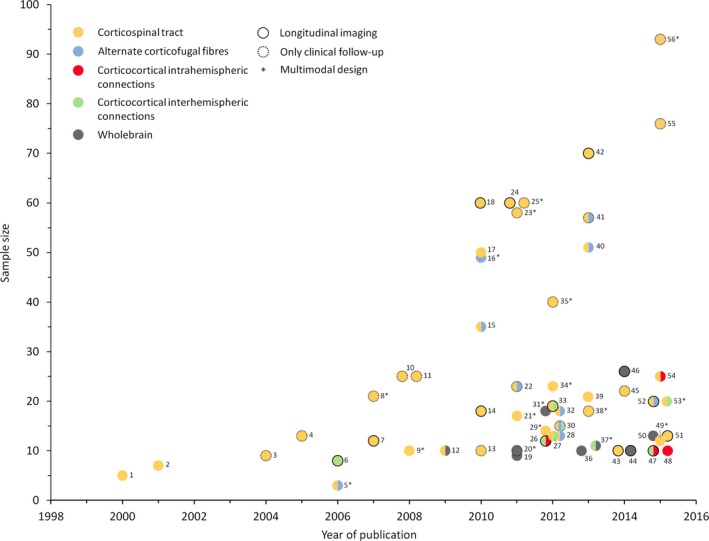
Synopsis of structural connectivity analyses. Individual studies considered in the present review are summarized with the year of publication, the sample size (patients) and their main focus of the structural analyses indicated by the color scheme. Cross‐sectional studies are represented by colored dots without any frame; a black frame represents studies with longitudinally repeated imaging. Cross‐sectional imaging studies with only clinical/behavioral follow‐up are indicated by dotted frames. Notably, the selection of studies included in this study is not supposed to be exhaustive but is rather made to illustrate previous and recent developments in structural connectivity analyses after stroke. In cases of multiple studies of similar sample sizes in 1 year, the representing dots were placed next to each other for illustration purposes. The references are numbered consecutively and listed below with the first author and the year of publication, et al. has been omitted for sake of readability. 1: Werring 200036; 2: Pierpaoli 200137; 3: Thomalla 200435; 4: Konishi 200551; 5: Newton 200661; 6: Gupta 200668; 7: Liang 200740; 8: Stinear 200780; 9: Schaechter 200817; 10: Jang 200847; 11: Nelles 200818; 12: Schaechter 200912; 13: Sterr 201050; 14: Radlinska 201041; 15: Lindenberg 201016; 16: Yeo and Jang 201059; 17: Zhu 201019; 18: Puig 201039; 19: Crofts 201124; 20: Bosnell 201148; 21: Qiu 201183; 22: Riley 201162; 23: Kwon 201181; 24: Puig 201145; 25: Nouri 201179; 26: Granziera 201230; 27: Borich 201253; 28: Schulz 201254; 29: Lotze 201278; 30: Lindenberg 201238; 31: Wang 201214; 32: Rüber 201258; 33: Radlinska 201269; 34: Carter 201274; 35: Stinear 201255; 36: Kalinosky 201386; 37: Chen and Schlaug 201371; 38: Vargas 201384; 39: Park 201323; 40: Kou 201320; 41: Phan 201321; 42: Puig 201346; 43: Groisser 201442; 44: Takenobu 201411; 45: Sterr 201449; 46: Kuceyeski 201477; 47: Lin 201531; 48: Schulz 201564; 49: Volz 201582; 50: Li 201572; 51: Song 201573; 52: Zheng and Schlaug 201560; 53: Liu 201570; 54: Schulz 201522; 55: Feng 201552; 56: Byblow 2015.85
Structural Connectivity Analyses of Corticospinal Pathways
The importance of plastic structural changes in the CST for motor stroke recovery has been assessed in detail by various studies. Stroke‐related microstructural damage to the ipsilesional CST in the cerebral peduncles has been found by cross‐sectional studies within the first 2 weeks,35 the first 2 to 6 months36 and after 1 year.37 Widespread ipsilesional FA reductions occur along the tract from the cerebral peduncle to subcortical white matter.38 Longitudinal data have indicated that FA reductions of the pontine ipsilesional CST were evident after 30 days, but not early after 12 h or 3 days and they have been associated with the motor outcome at the same time.39 For stroke patients with subcortical lesions involving the posterior limb of the internal capsule (PLIC), one study has suggested a progressive FA decrease above and below the lesion site from 1 week to 3 months after stroke. The amount of FA reduction has been correlated with the change in motor function.40 This surrogate of anterograde and retrograde white matter degeneration after stroke has been similarly found in another group of subacute patients with subcortical lesions, though a change in FA over time between 3 weeks and 6 months has not been detected.41 More recently, an analysis in stroke patients with a broad spectrum of cortical and subcortical lesions has revealed FA reductions particularly in the early subacute stage. However, only the subsequent subacute loss in FA has correlated with dexterity, an important factor of recovered hand function in the chronic stage. In contrast, the concurrent increase in axial diffusivity (AD) has been found to be a strong predictor of the degree of motor function in both the subacute and chronic phase.42 Hence, providing complementary information on structure–function relationships, different tensor‐derived diffusion metrics such as FA or AD might have differential value for prognosis of motor recovery.42 Similarly, such synergistic effects of different diffusion indices have been demonstrated comparing common diffusion tensor parameters and more elaborated techniques such as diffusion kurtosis imaging in stroke patients43 and in healthy aging.44 However, to what degree common diffusion metrics and the more recent and probably more tissue‐specific indices can add in structural connectivity analyses to better understand and predict motor function and recovery after stroke remains a topic of future work.
Alternative studies on cortical and subcortical stroke patients have related early CST damage at the level of the internal capsule45 or the cerebral peduncle35 to the motor outcome 3 months after stroke as well. Notably, a recent analysis has complemented these findings associating the integrity of the ipsilesional CST 1 month after the ictal event with long‐term outcome over 2 years.46 Similar findings have been reported for pontine stroke patients in which the status of the ipsilesional CST within the first month has been found to relate to the recovery over the 6 months subsequently.47 In the same way, an additional study has related ipsilesional CST integrity to short‐term improvements in a visuomotor‐tracking training.48 Aside from diffusivity‐related measures of CST connectivity, few studies have used the lesion load (i.e., spatial overlap between the stroke lesion and the CST derived from a healthy control group) to infer the level of damage. These studies have correlated the lesion load with residual motor function in the chronic stage of recovery19, 49, 50 or have found an association between the acute lesion load and motor recovery over 3 months.51, 52 In summary, the data have shown that ischemic strokes lead to time‐dependent secondary degeneration of ipsilesional corticospinal fibers both distal and proximal of the lesion, critically influencing residual motor function and recovery during rehabilitation. Comparing functional scores with imaging metrics, some studies have argued that structural connectivity indices might be better than clinical scores at certain time points in predicting recovery.45 In fact, rather than evaluating whether one or the other will be superior, we argue that it will be more important for future longitudinal studies to answer how both structural and clinical measures might interrelate with each other in terms of their contribution to motor recovery prediction after stroke. In this regard, open questions moreover concern which clinical scores might be of best value in complex prediction models, and to what extent their contribution might be time‐dependent given changing structural connectivity profiles after stroke.
Aside from the ipsilesional tract, structural connectivity changes have been reported for the contralesional corticospinal pathways. For example, decreased FA values have been detected bilaterally in severely impaired chronic stroke patients, whereas increased values have been found in patients with more favorable outcomes. Across both groups the CST status has correlated with motor function.12 This involvement of both CSTs has been similarly suggested in a recent study which has shown that only the mean FA value at the contralesional PLIC was significantly associated with residual motor function.53 These findings highlight one potentially critical point: Various studies have related the integrity indices of the ipsilesional CST to the value of the contralesional tract to account for intersubject variability in premorbid white matter microstructure.16, 35, 41, 54, 55 Despite that gross structural changes are naturally more evident in the ipsilesional than in the contralesional tracts, this approach neglects absolute and side‐specific CST alterations which might show differential relations to motor functioning and recovery after stroke as well. Hence, in future studies, for example, with more significantly impaired patients that are less likely to show cross‐correlations between the ipsi‐ and contralesional CST indices, more complex modeling will be needed to specifically adjust the target effects of the ipsilesional CST to the contralesional one and vice versa.
Structural Connectivity Analyses of Alternate Corticofugal Pathways
Aside from the CST, other descending pathways have gained growing interest in regard to structure–function relationships after stroke. Previous animal56 and human studies57 have hypothesized a relevant contribution of alternate motor fibers, such as the cortico‐rubro‐spinal and cortico‐reticulo‐spinal system, to support motor functioning in patients with CST damage. Confirming this hypothesis in patients, it has been shown that the structural integrity of the CST correlated better with residual motor function after stroke when the integrity of such alternate motor fibers were also considered in the model.16 More recent studies have revealed significant increases of white matter integrity in or surrounding the ipsilesional red nucleus during normal recovery after stroke.11, 58, 59 Interventional studies focusing on motor improvement during intensive training have associated baseline FA and RD values38 and also training‐related FA increases of the alternate motor fibers with functional improvement over time.60 It has been argued that the nature of the cortico‐rubro‐spinal system with crossed and uncrossed fibers from multiple cortical regions may potentially explain why such recovery‐ and training‐related plastic remodeling can be particularly detected and correlated with motor function compared to the CST.60 Despite these first stimulating results, the underlying processes for such plastic changes within alternate corticofugal pathways still remain very vague. While most studies have primarily focused on FA values, few studies have evaluated AD and RD aiming to provide additional information on the different microstructural components such as axons and myelin sheaths. The combination of larger sample sizes, more sophisticated models considering various diffusion indices simultaneously and including modern imaging techniques such as diffusion spectrum and magnetization transfer ratio imaging31 might be helpful to investigate this further.
As another example for alternate corticofugal fibers, animal studies have shown that the CST does not only receive inputs from the primary motor cortex but also from secondary motor areas such as the dorsal and ventral premotor cortex and the supplementary motor area. Increased brain activation in ipsilesional primary and secondary motor regions has been considered to reflect an increased reliance on undamaged ipsilesional corticospinal fibers.61 To investigate this hypothesis, trajectories originating from these areas have been reconstructed and their tract‐related lesion load20, 21, 61, 62 or white matter integrity54 have been correlated with motor function or training gains under therapy in chronic stroke patients. In detail, in the early stage after stroke the amount of damage to these different tracts has appeared to be related to the motor function after 3 months in a rather unspecific way.21 In contrast, in the chronic stage of recovery it has been shown that, aside from corticospinal fibers originating from the primary motor cortex, fibers from the dorsal premotor cortex have been specifically associated with motor function.54 Though, since other studies on tract‐related lesion overlap have reported either unspecific20 or no associations with baseline motor function but training gains after therapy,62 there is still ongoing debate to what extent secondary corticofugal fibers really relate to motor output after stroke and perhaps will help to infer further potential for rehabilitation.
Structural Connectivity Analyses of Corticocortical Connections
Various functional imaging studies have contributed to the understanding of widespread, time‐dependent changes in brain activation patterns with recovery‐related alterations of interregional corticocortical interactions between motor and nonmotor areas.1, 2 While structural connectivity analyses on the corticocortical level63 have already been used to infer structure–function relationships in healthy aging, such analyses are still rare with regard to motor recovery research after stroke.
With regard to intrahemispheric corticocortical interactions, previous functional imaging studies have shown that interactions between the primary motor cortex, the ventral premotor cortex and motor areas along the intraparietal sulcus are relevant for skilled hand function. In fact, a recent study has revealed that the integrity of ipsilesional parietofrontal pathways between the anterior intraparietal sulcus and the ventral premotor cortex along the superior longitudinal fascicle, as well as the ventral premotor and the primary motor cortex contribute to residual motor function in chronic stroke patients. Notably, this positive contribution was found in addition to that of the CST22 indicating that the combined analysis of different neuronal circuits will become progressively more important for a better understanding of the interrelations between corticospinal and corticocortical networks and their impact for stroke recovery. In good agreement, previous whole‐brain voxel‐wise analyses have also suggested that FA values within regions of the ipsilesional superior longitudinal fascicle are linked to residual motor function.12, 14 A functional role of corticocortical connections between the primary motor cortex and frontal secondary motor areas has also been found in well‐recovered chronic stroke patients with isolated subcortical lesions in which the integrity of the ipsilesional tract between the dorsal premotor cortex and the primary motor cortex has been associated with better performance.64 Recent diffusion spectrum imaging has shown that plastic changes in specific corticocortical motor connections of the contralesional hemisphere early after stroke are associated with subsequent recovery.30 It also shows the degree to which connectivity remodeling depends on axonal and/or myelin alterations as directly assessed by means of magnetization transfer ratio imaging.31
Given converging evidence that interhemispheric interactions between primary and secondary motor areas of the unaffected and the affected hemisphere play important roles,3, 65, 66, 67 it was also of particular interest to what degree the underlying interhemispheric structural connectivity via transcallosal commissural projections might be related to motor functioning and recovery after stroke. Indeed, studies have revealed that transcallosal motor fibers degenerate after stroke14, 68, 69, 70 depending on the amount of damage to the ipsilesional CST.69, 70 Furthermore, recent multimodal analyses in chronic stroke patients have shown that the disrupted interhemispheric, structural connection between the primary motor cortices might come along with enhanced resting‐state functional connectivity, potentially reflecting compensatory or reactive brain plasticity. This finding might be specific to well‐recovered70 but not to more severely affected patients.71 However, the small sample sizes of 20 and 11 patients, respectively, limit our ability to draw conclusions at this point. More importantly, the structural connectivity changes in transcallosal connections have also been related to the motor outcome after stroke.14, 38, 71 In contrast to previous studies involving chronic stroke patients with cortical and subcortical lesions,12 more recent longitudinal whole‐brain analyses in patients with subcortical and pontine lesions have found significant correlations for larger regions within the corpus callosum in the subacute and chronic stage, indicating that stroke locations and time after stroke might influence these structure–function relationships.72 Combining whole‐brain diffusion analysis, functional MRI and tractography, another study has related the white matter integrity of the transcallosal fibers connecting sensorimotor cortices, supplementary motor areas, ventral premotor cortices and parietal brains regions to the motor function and to increased activation of the contralesional hemisphere during simple finger movements. These results link CST damage, degeneration of transcallosal fibers, stronger bilateral recruitment of motor areas, and motor impairment.14 Aside from baseline motor function after stroke, structural connectivity metrics of transcallosal connections have been used to predict training gains under therapy. For instance, AD and RD values of fibers connecting the primary motor cortices have been related to functional improvement during an experimental rehabilitation trial for five consecutive days. Contrarily, the integrity of the CST has not been found to be a significant predictor in regard of its diffusivities, but its FA value38 confirming that diffusion metrics might differ in their predictive value for recovery during therapy.73
In summary, this data has enhanced the understanding of the functional role of intra and interhemispheric structural connections for motor recovery after stroke, in addition to the CSTs. However, as most studies have only used separate models for individual connections – probably due to small sample sizes – direct evidence for this additional role of specific corticocortical connections, independent from the status of the CST, is still rare.22, 74 Though, this approach of combined modeling might be promising to investigate how hypothesis‐driven structural connectivity information of specific tracts interrelates and how it adds to predict motor recovery after stroke.
Large‐Scale Structural Connectivity Analyses
In comparison to these hypothesis‐driven and rather tract‐specific analyses, several studies have considered the brain as a complex network of various functional nodes and edges at different cortical, subcortical and spinal levels. They have opted to primarily address the structural network characteristics at this larger scale using data‐driven, whole‐brain approaches and have related them to motor functioning and recovery. For instance, network “communicability,” a measure of information flow across a network, has been found to be reduced in regions surrounding the lesion and in homolog regions of the contralesional hemisphere.24 Together with whole‐brain data on white matter integrity12 and longitudinal cortical thickness data,75, 76 this argues for a secondary degeneration of fibers along with gray matter in remote regions, which are interconnected with the stroke lesion. Another approach is the combination of whole‐brain structural connectivity data from healthy participants and lesion information from stroke patients. Connectivity changes particularly in regions near the stroke lesions have been found to correlate with subsequent tissue loss.77
Multimodal Integration of Structural Connectivity Analyses
Providing synergistic or complementary information on brain plasticity to better understand stroke recovery, studies have increasingly combined structural connectivity analyses with other modalities for example TMS to probe excitability of the primary motor cortices55, 78, 79, 80, 81, 82 or functional imaging to assess regional brain activation14, 17, 61, 78, 80, 83, 84 or interregional coupling characteristics.74, 82 For instance, these studies have evidenced that the loss of integrity of the ipsilesional CST relates to an increase in task‐related brain activation in the contralesional primary sensorimotor cortex14, 17, 84 or bilateral dorsal premotor cortices,78 commonly seen in more impaired patients. Indeed, a small series has suggested that lesions to corticofugal tracts originating from secondary motor areas might explain increased functional recruitment of these areas after stroke.61 This would link structural changes, functional cortical reorganization, and motor output and recovery altogether.
Studies have further revealed that the structural and functional integrity of the CST measured by TMS are usually not interchangeable and might provide complementary information.78, 79 In this regard, it has been shown that not only the structurally but also functionally intact corticospinal system might be crucial both for residual motor function,82 spontaneous recovery55, 80, 81 and improvements following treatment.79 An interesting hierarchical prediction model has been built on a simple score of upper limb motor functioning with TMS metrics and FA analysis at the PLIC. Based on this model, 40 acute stroke patients could be sufficiently classified into four groups with varying outcomes over 6 months: The early clinical evaluation and the presence of TMS evoked motor potentials have been found to be relevant factors for complete or significant recovery, respectively. The structural analysis has allowed the additional classification of the more impaired patients without TMS evoked potentials into either the group with limited or no recovery. As argued by the authors, this early classification could be useful to define feasible rehabilitation goals.55, 80 A more recent study has confirmed this interaction between spontaneous motor recovery, functional and structural integrity measures of the CST indicating a proportional improvement by 70% of the maximum possible regardless of the initial impairment for patients with preserved TMS evoked motor potentials. In patients with absent motor potentials, the structural integrity of the CST has been found to predict subsequent recovery. Nevertheless, samples sizes of the regression analyses of the different subgroups were small, hence the findings should be interpreted with caution.85
Current Limitations, Future Directions, and Conclusion
In summary, structural brain imaging has substantially enhanced the understanding of stroke‐related network alterations and related them to motor functioning as well as recovery processes. In regard of the different networks addressed in the present review (Fig. 1), there is strong evidence that the integrity of the CST shows the most critical importance for impairment and recovery. However, studies have also argued that the structural properties of alternative networks at the corticofugal and corticocortical level will play significant roles as well. A number of limitations are worth considering. First, most of the studies were cross‐sectional; they have included patients in the chronic stage of recovery and were based on rather small sample sizes (Fig. 2). Few longitudinal studies have addressed the temporal change in structural connectivity and have also related it to motor recovery. Second, the majority of studies were focused only on the CST. Just a few studies have now addressed to what degree alternate corticofugal systems or distinct corticocortical commissural and associative connections might be involved in motor output or recovery after stroke as well. However, the sparse data for these networks make it difficult to prove or exclude a relevant contribution of these networks during stroke recovery at this point. Third, compared to the majority of unimodal studies, only a few studies have combined structural connectivity analyses with physiological measures or functional imaging data to explain residual motor function or recovery.
In thought of these limitations, future studies on structural connectivity analyses after stroke will have to address a number of important issues. First, more longitudinal studies across the acute, subacute and chronic stage of recovery are needed to investigate the temporal dynamics of structural connectivity changes in more detail. Importantly, only such analyses will differentiate to what extent the premorbid intersubject variance on one hand and stroke‐related structural alterations evoked by direct and remote lesion effects on the other hand might contribute to the structure–function associations after stroke. While the latter may most likely be in tracts with progressive prominent structural changes after stroke, the former might be important in tracts where changes early after stroke and during recovery are absent. Second, the longitudinal aspect should also be combined with a multimodal approach. Thereby, the multimodal dimension will refer to different techniques such as physiological connectivity data by means of TMS, resting‐state or task‐related connectivity data by means of functional imaging but also to the scale of connectivity level. For physiological connectivity data, additional research is needed to better understand how structural determinants of connections interrelate with such functional connectivity measures. In terms of the scale of connectivity level, current studies have either applied whole‐brain analyses or hypothesis‐driven, rather tract‐related approaches. In this context, future studies are needed to investigate to what extent, for example, graph‐theoretical large‐scale network characteristics might interrelate with tract‐specific connectivity metrics at the level of distinct neuronal circuits. Similarly, it will become more important how structural metrics of one specific tract or circumscribed network might contribute to structure–function relationships of other neuronal circuits, both for motor functioning and recovery prediction. Given the available data on the CST and intrahemispheric premotor‐motor connections or interhemispheric tracts between the primary motor cortices, one would hypothesize that the structural status of the CST may directly influence to what degree such corticocortical structural networks would contribute to motor functioning and recovery. More specifically, in patients with a damaged CST, the integrity of corticocortical connections, for instance, between the ipsilesional primary motor cortex and the dorsal64 or ventral premotor cortex,22 might gain a particular importance for preserved hand function or the potential for further recovery. In contrast, stroke patients with little lesion load to the CST might show a reduced dependence from such premotor–motor connections. Notably, such interactions between corticospinal and corticocortical networks have already been demonstrated for interhemispheric functional connectivity.74 Similar interactions might hold true for physiological measures gathered by means of TMS as well what would be another interesting topic for future work.
To make this more complex modeling in longitudinal and multimodal frameworks on higher dimensional data possible, studies with larger sample sizes are definitely a requirement. In this regard, recent data on fully automatized structural analyses might stimulate the field to develop approaches for efficient data analysis suitable for routine imaging. Though, how such multimodal analyses across multiple motor networks (Fig. 1) will address the statistical limitations such as significant cross‐correlations has yet to be clarified.
In the end, only the combined analysis of modality‐specific findings within a longitudinal framework can help to make the most of each technique promoting the understanding of their potential synergistic and complementary insights into a consistent picture of plastic brain changes after stroke. Together with network simulations based on empirical structural connectivity data from multiple corticofugal and corticocortical circuits, this may help to provide deeper insights into motor functioning and recovery after stroke in order to further pave the way for individually tailored treatment and rehabilitation protocols including noninvasive brain stimulation techniques.
Author Contributions
P. K. and R. S. were responsible for the selection of the literature, figure preparation, and drafting the manuscript. F. C. H. was responsible for review topic design and important critical revision of the manuscript.
Conflict of Interest
The authors certify that there are no potential conflicts of interest to disclose in regard to the form of the International Committee of Medical Journal Editors (http://www.icmje.org).
Acknowledgments
F. C. H. was supported by the German Research Foundation (SFB 936‐C4) and the Werner‐Otto‐Foundation (09/87). The authors thank Ms. Shirley Wang for her comments on the manuscript.
References
- 1. Grefkes C, Ward NS. Cortical reorganization after stroke: how much and how functional? Neuroscientist 2013;20:56–70. [DOI] [PubMed] [Google Scholar]
- 2. Stinear CM, Ward NS. How useful is imaging in predicting outcomes in stroke rehabilitation? Int J Stroke 2013;8:33–37. [DOI] [PubMed] [Google Scholar]
- 3. Hummel FC, Celnik P, Pascual‐Leone A, et al. Controversy: noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul 2008;1:370–382. [DOI] [PubMed] [Google Scholar]
- 4. Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed 2002;15:435–455. [DOI] [PubMed] [Google Scholar]
- 5. Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion‐weighted images. NMR Biomed 1995;8:333–344. [DOI] [PubMed] [Google Scholar]
- 6. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 1996;111:209–219. [DOI] [PubMed] [Google Scholar]
- 7. Shimony JS, McKinstry RC, Akbudak E, et al. Quantitative diffusion‐tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology 1999;212:770–784. [DOI] [PubMed] [Google Scholar]
- 8. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2007;4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dijkhuizen RM, van der Marel K, Otte WM, et al. Functional MRI and diffusion tensor imaging of brain reorganization after experimental stroke. Transl Stroke Res 2012;3:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van der Zijden JP, van der Toorn A, van der Marel K, Dijkhuizen RM. Longitudinal in vivo MRI of alterations in perilesional tissue after transient ischemic stroke in rats. Exp Neurol 2008;212:207–212. [DOI] [PubMed] [Google Scholar]
- 11. Takenobu Y, Hayashi T, Moriwaki H, et al. Motor recovery and microstructural change in rubro‐spinal tract in subcortical stroke. Neuroimage Clin 2014;4:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp 2009;30:3461–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang L, Xu H, Yu C. Brain connectivity plasticity in the motor network after ischemic stroke. Neural Plast 2013;2013:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang LE, Tittgemeyer M, Imperati D, et al. Degeneration of corpus callosum and recovery of motor function after stroke: a multimodal magnetic resonance imaging study. Hum Brain Mapp 2012;33:2941–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Behrens TEJ, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007;34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindenberg R, Renga V, Zhu LL, et al. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology 2010;74:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage 2008;39:1370–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nelles M, Gieseke J, Flacke S, et al. Diffusion tensor pyramidal tractography in patients with anterior choroidal artery infarcts. AJNR Am J Neuroradiol 2008;29:488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 2010;41:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kou N, Park C‐H, Seghier ML, et al. Can fully automated detection of corticospinal tract damage be used in stroke patients? Neurology 2013;80:2242–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phan TG, van der Voort S, Chen J, et al. Impact of corticofugal fibre involvement in subcortical stroke. BMJ Open 2013;3:e003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulz R, Koch P, Zimerman M, et al. Parietofrontal motor pathways and their association with motor function after stroke. Brain 2015;138:1949–1960. [DOI] [PubMed] [Google Scholar]
- 23. Park C, Kou N, Boudrias M‐H, et al. Assessing a standardised approach to measuring corticospinal integrity after stroke with DTI. Neuroimage Clin 2013;2:521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crofts JJ, Higham DJ, Bosnell R, et al. Network analysis detects changes in the contralesional hemisphere following stroke. Neuroimage 2011;54:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuch DS, Reese TG, Wiegell MR, et al. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 2002;48:577–582. [DOI] [PubMed] [Google Scholar]
- 26. Tuch DS. Q‐ball imaging. Magn Reson Med 2004;52:1358–1372. [DOI] [PubMed] [Google Scholar]
- 27. Wedeen VJ, Hagmann P, Tseng WYI, et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 2005;54:1377–1386. [DOI] [PubMed] [Google Scholar]
- 28. Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non‐negativity constrained super‐resolved spherical deconvolution. Neuroimage 2007;35:1459–1472. [DOI] [PubMed] [Google Scholar]
- 29. Adluru G, Gur Y, Anderson JS, et al. Assessment of white matter microstructure in stroke patients using NODDI. 2014 36th Annu. Int.Conf Proc IEEE Eng Med Biol Soc 2014;2014:742–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Granziera C, Daducci A, Meskaldji DE, et al. A new early and automated MRI‐based predictor of motor improvement after stroke. Neurology 2012;79:39–46. [DOI] [PubMed] [Google Scholar]
- 31. Lin Y‐C, Daducci A, Meskaldji DE, et al. Quantitative analysis of myelin and axonal remodeling in the uninjured motor network after stroke. Brain Connect 2015;5:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hepp‐Reymond MC, Wiesendanger M. Unilateral pyramidotomy in monkeys: effect on force and speed of a conditioned precision grip. Brain Res 1972;36:117–131. [DOI] [PubMed] [Google Scholar]
- 33. Wiesendanger M. Pyramidal tract function and the clinical “pyramidal syndrome”. Hum Neurobiol 1984;2:227–234. [PubMed] [Google Scholar]
- 34. Schulz R, Gerloff C, Hummel FC. Non‐invasive brain stimulation in neurological diseases. Neuropharmacology 2013;64:579–587. [DOI] [PubMed] [Google Scholar]
- 35. Thomalla G, Glauche V, Koch MA, et al. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage 2004;22:1767–1774. [DOI] [PubMed] [Google Scholar]
- 36. Werring DJ. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 2000;69:269–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 2001;13:1174–1185. [DOI] [PubMed] [Google Scholar]
- 38. Lindenberg R, Zhu LL, Rüber T, Schlaug G. Predicting functional motor potential in chronic stroke patients using diffusion tensor imaging. Hum Brain Mapp 2012;33:1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Puig J, Pedraza S, Blasco G, et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol 2010;31:1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liang Z, Zeng J, Liu S, et al. A prospective study of secondary degeneration following subcortical infarction using diffusion tensor imaging. J Neurol Neurosurg Psychiatry 2007;78:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Radlinska B, Ghinani S, Leppert IR, et al. Diffusion tensor imaging, permanent pyramidal tract damage, and outcome in subcortical stroke. Neurology 2010;75:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Groisser BN, Copen WA, Singhal AB, et al. Corticospinal tract diffusion abnormalities early after stroke predict motor outcome. Neurorehabil Neural Repair 2014;28:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hui ES, Fieremans E, Jensen JH, et al. Stroke assessment with diffusional kurtosis imaging. Stroke 2012;43:2968–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coutu JP, Chen JJ, Rosas HD, Salat DH. Non‐Gaussian water diffusion in aging white matter. Neurobiol Aging 2014;35:1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Puig J, Pedraza S, Blasco G, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol 2011;32:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Puig J, Blasco G, Daunis‐I‐Estadella J, et al. Decreased corticospinal tract fractional anisotropy predicts long‐term motor outcome after stroke. Stroke 2013;44:2016–2018. [DOI] [PubMed] [Google Scholar]
- 47. Jang SH, Bai D, Son SM, et al. Motor outcome prediction using diffusion tensor tractography in pontine infarct. Ann Neurol 2008;64:460–465. [DOI] [PubMed] [Google Scholar]
- 48. Bosnell RA, Kincses T, Stagg CJ, et al. Motor practice promotes increased activity in brain regions structurally disconnected after subcortical stroke. Neurorehabil Neural Repair 2011;25:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sterr A, Dean PJA, Szameitat AJ, et al. Corticospinal tract integrity and lesion volume play different roles in chronic hemiparesis and its improvement through motor practice. Neurorehabil Neural Repair 2014;28:335–343. [DOI] [PubMed] [Google Scholar]
- 50. Sterr A, Shen S, Szameitat AJ, Herron KA. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabil Neural Repair 2010;24:413–419. [DOI] [PubMed] [Google Scholar]
- 51. Konishi J, Yamada K, Kizu O, et al. MR tractography for the evaluation of functional recovery from lenticulostriate infarcts. Neurology 2005;64:108–113. [DOI] [PubMed] [Google Scholar]
- 52. Feng W, Wang J, Chhatbar PY, et al. Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol 2015; doi: 10.1002/ana.24510 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Borich MR, Mang C, Boyd LA. Both projection and commissural pathways are disrupted in individuals with chronic stroke: investigating microstructural white matter correlates of motor recovery. BMC Neurosci 2012;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schulz R, Park CH, Boudrias MH, et al. Assessing the integrity of corticospinal pathways from primary and secondary cortical motor areas after stroke. Stroke 2012;43:2248–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stinear CM, Barber PA, Petoe M, et al. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain 2012;135:2527–2535. [DOI] [PubMed] [Google Scholar]
- 56. Belhaj‐Saïf A, Cheney PD. Plasticity in the distribution of the red nucleus output to forearm muscles after unilateral lesions of the pyramidal tract. J Neurophysiol 2000;83:3147–3153. [DOI] [PubMed] [Google Scholar]
- 57. Fries W, Danek A, Witt TN. Motor responses after transcranial electrical stimulation of cerebral hemispheres with a degenerated pyramidal tract. Ann Neurol 1991;29:646–650. [DOI] [PubMed] [Google Scholar]
- 58. Ruber T, Schlaug G, Lindenberg R. Compensatory role of the cortico‐rubro‐spinal tract in motor recovery after stroke. Neurology 2012;79:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yeo SS, Jang SH. Changes in red nucleus after pyramidal tract injury in patients with cerebral infarct. Neurorehabilitation 2010;27:373–377. [DOI] [PubMed] [Google Scholar]
- 60. Zheng X, Schlaug G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Front Hum Neurosci 2015;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Newton JM, Ward NS, Parker GJM, et al. Non‐invasive mapping of corticofugal fibres from multiple motor areas – relevance to stroke recovery. Brain 2006;129:1844–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Riley JD, Le V, Der‐Yeghiaian L, et al. Anatomy of stroke injury predicts gains from therapy. Stroke 2011;42:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schulz R, Zimerman M, Timmermann JE, et al. White matter integrity of motor connections related to training gains in healthy aging. Neurobiol Aging 2014;35:1404–1411. [DOI] [PubMed] [Google Scholar]
- 64. Schulz R, Braass H, Liuzzi G, et al. White matter integrity of premotor–motor connections is associated with motor output in chronic stroke patients. Neuroimage Clin 2015;7:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rehme AK, Fink GR, Von Cramon DY, Grefkes C. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal fMRI. Cereb Cortex 2011;21:756–768. [DOI] [PubMed] [Google Scholar]
- 66. Ward NS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 2006;129:809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gerloff C, Bushara K, Sailer A, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain 2006;129:791–808. [DOI] [PubMed] [Google Scholar]
- 68. Gupta RK, Saksena S, Hasan KM, et al. Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: serial diffusion tensor imaging. J Magn Reson Imaging 2006;24:549–555. [DOI] [PubMed] [Google Scholar]
- 69. Radlinska BA, Blunk Y, Leppert IR, et al. Changes in callosal motor fiber integrity after subcortical stroke of the pyramidal tract. J Cereb Blood Flow Metab 2012;32:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu J, Qin W, Zhang J, et al. Enhanced interhemispheric functional connectivity compensates for anatomical connection damages in subcortical stroke. Stroke 2015;46:1045–1051. [DOI] [PubMed] [Google Scholar]
- 71. Chen JL, Schlaug G. Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front Neurol 2013;4:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Li Y, Wu P, Liang F, Huang W. The microstructural status of the corpus callosum is associated with the degree of motor function and neurological deficit in stroke patients. PLoS One 2015;10:e0122615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Song J, Nair VA, Young BM, et al. DTI measures track and predict motor function outcomes in stroke rehabilitation utilizing BCI technology. Front Hum Neurosci 2015;9:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Carter AR, Patel KR, Astafiev SV, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair 2012;26:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Duering M, Righart R, Wollenweber FA, et al. Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology 2015;84:1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cheng B, Schulz R, Bönstrup M, et al. Structural plasticity of remote cortical brain regions is determined by connectivity to the primary lesion in subcortical stroke. J Cereb Blood Flow Metab 2015;35:1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kuceyeski A, Kamel H, Navi BB, et al. Predicting future brain tissue loss from white matter connectivity disruption in ischemic stroke. Stroke 2014;45:717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lotze M, Beutling W, Loibl M, et al. Contralesional Motor cortex activation depends on ipsilesional corticospinal tract integrity in well‐recovered subcortical stroke patients. Neurorehabil Neural Repair 2012;26:594–603. [DOI] [PubMed] [Google Scholar]
- 79. Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology 2011;77:1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stinear CM, Barber PA, Smale PR, et al. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 2007;130:170–180. [DOI] [PubMed] [Google Scholar]
- 81. Jang S, Kwon Y, Son S, et al. Combined study of transcranial magnetic stimulation and diffusion tensor tractography for prediction of motor outcome in patients with corona radiata infarct. J Rehabil Med 2011;43:430–434. [DOI] [PubMed] [Google Scholar]
- 82. Volz LJ, Sarfeld A‐S, Diekhoff S, et al. Motor cortex excitability and connectivity in chronic stroke: a multimodal model of functional reorganization. Brain Struct Funct 2015;220:1093–1107. [DOI] [PubMed] [Google Scholar]
- 83. Qiu M, Darling WG, Morecraft RJ, et al. White matter integrity is a stronger predictor of motor function than BOLD response in patients with stroke. Neurorehabil Neural Repair 2011;25:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vargas P, Gaudron M, Valabrègue R, et al. Assessment of corticospinal tract (CST) damage in acute stroke patients: comparison of tract‐specific analysis versus segmentation of a CST template. J Magn Reson Imaging 2013;37:836–845. [DOI] [PubMed] [Google Scholar]
- 85. Byblow WD, Stinear CM, Barber PA, et al. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol 2015; doi: 10.1002/ana.24472 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 86. Kalinosky BT, Schindler‐Ivens S, Schmit BD. White matter structural connectivity is associated with sensorimotor function in stroke survivors. Neuroimage Clin 2013;2:767–781. [DOI] [PMC free article] [PubMed] [Google Scholar]


