Abstract
The Didymellaceae was established in 2009 to accommodate Ascochyta, Didymella and Phoma, as well as several related phoma-like genera. The family contains numerous plant pathogenic, saprobic and endophytic species associated with a wide range of hosts. Ascochyta and Phoma are morphologically difficult to distinguish, and species from both genera have in the past been linked to Didymella sexual morphs. The aim of the present study was to clarify the generic delimitation in Didymellaceae by combing multi-locus phylogenetic analyses based on ITS, LSU, rpb2 and tub2, and morphological observations. The resulting phylogenetic tree revealed 17 well-supported monophyletic clades in Didymellaceae, leading to the introduction of nine genera, three species, two nomina nova and 84 combinations. Furthermore, 11 epitypes and seven neotypes were designated to help stabilise the taxonomy and use of names. As a result of these data, Ascochyta, Didymella and Phoma were delineated as three distinct genera, and the generic circumscriptions of Ascochyta, Didymella, Epicoccum and Phoma emended. Furthermore, the genus Microsphaeropsis, which is morphologically distinct from the members of Didymellaceae, grouped basal to the Didymellaceae, for which a new family Microsphaeropsidaceae was introduced.
Key words: Ascochyta, Didymella, Multi-locus phylogeny, Phoma, Taxonomy
Taxonomic novelties: New family: Microsphaeropsidaceae Q. Chen, L. Cai & Crous
New genera: Allophoma Q. Chen & L. Cai, Calophoma Q. Chen & L. Cai, Heterophoma Q. Chen & L. Cai, Neoascochyta Q. Chen & L. Cai, Neodidymelliopsis Q. Chen & L. Cai, Nothophoma Q. Chen & L. Cai, Paraboeremia Q. Chen & L. Cai, Phomatodes Q. Chen & L. Cai, Xenodidymella Q. Chen & L. Cai
New names: Ascochytamedicaginicola var. medicaginicola Q. Chen & L. Cai, Didymellasenecionicola Q. Chen & L. Cai
New species: Allophomanicaraguensis Q. Chen & L. Cai, Phomaneerlandica Q. Chen & L. Cai, Stagonosporopsishelianthi Q. Chen & L. Cai
New combinations: Allophomalabilis (Sacc.) Q. Chen & L. Cai, All. minor (Aveskamp et al.) Q. Chen & L. Cai, All. piperis (Tassi) Q. Chen & L. Cai, All. tropica (R. Schneid. & Boerema) Q. Chen & L. Cai, All. zantedeschiae (Dippen.) Q. Chen & L. Cai, Ascochytaherbicola (Wehm.) Q. Chen & L. Cai, As. medicaginicola var. macrospora (Boerema et al.) Q. Chen & L. Cai, As. nigripycnidia (Boerema et al.) Q. Chen & L. Cai, As. phacae (Corbaz) Q. Chen & L. Cai, As. versabilis (Boerema et al.) Q. Chen & L. Cai, Boeremialilacis (Sacc.) Q. Chen & L. Cai, Calophomaaquilegiicola (M. Petrov) Q. Chen & L. Cai, Ca. clematidina (Thüm.) Q. Chen & L. Cai, Ca. clematidis-rectae (Petr.) Q. Chen & L. Cai, Ca. complanata (Tode) Q. Chen & L. Cai, Ca. glaucii (Brunaud) Q. Chen & L. Cai, Ca. vodakii (E. Müll.) Q. Chen & L. Cai, Didymellaacetosellae (A.L. Sm. & Ramsb.) Q. Chen & L. Cai, D. aliena (Fr.) Q. Chen & L. Cai, D. americana (Morgan-Jones & J.F. White) Q. Chen & L. Cai, D. anserina (Marchal) Q. Chen & L. Cai, D. aurea (Gruyter et al.) Q. Chen & L. Cai, D. bellidis (Neerg.) Q. Chen & L. Cai, D. boeremae (Gruyter) Q. Chen & L. Cai, D. calidophila (Aveskamp et al.) Q. Chen & L. Cai, D. chenopodii (P. Karst. & Har.) Q. Chen & L. Cai, D. coffeae-arabicae (Aveskamp et al.) Q. Chen & L. Cai, D. curtisii (Berk.) Q. Chen & L. Cai, D. dactylidis (Aveskamp et al.) Q. Chen & L. Cai, D. dimorpha (Aveskamp et al.) Q. Chen & L. Cai, D. eucalyptica (Sacc.) Q. Chen & L. Cai, D. gardeniae (S. Chandra & Tandon) Q. Chen & L. Cai, D. glomerata (Corda) Q. Chen & L. Cai, D. heteroderae (Boerema et al.) Q. Chen & L. Cai, D. longicolla (Aveskamp et al.) Q. Chen & L. Cai, D. mascrostoma (Mont.) Q. Chen & L. Cai, D. maydis (Arny & R.R. Nelson) Q. Chen & L. Cai, D. microchlamydospora (Aveskamp & Verkley) Q. Chen & L. Cai, D. molleriana (G. Winter) Q. Chen & L. Cai, D. musae (P. Joly) Q. Chen & L. Cai, D. negriana (Thüm.) Q. Chen & L. Cai, D. nigricans (P.R. Johnst. & Boerema) Q. Chen & L. Cai, D. pedeiae (Aveskamp et al.) Q. Chen & L. Cai, D. pinodella (L.K. Jones) Q. Chen & L. Cai, D. pomorum (Thüm.) Q. Chen & L. Cai, D. protuberans (Lév.) Q. Chen & L. Cai, D. rhei (Ellis & Everh.) Q. Chen & L. Cai, D. rumicicola (Boerema & Loer.) Q. Chen & L. Cai, D. sancta (Aveskamp et al.) Q. Chen & L. Cai, D. subglomerata (Boerema et al.) Q. Chen & L. Cai, D. subherbarum (Gruyter et al.) Q. Chen & L. Cai, D. viburnicola (Oudem.) Q. Chen & L. Cai, Epicoccumbrasiliense (Aveskamp et al.) Q. Chen & L. Cai, E. draconis (Berk. ex Cooke) Q. Chen & L. Cai, E. henningsii (Sacc.) Q. Chen & L. Cai, E. huancayense (Turkenst.) Q. Chen & L. Cai, E. plurivorum (P.R. Johnst.) Q. Chen & L. Cai, Heterophomaadonidis (Moesz) Q. Chen & L. Cai, H. nobilis (Kabát & Bubák) Q. Chen & L. Cai, H. novae-verbascicola (Aveskamp et al.) Q. Chen & L. Cai, H. poolensis (Taubenh.) Q. Chen & L. Cai, H. sylvatica (Sacc.) Q. Chen & L. Cai, Neoascochytadesmazieri (Cavara) Q. Chen & L. Cai, Neoa. europaea (Punith) Q. Chen & L. Cai, Neoa. exitialis (Morini) Q. Chen & L. Cai, Neoa. graminicola (Punith.) Q. Chen & L. Cai, Neoa. paspali (P.R. Johnst.) Q. Chen & L. Cai, Neodidymelliopsiscannabis (Aa & Boerema) Q. Chen & L. Cai, Neod. polemonii (Cooke) Q. Chen & L. Cai, Neod. xanthina (Sacc.) Q. Chen & L. Cai, Nothophomaanigozanthi (Tassi) Q. Chen & L. Cai, No. arachidis-hypogaeae (V.G. Rao) Q. Chen & L. Cai, No. gossypiicola (Gruyter) Q. Chen & L. Cai, No. infossa (Ellis & Everh.) Q. Chen & L. Cai, No. quercina (Syd.) Q. Chen & L. Cai, Paraboeremiaadianticola (Aa & Boerema) Q. Chen & L. Cai, Pa. putaminum (Speg.) Q. Chen & L. Cai, Pa. selaginellae (Sacc.) Q. Chen & L. Cai, Phomatodesaubrietiae (Moesz) Q. Chen & L. Cai, Phomat. nebulosa (Pers.) Q. Chen & L. Cai, Xenodidymellaapplanata (Niessl) Q. Chen & L. Cai, X. asphodeli ( E. Müll.) Q. Chen & L. Cai, X. catariae (Cooke & Ellis) Q. Chen & L. Cai, X. humicola (J.C. Gilman & E.V. Abbott) Q. Chen & L. Cai
Introduction
Although the first Phoma spp. were already described in 1821 (Sutton 1980), the genus was only officially introduced 60 years later by Saccardo (1880), the concept of which was emended by Boerema & Bollen (1975). Phoma has been shown to be highly polyphyletic with phoma-like species scattered in at least six families within the Pleosporales (Aveskamp et al. 2010). Although Boerema et al. (2004) subdivided the genus Phoma into nine sections (i.e. Phoma, Heterospora, Paraphoma, Peyronellaea, Phyllostictoides, Sclerophomella, Plenodomus, Macrospora and Pilosa) based on morphological characters (Boerema 1997), these classifications have been shown to be artificial and failed to reflect the natural evolutionary history of this group of fungi (Aveskamp et al., 2008, Aveskamp et al., 2010). Presently the monophyletic lineage anchored by its type species Phoma herbarum, is regarded as Phoma s. str., which belongs to the Didymellaceae (Aveskamp et al. 2010).
Results of a phylogenetic study including the type species of all nine Phoma sections and allied coelomycetous genera demonstrated that all nine sections grouped in the Pleosporales (de Gruyter et al. 2009). The type species of the sections Macrospora, Peyronellaea, Phoma, Phyllostictoides and Sclerophomella resided in Didymellaceae (De Gruyter et al., 2009, De Gruyter et al., 2012). However, the four other sections, namely Heterospora, Paraphoma, Pilosa and Plenodomus clustered in several distinct clades outside Didymellaceae, and were thus excluded from Phoma (De Gruyter et al., 2009, Aveskamp et al., 2010).
Approximately 70 % of the species recognised by Boerema et al. (2004) could be accommodated in Didymellaceae. The phylogenetic relationships of Phoma species in Didymellaceae, mainly from sections Macrospora, Peyronellaea, Phoma, Phyllostictoides and Sclerophomella were further assessed, resulting in many species being reclassified in existing genera (e.g. Didymella, Stagonosporopsis), or transferred to Boeremia, Epicoccum and Peyronellaea (Aveskamp et al. 2010). These results also revealed most morphological sections to be polyphyletic, the one exception being section Plenodomus (Aveskamp et al., 2010, De Gruyter et al., 2010, De Gruyter et al., 2012). Species originally classified in sections Heterospora, Paraphoma, Pilosa and Plenodomus were subsequently revised by De Gruyter et al., 2010, De Gruyter et al., 2012. Members of Phoma sect. Paraphoma were transferred to a range of genera including Coniothyrium (Coniothyriaceae), Paraphoma, Setophoma (Phaeosphaeriaceae), Pyrenochaeta and Pyrenochaetopsis (Cucurbitariaceae) (De Gruyter et al., 2010, De Gruyter et al., 2012). Furthermore, Phoma sect. Heterospora was elevated to generic rank in Leptosphaeriaceae (de Gruyter et al. 2012). Species of Phoma sect. Plenodomus were reclassified into Chaetosphaeronema (Phaeosphaeriaceae) (de Gruyter et al. 2010), Leptosphaeria, Paraleptosphaeria, Plenodomus and Subplenodomus (Leptosphaeriaceae) (de Gruyter et al. 2012). Finally, species of Phoma sect. Pilosa were determined to belong to Pleosporaceae (Aveskamp et al., 2010, De Gruyter et al., 2012).
The genus Ascochyta was established by Libert in 1830, and typified by As. pisi (Boerema & Bollen 1975). Ascochyta and Phoma have long been considered closely related since members from both genera are often highly similar in morphology, physiology, pathogenicity and nucleotide sequences (Aveskamp et al. 2010). Research efforts attempting to distinguish these genera have been carried out since Saccardoan times, using their substrate and morphological characters, such as presence or absence of conidial septa (Aveskamp et al. 2010). In Phoma, septate conidia are rare in vitro, although common in vivo (Aveskamp et al. 2008), whereas isolates of Ascochyta produce septate conidia both in vivo and in vitro (de Gruyter et al. 2009). Boerema & Bollen (1975) differentiated Phoma from Ascochyta based on differences in conidiogenesis and conidial septation. They emphasised that in Phoma conidia are produced from phialides with distinct collarettes (Boerema & Bollen 1975), and that conidial euseptation is a secondary process which occurs independently from conidiogenesis, namely after conidial secession (Boerema and Bollen, 1975, Aveskamp et al., 2010). In contrast, in Ascochyta conidia arise from the accumulation of annellations or from a gradually increasing collar of periclinal annellations, and conidial septation is an essential part of conidium development, which can be regarded as holoblastic (Boerema and Bollen, 1975, Aveskamp et al., 2010). Later Punithalingam (1979a) redefined Ascochyta, and reported that holoblastic conidiogenesis was temporary, whereas phialidic conidiogenesis remained functional at the completion of conidial development. He also concluded that conidial development and septation should not be used as taxonomic criteria for distinguishing species in these two genera.
In spite of these arguments, the taxonomy of these two genera remains confused. This is largely demonstrated by the high number of synonyms in this complex (Aveskamp et al. 2008). Furthermore, in recent studies the type species of the genus Ascochyta, As. pisi, also nested in the Didymellaceae (de Gruyter et al. 2009), close to the type species of Phoma (Peever et al., 2007, De Gruyter et al., 2009, Aveskamp et al., 2010). Because merging the genera Ascochyta and Phoma would prove highly unpopular among phytopathologists, both generic names are still in use, and their links to sexual genera in the Didymellaceae remain unresolved (Aveskamp et al. 2010).
Didymella was first used at the generic level by Saccardo in 1880, with the description of Didymella exigua (Holm, 1975, Corlett, 1981), which was later accepted as the type or lectotype species of the genus (Von Höhnel, 1918, Corbaz, 1957, Müller and von Arx, 1962, Holm, 1975, Von Arx and Müller, 1975). Didymella was originally accommodated in the Mycosphaerellaceae, and then placed in the Pleosporaceae, Phaeosphaeriaceae, Venturiaceae, or considered as incertae sedis in the Pleosporales (de Gruyter et al. 2009). In the study of de Gruyter et al. (2009), a new family Didymellaceae was introduced for the “Didymella clade”, which included most members of Phoma and related asexual genera. As a genus with phytopathological importance, Didymella is also in urgent need of taxonomic revision (Aveskamp et al. 2010), as it appears to be polyphyletic. The four sexual genera that have been linked to Phoma include Didymella, Leptosphaeria, Mycosphaerella and Pleospora (Boerema et al. 2004), while Ascochyta has sexual connections in both Didymella and Mycosphaerella (Corlett, 1981, Peever et al., 2007). In recent studies, however, it has been shown that the genus Didymella is the only genus that is correctly linked to Phoma s. str. (Woudenberg et al., 2009, Aveskamp et al., 2010) and Ascochyta (Chilvers et al., 2009, De Gruyter et al., 2009). Nevertheless, Didymella is still a poorly understood genus, with numerous species that remain phylogenetically unresolved. As both Ascochyta and Phoma have been regarded as polyphyletic, a proper study of the genera traditionally accommodating their sexual morphs is urgently needed (Aveskamp et al. 2010).
The genus Phoma is ubiquitous and species-rich, with species occurring on a diverse range of substrates, from soil to air, plants to animals, and even humans (Aveskamp et al., 2008, Aveskamp et al., 2010). Phoma is notorious because includes many important plant pathogen species, some of which are of quarantine concern (Aveskamp et al., 2008, Aveskamp et al., 2010, Chen et al., 2015). After the studies by Aveskamp et al. (2010) and De Gruyter et al., 2009, De Gruyter et al., 2012, significant progress has been made to clarify generic boundaries in Didymellaceae. However, nearly 70 Phoma species embedded in the Didymellaceae could not be assigned to definite genera due to a lack of phylogenetic support (Aveskamp et al. 2010). In previous molecular phylogenetic studies, partial small subunit nrDNA (18S, SSU) and partial large subunit nrDNA (28S, LSU) nucleotide sequences were used to resolve the relationships above family level (De Gruyter et al., 2009, De Gruyter et al., 2010, De Gruyter et al., 2012), with many species excluded from Phoma and Didymellaceae. As the LSU and SSU sequence data did not provide sufficient phylogenetic information to distinguish closely related genera nor species, Aveskamp et al. (2009a) sequenced the internal transcribed spacer regions 1 & 2 and intervening 5.8S nrDNA (ITS), and partial gene regions of β-tubulin (tub2) and gamma-actin (actA) to clarify the phylogeny of dictyochlamydospore-producing Phoma taxa. LSU and ITS combined with tub2 were used to infer a phylogeny for genera and species in Didymellaceae (Aveskamp et al. 2010). Although improved resolutions were obtained, most of the internal nodes in the trees remained unresolved, and it was concluded that more DNA loci should be employed to fully resolve closely related taxa in this family. In a subsequent study the RNA polymerase II second largest subunit (rpb2) gene was successfully applied in a combination with ITS, LSU and tub2 to distinguish closely related species in Phoma (Chen et al. 2015).
Given the complexities of Ascochyta, Didymella and Phoma, the objectives of this study were: 1) to determine the phylogenetic relationships of these genera using multi-locus sequence data, viz. LSU, ITS, rpb2 and tub2; 2) to delineate the phylogenetic lineages within Didymellaceae, and revise its taxonomy by adopting a polyphasic approach; 3) and to designate epitypes to stabilise the application of names within the family.
Materials and methods
Isolates and type specimens
Isolates used in this study included the majority used in Aveskamp et al. (2010). Furthermore, additional isolates previously identified as Ascochyta, Didymella and Phoma based solely on morphological characters, were also selected. In total, 287 strains were obtained from the culture collection of the CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands (CBS), and the Dutch National Plant Protection Organization, Wageningen, the Netherlands (PD) (Table 1). Freeze-dried isolates were revived overnight in 2 mL malt/peptone (50 % / 50 %) liquid medium and subsequently transferred to oatmeal agar (OA), 2 % malt extract agar (MEA) and potato dextrose agar (PDA) (recipes according to Crous et al. 2009), and incubated at room temperature. Some of the cultures were incubated under near-ultraviolet (UV) light (12 h light, 12 h dark) or on pine needle agar (PNA) (Smith et al., 1996, Su et al., 2012) to promote sporulation if necessary. Loan requests of type specimens were sent to 34 fungaria, viz. ABD, B, BHG, BP, BPI, BR, BRNM, DAR, E, FI, G, H, ILL, K, KIEL, L(U), LE, PAD, PAV, PC, PDD, PR, PRC, PRM, ROPV, S, SIENA, UPS, UV, VALPL, W, WU, Z and ZT. Additional specimens were loaned from BR, BPI, IMI, K, L, M, PDD, SIENA and ZT.
Table 1.
Isolates used in this study and their GenBank accession numbers. Newly generated sequences are indicated in bold.
| Species | Old name | Strain number1 | Status2 | Host, substrate | Country | GenBank accession numbers3 |
|||
|---|---|---|---|---|---|---|---|---|---|
| LSU | ITS | rpb2 | tub2 | ||||||
| Allophoma labilis | Phoma labilis | CBS 124.93; PD 87/269 | Solanum lycopersicum | The Netherlands | GU238091 | GU237765 | KT389552 | GU237619 | |
| All. minor | Phoma minor | CBS 325.82 | T | Syzygium aromaticum | Indonesia | GU238107 | GU237831 | KT389553 | GU237632 |
| All. nicaraguensis | CBS 506.91; PD 91/876; IMI 215229 | T | Coffea arabica | Nicaragua | GU238058 | GU237876 | KT389551 | GU237596 | |
| All. piperis | Phoma piperis | CBS 268.93; CBS 108.93; PD 88/720 | T | Peperomia pereskiifolia | The Netherlands | GU238129 | GU237816 | KT389554 | GU237644 |
| CBS 108.93; PD 90/2011 | Peperomia sp. | The Netherlands | GU238130 | GU237921 | KT389555 | GU237645 | |||
| All. tropica | Phoma tropica | CBS 436.75; DSM 63365 | T | Saintpaulia ionantha | Germany | GU238149 | GU237864 | KT389556 | GU237663 |
| All. zantedeschiae | Phoma zantedeschiae | CBS 131.93; PD 69/140 | Calla sp. | The Netherlands | GU238159 | FJ427084 | KT389557 | FJ427188 | |
| Didymella rabiei | CBS 229.32 | Cicer arietinum | Romania | KT389690 | KT389473 | KT389558 | KT389767 | ||
| Alternaia japonica | Alternaia japonica | CBS 118390 | Brassica chinensis | USA | KC584281 | KC584201 | KC584405 | — | |
| Ascochyta fabae | Ascochyta fabae | CBS 524.77 | Phaseolus vulgaris | Belgium | GU237963 | GU237880 | — | GU237526 | |
| CBS 649.71 | Vicia faba | The Netherlands | GU237964 | GU237902 | — | GU237527 | |||
| PD 83/492 | Phaseolus vulgaris | The Netherlands | GU237965 | GU237917 | — | GU237528 | |||
| As. herbicola | Phoma herbicola | CBS 629.97; PD 76/1017 | R | Water | USA | GU238083 | GU237898 | KP330421 | GU237614 |
| As. lentis | Ascochyta lentis | CBS 370.84; PD 81/783 | Lens culinaris | — | KT389691 | KT389474 | — | KT389768 | |
| As. medicaginicola var. macrospora | Phoma medicaginis var. macrospora | CBS 112.53 | T | Medicago sativa | USA | GU238101 | GU237749 | — | GU237628 |
| CBS 404.65; IMI 116999 | R | Medicago sativa | Canada | GU238102 | GU237859 | KP330423 | GU237629 | ||
| As. medicaginicola var. medicaginicola | Phoma medicaginis var. medicaginis | CBS 316.90 | Medicago sativa | Czech Republic | GU238103 | GU237828 | — | GU237630 | |
| As. nigripycnidia | Phoma nigripycnidia | CBS 116.96; PD 95/7930 | T | Vicia cracca | Russia | GU238118 | GU237756 | — | GU237637 |
| As. phacae | Didymella phacae | CBS 184.55 | T | Phaca alpina | Switzerland | KT389692 | KT389475 | — | KT389769 |
| As. pisi | Ascochyta pisi | CBS 122750; ATCC 201619 | Pisum sativum | USA | KT389694 | KT389477 | — | KT389771 | |
| CBS 122751; ATCC 201620 | Pisum sativum | Canada | KP330444 | KP330432 | EU874867 | KP330388 | |||
| CBS 122785; PD 78/517 | T | Pisum sativum | The Netherlands | GU237969 | GU237763 | — | GU237532 | ||
| CBS 126.54 | Pisum sativum | The Netherlands | EU754137 | GU237772 | DQ677967 | GU237531 | |||
| As. juglandis | CBS 108.49 | Juglans regia | The Netherlands | KT389693 | KT389476 | — | KT389770 | ||
| As. rabiei | As. rabiei | CBS 206.30 | — | — | KT389695 | KT389478 | KT389559 | KT389772 | |
| CBS 237.37 | T | Cicer arietinum | Bulgaria | KT389696 | KT389479 | — | KT389773 | ||
| CBS 534.65 | Cicer arietinum | India | GU237970 | GU237886 | KP330405 | GU237533 | |||
| Ascochyta sp. 1 | As. fabae | CBS 372.84; PD 80/1246 | Pisum sativum | Australia | KT389697 | KT389480 | — | KT389774 | |
| CBS 373.84; PD 80/1247 | Pisum sativum | Australia | KT389698 | KT389481 | KT389560 | KT389775 | |||
| Ascochyta sp. 2 | Didymella astragalina | CBS 113797 | Lathyrus vernus | Sweden | KT389699 | KT389482 | — | KT389776 | |
| As. syringae | Ascochyta syringae | CBS 545.72 | Syringa vulgaris | The Netherlands | KT389700 | KT389483 | — | KT389777 | |
| As. versabilis | Phoma versabilis | CBS 876.97; PD 82/1008 | R | Silene sp. | The Netherlands | GU238152 | GU237909 | KT389561 | GU237664 |
| As. viciae | Ascochyta viciae | CBS 451.68 | Vicia sepium | The Netherlands | KT389701 | KT389484 | KT389562 | KT389778 | |
| As. viciae-pannonicae | As. viciae-pannonicae | CBS 254.92 | Vicia pannonica | Czech Republic | KT389702 | KT389485 | — | KT389779 | |
| Bipolaris maydis | Bipolaris maydis | CBS 134.39; DSM 1149 | Zea mays | — | AY544645 | DQ491489 | DQ247790 | — | |
| Boeremia crinicola | Boeremia crinicola | CBS 109.79; PD 77/747 | R | Crinum powellii | The Netherlands | GU237927 | GU237737 | KT389563 | GU237489 |
| Boeremia diversispora | B. diversispora | CBS 102.80; IMI 331907; PD 79/61 | Phaseolus vulgaris | Kenya | GU237930 | GU237725 | KT389565 | GU237492 | |
| CBS 101194; PD 79/687; IMI 373349 | Phaseolus vulgaris | The Netherlands | GU237929 | GU237716 | KT389564 | GU237491 | |||
| B. exigua | Ascochyta cheiranthi | CBS 118.38 | Cheiranthus cheiri | Denmark | KT389706 | KT389489 | KT389582 | KT389783 | |
| As. ducometii | CBS 119.38 | Nicotiana tabacum | — | KT389707 | KT389490 | KT389583 | KT389784 | ||
| As. abelmoschi | CBS 107.21 | Abelmoschus esculentus | — | KT389708 | KT389491 | — | KT389785 | ||
| B. exigua var. coffeae | Boeremia exigua var. coffeae | CBS 119730 | Coffea arabica | Brazil | GU237942 | GU237759 | KT389567 | GU237504 | |
| CBS 109183; PD 2000/10506; IMI 300060 | R | Coffea arabica | Cameroon | GU237943 | GU237748 | KT389566 | GU237505 | ||
| B. exigua var. exigua | B. exigua var. exigua | CBS 431.74; PD 74/2447 | R | Solanum tuberosum | The Netherlands | EU754183 | FJ427001 | KT389569 | FJ427112 |
| B. exigua var. forsythiae | B. exigua var. forsythiae | CBS 101197; PD 95/721 | Forsythia sp. | The Netherlands | GU237931 | GU237718 | KT389570 | GU237493 | |
| CBS 101213; PD 92/959 | R | Forsythia sp. | The Netherlands | GU237932 | GU237723 | KT389571 | GU237494 | ||
| B. exigua var. gilvescens | B. exigua var. exigua | CBS 101150; PD 79/118 | Cichorium intybus | The Netherlands | EU754182 | GU237715 | KT389568 | GU237495 | |
| B. exigua var. heteromorpha | B. exigua var. heteromorpha | CBS 443.94 | T | Nerium oleander | Italy | GU237935 | GU237866 | KT389573 | GU237497 |
| CBS 101196; PD 79/176 | Nerium oleander | France | GU237934 | GU237717 | KT389572 | GU237496 | |||
| B. exigua var. linicola | B. exigua var. linicola | CBS 114.28 | Linum usitatissimum | The Netherlands | GU237937 | GU237752 | — | GU237499 | |
| CBS 116.76; ATCC 32332; IMI 197074; PD 75/544 | R | Linum usitatissimum | The Netherlands | GU237938 | GU237754 | KT389574 | GU237500 | ||
| Phoma nemophilae | CBS 248.38 | Nemophila insignis | The Netherlands | KT389703 | KT389486 | KT389575 | KT389780 | ||
| B. exigua var. populi | Boeremia exigua var. populi | CBS 100167; PD 93/217 | T | Populus (×) euramericana | The Netherlands | GU237939 | GU237707 | — | GU237501 |
| B. exigua var. pseudolilacis | B. exigua var. pseudolilacis | CBS 101207; PD 94/614 | T | Syringa vulgaris | The Netherlands | GU237941 | GU237721 | — | GU237503 |
| Ascochyta lamiorum | CBS 462.67 | Lamium maculatum | The Netherlands | KT389705 | KT389488 | — | KT389782 | ||
| As. lathyri | CBS 423.67 | Lathyrus sp. | The Netherlands | KT389704 | KT389487 | KT389576 | KT389781 | ||
| B. exigua var. viburni | Boeremia exigua var. viburni | CBS 100354; PD 83/448 | R | Viburnum opulus | The Netherlands | GU237944 | GU237711 | KT389577 | GU237506 |
| B. foveata | B. foveata | CBS 109176; PD 94/1394 | R | Solanum tuberosum | Bulgaria | GU237946 | GU237742 | KT389578 | GU237508 |
| B. hedericola | B. hedericola | CBS 367.91; PD 87/229 | R | Hedera helix | The Netherlands | GU237949 | GU237842 | KT389579 | GU237511 |
| B. lilacis | B. exigua var. lilacis | CBS 569.79; PD 72/741; IMI 331909 | R | Syringa vulgaris | The Netherlands | GU237936 | GU237892 | — | GU237498 |
| Ascochyta philadelphi | CBS 588.67 | Philadelphus sp. | The Netherlands | KT389709 | KT389492 | — | KT389786 | ||
| B. lycopersici | Boeremia lycopersici | CBS 378.67; PD 67/276 | R | Solanum lycopersicum | The Netherlands | GU237950 | GU237848 | KT389580 | GU237512 |
| B. noackiana | B. noackiana | CBS 101203; PD 79/1114 | Phaseolus vulgaris | Colombia | GU237953 | GU237720 | KT389581 | GU237515 | |
| CBS 100353; PD 87/718 | R | Phaseolus vulgaris | Guatemala | GU237952 | GU237710 | — | GU237514 | ||
| B. sambuci-nigrae | B. sambuci-nigrae | CBS 629.68; CECT 20048; IMI 331913; PD 67/753 | T | Sambucus nigra | The Netherlands | GU237955 | GU237897 | — | GU237517 |
| B. strasseri | B. strasseri | CBS 126.93; PD 73/642 | Mentha sp. | The Netherlands | GU237956 | GU237773 | KT389584 | GU237518 | |
| B. telephii | B. telephii | CBS 760.73; PD 71/1616 | R | Sedum telephium | The Netherlands | GU237959 | GU237905 | — | GU237521 |
| CBS 109175; PD 79/524 | R | Sedum telephium | The Netherlands | GU237958 | GU237741 | KT389585 | GU237520 | ||
| Calophoma aquilegiicola | Ascochyta aquilegiae | CBS 107.31 | Aquilegia sp. | — | KT389710 | KT389493 | — | KT389787 | |
| Phoma aquilegiicola | CBS 107.96; PD 73/598 | R | Aconitum pyramidale | The Netherlands | GU238041 | GU237735 | KT389586 | GU237581 | |
| Phoma aquilegiicola | CBS 108.96; PD 79/611 | R | Aquilegia sp. | The Netherlands | GU238042 | GU237736 | — | GU237582 | |
| Phoma aquilegiicola | CBS 109.96; PD 83/832 | Aquilegia sp. | The Netherlands | KT389711 | KT389494 | — | KT389788 | ||
| Phoma aquilegiicola | CBS 116402 | Thalictrum dipterocarpum | New Zealand | KT389712 | KT389495 | — | KT389789 | ||
| Ca. clematidina | Phoma clematidina | CBS 102.66 | Clematis sp. | UK | FJ515630 | FJ426988 | KT389587 | FJ427099 | |
| CBS 108.79; PD 78/522 | T | Clematis sp. | The Netherlands | FJ515632 | FJ426989 | KT389588 | FJ427100 | ||
| Ca. clematidis-rectae | Phoma clematidis-rectae | CBS 507.63; PD 07/03486747; MUCL 9574 | Clematis sp. | The Netherlands | FJ515647 | FJ515606 | KT389589 | FJ515624 | |
| Ca. complanata | Phoma complanata | CBS 268.92 = PD 75/3 | Angelica sylvestris | The Netherlands | EU754180 | FJ515608 | GU371778 | FJ515626 | |
| CBS 100311 | Heracleum sphondylium | The Netherlands | EU754181 | GU237709 | KT389590 | GU237594 | |||
| Ca. glaucii | Phoma glaucii | CBS 112.96; PD 79/765 | Dicentra sp. | The Netherlands | GU238077 | GU237750 | — | GU237610 | |
| CBS 114.96; PD 94/888 | Chelidonium majus | The Netherlands | FJ515649 | FJ515609 | — | FJ515627 | |||
| Calophoma sp. 1 | Didymella vincetoxici | CBS 186.55 | Vincetoxicum officinale | Switzerland | KT389713 | KT389496 | — | KT389790 | |
| Ca. vodakii | D. vodakii | CBS 173.53 | T | Hepatica triloba | Switzerland | KT389714 | KT389497 | — | KT389791 |
| Coniothyrium cartei | Coniothyrium cartei | CBS 105.91 | Quercus robur | Germany | GQ387594 | JF740181 | KT389591 | KF252700 | |
| Co. glycines | C. glycines | CBS 124141 | Glycine max | Zimbabwe | GQ387598 | JF740185 | — | KF252702 | |
| Co. palmarum | C. palmarum | CBS 400.71 | Chamaerops humilis | Italy | EU754153 | AY720708 | KT389592 | KT389792 | |
| Co. telephii | C. telephii | CBS 188.71 | Air | Finland | GQ387599 | JF740188 | KT389593 | KT389793 | |
| Cucurbitaria berberidis | Cucurbitaria berberidis | CBS 363.93 | Berberis vulgaris | The Netherlands | GQ387606 | JF740191 | — | KT389794 | |
| Didymella acetosellae | Phoma acetosellae | CBS 179.97 | Rumex hydrolapathum | The Netherlands | GU238034 | GU237793 | KP330415 | GU237575 | |
| D. aliena | Phoma aliena | CBS 379.93; PD 82/945 | Berberis sp. | The Netherlands | GU238037 | GU237851 | KP330416 | GU237578 | |
| D. americana | Peyronellaea americana | CBS 185.85; PD 80/1191 | R | Zea mays | USA | GU237990 | FJ426972 | KT389594 | FJ427088 |
| CBS 568.97; ATCC 44494; PD 94/1544 | Glycine max | USA | GU237991 | FJ426974 | — | FJ427090 | |||
| D. anserina | Phoma radicis-callunae | CBS 253.80 | — | Germany | KT389715 | KT389498 | KT389595 | KT389795 | |
| CBS 285.29 | Calluna sp. | UK | KT389716 | KT389499 | — | KT389796 | |||
| Peyronellaea anserina | CBS 360.84 | R | Potato flour | The Netherlands | GU237993 | GU237839 | KT389596 | GU237551 | |
| Phoma radicis-callunae | CBS 397.65 | Plastic | Germany | KT389717 | KT389500 | KT389597 | KT389797 | ||
| D. arachidicola | Peyronellaea arachidicola | CBS 333.75; ATCC 28333; IMI 386092; PREM 44889 | T | Arachis hypogaea | South Africa | GU237996 | GU237833 | KT389598 | GU237554 |
| D. aurea | Pe. aurea | CBS 269.93; PD 78/1087 | T | Medicago polymorpha | New Zealand | GU237999 | GU237818 | KT389599 | GU237557 |
| D. bellidis | Phoma bellidis | CBS 714.85; PD 74/265 | R | Bellis perennis | The Netherlands | GU238046 | GU237904 | KP330417 | GU237586 |
| PD 94/886 | Bellis sp. | The Netherlands | GU238047 | GU237923 | — | GU237587 | |||
| D. boeremae | Phoma boeremae | CBS 109942; PD 84/402 | T | Medicago littoralis cv. Harbinger | Australia | GU238048 | FJ426982 | KT389600 | FJ427097 |
| D. calidophila | Phoma calidophila | CBS 448.83 | T | Soil | Egypt | GU238052 | FJ427059 | — | FJ427168 |
| PD 84/109 | Cucumis sativus | The Netherlands | GU238053 | FJ427060 | — | FJ427169 | |||
| D. chenopodii | Phoma chenopodiicola | CBS 128.93; PD 79/140 | R | Chenopodium quinoa cv. Sajana | Peru | GU238055 | GU237775 | KT389602 | GU237591 |
| D. coffeae-arabicae | Peyronellaea coffeae-arabicae | CBS 123380; PD 84/1013 | T | Coffea arabica | Ethiopia | GU238005 | FJ426993 | KT389603 | FJ427104 |
| D. curtisii | Pe. curtisii | CBS 251.92; PD 86/1145 | R | Nerine sp. | The Netherlands | GU238013 | FJ427038 | — | FJ427148 |
| PD 92/1460 | Sprekelia sp. | The Netherlands | GU238012 | FJ427041 | KT389604 | FJ427151 | |||
| D. dactylidis | Phoma dactylidis | CBS 124513; PD 73/1414 | T | Dactylis glomerata | USA | GU238061 | GU237766 | — | GU237599 |
| D. dimorpha | Phoma dimorpha | CBS 346.82 | T | Opuntiae sp | Spain | GU238068 | GU237835 | — | GU237606 |
| D. eucalyptica | Peyronellaea eucalyptica | CBS 377.91; PD 79/210 | R | Eucalyptus sp. | Australia | GU238007 | GU237846 | KT389605 | GU237562 |
| D. exigua | Didymella exigua | CBS 183.55 | T | Rumex arifolius | France | EU754155 | GU237794 | EU874850 | GU237525 |
| D. gardeniae | Peyronellaea gardeniae | CBS 626.68; IMI 108771 | T | Gardenia jasminoides | India | GQ387595 | FJ427003 | KT389606 | FJ427114 |
| D. glomerata | Pe. glomerata | CBS 133.72 | Fresco in church | Romania | KT389718 | FJ427004 | — | FJ427115 | |
| CBS 528.66; PD 63/590 | R | Chrysanthemum sp. | The Netherlands | EU754184 | FJ427013 | GU371781 | FJ427124 | ||
| D. heteroderae | Pe. heteroderae | CBS 109.92; PD 73/1405 | T | Undefined food material | The Netherlands | GU238002 | FJ426983 | KT389601 | FJ427098 |
| D. lethalis | Pe. lethalis | CBS 103.25 | — | — | GU238010 | GU237729 | KT389607 | GU237564 | |
| D. longicolla | Phoma longicolla | CBS 124514; PD 80/1189 | T | Opuntia sp. | Spain | GU238095 | GU237767 | — | GU237622 |
| D. mascrostoma | Phoma mascrostoma var. mascrostoma | CBS 482.95 | Larix decidua | Germany | GU238099 | GU237869 | KT389609 | GU237626 | |
| CBS 529.66; PD 66/521 | R | Malus sylvestris | The Netherlands | GU238098 | GU237885 | — | GU237625 | ||
| Phoma mascrostoma var. incolorata | CBS 223.69 | R | Acer pseudoplatanus | Switzerland | GU238096 | GU237801 | KT389608 | GU237623 | |
| Phoma libertiana | CBS 247.38 | Pinus nigra var. astriaca | — | KT389719 | KT389501 | — | KT389798 | ||
| D. maydis | Peyronellaea maydis | CBS 588.69 | T | Zea mays | USA | EU754192 | FJ427086 | GU371782 | FJ427190 |
| D. microchlamydospora | Phoma microchlamydospora | CBS 105.95 | T | Eucalyptus sp. | UK | GU238104 | FJ427028 | KP330424 | FJ427138 |
| D. molleriana | Phoma digitalis | CBS 229.79; LEV 7660 | R | Digitalis purpurea | New Zealand | GU238067 | GU237802 | KP330418 | GU237605 |
| CBS 109179; PD 90/835-1 | Digitalis sp. | The Netherlands | GU238066 | GU237744 | — | GU237604 | |||
| D. musae | Peyronellaea musae | CBS 463.69 | R | Mangifera indica | India | GU238011 | FJ427026 | — | FJ427136 |
| D. negriana | Phoma negriana | CBS 358.71 | R | Vitis vinifera | Germany | GU238116 | GU237838 | KT389610 | GU237635 |
| D. nigricans | Peyronellaea australis | CBS 444.81; PDDCC 6546 | T | Actinidia chinensis | New Zealand | GU238000 | GU237867 | — | GU237558 |
| PD 77/919 | Actinidea chinensis | New Zealand | GU238001 | GU237915 | KT389611 | GU237559 | |||
| D. pedeiae | Phoma pedeiae | CBS 124517; PD 92/612A | T | Schefflera elegantissima | The Netherlands | GU238127 | GU237770 | KT389612 | GU237642 |
| D. pinodella | Peyronellaea pinodella | CBS 318.90; PD 81/729 | Pisum sativum | The Netherlands | GU238016 | FJ427051 | — | FJ427161 | |
| CBS 531.66 | Trifolium pretense | USA | GU238017 | FJ427052 | KT389613 | FJ427162 | |||
| D. pinodes | Pe. pinodes | CBS 525.77 | T | Pisum sativum | Belgium | GU238023 | GU237883 | KT389614 | GU237572 |
| D. pomorum | Pe. pomorum var. circinata | CBS 285.76; ATCC 26241; IMI 176742; VKM F-1843 | Heracleum dissectum | Russia | GU238025 | FJ427053 | KT389615 | FJ427163 | |
| Pe. pomorum var. cyanea | CBS 388.80 | Triticum sp. | South Africa | GU238027 | FJ427055 | KT389617 | FJ427165 | ||
| Pe. pomorum var. pomorum | CBS 539.66; ATCC 16791; IMI 122266; PD 64/914 | R | Polygonum tataricum | The Netherlands | GU238028 | FJ427056 | KT389618 | FJ427166 | |
| Phoma triticina | CBS 354.52 | Triticum spelta | Switzerland | KT389720 | KT389502 | KT389616 | KT389799 | ||
| D. protuberans | Peyronellaea alectorolophi | CBS 132.96; PD 93/853 | Rhinanthus major | The Netherlands | GU237989 | GU237778 | — | GU237550 | |
| Pe. obtusa | CBS 377.93; PD 80/976 | Daucus carota | The Netherlands | GU238014 | GU237847 | KT389619 | GU237565 | ||
| CBS 391.93; PD 80/87 | Spinacia oleracea | The Netherlands | GU238015 | GU237858 | KT389621 | GU237566 | |||
| Pe. protuberans | CBS 381.96; PD 71/706 | T | Lycium halifolium | The Netherlands | GU238029 | GU237853 | KT389620 | GU237574 | |
| D. rhei | Phoma rhei | CBS 109177; LEV 15165; PD 2000/9941 | R | Rheum rhaponticum | New Zealand | GU238139 | GU237743 | KP330428 | GU237653 |
| D. rumicicola | Phoma rumicicola | CBS 683.79; LEV 15094 | T | Rumex obtusifolius | New Zealand | KT389721 | KT389503 | KT389622 | KT389800 |
| D. sancta | Peyronellaea sancta | CBS 281.83 | T | Ailanthus altissima | South Africa | GU238030 | FJ427063 | KT389623 | FJ427170 |
| D. senecionicola | Phoma senecionis | CBS 160.78; LEV 11451 | R | Senecio jacobaea | New Zealand | GU238143 | GU237787 | — | GU237657 |
| Didymella sp. 1 | Didymella adianticola | CBS 379.96 | Pteris sp. | The Netherlands | KT389722 | KT389504 | KT389624 | KT389801 | |
| Didymella sp. 2 | Ascochyta pyrethri | CBS 115.58; DSM 62044 | Chrysanthemum roseum | Germany | KT389723 | KT389505 | KT389625 | KT389802 | |
| D. subglomerata | Peyronellaea subglomerata | CBS 110.92; PD 76/1010 | R | Triticum sp. | USA | GU238032 | FJ427080 | KT389626 | FJ427186 |
| D. subherbarum | Phoma subherbarum | CBS 249.92; PD 78/1088 | Solanum sp. | Peru | GU238144 | GU237808 | — | GU237658 | |
| CBS 250.92; DAOM 171914; PD 92/371 | T | Zea mays | Canada | GU238145 | GU237809 | — | GU237659 | ||
| D. viburnicola | Phoma viburnicola | CBS 523.73; PD 69/800 | R | Viburnum cassioides | The Netherlands | GU238155 | GU237879 | KP330430 | GU237667 |
| Epicoccum brasiliense | Phoma brasiliensis | CBS 120105 | T | Amaranthus sp. | Brazil | GU238049 | GU237760 | KT389627 | GU237588 |
| E. draconis | Phoma draconis | CBS 186.83; PD 82/47 | R | Dracaena sp. | Rwanda | GU238070 | GU237795 | KT389628 | GU237607 |
| E. henningsii | Phoma henningsii | CBS 104.80; PD 74/1017 | R | Acacia mearnsii | Kenya | GU238081 | GU237731 | KT389629 | GU237612 |
| E. huancayense | Phoma huancayensis | CBS 105.80; PD 75/908 | T | Solanum sp. | Peru | GU238084 | GU237732 | KT389630 | GU237615 |
| E. nigrum | Epicoccum nigrum | CBS 125.82; IMI 331914; CECT 20044 | Human toenail | The Netherlands | GU237974 | FJ426995 | KT389631 | FJ427106 | |
| CBS 173.73; ATCC 24428; IMI 164070 | T | Dactylis glomerata | USA | GU237975 | FJ426996 | KT389632 | FJ427107 | ||
| E. pimprinum | E. pimprinum | CBS 246.60; ATCC 22237; ATCC 16652; IMI 81601 | T | Soil | India | GU237976 | FJ427049 | — | FJ427159 |
| PD 77/1028 | Soil | India | GU237977 | FJ427050 | KT389633 | FJ427160 | |||
| E. plurivorum | Phoma plurivora | CBS 558.81; PDDCC 6873 | T | Setaria sp. | New Zealand | GU238132 | GU237888 | KT389634 | GU237647 |
| E. sorghinum | Epicoccum sorghinum | CBS 179.80; PD 76/1018 | Sorghum vulgare | Puerto Rico | GU237978 | FJ427067 | KT389635 | FJ427173 | |
| CBS 627.68; PD 66/926 | Citrus sp. | France | GU237979 | FJ427072 | KT389636 | FJ427178 | |||
| Heterophoma adonidis | Didymella adonidis | CBS 114309; UPSC 2982 | Adonis vernalis | Sweden | KT389724 | KT389506 | KT389637 | KT389803 | |
| H. dictamnicola | Phoma dictamnicola | CBS 507.91; PD 74/148 | Dictamnus albus | The Netherlands | GU238065 | GU237877 | KT389638 | GU237603 | |
| H. novae-verbascicola | Phoma novae-verbascicola | CBS 127.93; PD 92/347 | Verbascum densiflorum | The Netherlands | GU238120 | GU237774 | — | GU237639 | |
| H. poolensis | Phoma poolensis | CBS 113.20; PD 92/774 | — | — | GU238119 | GU237751 | — | GU237638 | |
| CBS 116.93; PD 71/884 | Antirrhinum majus | The Netherlands | GU238134 | GU237755 | — | GU237649 | |||
| H. sylvatica | Phoma sylvatica | CBS 874.97; PD 93/764 | Melampyrum pratense | The Netherlands | GU238148 | GU237907 | — | GU237662 | |
| Leptosphaeria conoidea | Leptosphaeria conoidea | CBS 616.75; ATCC 32813; IMI 199777; PD 74/56 | Lunaria annua | The Netherlands | JF740279 | JF740201 | KT389639 | KT389804 | |
| Leptosphaeria doliolum | Leptosphaeria doliolum | CBS 505.75 | T | Urtica dioica | The Netherlands | GQ387576 | JF740205 | KT389640 | JF740144 |
| Leptosphaerulina americana | Leptosphaerulina americana | CBS 213.55 | Trifolium pratense | USA | GU237981 | GU237799 | KT389641 | GU237539 | |
| L. arachidicola | L. arachidicola | CBS 275.59; ATCC 13446 | Arachis hypogaea | Taiwan, China | GU237983 | GU237820 | — | GU237543 | |
| L. australis | L. australis | CBS 317.83 | Eugenia aromatica | Indonesia | EU754166 | GU237829 | GU371790 | GU237540 | |
| L. trifolii | L. trifolii | CBS 235.58 | Trifolium sp. | The Netherlands | GU237982 | GU237806 | — | GU237542 | |
| Macroventuria anomochaeta | Macroventuria anomochaeta | CBS 502.72 | Medicago sativa | South Africa | GU237985 | GU237873 | — | GU237545 | |
| CBS 525.71 | T | Decayed canvas | South Africa | GU237984 | GU237881 | GU456346 | GU237544 | ||
| Ma. wentii | Ma. wentii | CBS 526.71 | T | Plant litter | USA | GU237986 | GU237884 | KT389642 | GU237546 |
| Microsphaeropsis olivacea | Microsphaeropsis olivacea | CBS 233.77 | Pirus laricio | France | GU237988 | GU237803 | KT389643 | GU237549 | |
| CBS 432.71 | Sarothamnus sp. | The Netherlands | GU237987 | GU237863 | — | GU237548 | |||
| Mi. proteae | Mi. proteae | CBS 111319; CPC 1425 | Protea nitida | South Africa | JN712563 | JN712497 | — | JN712650 | |
| Neoascochyta desmazieri | Ascochyta desmazieri | CBS 247.79 | Gramineae | Austria | KT389725 | KT389507 | — | KT389805 | |
| As. desmazieri | CBS 297.69 | T | Lolium perenne | Germany | KT389726 | KT389508 | KT389644 | KT389806 | |
| As. agrostidis | CBS 758.97 | Hay | Norway | KT389727 | KT389509 | — | KT389807 | ||
| Neoa. europaea | As. hordei var. europaea | CBS 819.84 | Hordeum vulgare | Germany | KT389728 | KT389510 | KT389645 | KT389808 | |
| CBS 820.84 | T | Hordeum vulgare | Germany | KT389729 | KT389511 | KT389646 | KT389809 | ||
| Neoa. exitialis | Didymella arcuata | CBS 118.40 | — | — | KT389732 | KT389514 | KT389647 | KT389812 | |
| D. exitialis | CBS 389.86 | Triticum aestivum | Switzerland | KT389733 | KT389515 | KT389648 | KT389813 | ||
| Ascochyta avenae | CBS 811.84 | Secale cereale | Germany | KT389734 | KT389516 | — | KT389814 | ||
| As. avenae | CBS 812.84 | Hordeum vulgare | Germany | KT389735 | KT389517 | — | KT389815 | ||
| As. skagwayensis | CBS 110124 | Triticum sp. | The Netherlands | KT389730 | KT389512 | — | KT389810 | ||
| As. allii | CBS 113693; UPSC 1929 | Allium sp. | Sweden | KT389731 | KT389513 | — | KT389811 | ||
| Neoa. graminicola | As. sorghi | CBS 301.69 | Lolium multiflorum | Germany | KT389737 | KT389519 | KT389650 | KT389817 | |
| Didymella exitialis | CBS 447.82 | Triticum aestivum | Germany | KT389738 | KT389520 | — | KT389818 | ||
| Ascochyta graminea | CBS 586.79 | Hordeum vulgare | Belgium | KT389739 | KT389521 | — | KT389819 | ||
| As. hordei var. americana | CBS 815.84 | Hordeum vulgare | Germany | KT389740 | KT389522 | — | KT389820 | ||
| As. hordei var. americana | CBS 816.84 | Hordeum vulgare | Germany | KT389741 | KT389523 | KT389651 | KT389821 | ||
| Didymella graminicola | CBS 102789 | R | Lolium perenne | New Zealand | KT389736 | KT389518 | KT389649 | KT389816 | |
| Neoa. paspali | Phoma paspali | CBS 560.81; PD 92/1569 | T | Paspalum dilatatum | New Zealand | GU238124 | FJ427048 | KP330426 | FJ427158 |
| Neoascochyta sp. 1 | Ascochyta hordei | CBS 112524 | Triticum aestivum | Argentina | KT389742 | KT389524 | — | KT389822 | |
| Neoascochyta sp. 2 | Didymella graminicola | CBS 516.81 | Oryza sativa | Italy | KT389743 | KT389525 | KT389653 | KT389823 | |
| Neoascochyta sp. 3 | Ascochyta festucae | CBS 689.97 | Hay | Norway | KT389744 | KT389526 | KT389654 | KT389824 | |
| Neoascochyta sp. 4 | As. hordei var. hordei | CBS 544.74 | Triticum aestivum | South Africa | EU754134 | GU237887 | KT389652 | GU237488 | |
| Neoascochyta sp. 5 | As. brachypodii | CBS 876.72 | Straw | South Africa | KT389745 | KT389527 | — | KT389825 | |
| Neodidymelliopsis cannabis | Didymella urticicola | CBS 121.75; ATCC 32164; IMI 194767; PD 73/584 | T | Urtica dioica | The Netherlands | GU237972 | GU237761 | — | GU237535 |
| D. cannabis | CBS 234.37 | Cannabis sativa | — | GU237961 | GU237804 | KP330403 | GU237523 | ||
| D. eupyrena | CBS 591.67 | Urtica dioica | The Netherlands | KT389746 | KT389528 | — | KT389826 | ||
| D. cannabis | CBS 629.76 | Packing material | The Netherlands | KT389747 | KT389529 | — | KT389827 | ||
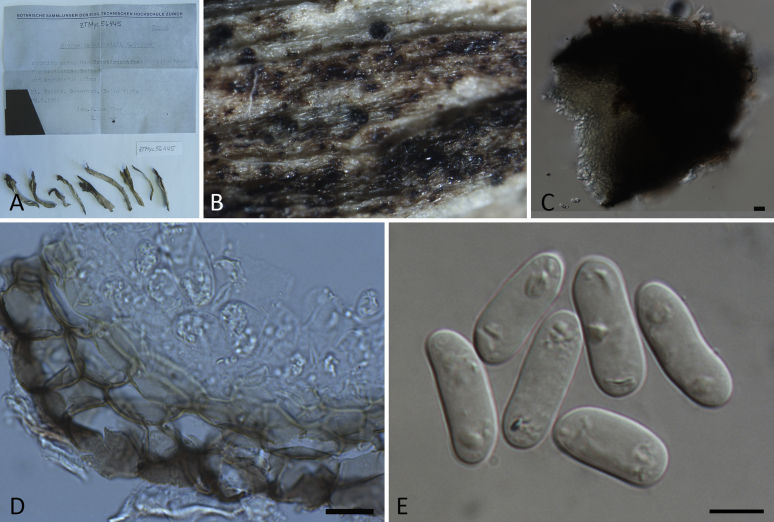
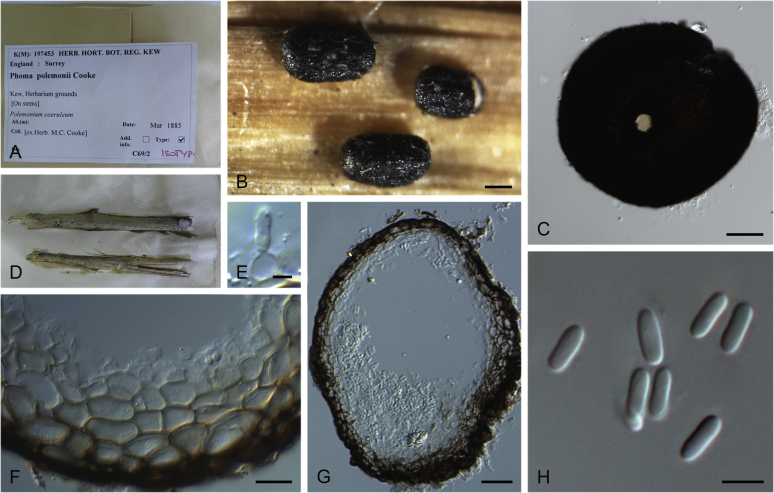
| Neod. polemonii | Ascochyta polemonii | CBS 375.67 | Polemonium caeruleum | The Netherlands | KT389748 | KT389530 | — | KT389828 | |
| Phoma polemonii | CBS 109181; PD 83/757 | T | Polemonium caeruleum | The Netherlands | GU238133 | GU237746 | KP330427 | GU237648 | |
| Neodidymelliopsis sp. 1 | Ascochyta achlydis | CBS 256.77 | Achlys triphylla | Canada | KT389749 | KT389531 | — | KT389829 | |
| Neodidymelliopsis sp. 2 | As. scotinospora | CBS 382.96 | Soil in desert | Israel | KT389750 | KT389532 | — | KT389830 | |
| Neod. xanthina | As. aquilegiae | CBS 168.70 | Delphinium sp. | The Netherlands | KT389751 | KT389533 | — | KT389831 | |
| Phoma xanthina | CBS 383.68 | T | Delphinium sp. | The Netherlands | GU238157 | GU237855 | KP330431 | GU237668 | |
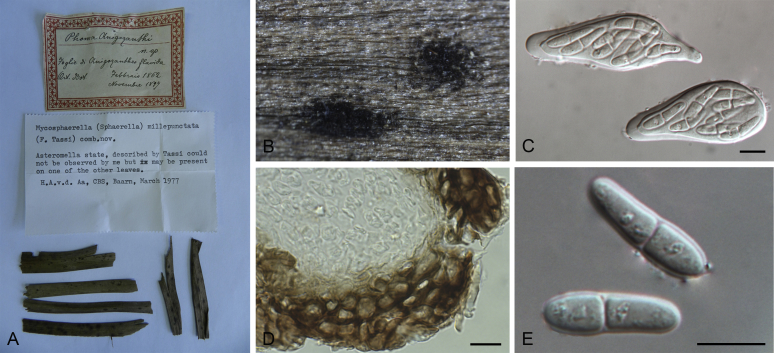
| Nothophoma anigozanthi | Phoma anigozanthi | CBS 381.91; PD 79/1110 | T | Anigozanthus maugleisii | The Netherlands | GU238039 | GU237852 | KT389655 | GU237580 |
| No. arachidis-hypogaeae | Phoma arachidis-hypogaeae | CBS 125.93; PD 77/1029 | R | Arachis hypogaea | India | GU238043 | GU237771 | KT389656 | GU237583 |
| No. gossypiicola | Phoma gossypiicola | CBS 377.67 | Gossypium sp. | USA | GU238079 | GU237845 | KT389658 | GU237611 | |
| No. infossa | Phoma infossa | CBS 123395 | T | Fraxinus pennsylvanica | Argentina | GU238089 | FJ427025 | KT389659 | FJ427135 |
| No. quercina | Phoma fungicola | CBS 633.92; ATCC 36786; VKM MF-325 | Microsphaera alphitoides from Quercus sp. | Ukraine | EU754127 | GU237900 | KT389657 | GU237609 | |
| Ophiosphaerella herpotricha | Ophiosphaerella herpotricha | CBS 620.86 | Bromus erectus | Switzerland | DQ678062 | KF498728 | DQ677958 | — | |
| Paraboeremia adianticola | Didymella adianticola | CBS 187.83; PD 82/128 | Polystichum adiantiforme | USA | GU238035 | GU237796 | KP330401 | GU237576 | |
| CBS 260.92; PD 86/1103 | Pteris ensiformis | — | KT389752 | KT389534 | — | KT389832 | |||
| Pa. putaminum | Phoma putaminum | CBS 130.69; CECT 20054; IMI 331916 | R | Malus sylvestris | Denmark | GU238138 | GU237777 | — | GU237652 |
| CBS 372.91; PD 75/960 | R | Ulmus sp. | The Netherlands | GU238137 | GU237843 | — | GU237651 | ||
| Pa. selaginellae | Phoma selaginellicola | CBS 122.93; PD 77/1049 | T | Selaginella sp. | The Netherlands | GU238142 | GU237762 | — | GU237656 |
| Paraleptosphaeria nitschkei | Paraleptosphaeria nitschkei | CBS 306.51 | T | Cirsium spinosissimum | Switzerland | JF740308 | JF740239 | KT389660 | KT389833 |
| Phaeosphaeria ammophilae | Phaeosphaeria ammophilae | CBS 114595 | Ammophila arenaria | Sweden | GU301859 | KF766146 | GU371724 | — | |
| Phaeosphaeriopsis triseptata | Phaeosphaeriopsis triseptata | MFLUCC 13-0347 | Ruscus aculeatus | Italy | KJ522480 | KJ522476 | KJ522486 | — | |
| Phoma neerlandica | CBS 134.96; PD 84/676 | T | Delphinium sp. | The Netherlands | KT389753 | KT389535 | KT389661 | KT389834 | |
| Phoma herbarum | Phoma cruris-hominis | CBS 377.92; IMI 213845 | Human leg | The Netherlands | KT389756 | KT389536 | KT389663 | KT389837 | |
| Phoma herbarum | CBS 502.91; PD 82/276 | Nerium sp. | The Netherlands | GU238082 | GU237874 | KP330419 | GU237613 | ||
| Phoma herbarum | CBS 615.75; PD 73/665; IMI 199779 | R | Rosa multiflora cv. Cathayensis | The Netherlands | EU754186 | FJ427022 | KP330420 | FJ427133 | |
| Atradidymella muscivora | CBS 127589; UAMH 10909 | Polytrichum juniperinum | USA | KT389757 | KT389539 | KT389664 | KT389838 | ||
| Phoma acuum | CBS 274.37 | Picea excelsa | UK | KT389754 | KT389537 | KT389662 | KT389835 | ||
| Leptosphaeria millefolii | CBS 304.51 | Achillea millefolium | Switzerland | KT389755 | KT389538 | — | KT389836 | ||
| Phomatodes aubrietiae | Phoma aubrietiae | CBS 383.67; PD 65/223 | R | Aubrietia hybrida cv. Superbissima | The Netherlands | GU238044 | GU237854 | — | GU237584 |
| CBS 627.97; PD 70/714 | T | Aubrietia sp. | The Netherlands | GU238045 | GU237895 | KT389665 | GU237585 | ||
| Phomat. nebulosa | Phoma nebulosa | CBS 117.93; PD 83/90 | Mercurialis perennis | The Netherlands | GU238114 | GU237757 | KP330425 | GU237633 | |
| CBS 100191 | Thlaspi arvense | Poland | KP330446 | KP330434 | KT389666 | KP330390 | |||
| CBS 740.96 | Armoracia rusticana | The Netherlands | KT389758 | KT389540 | KT389667 | KT389839 | |||
| Plenodomus biglobosus | Plenodomus biglobosus | CBS 532.66; PD 65/911 | Brassica sp. | The Netherlands | KT389759 | KT389541 | KT389668 | KT389840 | |
| Plen. lingam | Plen. lingam | CBS 275.63 | Brassica sp. | UK | JF740306 | JF740234 | KT389669 | KT389841 | |
| Pleospora betae | Pleospora betae | CBS 523.66 | Beta vulgaris | The Netherlands | EU754179 | FJ426981 | KT389670 | KT389842 | |
| Pleo. herbarum | Pleo. herbarum | CBS 191.86 | T | Medicago sativa | India | GU238160 | KC584239 | KC584471 | — |
| Pleo. typhicola | Pleo. typhicola | CBS 132.69 | Typha angustifolia | The Netherlands | JF740325 | JF740105 | KC584505 | KT389843 | |
| Pyrenochaeta cava | Pyrenochaeta cava | CBS 257.68; CECT 20043; IMI 331911 | Soil from wheat-field | Germany | EU754199 | JF740260 | — | KT389844 | |
| Pyrenochaeta nobilis | Pyrenochaeta nobilis | CBS 407.76 | T | Laurus nobilis | Italy | EU754206 | NR_103598 | DQ677991 | KT389845 |
| Pyrenochaetopsis pratorum | Pyrenochaetopsis pratorum | CBS 445.81 | T | Lolium perenne | New Zealand | GU238136 | NR_111623 | KT389671 | KT389846 |
| Pyrenophora phaeocomes | Pyrenophora phaeocomes | DAOM 222769 | Calamagrostis villosa | Switzerland | JN940093 | JN943649 | DQ497614 | — | |
| Setomelanomma holmii | Setomelanomma holmii | CBS 110217 | Picea pungens | USA | GQ387633 | KT389542 | GU371800 | — | |
| Sporomiella minima | Sporomiella minima | CBS 524.50 | Dung of goat | Panama | DQ678056 | KT389543 | DQ677950 | — | |
| Stagonosporopsis actaeae | Stagonosporopsis actaeae | CBS 106.96; PD 94/1318 | T | Actaea spicata | The Netherlands | GU238166 | GU237734 | KT389672 | GU237671 |
| Didymella hellebori | CBS 114303; UPSC 2962 | Actaea spicata | Sweden | KT389760 | KT389544 | — | KT389847 | ||
| S. ajacis | S. ajacis | CBS 177.93; PD 90/115 | T | Delphinium sp. | Kenya | GU238168 | GU237791 | KT389673 | GU237673 |
| S. andigena | S. andigena | CBS 101.80; PD 75/909; IMI 386090 | R | Solanum sp. | Peru | GU238169 | GU237714 | — | GU237674 |
| CBS 269.80; PD 75/914 | Solanum sp. | Peru | GU238170 | GU237817 | — | GU237675 | |||
| S. artemisiicola | S. artemisiicola | CBS 102636; PD 73/1409 | R | Artemisia dracunculus | France | GU238171 | GU237728 | KT389674 | GU237676 |
| S. astragali | S. astragali | CBS 178.25; MUCL 9915 | R | Astragalus sp. | — | GU238172 | GU237792 | — | GU237677 |
| S. caricae | S. caricae | CBS 248.90 | Carica papaya | Chile | GU238175 | GU237807 | — | GU237680 | |
| CBS 282.76 | Brassica sp. | Indonesia | GU238177 | GU237821 | — | GU237682 | |||
| S. chrysanthemi | S. chrysanthemi | CBS 500.63; MUCL 8090 | R | Chrysanthemum indicum | Germany | GU238190 | GU237871 | — | GU237695 |
| CBS 137.96; PD 84/75 | R | Chrysanthemum indicum | The Netherlands | GU238191 | GU237783 | — | GU237696 | ||
| S. crystalliniformis | S. crystalliniformis | CBS 713.85; ATCC 76027; PD 83/826 | T | Solanum lycopersicum | Colombia | GU238178 | GU237903 | KT389675 | GU237683 |
| S. cucurbitacearum | S. cucurbitacearum | CBS 133.96;PD 79/127 | Cucumis sp. | New Zealand | GU238181 | GU237780 | KT389676 | GU237686 | |
| S. dennisii | S. dennisii | CBS 631.68; PD 68/147 | T | Solidago floribunda | The Netherlands | GU238182 | GU237899 | KT389677 | GU237687 |
| S. dorenboschii | S. dorenboschii | CBS 426.90; IMI 386093; PD 86/551 | T | Physostegia virginiana | The Netherlands | GU238185 | GU237862 | KT389678 | GU237690 |
| S. helianthi | CBS 200.87 | T | Helianthus annuus | Italy | KT389761 | KT389545 | KT389683 | KT389848 | |
| S. heliopsidis | S. heliopsidis | CBS 109182; PD 74/231 | R | Heliopsis patula | The Netherlands | GU238186 | GU237747 | KT389679 | GU237691 |
| S. hortensis | S. hortensis | CBS 104.42 | R | — | The Netherlands | GU238198 | GU237730 | KT389680 | GU237703 |
| CBS 572.85; PD 79/269 | R | Phaseolus vulgaris | The Netherlands | GU238199 | GU237893 | KT389681 | GU237704 | ||
| S. inoxydabilis | S. inoxydabilis | CBS 425.90; PD 81/520 | T | Chrysanthemum parthenii | The Netherlands | GU238188 | GU237861 | KT389682 | GU237693 |
| S. loticola | S. loticola | CBS 562.81; PDDCC 6884 | T | Lotus pedunculatus | New Zealand | GU238192 | GU237890 | KT389684 | GU237697 |
| S. lupini | S. lupini | CBS 101494; PD 98/5247 | T | Lupinus albus | UK | GU238194 | GU237724 | KT389685 | GU237699 |
| S. oculo-hominis | S. oculo-hominis | CBS 634.92; IMI 193307 | T | Human corneal ulcer | USA | GU238196 | GU237901 | KT389686 | GU237701 |
| S. rudbeckiae | S. rudbeckiae | CBS 109180; PD 79/175 | R | Rudbeckia bicolor | The Netherlands | GU238197 | GU237745 | — | GU237702 |
| S. tanaceti | S. tanaceti | CBS 131484 | T | Tanacetum cinerariifolium | Australia | JQ897461 | NR_111724 | — | JQ897496 |
| S. trachelii | S. trachelii | CBS 379.91; PD 77/675 | R | Campanula isophylla | The Netherlands | GU238173 | GU237850 | KT389687 | GU237678 |
| CBS 384.68 | R | Campanula isophylla | Sweden | GU238174 | GU237856 | — | GU237679 | ||
| S. valerianellae | S. valerianellae | CBS 273.92; PD 82/43 | Valerianella locusta | The Netherlands | GU238200 | GU237819 | — | GU237705 | |
| CBS 329.67; PD 66/302 | T | Valerianella locusta var. oleracea | The Netherlands | GU238201 | GU237832 | — | GU237706 | ||
| Subplenodomus violicola | Subplenodomus violicola | CBS 306.68 | Viola tricolor | The Netherlands | GU238156 | FJ427083 | — | KT389849 | |
| Xenodidymella applanata | Didymella applanata | CBS 195.36 | T | Rubus idaeus | The Netherlands | KT389764 | KT389548 | — | KT389852 |
| CBS 205.63 | Rubus idaeus | The Netherlands | GU237998 | GU237798 | KP330402 | GU237556 | |||
| CBS 115577 | Rubus idaeus | Sweden | KT389762 | KT389546 | KT389688 | KT389850 | |||
| CBS 115578 | Rubus arcticus nothossp. stellarcticus | Sweden | KT389763 | KT389547 | — | KT389851 | |||
| X. asphodeli | D. asphodeli | CBS 375.62 | T | Asphodelus albus | France | KT389765 | KT389549 | KT389689 | — |
| CBS 499.72 | Asphodelus ramosus | Italy | KT389766 | KT389550 | — | KT389853 | |||
| X. catariae | D. catariae | CBS 102635; PD 77/1131 | Nepeta catenaria | The Netherlands | GU237962 | GU237727 | KP330404 | GU237524 | |
| X. humicola | Phoma humicola | CBS 220.85; PD 71/1030 | R | Franseria sp. | USA | GU238086 | GU237800 | KP330422 | GU237617 |
ATCC: American Type Culture Collection, Virginia, USA; CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CECT: Colección Española de Cultivos Tipo, Valencia University, Spain; CPC: Culture collection of Pedro Crous, housed at CBS; DAOM: Canadian Collection of Fungal Cultures, Ottawa, Canada; DSM: Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK; LEV: Plant Health and Diagnostic Station, Auckland, New Zealand; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; MUCL: Mycotheque de l'Universite catholique de Louvain, Louvain-la-Neuve, Belgium; PD: Plant Protection Service, Wageningen, the Netherlands; PDDCC: Plant Diseases Division Culture Collection, Auckland, New Zealand; PREM: National Collection of Fungi: Culture Collection, Pretoria, South Africa; UAMH: University of Alberta Microfungus Collection and Herbarium, Canada; UPSC: Uppsala University Culture Collection, Sweden; VKM: All-Russian Collection of Microorganisms, Pushchino, Russia.
T: ex-type strain; R: representative strain.
ITS: internal transcribed spacer regions 1 & 2 including 5.8S nrDNA gene; LSU: 28S large subunit of the nrRNA gene; rpb2: RNA polymerase II second largest subunit; tub2: ß-tubulin.
Morphology
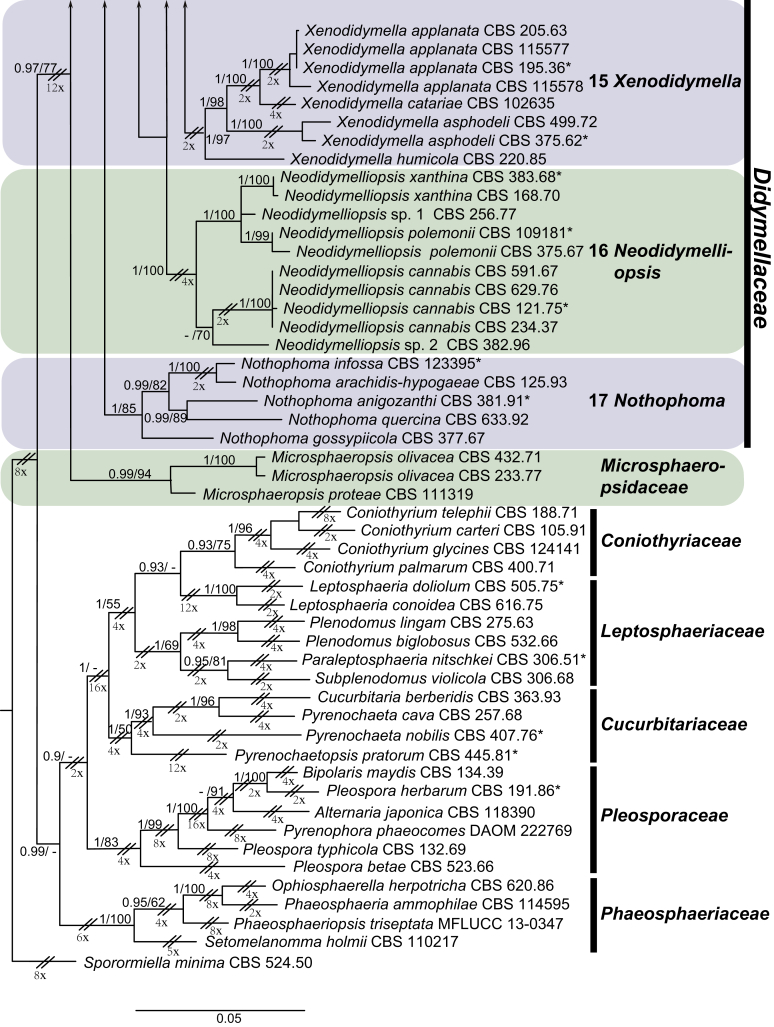
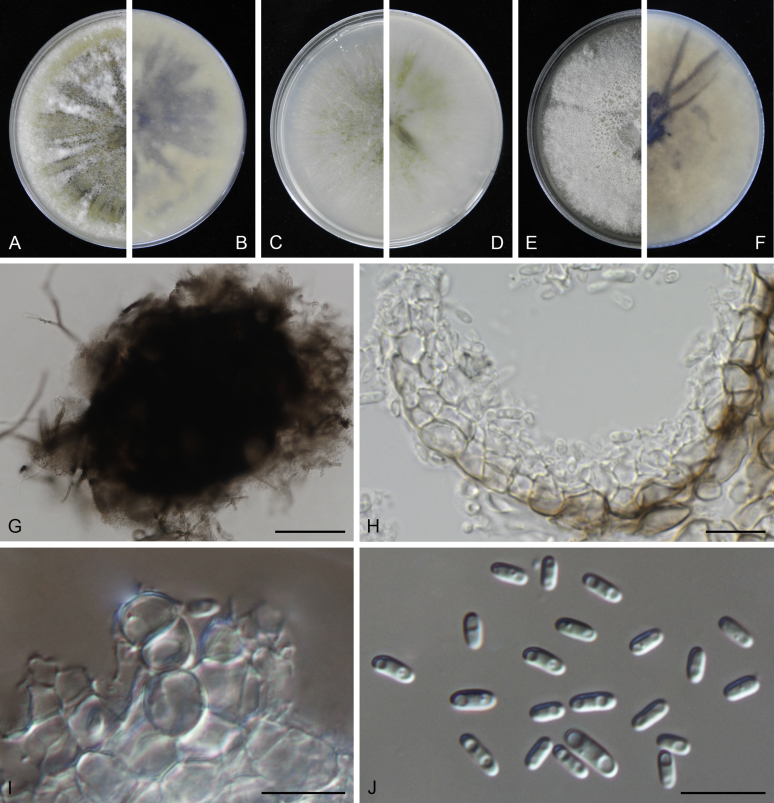
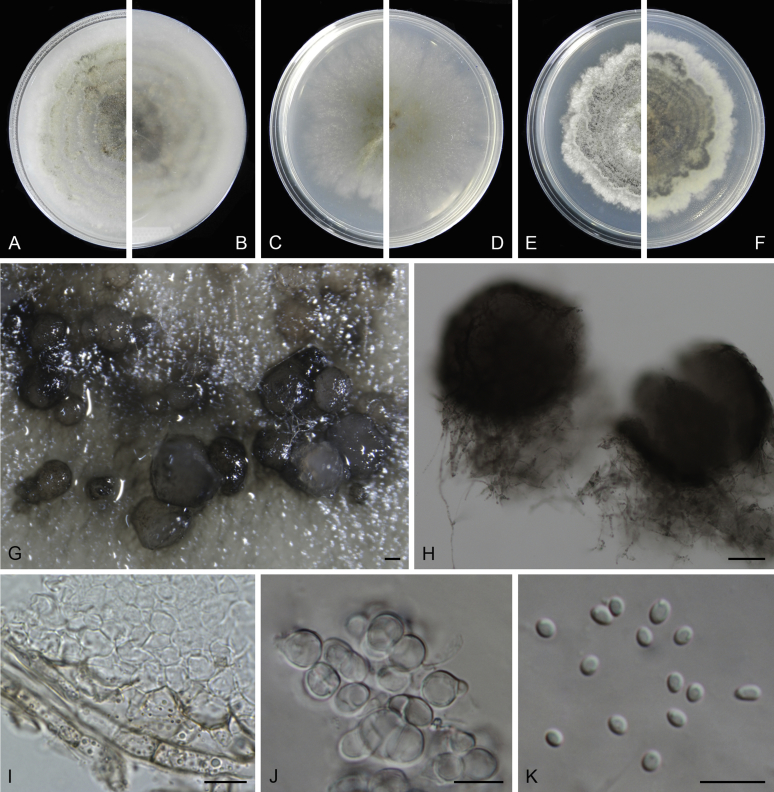
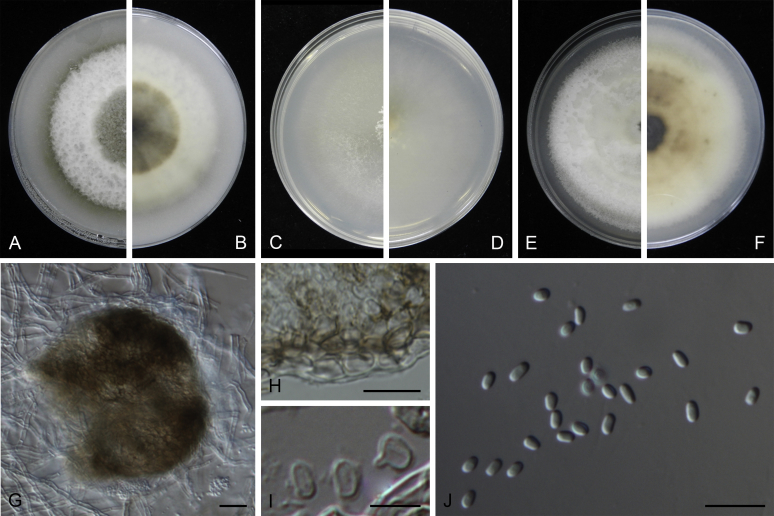
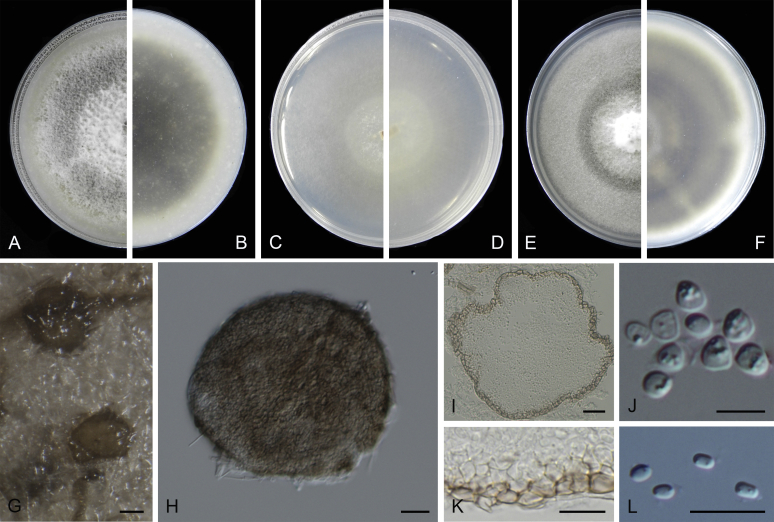
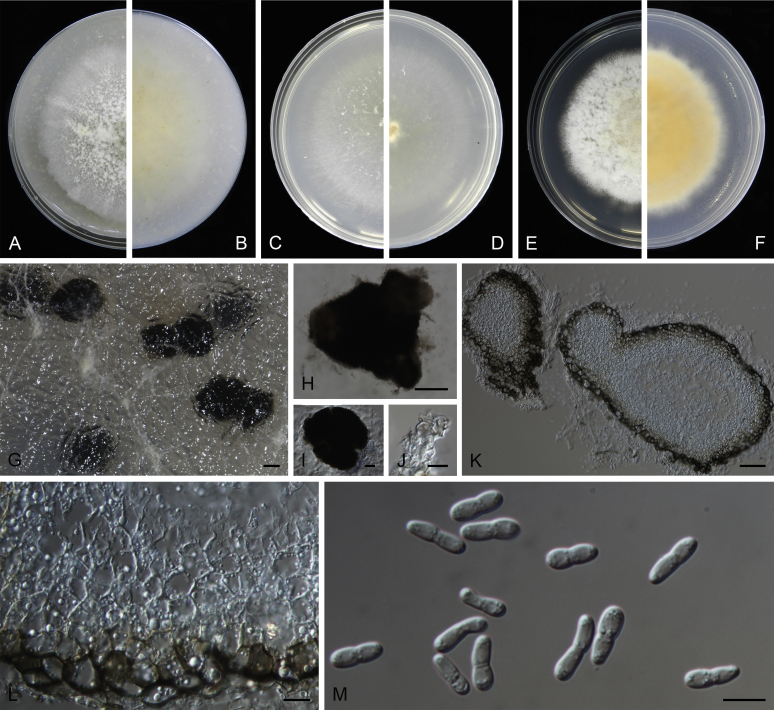
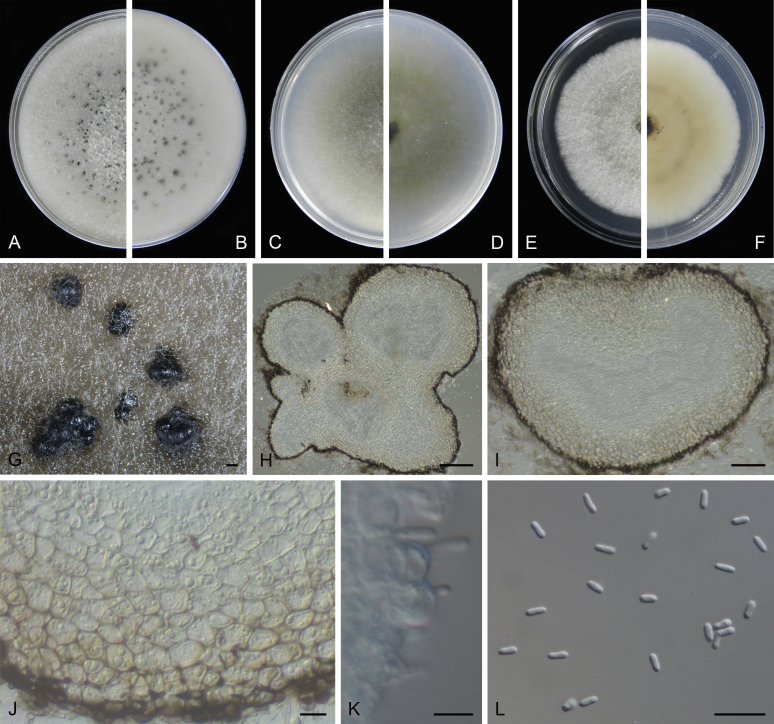
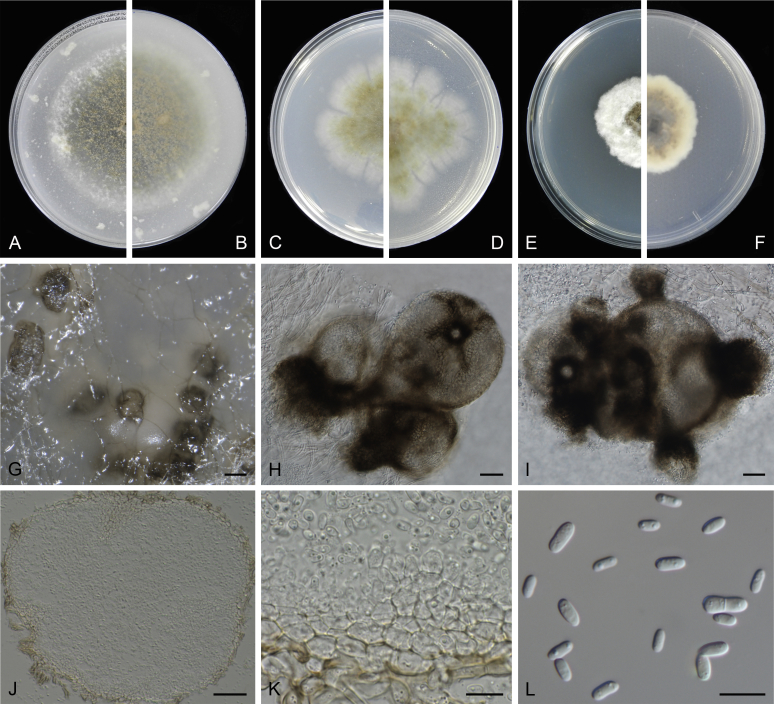
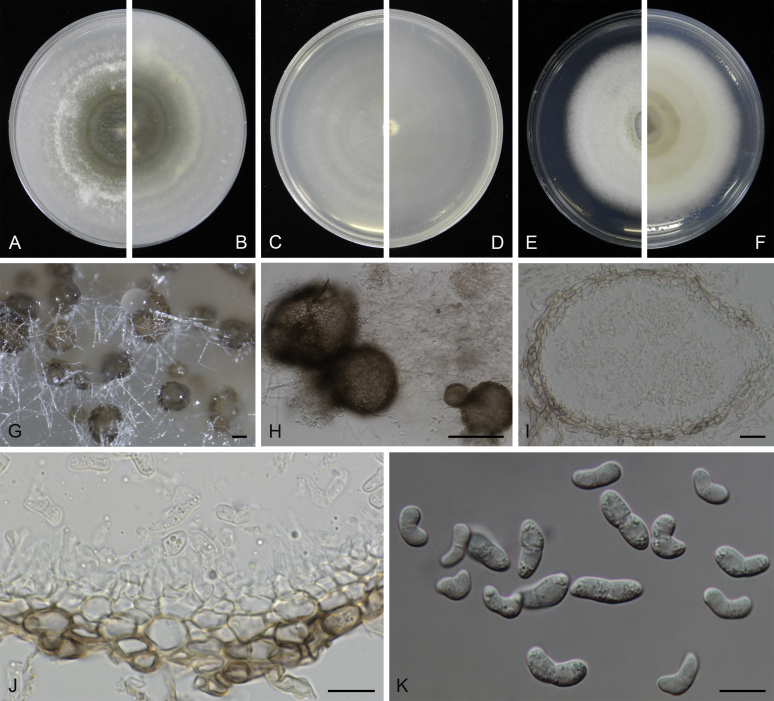
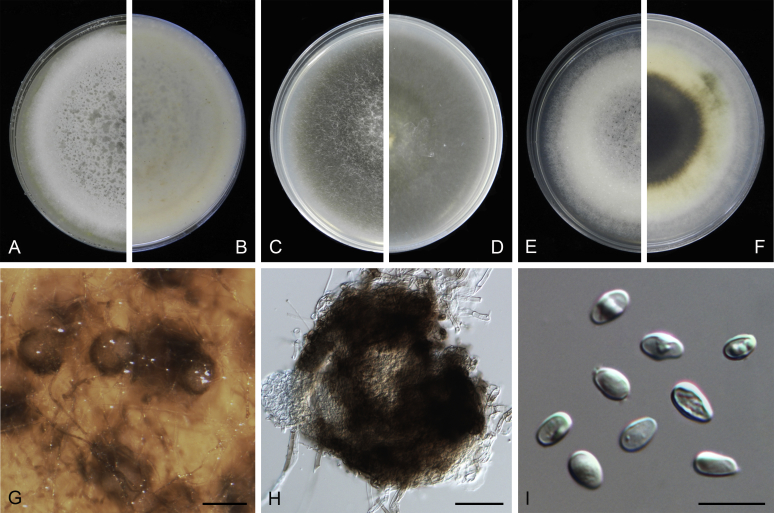
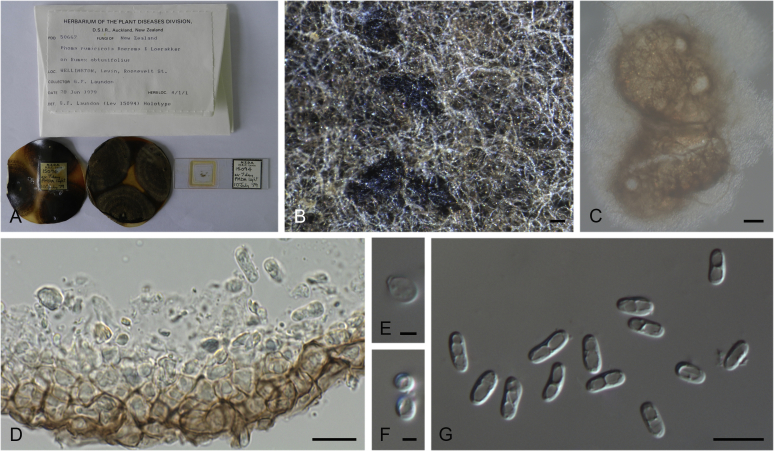
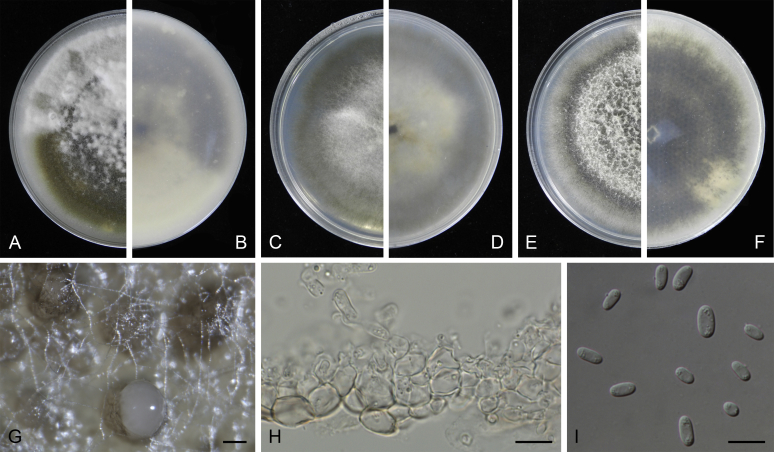
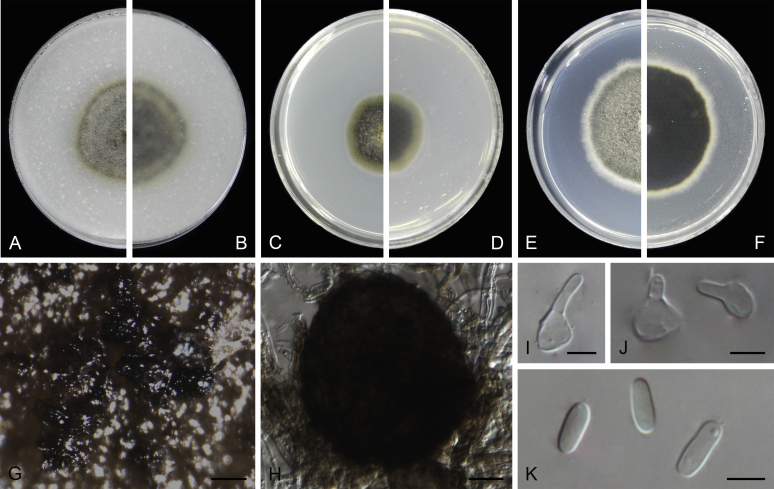
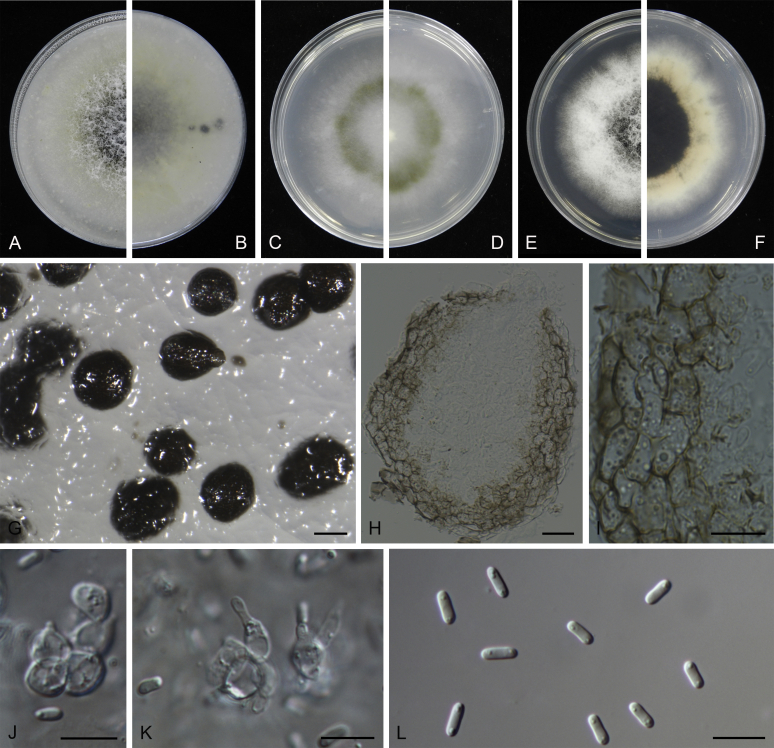
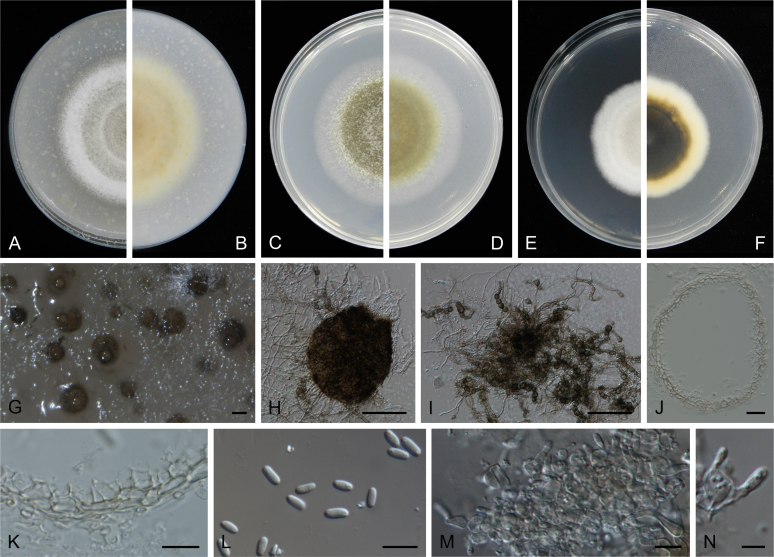
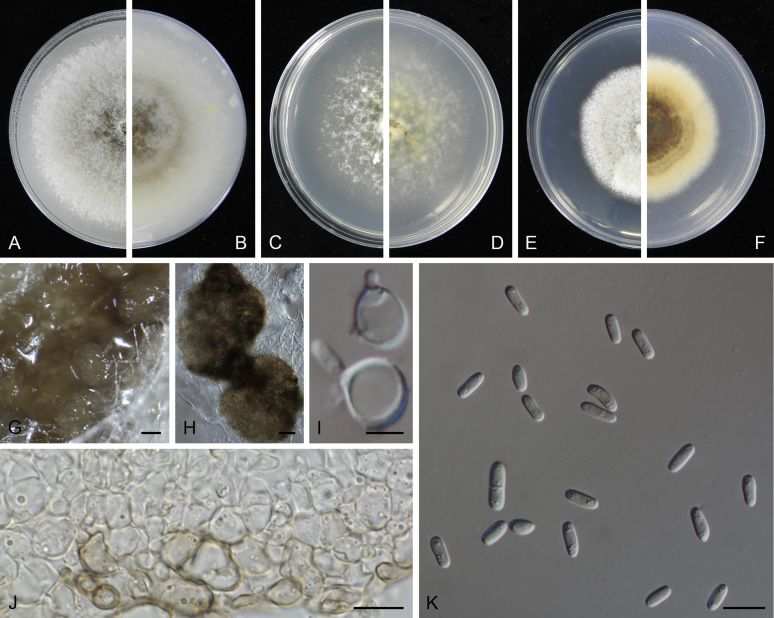
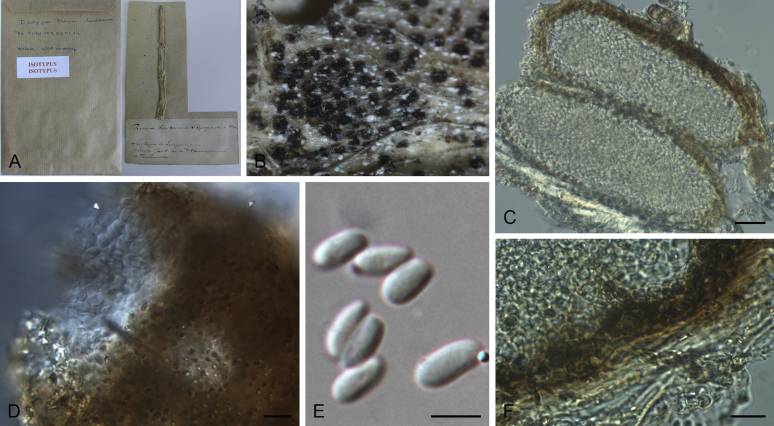
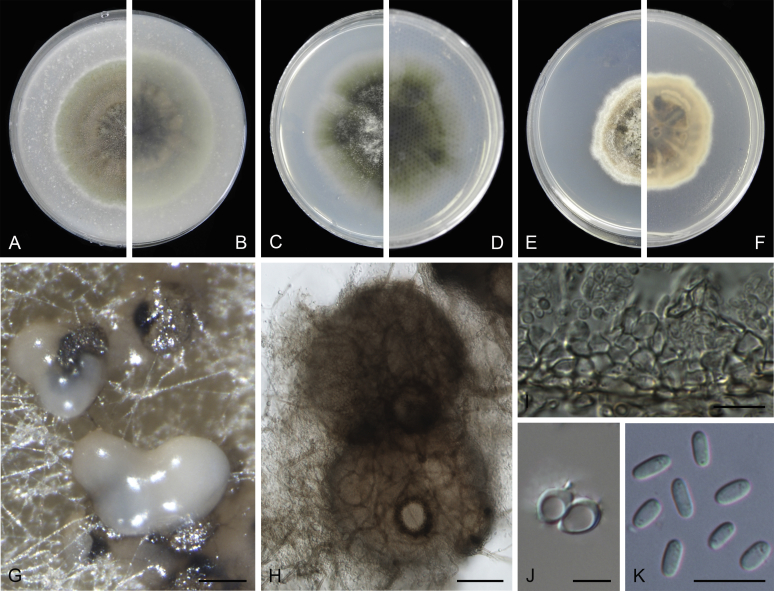
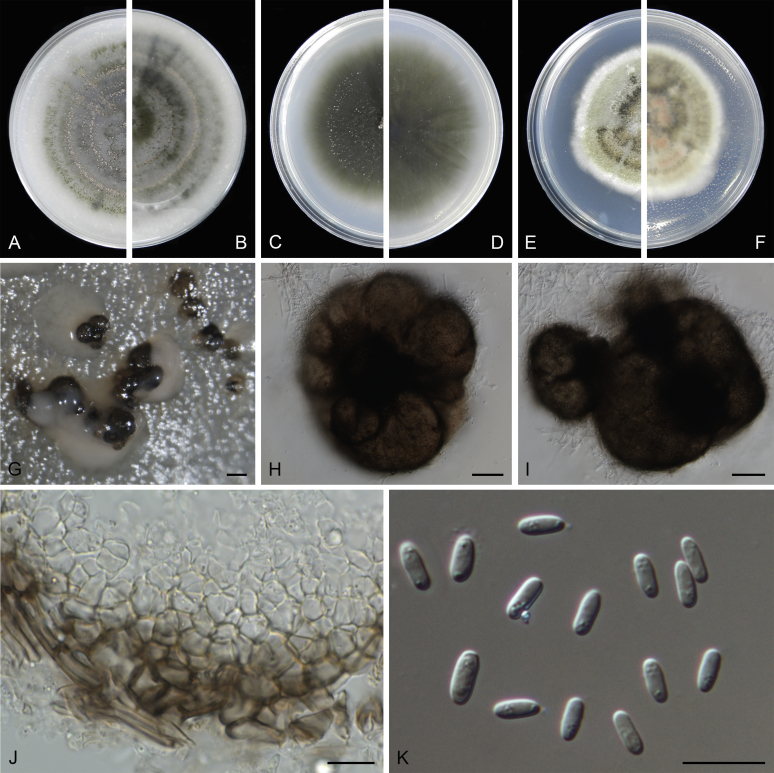
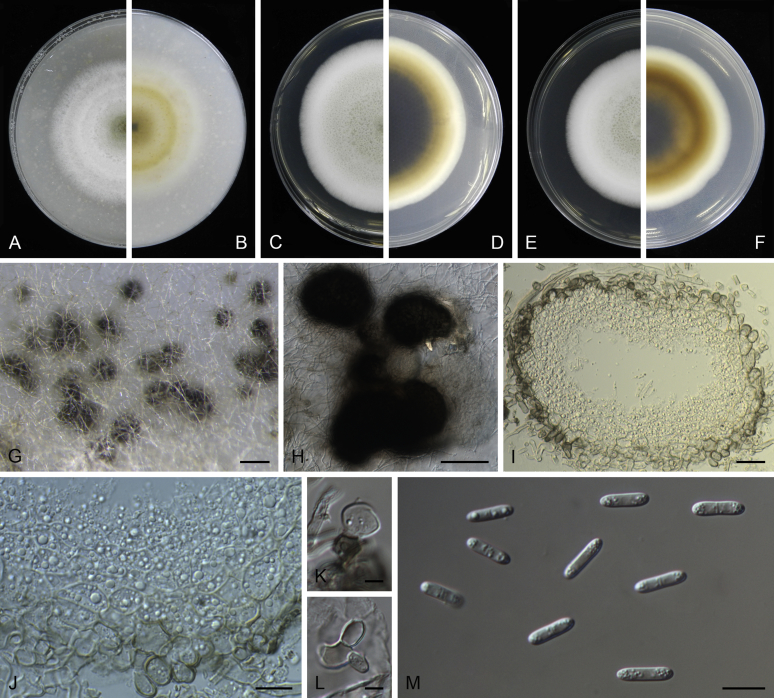
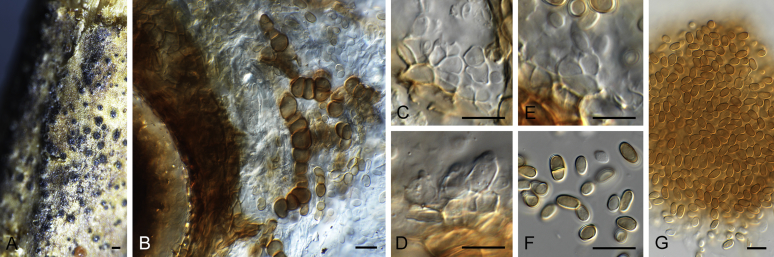
Morphological studies of living cultures were conducted following the methods described by Boerema et al. (2004) for the cultures grown on MEA, OA and PDA. Colony diameters were measured after 7 d, and colony morphologies determined after 14 d of incubation. Colony colours on the surface and reverse of inoculated Petri dishes were assessed according to the colour charts of Rayner (1970). Micromorphological descriptions and measurements for 30 replicates of relevant features were carried out from mature conidiomata and conidia mounted in water (Aveskamp et al., 2010, Chen et al., 2015). For conidiomatal pycnidia, pycnidial walls and conidiogenous cells, measurements were taken from 5–10 samples. Observations were conducted with a Leica M125 dissecting microscope and with a Zeiss Axio Imager A2 compound microscope under differential interference contrast (DIC) illumination. Sections of pycnidia were prepared using a Leica CM1950 freezing microtome, to study the anatomy of pycnidial walls and the morphology of conidiogenous cells (Aveskamp et al., 2010, Chen et al., 2015). The NaOH spot test was carried out on MEA cultures to detect the production of metabolite E (Boerema et al. 2004). For the fungarium specimens studied, pycnidia and ascomata were rehydrated in 10 % lactic acid or 5 % KOH for examination. Observations and sections of these materials were conducted using the same methods as described for cultures above.
DNA isolation, PCR amplification and sequencing
Genomic DNA was extracted following the protocol of Cubero et al. (1999), from fungal mycelium growing on MEA. Some of the DNAs were provided by the authors of Aveskamp et al. (2010; Utrecht, the Netherlands), which were extracted using the UltraClean Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Carlsbad, CA, USA). The LSU region was amplified with the primer pair LR0R (Rehner & Samuels 1994) and LR7 (Vilgalys & Hester 1990), the ITS region with V9G (de Hoog & Gerrits van den Ende 1998) and ITS4 (White et al. 1990), the tub2 region with the primers Btub2Fd and Btub4Rd (Woudenberg et al. 2009), and the rpb2 region with RPB2-5F2 (Sung et al. 2007) and fRPB2-7cR (Liu et al. 1999), respectively. The PCR amplifications were performed in a total volume of 25 μL containing 2.5 μL 10× EasyTaq Buffer (TransGen Biotech, Beijing, China), 50 μM dNTPs, 0.1 μM of each primer, 0.75 U Taq DNA polymerase and 1–10 ng genomic DNA. PCR conditions for LSU, ITS and tub2 were set as follows: an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation, annealing and extension, and a final extension step at 72 °C for 10 min. For the LSU amplification, the 35 cycles consisted of 45 s at 95 °C, 45 s at 48 °C and 2 min at 72 °C; for the ITS 30 s at 95 °C, 30 s at 48 °C and 80 s at 72 °C; and for the tub2 region 30 s at 95 °C, 30 s at 52 °C and 80 s at 72 °C. The PCR program for rpb2 amplification consisted of 5 cycles of 45 s at 94 °C, 45 s at 60 °C and 2 min at 72 °C, then 5 cycles with a 58 °C annealing temperature and 30 cycles with a 54 °C annealing temperature (Woudenberg et al. 2013). Sequencing was conducted by the Omega Genetics Company (Beijing, China) using the PCR primers and the additional internal sequence primer LR5 (Vilgalys & Hester 1990) for LSU.
Phylogenetic analyses
Sequences from each primer combination were used to obtain consensus sequences with MEGA v. 6.0 (Tamura et al. 2013). Reference sequences from Aveskamp et al. (2010) were downloaded from GenBank, and are listed in Table 1. Alignments of all consensus sequences, as well as the reference sequences were generated with MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/index.html; Katoh & Standley 2013), and were improved manually when necessary. Ambiguous regions were excluded from the analyses and gaps were treated as missing data. A 70 % neighbour-joining (NJ) reciprocal bootstrap method with maximum-likelihood distance was applied to check the congruence of the individual loci in the multi-locus dataset (Mason-Gamer & Kellogg 1996). Phylogenetic analyses of both individual and combined aligned data consisted of Bayesian and maximum-likelihood analyses.
MrModeltest v. 2.3 (Nylander 2004) was used to determine the best nucleotide substitution model settings for each locus. The Bayesian analyses of the combined four-locus dataset and individual locus data were performed with MrBayes v. 3.2.1 (Ronquist et al. 2012) based on the results of the MrModeltest. The Markov Chain Monte Carlo sampling (MCMC) analysis of four chains started in parallel from a random tree topology. The number of generations was set at 10 million and the run was stopped automatically when the average standard deviation of split frequencies fall below 0.01. Trees were saved each 1 000 generations. Burn-in was set at 25 % after which the likelihood values were stationary and the remaining trees were used to calculate posterior probabilities. Maximum-likelihood analyses including 1 000 bootstrap replicates were conducted using RAxML v. 7.2.6 (Stamatakis & Alachiotis 2010). A general time reversible model (GTR) was applied with a gamma-distributed rate variation. Novel sequences generated in this study were deposited in GenBank (Table 1), the final matrices used for phylogenetic analyses in TreeBASE (www.treebase.org; accession number: S18162), and novel taxonomic descriptions and nomenclature in MycoBank (www.MycoBank.org; Crous et al. 2004).
Results
Phylogenetic analyses
The final concatenated alignment contained 286 ingroup taxa with a total of 2 620 characters including gaps (966 characters for LSU, 648 for ITS, 395 for tub2 and 599 for rpb2) of which 883 were unique site patterns (45 for LSU, 270 for ITS, 216 for tub2 and 352 for rpb2), and Sporormiella minima (CBS 524.50) served as the outgroup taxon. The first 57 and the last 342 characters including gaps of the original LSU alignment was excluded from the analyses as these regions are unalignable. The general time reversible model with inverse gamma rates (GTR + I + G) was determined to be the best for all four loci by MrModeltest. The LSU, ITS, tub2 and rpb2 sequence datasets did not show any conflicts in the tree topologies for the 70 % reciprocal bootstrap trees, which allowed to combine the four loci for the multi-locus analysis.
The single locus phylogenies of LSU and ITS display low resolution at both generic and species level. The LSU phylogeny was only able to distinguish Boeremia, Calophoma, Leptosphaerulina, Macroventuria, Neoascochyta and Neodidymelliopsis clades, but failed for the other 11 genera. The ITS phylogeny was only able to distinguish 9 of 17 generic clades and failed for Allophoma, Ascochyta, Didymella, Epicoccum, Heterophoma, Macroventuria, Nothophoma and Xenodidymella. The rpb2 phylogeny was able to distinguish all 17 generic clades and with good resolution of species among these genera. The tub2 phylogeny was able to distinguish 13 of 17 generic clades and failed for Allophoma, Ascochyta, Calophoma and Stagonosporopsis.
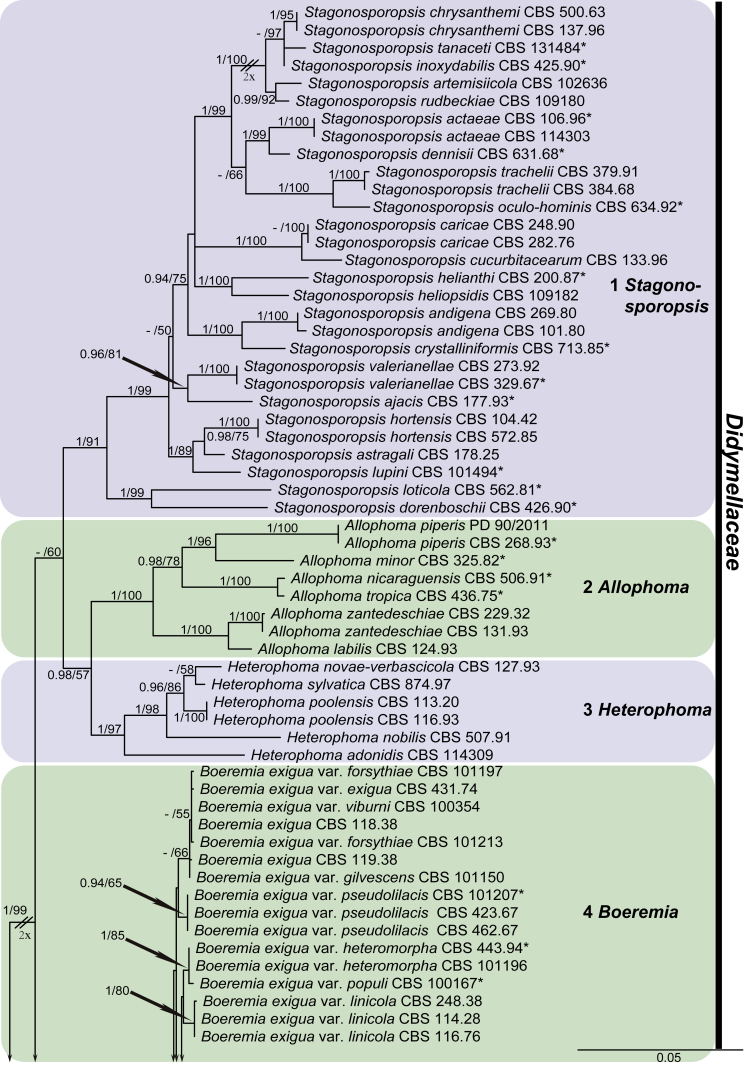
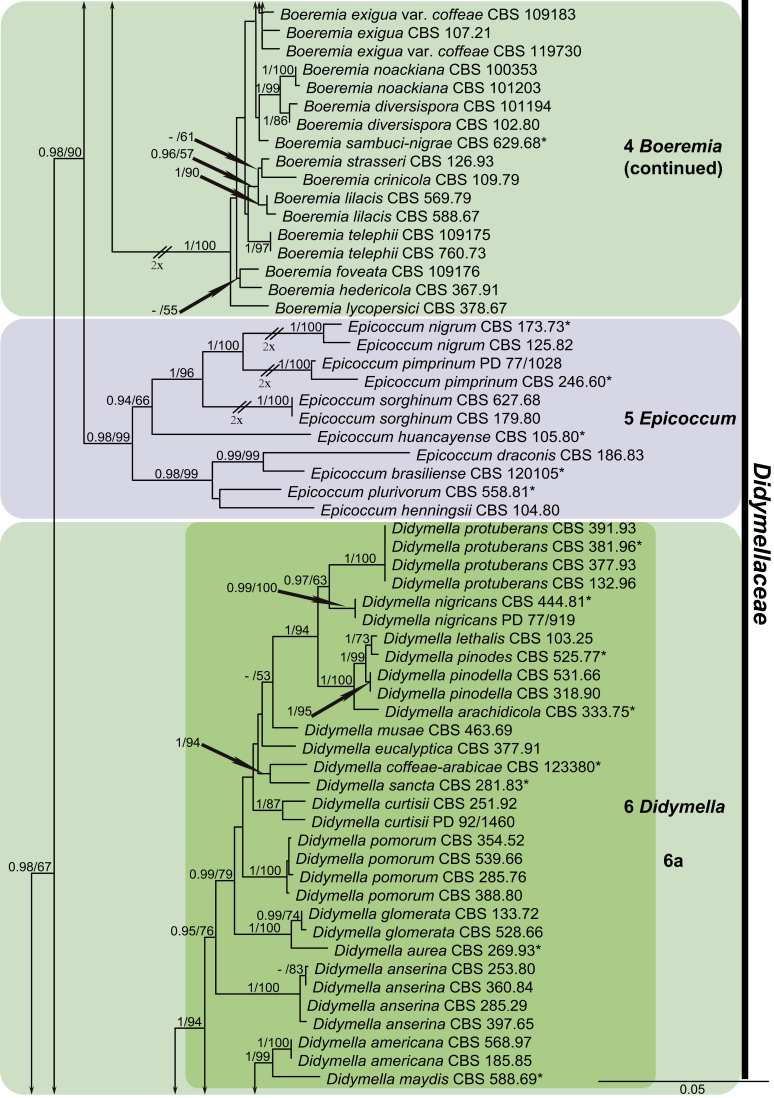
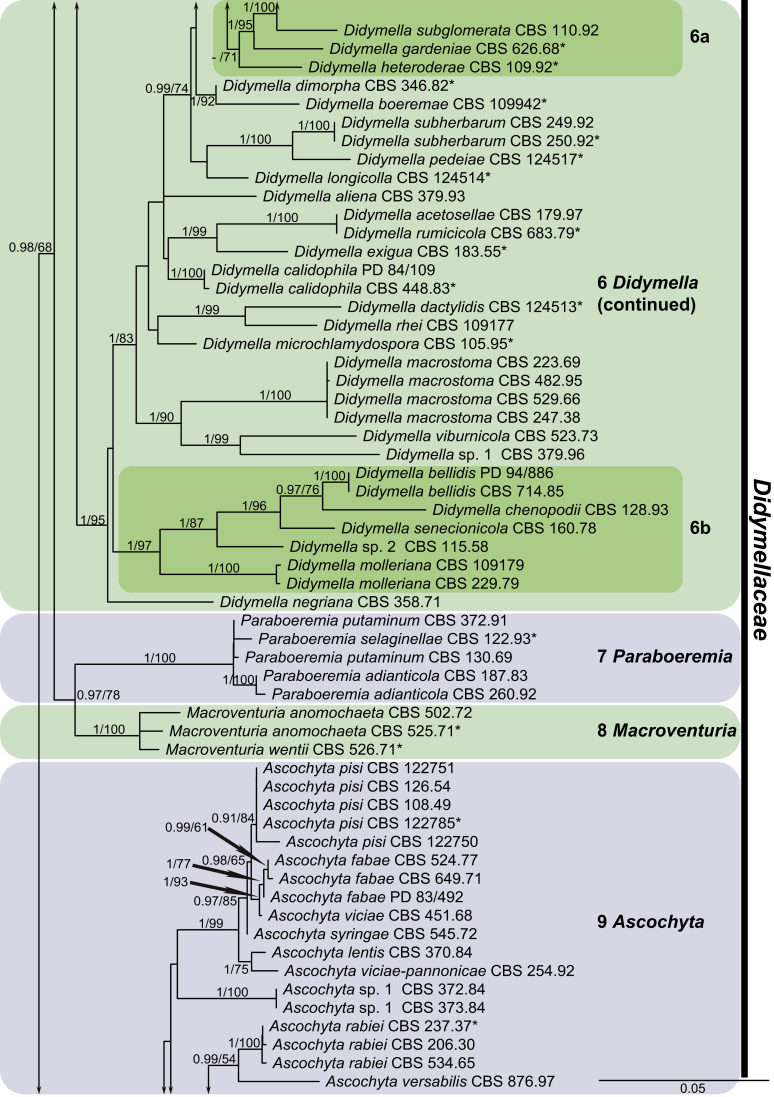
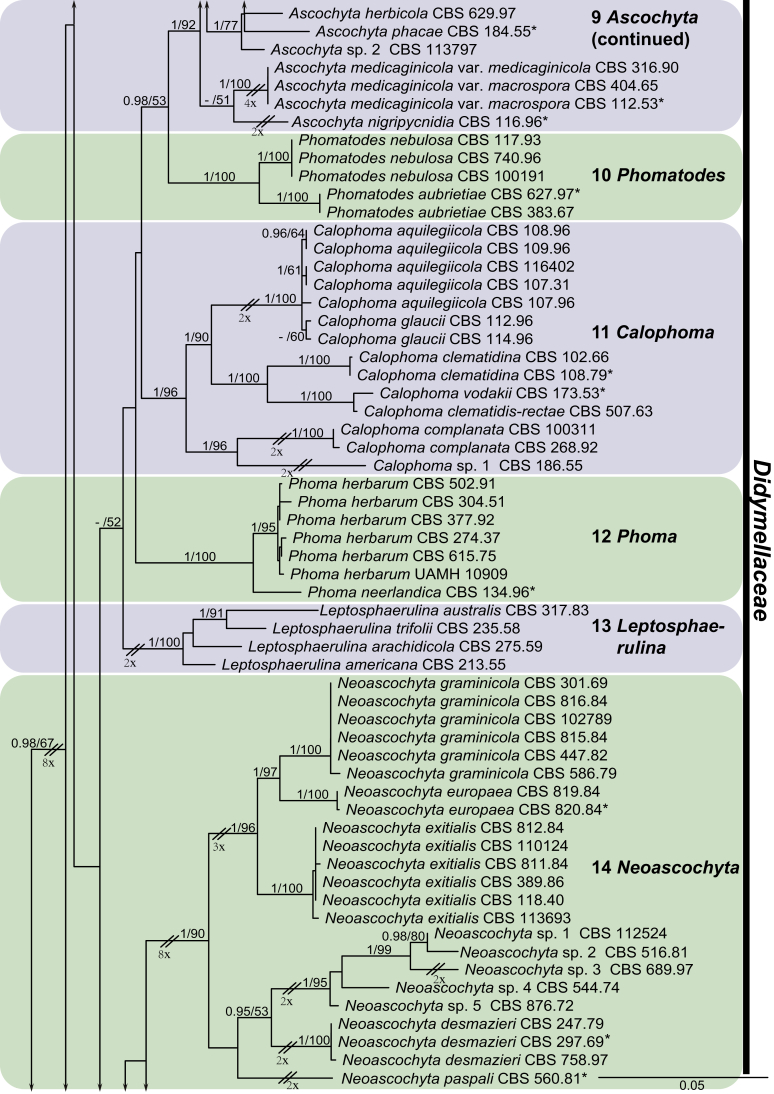
For the multi-locus analyses, a total of 12 858 trees were sampled after the burn-in with a stop value of 0.01. The topology of the BI tree confirmed that of ML tree for the distinctions of 17 well supported monophyletic clades, and therefore only the ML consensus tree with Bayesian posterior probabilities (BPP) and RAxML bootstrap support (MLBS) values are indicated in Fig. 1. Clustering basal in the four-locus tree (Fig. 1) were the outgroup taxon Sporormiella minima (CBS 524.50) and five monophyletic groups representing the five other families in Pleosporales close to Didymellaceae, namely Coniothyriaceae (BPP = 0.93; MLBS = 75 %) comprising four species, Coniothyrium carteri, Co. glycines, Co. palmarum and Co. telephii; Leptosphaeriaceae (BPP = 1; MLBS = 69 %) containing six species, Leptosphaeria conoidea, Leptosphaeria doliolum, Paraleptosphaeria nitschkei, Plenodomus biglobosus, Plen. lingam and Subplenodomus violicola; Cucurbitariaceae (BPP = 1; MLBS = 50 %) comprising four species, Cucurbitaria berberidis, Pyrenochaeta cava, Pyrenochaeta nobilis and Pyrenochaetopsis pratorum; Pleosporaceae (BPP = 1; MLBS = 83 %) comprising six species, Alternaria japonica, Bipolaris maydis, three Pleospora species, viz. Pleospora betae, Pleo. herbarum and Pleo. typhicola, and Pyrenophora phaeocomes; and Phaeosphaeriaceae (BPP = 1; MLBS = 100 %) comprising four species, Ophiosphaerella herpotricha, Phaeosphaeria ammophilae, Phaeosphaeriopsis triseptata and Setomelanomma holmii.
Fig. 1.
Phylogenetic tree inferred from a Maximum likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tub2 sequences of 287 strains representing Didymellaceae and allied families. The RAxML bootstrap support values (MLBS) and Bayesian posterior probabilities (BPP) are given at the nodes (BPP/MLBS). Some branches were shortened to fit them to the page – these are indicated by two diagonal lines with the number of times a branch was shortened indicated next to the lines. Ex-type strains are marked by an asterisk (*). The tree was rooted to Sporormiella minima (CBS 524.50).
The remaining ingroup could be divided into a basal Microsphaeropsis clade (BPP = 0.99; MLBS = 94 %, three isolates including the type species of Microsphaeropsis, Mi. olivacea) and the main Didymellaceae clade (BPP = 0.98; MLBS = 67 %). In the Didymellaceae clade, 17 well-supported monophyletic lineages were resolved, of which eight represent existing genera, and the remaining nine are described as new genera.
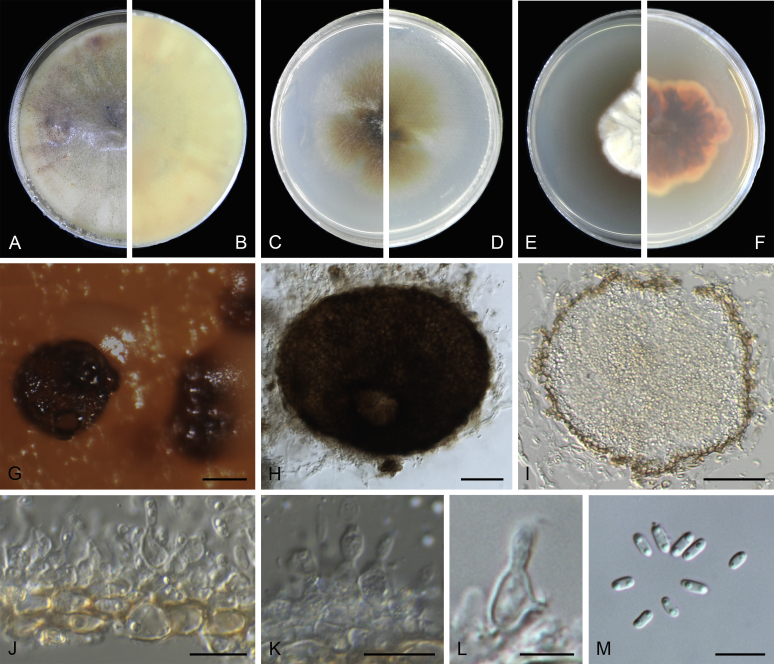
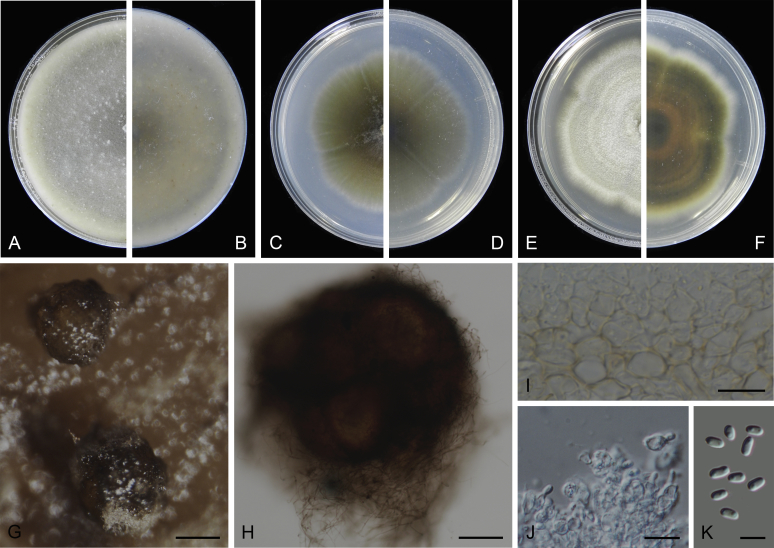
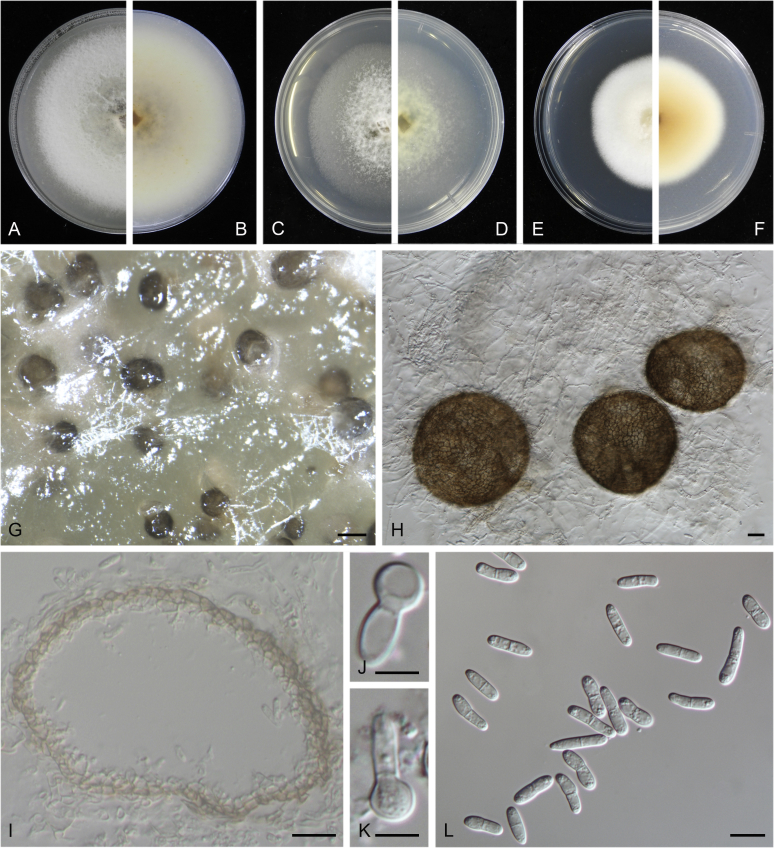
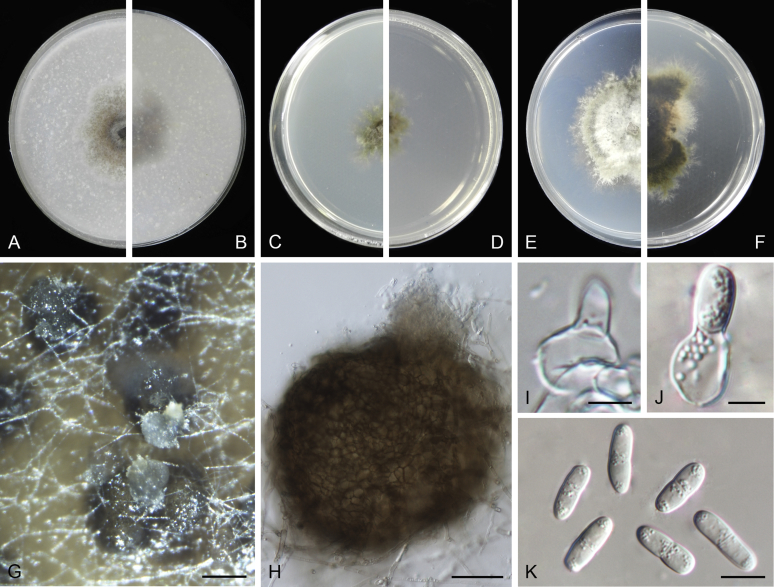
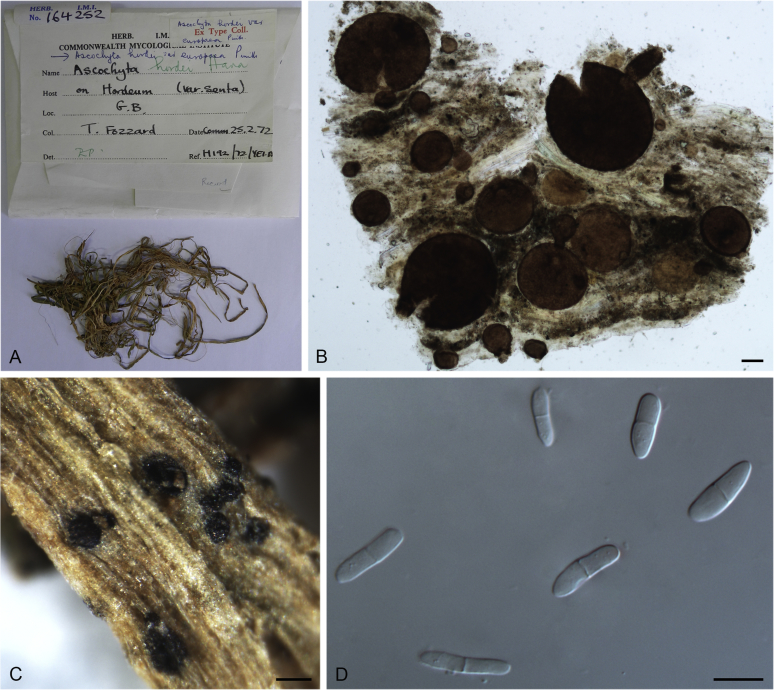
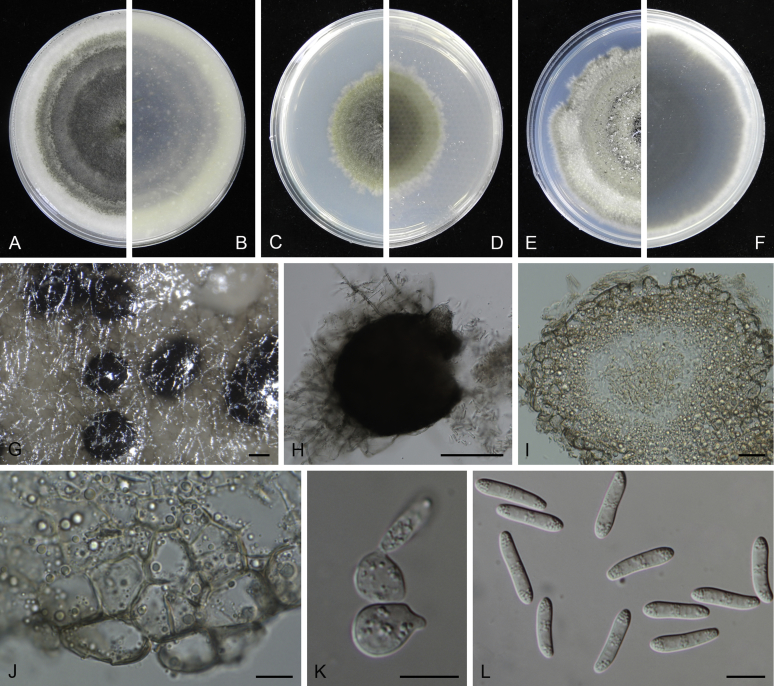
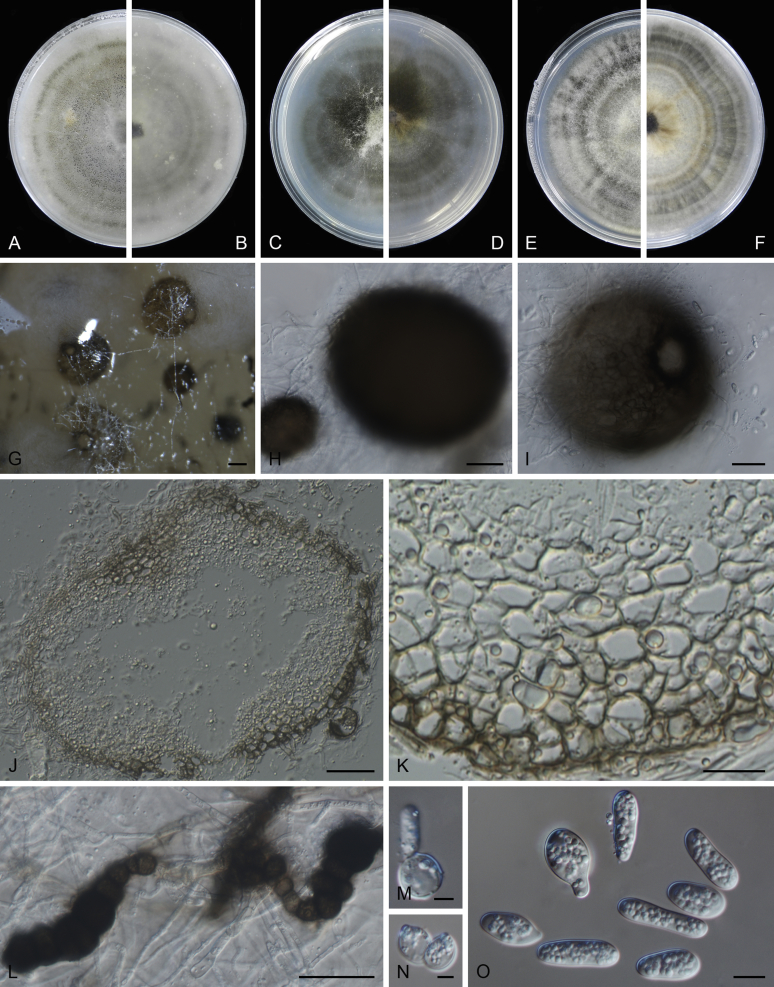
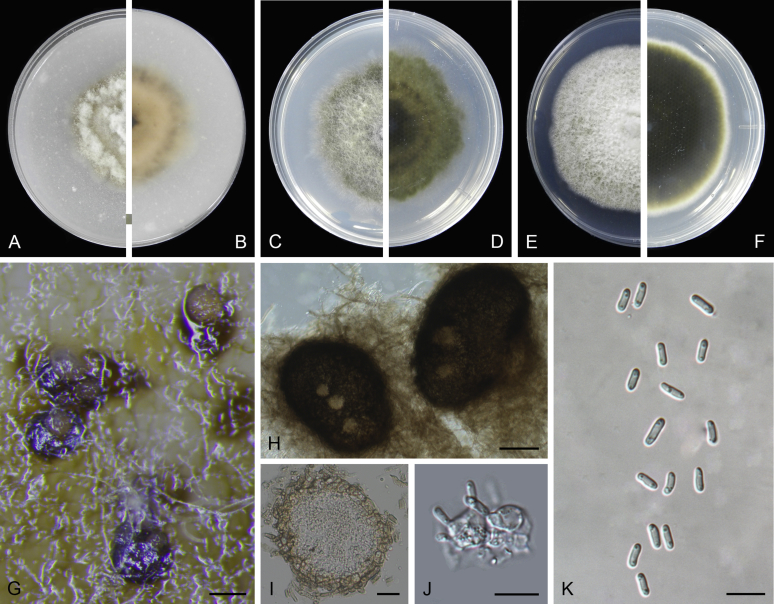
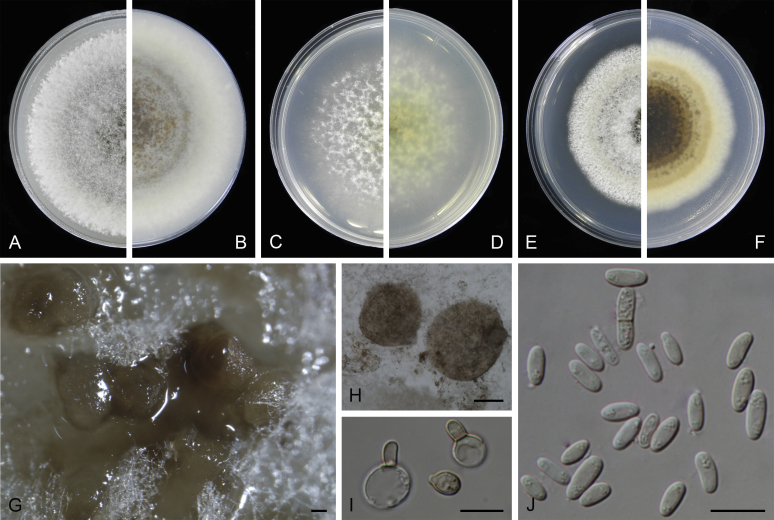
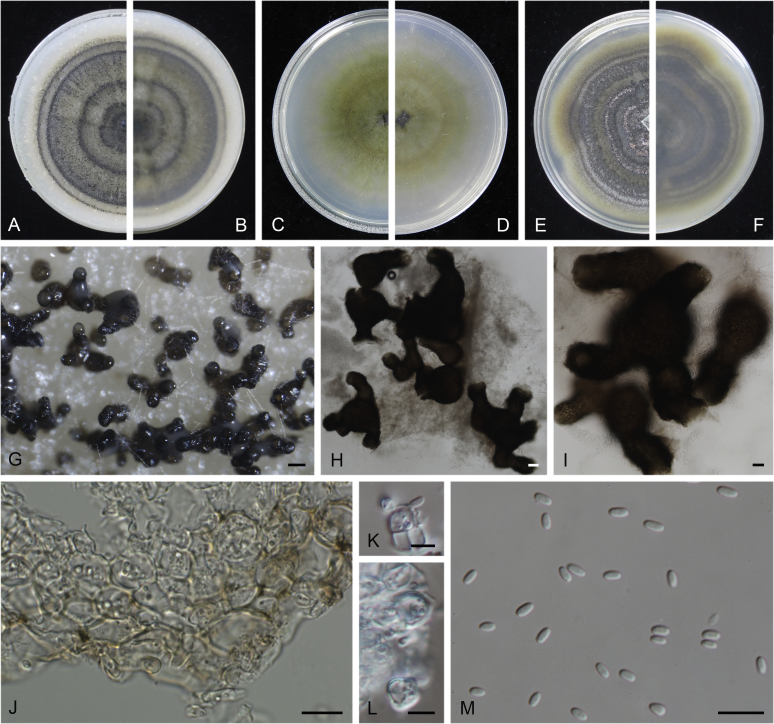
At the most terminal position, a well-supported clade, Clade 1 (BPP = 1; MLBS = 91 %, 29 isolates) accommodated all the species of the genus Stagonosporopsis, which was in congruence with the results of Aveskamp et al. (2010). Clade 2 (BPP = 1; MLBS = 100 %, eight isolates) comprised five “Phoma” species and a novel species, which formed a novel genus Allophoma, i.e. All. nicaraguensis, All. labilis (syn. Phoma labili), All. minor (syn. Phoma minor), All. piperis (syn. Phoma piperis), All. tropica (syn. Phoma tropica), and All. zantedeschiae (syn. Phoma zantedeschiae). Clade 3 (BPP = 1; MLBS = 97 %, six isolates) comprised five species accommodated in a novel genus Heterophoma, i.e. H. adonidis (syn. Didymella adonidis), H. nobilis (syn. Ascochyta nobilis), H. novae-verbascicola (syn. Phoma novae-verbascicola), H. poolensis (syn. Phoma poolensis), and H. sylvatica (syn. Phoma sylvatica). In congruence with the study of Aveskamp et al. (2010), the Boeremia species grouped in a well-defined cluster. Clade 4 (BPP = 1; MLBS = 100 %, 33 isolates), including B. exigua varieties and 10 other Boeremia species. Clade 5 (BPP = 0.98; MLBS = 99 %, 11 isolates) included three species of the genus Epicoccum, E. nigrum, E. pimprinum and E. sorghinum, and another five species of Phoma which were recombined into this genus, E. brasiliense (syn. Phoma brasiliensis), E. draconis (syn. Phoma draconis), E. henningsii (syn. Phoma henningsii), E. huancayense (syn. Phoma huancayensis) and E. plurivorum (syn. Phoma plurivora). Clade 6 (BPP = 1; MLBS = 95 %, 62 isolates) accommodated the type genus of the family Didymellaceae, Didymella, with the type species D. exigua (CBS 183.55). The subclade 6a accommodated 20 taxa belonging to the recently resurrected genus Peyronellaea, which were recombined into the genus Didymella. The subclade 6b comprised a cluster containing D. bellidis (syn. Phoma bellidis), D. chenopodii (syn. Phoma chenopodiicola), D. molleriana (syn. Phoma digitalis), D. senecionicola (syn. Phoma senecionis) and an isolate received as “Ascochyta pyrethri” (CBS 115.58). Between these two subclades there were several small groups comprised of D. acetosellae (syn. Phoma acetosellae), D. aliena (syn. Phoma aliena), D. boeremae (syn. Phoma boeremae), D. calidophila (syn. Phoma calidophila), D. dactylidis (syn. Phoma dactylidis), D. dimorpha (syn. Phoma dimorpha), the aforementioned D. exigua, D. longicolla (syn. Phoma longicolla), D. mascrostoma (syn. Phoma mascrostoma var. mascrostoma), D. microchlamydospora (syn. Phoma microchlamydospora), D. pedeiae (syn. Phoma pedeiae), D. rhei (syn. Phoma rhei), D. rumicicola (syn. Phoma rumicicola), D. subherbarum (syn. Phoma subherbarum), D. viburnicola (syn. Phoma viburnicola), an isolate received as “Phoma libertiana” (CBS 247.38) and an isolate representing a single lineage (CBS 379.96). Clade 7 (BPP = 1; MLBS = 100 %) comprised five isolates representing three species, which belong to a newly introduced genus Paraboeremia, namely Pa. adianticola (syn. D. adianticola), Pa. putaminum (syn. Phoma putaminum), and Pa. selaginellae (syn. Phoma selaginellicola). Clade 8 (BPP = 1; MLBS = 100 %) contained three isolates of Macroventuria including the generic type, Ma. anomochaeta. Clade 9 (BPP = 1; MLBS = 92 %, 25 isolates) accommodated the genus Ascochyta with its type species, As. pisi, and other Ascochyta species, As. fabae, As. herbicola (syn. Phoma herbicola), As. lentis, As. medicaginicola var. macrospora (syn. Phoma medicaginis var. macrospora), As. medicaginicola var. medicaginicola (syn. Phoma medicaginis var. medicaginis), As. nigripycnidia (syn. Phoma nigripycnidia), As. rabiei, As. syringae, As. versabilis (syn. Phoma versabilis), As. viciae, As. viciae-pannonicae, As. phacae and three isolates representing two insufficiently known species (CBS 372.84, CBS 373.84, CBS 113797). Two species that produced phoma-like conidia were embedded in clade 10 (BPP = 1; MLBS = 100 %, five isolates), which is proposed here as a new genus, Phomatodes, including Phomat. aubrietiae (syn. Phoma aubrietiae) and Phomat. nebulosa (syn. Phoma nebulosa). The majority of the isolates that clustered in clade 11 (BPP = 1; MLBS = 96 %, 14 isolates) were identified as “Phoma” sp., and a new generic name Calophoma is introduced below for this clade, which comprised five accepted species, Ca. aquilegiicola (syn. Phoma aquilegiicola), Ca. clematidina (syn. Phoma clematidina), Ca. clematidis-rectae (syn. Phoma clematidis-rectae), Ca. complanata (syn. Phoma complanata), Ca. glaucii (syn. Phoma glaucii), Ca. vodakii (syn. D. vodakii) and an insufficiently known species (CBS 186.55). Clade 12 (BPP = 1; MLBS = 100 %, seven isolates) accommodated the genus Phoma, including the generic type, Phoma herbarum and its sexual morph (based on Atradidymella muscivora strain UAMH 10909), and a new species Phoma neerlandica. Clade 13 (BPP = 1; MLBS = 100 %) comprised four isolates of Leptosphaerulina, including its type species, L. australis. Clade 14 (BPP = 1; MLBS = 90 %, 23 isolates) comprised a “Phoma” isolate and 22 isolates formerly identified as “Ascochyta”, and a “Didymella” species, most of which were subjected to molecular analysis for the first time. A new generic name Neoascochyta is proposed below for these taxa. These included Neoa. desmazieri (syn. Ascochyta desmazieri), Neoa. exitialis (syn. Didymella exitialis), Neoa. graminicola (syn. Didymella graminicola), Neoa. europaea (syn. As. hordei var. europaea), Neoa. paspali (syn. Phoma paspali) and five insufficiently known isolates (CBS 516.81, CBS 544.74, CBS 689.97, CBS 876.72 and CBS 112524). Clade 15 (BPP = 1; MLBS = 97 %, eight isolates) accommodated a newly established sexual genus, Xenodidymella, including X. applanata (syn. Didymella applanata), X. asphodeli (syn. D. asphodeli), X. catariae (syn. D. catariae) and X. humicola (syn. Phoma humicola). Clade 16 (BPP = 1; MLBS = 100 %) contained 10 isolates initially classified in the genera Ascochyta and Didymella, as well as Phoma, and for this well-supported cluster the new generic name Neodidymelliopsis is proposed below, including six species, Neod. cannabis (syn. D. cannabis), Neod. polemonii (syn. Phoma polemonii), Neod. xanthina (syn. Phoma xanthina) and two insufficiently known isolates (CBS 256.77, CBS 382.96). Clade 17 (BPP = 1; MLBS = 85 %, five isolates) contained five species that were accommodated in a new genus proposed below, Nothophoma, namely No. anigozanthi (syn. Phoma anigozanthi), No. arachidis-hypogaeae (syn. Phoma arachidis-hypogaeae), No. quercina (syn. Phoma fungicola), No. gossypiicola (syn. Phoma gossypiicola) and No. infossa (syn. Phoma infossa).
Taxonomy
Phylogenetic analyses based on the combined LSU, ITS, tub2 and rpb2 sequences resolved a total of 24 clades, in which 17 clades including 162 taxa belonged to the Didymellaceae. With morphological examination of the type specimens and isolates, nine new genera, three new species, 84 new combinations, two new names and 11 epitypifications and seven neotypifications are proposed below. All recognised clades are treated, and the novelties, as well as epitypifications and neotypifications are described and illustrated below. The main morphological characters of accepted genera in Didymellaceae were provided in Table 2. The identity of several species and / or isolates could not be resolved, mostly because the type materials were unavailable for study. Their identities remain uncertain and will be resolved in future studies. The genus Microsphaeropsis grouped basal to the Didymellaceae, for which a new family Microsphaeropsidaceae was introduced.
Table 2.
Overview of the main characters of genera in the Didymellaceae.
| Genera | Asexual morph |
Sexual morph |
|||
|---|---|---|---|---|---|
| Conidia | Septa | Chlamydospores | Ascospores | Septa | |
| Allophoma | ovoid, oblong, ellipsoidal to cylindrical, or slightly allantoid | aseptate | – | – | – |
| Ascochyta | ovoid, oblong, subcylindrical, ellipsoidal, cymbiform, allantoid | 0–1(–3) | unicellular or multicellular | ovoid to ellipsoidal, slightly biconic | 1 or 3 |
| Boeremia | variable in shape | 0–1(–2) | – | ellipsoidal | 1 |
| Calophoma | subglobose, subcylindrical, ellipsoidal, somewhat obclavate-fusiform | 0–1 | unicellular or multicellular | – | – |
| Didymella | ellipsoidal to subglobose, cylindrical, oblong, ovoid, sometimes allantoid | aseptate | unicellular or multicellular | ellipsoidal to cymbiform | 1 or multiseptate |
| Epicoccum | ovoid, ellipsoidal to oblong, (sub-)cylindrical; epicoccoid conidia: multicellular-phragmosporous, subglobose-pyriform | aseptate; septa being obscured by the dark verrucose wall | unicellular or multicellular | – | – |
| Heterophoma | ellipsoidal, oblong, cylindrical, reniform, or slightly allantoid | 0–1(–2) | unicellular | – | – |
| Leptosphaerulina | – | – | – | muriform, oblong, ellipsoidal to obovoid, subfusoid | 1(–6) |
| Macroventuria | – | – | – | ellipsoidal | 1 |
| Neoascochyta | fusoid to cylindrical, obclavate-ovoid to ellipsoidal | 0–1 | – | cylindrical to ovoid, ellipsoidal | 1 |
| Neodidymelliopsis | ovoid to ellipsoidal, cylindrical, allantoid | 0–1 | unicellular or multicellular | subovoid to oblong, ellipsoidal | 1(–3) |
| Nothophoma | ovoid, oblong to ellipsoidal | aseptate | – | – | – |
| Paraboeremia | ellipsoidal | aseptate | – | subcylindrical | 1 |
| Phoma | oblong to cylindrical, ellipsoidal, sometimes fusiform | aseptate | – | fusiform | 1 |
| Phomatodes | cylindrical to allantoid | aseptate | – | – | – |
| Stagonosporopsis | ellipsoidal to subglobose | 0–3 | – | ellipsoidal, fusiform or obovoid | 1 |
| Xenodidymella | ellipsoidal to allantoid, subcylindrical, oblong, pyriform | 0–1 | unicellular | obovoid to oblong, clavate, ellipsoidal | 1 |
Treatment of monophyletic lineages
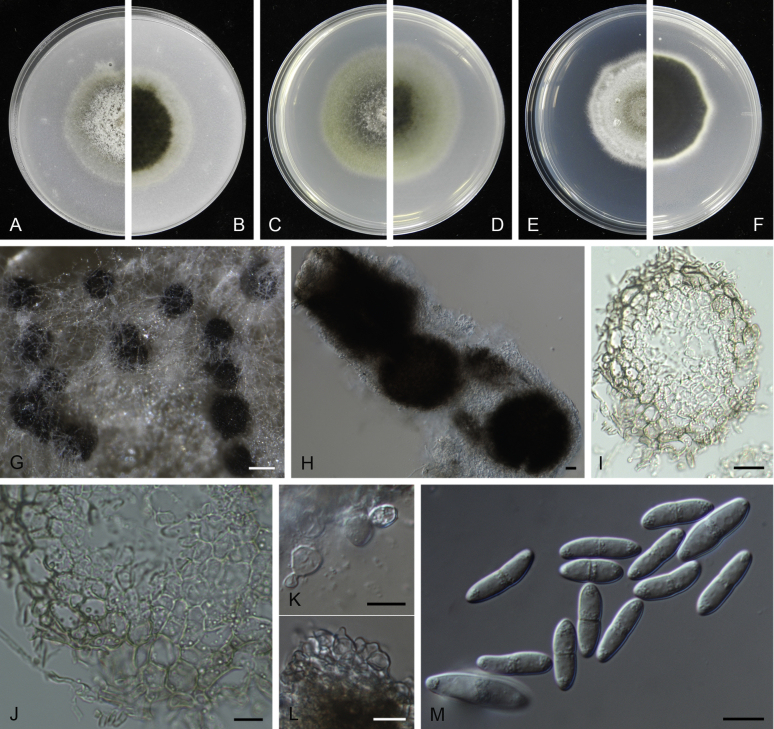
Clade 1: Stagonosporopsis
Stagonosporopsis Died. emend. Aveskamp et al., Stud. Mycol. 65: 44. 2010.
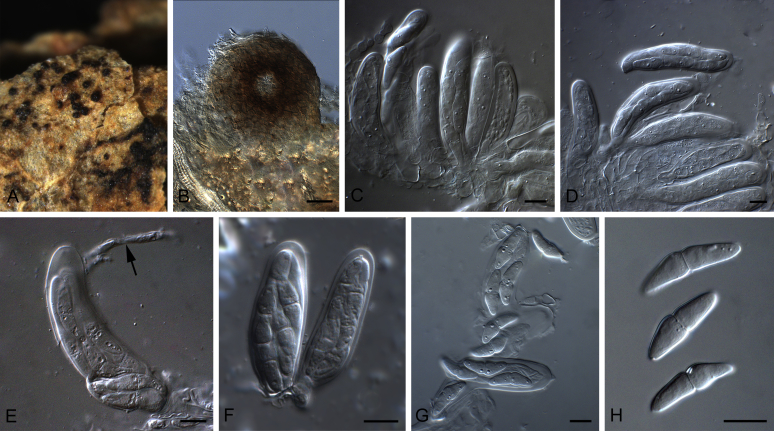
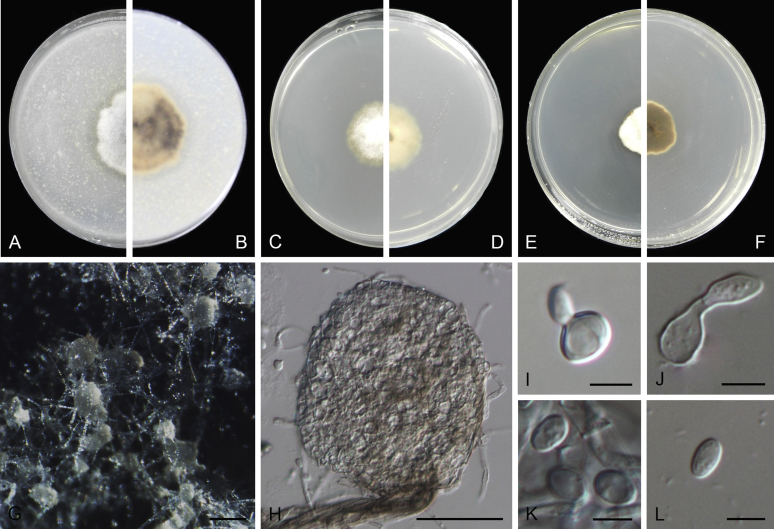
Conidiomata pycnidial, globose to subglobose, superficial on or immersed into the agar, solitary or confluent, ostiolate or poroid. Pycnidial wall pseudoparenchymatous, 2–6-layered, with an outer wall composed of 1–3 layers of brown olivaceous cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform or doliiform. Conidia often dimorphic: majority aseptate, hyaline, ellipsoidal to subglobose, thin- and smooth-walled. Conidia of the second type smaller in size, can be produced both in vivo and in vitro in the same pycnidia, unicellular or with up to 3 septa. Ascomata pseudothecial, if present, occurring only in vivo, globose to subglobose, sometimes with a somewhat conical neck. Asci cylindrical or subclavate, 8-spored, biseriate. Ascospores ellipsoidal, fusiform or obovoid, 1-septate, guttulate (from Aveskamp et al. 2010).
Type species: Stagonosporopsis hortensis (Sacc. & Malbr.) Petr., Ann. Mycol. 19: 21. 1921.
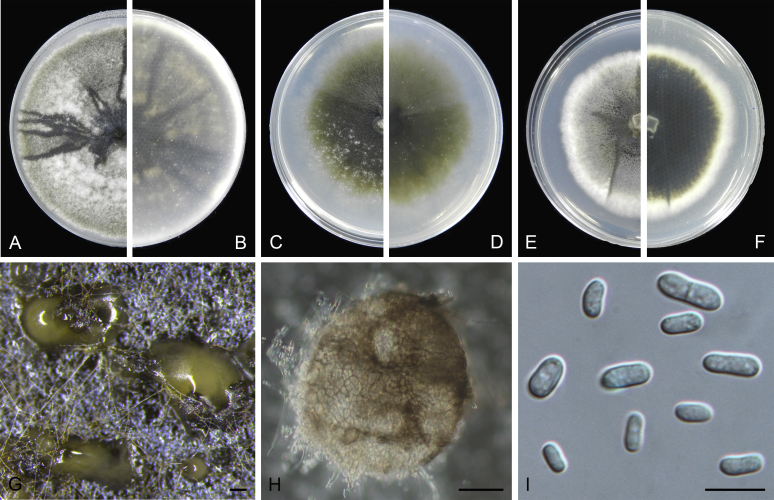
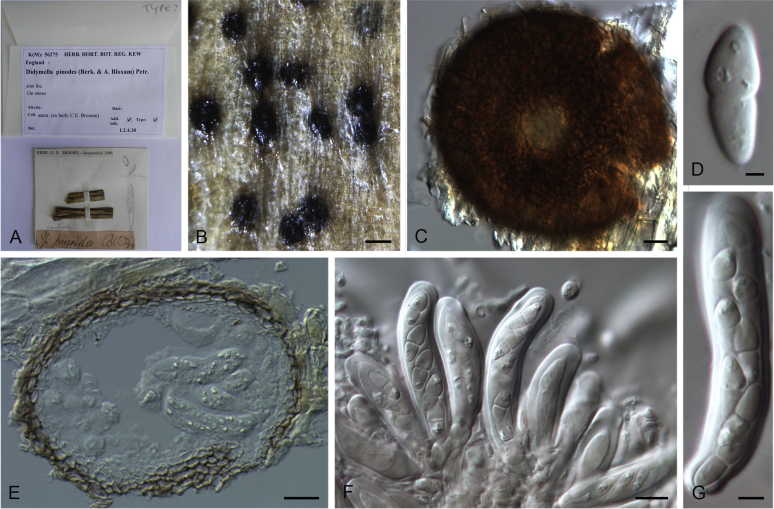
Stagonosporopsis actaeae (Allesch.) Died., Ann. Mycol. 10: 141. 1912.
Basionym: Actinonema actaeae Allesch., Ber. Bayer. Bot. Ges. 5: 7. 1897.
= Phoma actaeae Boerema et al., Persoonia 16: 347. 1997.
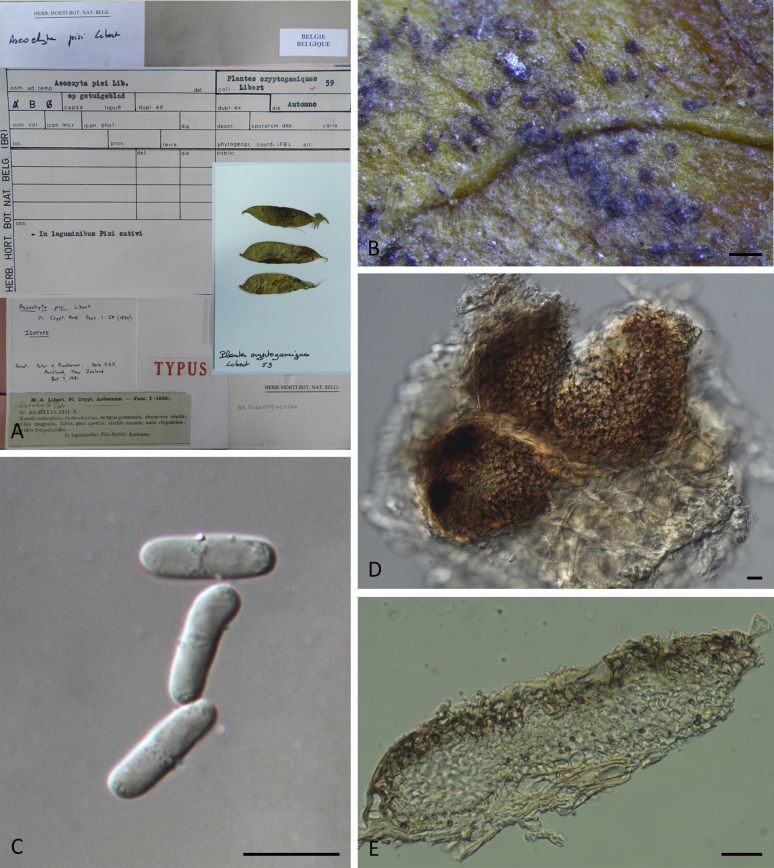
Specimens examined: Sweden, Uppland, Dalby par., Jerusalem, from Actaea spicata, 16 Jun. 1989, K. & L. Holm, CBS 114303 = UPSC 2962. The Netherlands, Limburg, Schaersbergerbos, from a leaf spot of Actaea spicata, 22 Sep. 1994 (holotype of Phoma actaeae L 992.167-501, culture ex-holotype CBS 106.96 = PD 94/1318).
Notes: Isolate CBS 114303, received as “Didymella hellebori”, was also isolated from the same host as the holotype of Stagonosporopsis actaeae, and is genetically identical to CBS 106.96 in all sequenced loci. It appears that CBS 114303 represents the sexual morph for S. actaeae.
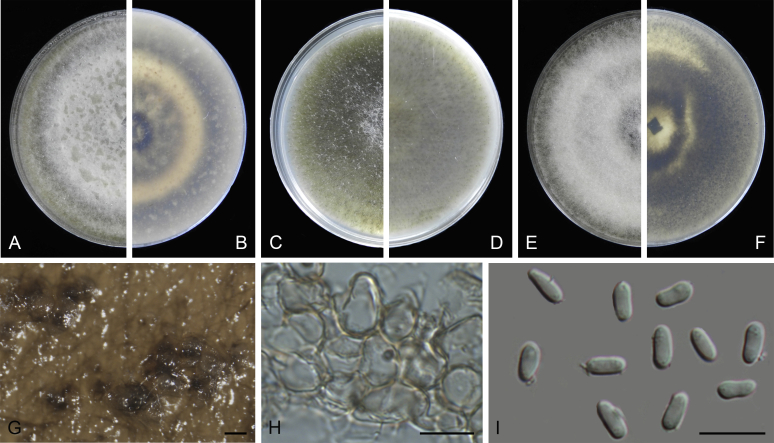
Stagonosporopsis ajacis (Thüm.) Aveskamp et al., Stud. Mycol. 65: 44. 2010.
Basionym: Phyllosticta ajacis Thüm., Boll. Soc. Adriat. Sci. Nat. Trieste 6: 329. 1880.
= Phoma ajacis Aa & Boerema, Persoonia 15: 383. 1993.
Specimen examined: Kenya, from Delphinium sp., 1990, Hopman (neotype of Phoma ajacis L 993.034.225, culture ex-neotype CBS 177.93 = PD 90/115).
Stagonosporopsis andigena (Turkenst.) Aveskamp et al., Stud. Mycol. 65: 44. 2010.
Basionym: Phoma andigena Turkenst., Persoonia 16: 131. 1995.
Specimens examined: Peru, Dep. Junin, Huancayo, near Valle del Mantaro, from a leaf of Solanum sp., deposited in CBS Jan. 1980, G.H. Boerema, CBS 101.80 = PD 75/909 = IMI 386090; Dep. Junin, Huancayo, near Valle del Mantaro, from a leaf of Solanum sp., 1975, L.J. Turkensteen, CBS 269.80 = PD 75/914.
Stagonosporopsis artemisiicola (Hollós) Aveskamp et al., Stud. Mycol. 65: 44. 2010.
Basionym: Phoma artemisiicola Hollós, Mat. Term. Közlem. 35: 40. 1926. (as “artemisaecola”)
Specimen examined: France, from a stem base of Artemisia dracunculus, deposited in CBS Mar. 2000, CBS 102636 = PD 73/1409.
Stagonosporopsis astragali (Cooke & Harkn.) Aveskamp et al., Stud. Mycol. 65: 45. 2010.
Basionym: Phoma astragali Cooke & Harkn., Grevillea 13: 111. 1885.
Specimen examined: Unknown origin, from Astragalus sp., deposited in CBS Sep. 1925, A.W. Archer, CBS 178.25 = MUCL 9915.
Stagonosporopsis caricae (Syd. & P. Syd.) Aveskamp et al., Stud. Mycol. 65: 45. 2010.
Basionym: Mycosphaerella caricae Syd. & P. Syd., Ann. Mycol. 11: 403. 1913.
= Ascochyta caricae-papayae Tarr., The fungi and plant diseases of Sudan: 53. 1955.
≡ Phoma caricae-papayae (Tarr.) Punith., Trans Brit. Mycol. Soc. 75: 340. 1980.
= Phoma caricae Punith., C.M.I. Descript. Pathog. Fungi Bact. 634: 1. 1979.
Specimens examined: Chile, from fruit of Carica papaya, deposited in CBS Jun. 1990, CBS 248.90. Indonesia, Java, Segunung, from Brassica sp., Feb. 1976, H. Vermeulen, CBS 282.76.
Stagonosporopsis chrysanthemi (F. Stevens) Crous et al., Australas. Pl. Pathol. 41: 681. 2012.
Basionym: Ascochyta chrysanthemi F. Stevens, Bot. Gaz. 44: 246. 1907.
= Mycosphaerella ligulicola K.F. Baker et al., Phytopathology 39: 799. 1949.
≡ Didymella ligulicola (K.F. Baker et al.) Arx, Beitr. Kryptogamenfl. Schweiz. 11: 364. 1962.
≡ Didymella ligulicola var. ligulicola (K.F. Baker et al.) Arx, Stud. Mycol. 32: 9. 1990.
≡ Stagonosporopsis ligulicola var. ligulicola (K.F. Baker et al.) Aveskamp et al., Stud. Mycol. 65: 46. 2010.
= Phoma ligulicola var. ligulicola Boerema, Stud. Mycol. 32: 9. 1990.
Specimens examined: Germany, Berlin, from Chrysanthemum indicum, deposited in CBS Dec. 1963, R. Schneider, CBS H-11952, culture CBS 500.63 = MUCL 8090. The Netherlands, near Lisse, from a leaf of Chrysanthemum indicum, deposited in CBS Feb. 1996, CBS 137.96 = PD 84/75.
Stagonosporopsis crystalliniformis (Loer. et al.) Aveskamp et al., Stud. Mycol. 65: 45. 2010.
Basionym: Phoma andina var. crystalliniformis Loer. et al., Fitopatología 21: 100. 1986.
≡ Phoma crystalliniformis (Loer. et al.) Noordel. & Gruyter, Mycol. Res. 97: 1344. 1993.
Specimens examined: Colombia, Antioquia, Rionegro, from a stem base of Lycopersicon esculentum, 1983, R. Navarro (holotype CBS H-3926, culture ex-holotype CBS 713.85 = ATCC 76027 = PD 83/826).
Stagonosporopsis cucurbitacearum (Fr.) Aveskamp et al., Stud. Mycol. 65: 45. 2010.
Basionym: Sphaeria cucurbitacearum Fr., Syst. Mycol. 2: 502. 1823.
≡ Phoma cucurbitacearum (Fr.) Sacc., Syll. Fung. 3: 148. 1884.
= Sphaeria bryoniae Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 112. 1870.
≡ Didymella bryoniae (Fuckel) Rehm, Ber. Naturhist. Vereins Augsburg 26: 27. 1881.
Specimen examined: New Zealand, from Cucumis sp., deposited in CBS May 1996, CBS 133.96 = PD 79/127.
Stagonosporopsis dennisii Boerema et al., Persoonia 16: 350. 1997. Fig. 2.
Fig. 2.
Stagonosporopsis dennisii (CBS 631.68). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidium. H. Section of pycnidial wall. I. Conidiogenous cells. J. Conidia. Scale bars: G = 100 μm; H–J = 10 μm.
= Phoma dennisii Boerema, Trans. Brit. Mycol. Soc. 67: 307. 1976.
Description from ex-epitype culture (CBS 631.68): Conidiomata pycnidial, confluent, subglobose, glabrous, superficial on or immersed into the agar, (110–)170–400 × (110–)130–275(–300) μm. Ostioles 1–2, slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous, composed of oblong to isodiametric cells, 2–3 layers, 11–14 μm thick. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 5–8.5 × 3.5–7(–9.5) μm. Conidia ellipsoidal to cylindrical, thin-walled, smooth, aseptate, 3.5–5.5 × 1.5–3.5 μm, egutullate or sometimes with 1–3 small guttules. Conidial matrix cream to buff.
Culture characteristics: Colonies on OA, 65–70 mm diam after 7 d, margin regular, in some sectors covered by floccose aerial mycelia, white to greenish olivaceous; reverse olivaceous, buff in some sectors. Colonies on MEA 65–70 mm diam after 7 d, margin regular, aerial mycelium sparse, white to pale olivaceous; reverse white, pale olivaceous near the centre. Colonies on PDA, 70–75 mm diam after 7 d, margin regular, floccose aerial mycelium covering the whole colony, white to pale grey; reverse hazel with some sectors in brown olivaceous. NaOH spot test: a slight reddish discolouration on MEA.
Specimens examined: The Netherlands, Arnhem, from a stem of Solidago floribunda, deposited in CBS Sep. 1968 (epitype designated here HMAS 246703, MBT202490, culture ex-epitype CBS 631.68 = PD 68/147); Wageningen, from dead stems of Solidago virgaurea, Oct. 1976, M.M.J. Dorenbosch (holotype L 996, 047-028).
Notes: This fungus was originally described from dead stems of Solidago virgaurea, with conidia 2.5–8.5 × 1–3.5 μm (Boerema 1976). The epitype from Solidago floribunda agrees well in morphology with the type material as conidia are aseptate, measuring 3.5–5.5 × 1.5–3.5 μm.
Stagonosporopsis dorenboschii (Noordel. & Gruyter) Aveskamp et al., Stud. Mycol. 65: 45. 2010.
Basionym: Phoma dorenboschii Noordel. & Gruyter, Persoonia 15: 83. 1992.
Specimen examined: The Netherlands, Rijnsburg, from Physostegia virginiana, deposited in CBS Oct. 1990, M.E. Noordeloos (holotype L 988.202-121, isotype CBS H-7604, culture ex-holotype CBS 426. 90 = IMI 386093 = PD 86/551).
Stagonosporopsis heliopsidis (H.C. Greene) Aveskamp et al., Stud. Mycol. 65: 45. 2010.
Basionym: Phyllosticta heliopsidis H.C. Greene, Trans. Wisconsin Acad. Sci. 50: 158. 1961.
≡ Phoma heliopsidis (H.C. Greene) Aa & Boerema, Persoonia 18: 40. 2002.
Specimen examined: The Netherlands, from Heliopsis patula, deposited in CBS Jan. 2001, H. de Gruyter, CBS 109182 = PD 74/231.
Stagonosporopsis hortensis (Sacc. & Malbr.) Petr., Ann. Mycol. 19: 21. 1921.
Basionym: Hendersonia hortensis Sacc. & Malbr., Michelia 2: 629. 1882.
= Phoma subboltshauseri Boerema et al., Persoonia 16: 360. 1997.
= Ascochyta boltshauseri Sacc. Z. Pflanzenkrankh. 1: 136. 1891.
≡ Stagonosporopsis boltshauseri (Sacc.) Died., Ann Mycol. 10: 141. 1912.
Specimen examined: The Netherlands, from an unknown substrate, deposited in CBS Mar. 1942, N. Hubbeling, CBS 104.42; from Phaseolus vulgaris, deposited in CBS Sep. 1985, G.H. Boerema, culture CBS 572.85 = PD 79/269.
Note: As no generic type was designated by Diedicke (1912) when he established the genus Stagonosporopsis, S. hortensis was chosen as the type for this genus (Boerema and Verhoeven, 1979, Vaghefi et al., 2012).
Stagonosporopsis inoxydabilis (Boerema) Crous et al., Australas. Pl. Pathol. 41: 682. 2012.
Basionym: Didymella ligulicola var. inoxydabilis Boerema, Stud. Mycol. 32: 9. 1990.
≡ Stagonosporopsis ligulicola var. inoxydabilis (Boerema) Aveskamp et al., Stud. Mycol. 65: 45. 2010.
= Phoma ligulicola var. inoxydabilis Boerema, Stud. Mycol. 32: 10. 1990.
Description and illustration (Vaghefi et al. 2012).
Specimen examined: The Netherlands, from Chrysanthemum parthenii, deposited in CBS Oct. 1990, (holotype CBS H-7611, culture ex-holotype CBS 425.90 = PD 81/520).
Stagonosporopsis helianthi Q. Chen & L. Cai, sp. nov. MycoBank MB814078. Fig. 3.
Fig. 3.
Stagonosporopsis helianthi (CBS 200.87). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidia. I. Section of pycnidial wall. J. Conidiogenous cells. K. Conidia. Scale bars: G = 200 μm; H = 100 μm; I–K = 10 μm.
Etymology: Name after the host genus from which it was collected, Helianthus.
Description from ex-holotype culture (CBS 200.87): Conidiomata pycnidial, solitary or aggregated, subglobose, glabrous or covered with hyphal outgrows, mostly produced on the agar surface, sometimes immersed, 350–550 × 330–550 μm. Ostiole single, slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 2–4 layered, 13–25 μm thick, composed of isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, 6–10.5 × 6.5–10 μm. Conidia broadly ellipsoidal, hyaline, smooth- and thin-walled, aseptate, 2–4 × 2–3 μm, with 0–3 guttules. Conidial matrix whitish cream.
Culture characteristics: Colonies on OA, 45–50 mm diam after 7 d, margin regular, aerial mycelia sparse, abundant pycnidia semi-immersed in concentric rings, pale grey to olivaceous; reverse concolourous. Colonies on MEA 30–35 mm diam after 7 d, margin regular, aerial mycelium sparse, wooly, white, pale olivaceous near the centre; reverse concolourous. Colonies on PDA, 45–50 mm diam after 7 d, margin regular, floccose, pycnidia produced in concentric rings, grey, white near the colony margin and somewhat olivaceous near the centre; reverse dark grey in concentric rings, white near the margin and buff near the centre. NaOH spot test: a slight greenish discolouration on MEA, reddish near the margin.
Specimen examined: Italy, Perugia, from Helianthus annuus, deposited in CBS Mar. 1987 (holotype HMAS 246704, culture ex-holotype CBS 200.87).
Notes: Isolate CBS 200.87 was received as “Didymella lophospora”, which was isolated from Helianthus annuus, and is different from the original host of D. lophospora (Pteridium aquilinum). The type material of D. lophospora was not obtained from the fungaria consulted (see Materials and Methods). Although we did not observe the sexual morph of CBS 200.87, we consider this isolate to represent a different species from D. lophospora, because they are from different host families, and there is no record of an asexual morph of D. lophospora to compare with our isolate CBS 200.87. Therefore we introduce a new species, Stagonosporopsis helianthi based on CBS 200.87. Stagonosporopsis helianthi was resolved in a sister clade to S. heliopsidis (CBS 109182), and is significantly different from S. heliopsidis in morphology: pycnidia (ca. 350–550 μm diam in S. helianthi vs. 70–300 μm diam in S. heliopsidis), conidiogenous cells (6–10.5 × 6.5–10 μm in S. helianthi vs. 4–8 × 4–8 μm in S. heliopsidis), and conidia (2–4 × 2–3 μm in S. helianthi vs. 6–8 × 1.5–3 μm in S. heliopsidis).
Stagonosporopsis loticola (Died.) Aveskamp et al., Stud. Mycol. 65: 46. 2010.
Basionym: Phoma loticola Died., Kryptog.-Fl. Mark Brandenburg. 9: 152. 1912.
= Phoma lotivora P.R. Johnst., New Zealand J. Bot. 19: 178. 1981
Specimen examined: New Zealand, Auckland, Mt. Albert, from Lotus pedunculatus, May 1980, P.R. Johnston (isotype CBS H-7612, culture ex-isotype CBS 562.81 = PDDCC 6884).
Stagonosporopsis lupini (Boerema & R. Schneid.) Boerema et al., Persoonia 17: 283. 1999.
Basionym: Ascochyta lupini Boerema & R. Schneid., Verslagen Meded. Plantenziektenk. Dienst Wageningen 162: 28. 1984.
= Phoma schneiderae Boerema et al., Persoonia 17: 282. 1999.
Specimen examined: UK, Cambridgeshire, Mepal, from Lupinus albus, Apr. 1998 (holotype of Phoma schneiderae L 998.099.105, culture ex-holotype CBS 101494 = PD 98/5247).
Stagonosporopsis oculo-hominis (Punith.) Aveskamp et al., Stud. Mycol. 65: 46. 2010.
Basionym: Phoma oculi-hominis Punith., Trans. Brit. Mycol. Soc. 67: 142. 1976. (as “oculo-hominis”)
≡ Phoma dennisii var. oculo-hominis (Punith.) Boerema et al., Persoonia 16: 351. 1997.
Specimen examined: USA, Tennessee, Nashville, from a man's corneal ulcer, Apr. 1975, Y.M. Clayton (culture ex-holotype CBS 634.92 = IMI 193307).
Stagonosporopsis rudbeckiae (Fairm.) Aveskamp et al., Stud. Mycol. 65: 46. 2010.
Basionym: Phoma rudbeckiae Fairm., Proc. Rochester Acad. Sci. 1: 51. 1890.
Specimen examined: The Netherlands, from Rudbeckia bicolor, deposited in CBS Jan. 2001, H. de Gruyter, CBS 109180 = PD 79/175.
Stagonosporopsis tanaceti Vaghefi et al., Australas. Pl. Pathol. 41: 682. 2012.
Specimen examined: Australia, Northern Tasmania, Scottsdale, from Tanacetum cinerariifolium, S.J. Pethybridge (holotype CBS H-20947, culture ex-holotype CBS 131484).
Stagonosporopsis trachelii (Allesch.) Aveskamp et al., Stud. Mycol. 65: 46. 2010.
Basionym: Phoma trachelii Allesch., Hedwigia 34: 259. 1895.
= Ascochyta bohemica Kabát & Bubák, Hedwigia 44: 352. 1905.
≡ Stagonosporopsis bohemica (Kabát & Bubák) Boerema et al., Persoonia 16: 361. 1997.
Description and illustrations (Vaghefi et al. 2012).
Specimens examined: Sweden, Svalöv, from Campanula isophylla, deposited in CBS May 1968, W. Södergren, CBS H-8972, culture CBS 384.68. The Netherlands, from a leaf of Campanula isophylla, deposited in CBS Jun. 1991, CBS 379.91 = PD 77/675.
Stagonosporopsis valerianellae (Gindrat et al.) Aveskamp et al., Stud. Mycol. 65: 46. 2010.
Basionym: Phoma valerianellae Gindrat et al., Rev. Hort. Suisse. 40: 350. 1967.
Specimens examined: The Netherlands, Wageningen, from Valerianella locusta var. oleracea, deposited in CBS Jul. 1967, G.H. Boerema (holotype L 965.300.24, isotype CBS H-7631, culture ex-isotype CBS 329.67 = PD 66/302); from Valerianella locusta, deposited in CBS Jun. 1992, J. de Gruyter, CBS 273.92 = PD 82/43.
Clade 2: Allophoma
Allophoma Q. Chen & L. Cai, gen. nov. MycoBank MB814058.
Etymology: Allo = allos in Greek, different; phoma-like conidia.
Conidiomata pycnidial, globose to flask-shaped, superficial on or immersed into the agar, solitary or confluent, ostiolate. Pycnidial wall pseudoparenchymatous, 2–5-layered. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, sometimes flask-shaped or isodiametric. Conidia variable in shape and size, hyaline, thin-walled, smooth, aseptate, i.e. ovoid, oblong, ellipsoidal to cylindrical, or slightly allantoid, mostly guttulate.
Type species: Allophoma tropica (R. Schneid. & Boerema) Q. Chen & L. Cai.
Allophoma labilis (Sacc.) Q. Chen & L. Cai, comb. nov. MycoBank MB814068.
Basionym: Phoma labilis Sacc., Michelia 2: 341. 1881.
Description (de Gruyter et al. 1993).
Specimen examined: The Netherlands, Barendrecht, from a stem of Lycopersicon esculentum, deposited in CBS Jan 1993, J. de Gruyter, CBS 124.93 = PD 87/269.
Allophoma minor (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814069.
Basionym: Phoma minor Aveskamp et al., Stud. Mycol. 65: 42. 2010.
Description and illustration (Aveskamp et al. 2010).
Specimen examined: Indonesia, Sumatra, from Syzygium aromaticum, Apr. 1982, R. Kasim (holotype CBS H-20236, culture ex-holotype CBS 325.82).
Allophoma nicaraguensis Q. Chen & L. Cai, sp. nov. MycoBank MB814067. Fig. 4.
Fig. 4.
Allophoma nicaraguensis (CBS 506.91). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidium. H. Section of pycnidial wall. I. Conidiogenous cells. J. Conidia. Scale bars: G = 20 μm; H, J = 10 μm; I = 5 μm.
Etymology: Epithet refers to the country of origin, Nicaragua.
Description from ex-holotype culture (CBS 506.91): Conidiomata pycnidial, solitary, globose to flask-shaped, glabrous, semi-immersed or immersed, 30–150(–180) × 28–120(–165) μm. Ostiole single, slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 3–5-layered, 8–12 μm thick, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 3–4.5 × 3.5–4.5(–5.5) μm. Conidia ellipsoidal to oblong, thin-walled, smooth, aseptate, 2.5–4 × 1.5–2.5 μm, egutullate or sometimes with 1(–3) small guttules. Conidial matrix whitish.
Culture characteristics: Colonies on OA, 45–50 mm diam after 7 d, margin regular, floccose, greenish olivaceous, white near the margins; reverse white, olivaceous near the centre. Colonies on MEA 45–50 mm diam after 7 d, margin regular, aerial mycelium sparse, white to pale olivaceous; reverse white, pale olivaceous near the centre. Colonies on PDA, 45–50 mm diam after 7 d, margin regular, floccose, white to pale olivaceous; reverse white to pale brown, olivaceous near the centre. NaOH test negative.
Specimen examined: Nicaragua, from a twig of Coffea arabica, deposited in CBS Sep. 1991, J. de Gruyter (holotype HMAS 246701, culture ex-holotype CBS 506.91 = PD 91/876 = IMI 215229).
Notes: Since isolate CBS 506.91 was collected from Coffea arabica, the same host as Phoma costaricensis, this isolate was initially identified as “P. costaricensis”. However, its conidia (2.5–4 × 1.5–2.5 μm) were found to differ from the original description of P. costaricensis [5–6(–7) × 2–3 μm; Echandi 1957]. We therefore introduced a new species, All. nicaraguensis, to accommodate this isolate. Allophoma nicaraguensis showed a close phylogenetic relationship with All. tropica (syn. Phoma tropica). However, the pycnidia of All. tropica (100–300 μm) are larger than All. nicaraguensis (50–150 μm), and with more conspicuous ostioles (1–5), as compared to a single ostiole in All. nicaraguensis (Boerema et al. 2004).
Allophoma piperis (Tassi) Q. Chen & L. Cai, comb. nov. MycoBank MB814070. Fig. 5.
Fig. 5.
Allophoma piperis (CBS 268.93). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidium. I. Section of pycnidium. J. Conidiogenous cells. K. Section of pycnidial wall. L. Conidia. Scale bars: G = 100 μm; H–I = 20 μm; J = 5 μm; K–L = 10 μm.
Basionym: Phyllosticta piperis Tassi, Bull. Labor. Ort. Bot. Siena 3: 28. 1900.
≡ Phoma piperis (Tassi) Aa & Boerema, Persoonia 15: 398. 1993.
Description from holotype (N 354): Leaf spots elliptical to circular, brown to black. Conidiomata pycnidial, on leaves of Peperomia pereskifolia, solitary, subglobose, 115–245 × 85–230 μm. Ostiole single, slightly papillate. Pycnidial wall pseudoparenchymatous, composed of isodiametric cells. Conidiogenous cells phialidic, hyaline, simple, smooth, doliiform. Conidia ellipsoidal to ovoid, thin-walled, smooth, aseptate, 3.5–5.5 × 1.5–2.5 μm, with 1–2 large guttules.
Description from ex-epitype culture (CBS 268.93): Conidiomata pycnidial, solitary, globose to subglobose, glabrous or with some hyphal outgrowths, on the agar surface, 110–240 × 100–200 μm. Ostiole single, slightly papillate. Pycnidial wall pseudoparenchymatous, composed of oblong to isodiametric cells, 3–5 layers, 7.5–12.5 μm thick. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 2.5–3.5 × 2–3 μm. Conidia oblong to ellipsoidal, thin-walled, smooth, aseptate, 2.5–4 × 1.5–2.5 μm, with 2 polar guttules. Conidial exudates not recorded.
Culture characteristics: Colonies on OA, 40–45 mm diam after 7 d, margin regular, covered by floccose aerial mycelia, dull green, pale grey olivaceous near the colony margin; reverse olivaceous. Colonies on MEA 35–40 mm diam after 7 d, margin regular, aerial mycelium sparse, white, pale green near the centre; reverse concolourous. Colonies on PDA, 40–45 mm diam after 7 d, margin regular, covered by densely grey felty aerial mycelium, pycnidia in a concentric ring; reverse dull green to olivaceous. NaOH test negative.
Specimens examined: Italy, from leaves of Piper longum, Mar. 1899 (holotype N 354 in SIENA). The Netherlands, Tiel, from a leaf of Peperomia pereskiifolia, deposited in CBS Apr 1993, J. de Gruyter (epitype designated here HMAS 246702, MBT202493, culture ex-epitype CBS 268.93 = PD 88/720); Ressen, from Peperomia pereskiifolia, deposited in CBS Jan. 1993, J. de Gruyter, CBS 108.93 = PD 90/2011.
Notes: The holotype of Phoma piperis was described from Piper longum collected in Italy, with conidia measuring 3.5–5.5 × 1.5–2.5 μm. De Gruyter et al. (1993) reported a similar conidial size of 3–5 × 1.5 μm based on an authentic strain CBS 268.93, which was from the Netherlands and from Peperomia pereskiifolia, another host genus in Piperaceae. The collection HMAS 246702 (living culture CBS 268.93) is from the same host family, and the conidia we observed (2.5–4 × 1.5–2.5 μm) generally agree with the type material and that of de Gruyter et al. (1993). We thus designated HMAS 246702 as epitype. Allophoma piperis was reported as a pathogen that caused leaf spots of Piper spp., especially Piper longus, and sometimes also infected Peperomia spp. (de Gruyter et al. 1993).
Allophoma tropica (R. Schneid. & Boerema) Q. Chen & L. Cai, comb. nov. MycoBank MB814071.
Basionym: Phoma tropica R. Schneid. & Boerema, Phytopathol. Z. 83: 361. 1975.
Description (de Gruyter & Noordeloos 1992).
Specimen examined: Germany, Horrheim, from Saintpaulia ionantha, deposited in CBS Aug. 1975, R. Schneider (isotype CBS H-7629, culture ex-isotype CBS 436.75 = DSM 63365).
Allophoma zantedeschiae (Dippen.) Q. Chen & L. Cai, comb. nov. MycoBank MB814072.
Basionym: Phoma zantedeschiae Dippen., S. African J. Sci. 28: 284. 1931.
= Phyllosticta richardiae F.T. Brooks, Ann. Appl. Biol.: 18. 1932.
Description (Boerema 1993).
Specimens examined: Romania, from Cicer arietinum, deposited in CBS Apr. 1932, T. Savulescu, CBS 229.32. The Netherlands, from a bulb of Zantedeschiae sp., deposited in CBS Jan 1993, J, de Gruyter, CBS 131.93 = PD 69/140.
Notes: The isolate CBS 229.32 was received as “Didymella rabiei”. It is however genetically distinct from other strains of D. rabiei (CBS 206.30, CBS 237.37 and CBS 534.65), but identical to the authentic strain of Phoma zantedeschiae (CBS 131.93) based on four sequenced loci.
Clade 3: Heterophoma
Heterophoma Q. Chen & L. Cai, gen. nov. MycoBank MB814059.
Etymology: Heter = έτερος in Greek, other; morphologically similar to but phylogenetically different from Phoma.
Conidiomata pycnidial, globose to subglobose, superficial on or immersed into the agar, solitary or confluent, ostiolate. Pycnidial wall pseudoparenchymatous, 5–12-layered. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform. Conidia variable in shape and size, hyaline, thin-walled, smooth, 0–1(–2) septate, i.e. ellipsoidal, oblong, cylindrical, reniform, or slightly allantoid, mostly guttulate. Chlamydospores unicellular, globose, intercalary in chains, olivaceous.
Type species: Heterophoma sylvatica (Sacc.) Q. Chen & L. Cai.
Heterophoma adonidis (Moesz) Q. Chen & L. Cai, comb. nov. MycoBank MB814073. Fig. 6.
Fig. 6.
Heterophoma adonidis (CBS 114309). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H–I. Pycnidia. J. Conidiogenous cells. K. Section of pycnidia. L. Section of pycnidial wall. M. Conidia. Scale bars: G = 200 μm; H = 100 μm; K = 50 μm; I = 20 μm; J, L, M = 10 μm.
Basionym: Didymella adonidis Moesz, Bot. Közlem. 8: 219. 1909.
Description from culture (CBS 114309): Conidiomata pycnidial, solitary or aggregated, (sub-)globose, glabrous or with some hyphal outgrows, superficial and immersed, later developing to black subglobose or irregular conidiomata and with a short wide elongated neck around the ostiole, (85–)100–400(–450) × (80–)100–245 μm. Ostiole single, slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 6–8 layered, 27–35 μm thick, composed of isodiametric cells, outer wall 2–3–layered, pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 4.5–8.5 × 4.5–8(–9) μm. Conidia oblong to cylindrical, hyaline, thin-walled, smooth, often uniseptate, 10.5–16.5 × 3–4 μm, always somewhat constricted at the septum, with 5–15 guttules per cell. Conidial matrix yellowish.
Culture characteristics: Colonies on OA, 35–40 mm diam after 7 d, margin regular, floccose, white, pale olivaceous near the centre, flat near the margin; reverse buff. Colonies on MEA 40–45 mm diam after 7 d, margin regular, aerial mycelium sparse, white to pale olivaceous; reverse white, pale olivaceous near the centre. Colonies on PDA, 40–45 mm diam after 7 d, margin regular, floccose, white or somewhat buff; reverse pale saffron. NaOH spot test: a luteous discolouration on MEA, later changing to three colour layers, via dull green, dark brown to reddish, from the centre to outer ring.
Specimen examined: Sweden, Öland, Mörbylilla, on Adonis vernalis, Jun. 1989, K. & L. Holm, CBS 114309 = UPSC 2982.
Notes: The holotype of Didymella adonidis was on Adonis vernalis from Hungary, and could not be located from BP or MICH for examination. The culture CBS 114309, isolated from same host from Sweden, was deposited in CBS under the name “Didymella adonidis”. The original description of D. adonidis only had details of a sexual morph, with asci clavate, 50–66 × 12–13 μm and uniseptate ascospores, oblong-ellipsoidal, 19–26.5 × 3–5 μm. CBS 114309 was however, strictly asexual in culture.
Heterophoma nobilis (Kabát & Bubák) Q. Chen & L. Cai, comb. nov. MycoBank MB814074.
Basionym: Ascochyta nobilis Kabát & Bubák, Oesterr. Bot. Z. 54: 3. 1904.
≡ Phoma dictamnicola Boerema et al., Persoonia 15: 90. 1992.
Description (de Gruyter & Noordeloos 1992).
Specimen examined: The Netherlands, Arnhem, from a stem of Dictamnus albus, deposited in CBS Sep. 1991, J. de Gruyter, CBS 507.91 = PD 74/148.
Notes: Heterophoma nobilis is the only species that produces chlamydospores in this genus, and its conidia are more variable in size and shape in vivo than those in vitro. This species was originally described in the genus Ascochyta based on its large, septate conidia, and later replaced by a new name Phoma dictamnicola by de Gruyter & Noordeloos (1992).
Heterophoma novae-verbascicola (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814075.
Basionym: Phoma novae-verbascicola Aveskamp et al., Stud. Mycol. 65: 41. 2010.
Description (de Gruyter et al. 1993).
Specimens examined: The Netherlands, Zeist, Abburg nursery, from Verbascum sp. (holotype L 9893.00.134); Haarlem, from dead stem material of Verbascum densiflorum, deposited in CBS Jan 1993, J, de Gruyter, CBS 127.93 = PD 92/347.
Heterophoma poolensis (Taubenh.) Q. Chen & L. Cai, comb. nov. MycoBank MB814076.
Basionym: Phoma poolensis Taubenh., Dis. Greenhouse Crops: 203. 1919.
Description (de Gruyter et al. 1993).
Specimens examined: The Netherlands, Bennekom, from a stem of Antirrhinum majus, deposited in CBS Jan 1993, J. de Gruyter, CBS 116.93 = PD 71/884. Unknown origin, from unknown substrate, deposited in CBS Aug. 1920, E.M. Smiley, CBS 113.20 = PD 92/774.
Note: According to the records in the USDA database, this is the only species in Phoma s. lat. that is reported to be associated with Antirrhinum sp. (Farr & Rossman 2015).
Heterophoma sylvatica (Sacc.) Q. Chen & L. Cai, comb. nov. MycoBank MB814077. Fig. 7.
Fig. 7.
Heterophoma sylvatica (CBS 874.97). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H–I. Section of pycnidia. J. Section of pycnidial wall. K. Conidiogenous cells. L. Conidia. Scale bars: G = 200 μm; H = 100 μm; I = 50 μm; J, L = 10 μm; K = 5 μm.
Basionym: Phoma sylvatica Sacc., Michelia 2: 337. 1881.
Description from ex-neotype culture (CBS 874.97): Conidiomata pycnidial, solitary or confluent, globose to subglobose, with some hyphal outgrows, superficial on and immersed into the agar, 110–330 μm diam. Ostiole mainly single, occasionally two ostiolate, non-papillate or slightly papillate. Pycnidial wall 5–9(–20)-layered, outer layers pigmented. Conidiogenous cells phialidic, hyaline, smooth, bottle-shaped, 3–6 × 3–6 μm. Conidia cylindrical, sometimes slightly allantoid, smooth- and thin-walled, aseptate, 3.5–6 × 1–2 μm, with 2 small polar guttules. Conidial exudates not recorded.
Culture characteristics: Colonies on OA, 65–75 mm diam after 7 d, margin regular to slightly irregular, floccose, pale olivaceous grey, black pycnidia visible; reverse concolourous. Colonies on MEA 60–65 mm diam after 7 d, margin regular to slightly irregular, woolly, dull green to (pale) olivaceous grey; reverse greenish olivaceous to dull green, partly with vinaceous buff tinges, olivaceous black near the centre. Colonies on MEA, 55–60 mm diam after 7 d, margin irregular, with compact, woolly to floccose, pale olivaceous grey to olivaceous, staining the agar in sienna to scarlet due to the production of a diffusible pigment; reverse olivaceous to sepia. NaOH spot test: a greenish discolouration on MEA, later changing to red (from Boerema & de Gruyter 1998).
Specimen examined: The Netherlands, Wageningen, from a stem of Melampyrum pratense, deposited in CBS Jun. 1997 (neotype designated here HMAS 246700, MBT202494, culture ex-neotype CBS 874.97 = PD 93/764).
Notes: The holotype of Phoma sylvatica was not located in any of the fungaria consulted, and is considered lost. Here we designate CBS 874.97 as neotype, as conidial size of the neotype (3.5–6 × 1–2 μm) agrees well with the original description of Phoma sylvatica (4 × 1 μm). Although H. sylvatica is morphologically similar to H. novae-verbascicola, H. sylvatica was frequently reported on Melampyrum spp. (Boerema & de Gruyter 1998), while H. novae-verbascicola occurs on Verbascum spp. (Aveskamp et al. 2010). In the phylogenetic tree, they are clearly distinct from each other, forming two sister clades.
Clade 4: Boeremia
Boeremia Aveskamp et al., Stud. Mycol. 65: 36. 2010.
Conidiomata pycnidial, variable in shape and size, mostly globose to subglobose, superficial on or immersed into the agar, solitary or confluent. Ostioles 1–2(–3), non-pappillate or pappillate, lined internally with a hyaline cells when mature. Pycnidial wall pseudoparenchymatous, 2–8-layered, outer wall 1–3-layered, brown pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform. Conidia variable in shape, hyaline, smooth- and thin-walled, mainly aseptate, but regularly 1(–2)-septate larger conidia may be found. Ascomata pseudothecial, only recorded in one species in vivo, subglobose. Asci cylindrical or subclavate, always 8-spored, biseriate. Ascospores ellipsoidal, 1-septate (from Aveskamp et al. 2010).
Type species: Boeremia exigua (Desm.) Aveskamp et al., Stud. Mycol. 65: 36. 2010.
Boeremia crinicola (Siemasko) Aveskamp et al., Stud. Mycol. 65: 37. 2010.
Basionym: Phyllosticta crinicola Siemasko, Acta Soc. Bot. Poloniae 1: 22. 1923.
≡ Phoma crinicola (Siemasko) Boerema, Verslagen Meded. Plantenziektenk. Dienst Wageningen 153: 18. 1979.
Specimen examined: The Netherlands, Haarlem, from a bulb of Crinum powellii, Mar. 1976, G.H. Boerema, CBS H-16198, culture CBS 109.79 = PD 77/747.
Boeremia diversispora (Bubák) Aveskamp et al., Stud. Mycol. 65: 37. 2010.
Basionym: Phoma diversispora Bubák, Oesterr. Bot. Z. 55: 78. 1905.
≡ Phoma exigua var. diversispora (Bubák) Boerema, Gewasbescherming 11: 122. 1980.
Specimens examined: Kenya, from a pod of Phaseolus vulgaris, 1979, G.H. Boerema, CBS H-16308, CBS 102.80 = CECT 20049 = IMI 331907 = PD 79/61. The Netherlands, near Tilburg, from Phaseolus vulgaris, deposited in CBS Sep. 1998, J. de Gruyter, CBS 101194 = PD 79/687 = IMI 373349.
Boeremia exigua (Desm.) Aveskamp et al., Stud. Mycol. 65: 36. 2010.
Specimen examined: Denmark, from necrotic stems of Cheiranthus cheiri, Apr. 1938, CBS 118.38. Unknown origin, from Nicotiana tabacum, deposited in CBS Jun. 1938, R. Fourmont, CBS 119.38; from Abelmoschus esculentus, deposited in CBS Feb. 1921, L.L. Harter, CBS 107.21.
Notes: CBS 118.38 and CBS 119.38, received as “Ascochyta cheiranthi” and “Ascochyta ducometii”, clustered together with Boeremia exigua var. exigua (CBS 431.74), B. exigua var. forsythiae (CBS 101197, CBS 101213), and B. exigua var. viburni (CBS 100354) in the phylogenetic tree (Fig. 1). Therefore, these two isolates were reidentified as B. exigua here. Ascochyta cheiranthi and As. ducometii might be synonyms of B. exigua, but this needs to be confirmed by examining the type specimens.
Isolate CBS 107.21 was received as “Ascochyta abelmoschi” and is from the original host of A. abelmoschi (Abelmoschus esculentus). It clustered in a single lineage, which is distinct from other varieties in the B. exigua clade (Fig. 1), and might represent a new variety.
Boeremia exigua var. coffeae (Henn.) Aveskamp et al., Stud. Mycol. 65: 37. 2010.
Basionym: Ascochyta coffeae Henn., Hedwigia 41: 307. 1902; non Phoma coffeae Delacr. 1897.
= Ascochyta tarda R.B. Stewart, Mycologia 49: 430. 1957.
≡ Phoma tarda (R.B. Stewart) H. Verm., Coffee Berry Dis. Kenya: 14. 1979.
Specimens examined: Brazil, Patrocínio, from leaf of Coffea arabica, deposited in CBS by L.H. Pfenning, CBS 119730. Cameroon, Bemenda, from Coffea arabica, deposited in CBS Jan. 2001, H. de Gruyter, CBS 109183 = PD 2000/10506 = IMI 300060.
Boeremia exigua var. exigua (Desm.) Aveskamp et al., Stud. Mycol. 65: 37. 2010.
Basionym: Phoma exigua Desm., Ann. Sci. Nat. Bot. III 11: 282. 1849.
Specimens examined: The Netherlands, Emmeloord, from a tuber of Solanum tuberosum, deposited in CBS Jul. 1974, G.H. Boerema, CBS 431.74 = PD 74/2447; from a graft of Ulmus, 1961, H.M. Heybroek, CBS 373.61.
Boeremia exigua var. forsythiae (Sacc.) Aveskamp et al., Stud. Mycol. 65: 37. 2010.
Basionym: Phyllosticta forsythiae Sacc., Michelia 1: 93. 1877.
≡ Ascochyta forsythiae (Sacc.) Höhn., Verh. Naturf. Vereins Brünn 47: 36. 1909.
≡ Phoma exigua var. forsythiae (Sacc.) Aa et al., Persoonia 17: 452. 2000.
Specimens examined: The Netherlands, from Forsythia sp., deposited in CBS Sep. 1998, J. de Gruyter, CBS 101213 = PD 92/959; from Forsythia sp., deposited in CBS Sep. 1998, J. de Gruyter, CBS 101197 = PD 95/721.
Boeremia exigua var. gilvescens Aveskamp et al., Stud. Mycol. 65: 37. 2010.
Specimens examined: The Netherlands, Baarn, from leaves of Dactylis purpurea, 1970, H.A. van der Aa (holotype CBS H-16281, culture ex-holotype CBS 761.70); Emmeloord, from Cichorium intybus, deposited in CBS Sep. 1998, H. de Gruyter, CBS 101150 = PD 79/118.
Boeremia exigua var. heteromorpha (Schulzer & Sacc.) Aveskamp et al., Stud. Mycol. 65: 38. 2010. Fig. 8.
Fig. 8.
Boeremia exigua var. heteromorpha (CBS 443.94). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H–I. Pycnidia. J. Section of pycnidia. K. Section of pycnidial wall. L. Conidia. Scale bars: G = 200 μm; H–I = 40 μm; J = 50 μm; K–L = 10 μm.
Basionym: Phoma heteromorpha Schulzer & Sacc., Hedwigia 23: 107. 1884.
≡ Phoma exigua var. heteromorpha (Schulzer & Sacc.) Noordel. & Boerema, Verslagen Meded. Plantenziektenk. Dienst Wageningen 166: 109. 1989.
Description from ex-neotype culture (CBS 443.94): Conidiomata pycnidial, solitary or aggregated, globose to subglobose, glabrous or with few hyphal outgrows, superficial and immersed, later developing to irregular conidiomata and with a short broad elongated neck, 120–320 × 105–285 μm. Ostioles 1–4(–5), on a short elongated neck. Pycnidial wall pseudoparenchymatous 3–8-layered, 16–50 μm thick, composed of oblong to isodiametric cells, outer wall 2–3-layered, pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 3–8 × 3–5.5 μm. Conidia ovoid, ellipsoidal to cylindrical, thin-walled, smooth, mainly aseptate, occasionally 1–2 septate, 4.5–8(–10.5) × 2.5–4 μm, with (0–)2–8 minute guttules. Conidial matrix buff.
Culture characteristics: Colonies on OA, 45–50 mm diam after 7 d, margin regular, floccose, white, honey to pale olivaceous near the centre; reverse concolourous. Colonies on MEA 40–45 mm diam after 7 d, margin irregular, aerial mycelium sparse, white to pale olivaceous; reverse concolourous. Colonies on PDA, 15–20 mm diam after 7 d, margin regular, floccose, white, brown near the centre; reverse buff to brown, white near the margin. NaOH spot test: a greenish discolouration on MEA, later changing to reddish near the margin.
Specimens examined: France, Antibes, from Nerium oleander, deposited in CBS Sep. 1998, J. de Gruyter, CBS 101196 = PD 79/176. Italy, Perugia, from Nerium oleander, deposited in CBS Aug. 1994, A. Zazzerini (neotype designated here HMAS 246695, MBT202495, culture ex-neotype CBS 443.94).
Notes: The type specimen of Phoma heteromorpha could not be located, and is presumed lost. Conidia of the neotype are mostly aseptate, 4.5–8(–10.5) × 2.5–4 μm, which agree well with the original description. Boeremia exigua var. heteromorpha clustered with B. exigua var. populi in the phylogenetic tree, but B. exigua var. heteromorpha occurred on Nerium oleander, while B. exigua var. populi on Populus and Salix spp. respectively (Boerema et al. 2004).
Boeremia exigua var. linicola (Naumov & Vassiljevsky) Aveskamp et al., Stud. Mycol. 65: 39. 2010.
Basionym: Ascochyta linicola Naumov & Vassiljevsky, Mater. Mikol. Fitopatol. 5: 3. 1926.
≡ Phoma exigua var. linicola (Naumov & Vassiljevsky) P.W.T. Maas, Netherlands J. Pl. Pathol. 71: 118. 1965.
Specimens examined: The Netherlands, Flevoland, from a stem of Linum usitatissimum, deposited in CBS Feb. 1976, G.H. Boerema, CBS 116.76 = ATCC 32332 = CECT 20022 = CECT 20023 = IMI 197074 = PD 75/544; Wageningen, from seeds of Nemophila insignis, deposited in CBS Oct. 1938, P. Neergaard, CBS 248.38; Zierikzee, from Linum usitatissimum, deposited in CBS Dec.1928, H.A. Diddens, CBS 114.28.
Notes: Isolate CBS 248.38, deposited as “Phoma nemophilae”, clustered with authentic cultures of B. exigua var. linicola (CBS 114.28, CBS 116.76) in the phylogenetic tree. The LSU, ITS, tub2 and rpb2 loci sequences proved to be identical among these three strains originating from the Netherlands. It is therefore concluded that the materials studied belong to the same variety, B. exigua var. linicola.
Boeremia exigua var. populi (Gruyter & P. Scheer) Aveskamp et al., Stud. Mycol. 65: 39. 2010.
Basionym: Phoma exigua var. populi Gruyter & P. Scheer, J. Phytopathol. 146: 413. 1998.
Specimen examined: The Netherlands, Deil, from a twig of Populus (×) euramericana cv. Robusta, deposited in CBS Nov. 1997 (holotype L 995.263.325, culture ex-holotype CBS 100167 = PD 93/217).
Boeremia exigua var. pseudolilacis Aveskamp et al., Stud. Mycol. 65: 39. 2010.
Specimens examined: The Netherlands, Baarn, from leaf spots in Lamium maculatum, deposited in CBS Nov. 1967, CBS 462.67; Baarn, from leaf spots of Lathyrus sp., deposited in CBS Oct. 1967, H.A. van der Aa, CBS H-9059, culture CBS 423.67; near Boskoop, from Syringa vulgaris, deposited in CBS Sep. 1998, J. de Gruyter (holotype CBS H-20371, culture ex-holotype CBS 101207 = PD 94/614).
Notes: Isolates CBS 462.67 and CBS 423.67 were initially deposited as “Ascochyta lamiorum” and “Ascochyta lathyri” respectively. But these two isolates grouped with the ex-type culture of B. exigua var. pseudolilacis (CBS 101207) in the phylogenetic tree with all four sequenced loci being identical. Therefore, we concluded that CBS 462.67 and CBS 423.67 belong to a same variety B. exigua var. pseudolilacis.
Boeremia exigua var. viburni (Roum. ex. Sacc.) Aveskamp et al., Stud. Mycol. 65: 39. 2010.
Basionym: Ascochyta viburni Roum. ex. Sacc., Syll. Fung. 3: 387. 1884.
≡ Phoma viburni (Roum. ex. Sacc.) Boerema & M.J. Griffin, Trans. Brit. Mycol. Soc. 63: 110. 1974.
≡ Phoma exigua var. viburni (Roum. ex. Sacc.) Boerema, J. Phytopathol. 146: 414. 1998.
Specimen examined: The Netherlands, Boskoop, from Viburnum opulus, deposited in CBS Jan 1998, CBS 100354 = PD 83/448.
Boeremia foveata (Foister) Aveskamp et al., Stud. Mycol. 65: 40. 2010.
Basionym: Phoma foveata Foister, Trans. & Proc. Bot. Soc. Edinburgh 33: 66. 1940.
Specimen examined: Bulgaria, from a tuber of Solanum tuberosum, deposited in CBS Jan. 2001, H. de Gruyter, CBS 109176 = CECT 2828 = PD 94/1394.
Boeremia hedericola (Durieu & Mont.) Aveskamp et al., Stud. Mycol. 65: 40. 2010.
Basionym: Phyllosticta hedericola Durieu & Mont., Flore d'Algérie Cryptog. 1: 611. 1849. (as “hederaecola”; see also Sylloge Pl. crypt.: 279. 1856.)
≡ Phoma hedericola (Durieu & Mont.) Boerema, Trans. Brit. Mycol. Soc. 67: 295. 1976.
Specimens examined: The Netherlands, from Hedera helix, deposited in CBS Jun. 1991, J. de Gruyter, CBS 367.91 = PD 87/229.
Boeremia lilacis (Sacc.) Q. Chen & L. Cai, comb. et stat. nov. MycoBank MB814751.
Basionym: Phoma herbarum f. lilacis Sacc., Michelia 2: 93. 1880.
≡ Phoma exigua var. lilacis (Sacc.) Boerema, Phytopathol. Medit. 18: 105. 1980.
≡ Boeremia exigua var. lilacis (Sacc.) Aveskamp et al., Stud. Mycol. 65: 38. 2010.
Specimen examined: The Netherlands, Baarn, from leaf spots of Philadelphus sp., Nov. 1967, H.A. van der Aa, CBS H-9070, culture CBS 588.67; Wageningen, from a twig of Syringa vulgaris, deposited in CBS Aug. 1979, G.H. Boerema, CBS H-163131, culture CBS 569.79 = PD 72/741 = CECT 20050 = IMI 331909.
Notes: This taxon was elevated to species level based on the multi-locus phylogeny of the Boeremia exigua varieties (Berner et al. 2015). A single isolate deposited as “Ascochyta philadelphi” was re-identified as B. lilacis in this study. The name As. philadelphi might need to be synonymised, but since the type was not obtained for comparison, this awaits confirmation in future study.
Boeremia lycopersici (Cooke) Aveskamp et al., Stud. Mycol. 65: 40. 2010.
Basionym: Phoma lycopersici Cooke, Grevelia 13: 94. 1885.
= Didymella lycopersici Kleb., Z. Pflanzenkrankh. 31: 9. 1921.
Specimen examined: The Netherlands, Heerde, from fruit of Lycopersicon esculentum, deposited in CBS Aug. 1967, G.H. Boerema, CBS 378.67 = PD 67/276.
Boeremia noackiana (Allesch.) Aveskamp et al., Stud. Mycol. 65: 40. 2010. Fig. 9.
Fig. 9.
Boeremia noackiana (CBS 101203). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Colonies sporulating on OA.H. Pycnidium. I. Conidia. Scale bars: G = 200 μm; H = 50 μm; I = 10 μm.
Basionym: Phyllosticta noackiana Allesch., Bol. Técn. Inst. Agron. Estado São Paulo 9: 85. 1898.
≡ Phoma exigua var. noackiana (Allesch.) Aa, Boerema & Gruyter, Persoonia 17: 450. 2000.
Description from ex-epitype culture (CBS 101203): Conidiomata pycnidial, solitary or confluent, globose to subglobose, covered with hyphal outgrowths, semi-immersed or immersed, 130–315(–345) × 110–265(–310) μm. Ostioles 1–2, slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous 3–5-layered, 6–12 μm thick, composed of oblong to isodiametric cells, outer cell layer brown. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to flask-shaped, 3–5 × 2–3.5 μm. Conidia ellipsoidal to oblong, sometimes allantoid, hyaline, thin-walled, smooth, mainly aseptate, 4.5–8.5 × 2–3 μm, but occasionally 1-septate, 8–13 × 3.5–5 μm, with small guttules. Conidial matrix yellowish.
Culture characteristics: Colonies on OA, 45–50 mm diam after 7 d, margin regular, covered by white, wooly aerial mycelia, olivaceous to iron grey, with dendritic leaden-black zones; reverse buff to olivaceous, with some leaden-black zones. Colonies on MEA 25–30 mm diam after 7 d, margin regular, white aerial mycelium sparse, olivaceous to greenish olivaceous; reverse concolourous. Colonies on PDA, 25–30 mm diam after 7 d, margin regular, felty, pale olivaceous, white near the margin; reverse olivaceous, white near the margin. NaOH spot test: a brown discolouration on MEA.
Specimens examined: Brazil, Brasilien, Campinas, from Phaseolus sp., Mar. 1897, F. Noack (holotype F52544). Colombia, from Phaseolus vulgaris, deposited in CBS Sep. 1998, J. de Gruyter (epitype designated here HMAS 246697, MBT202496, culture ex-epitype CBS 101203 = PD 79/1114). Guatemala, from Phaseolus vulgaris, deposited in CBS Jan. 1998, IPO Wageningen, CBS 100353 = PD 87/718.
Notes: Boeremia noackiana was formerly treated as a variety of Phoma exigua (van der Aa et al. 2000), but in our analysis it appears to be genetically distinct from the Phoma exigua complex, which is in congruence with the results of Aveskamp et al. (2010), who elevated it to species level. The type specimen of Phyllosticta noackiana is preserved in B, and conidia of this species were described as oblong, 4–6 × 2 μm (Saccardo 1902). The morphological characters of HMAS 246697 agree well with those of the representative culture of this species reported by van der Aa et al. (2000). Here we designate HMAS 246697 as its epitype because it agrees well with the original description with regard to morphology, host and locality.
Boeremia sambuci-nigrae (Sacc.) Aveskamp et al., Stud. Mycol. 65: 40. 2010.
Basionym: Phoma herbarum f. sambuci-nigrae Sacc., Syll. Fung. 3: 133. 1884.
≡ Phoma exigua var. sambuci-nigrae (Sacc.) Boerema & Höweler, Persoonia 5: 26. 1967.
≡ Phoma sambuci-nigrae (Sacc.) E. Monte, Bridge & B. Sutton, Mycopathologia 115: 102. 1991.
Specimen examined: The Netherlands, Wageningen, from a leaf of Sambucus nigra, deposited in CBS Sep. 1968 (lectotype CBS H-16314, culture ex-lectotype CBS 629.68 = CECT 20048 = IMI 331913 = PD 67/753).
Boeremia strasseri (Moesz) Aveskamp et al., Stud. Mycol. 65: 40. 2010. Fig. 10.
Fig. 10.
Boeremia strasseri (CBS 126.93). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia producing on OA. H. Section of pycnidial wall. I. Conidia. Scale bars: G = 100 μm; H–I = 10 μm.
Basionym: Phoma strasseri Moesz, Bot. Közlem. 22: 45. 1924.
Description from ex-neotype culture (CBS 126.93): Conidiomata pycnidial, solitary or confluent, globose to subglobose, glabrous or covered with hyphae, semi-immersed or immersed, (145–)175–330(–355) × 125–320 μm. Ostioles 1–3, slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous, composed of oblong to isodiametric cells, 5–7 layers, 15–30 μm thick. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 4–7 × (2.5–)3.5–5.5 μm. Conidia ellipsoidal to cylindrical, hyaline, thin-walled, smooth, aseptate, 4–7 × 2–3 μm, with 2–4 polar guttules. Conidial matrix whitish.
Culture characteristics: Colonies on OA, 60–65 mm diam after 7 d, margin regular, felty, pale grey olivaceous; reverse olivaceous near the margin, towards the centre of colony becoming buff, pale olivaceous to olivaceous. Colonies on MEA 65–70 mm diam after 7 d, margin regular, aerial mycelium sparse, greenish olivaceous; reverse concolourous. Colonies on PDA, 70–75 mm diam after 7 d, margin regular, floccose, white; reverse olivaceous with buff tinge in some sections. NaOH spot test: a brown discolouration on MEA.
Specimen examined: The Netherlands, Arnhem, from a stem of Mentha sp., deposited in CBS Jan 1993, J, de Gruyter (neotype designated here HMAS 246698, MBT202497, culture ex-neotype CBS 126.93 = PD 73/642).
Notes: This species was initially described as Phoma menthae Strasser. However, this name was illegitimate and thus replaced by a new name, Phoma strasseri (Moesz 1925). The type specimen of this species could not be located, and is considered lost. The holotype was on Mentha silvestris collected from Austria, with conidia measuring 4–5 × 3–3.5 μm (Moesz 1925). Strain CBS 126.93 was also from Mentha sp., with conidia measuring 4–7 × 2–3 μm, which is in general agreement with the original description. Hence the specimen HMAS 246698 (ex CBS 126.93) is designated as neotype.
This species is phylogenetically and morphological similar to B. crinicola, but B. strasseri is only known from Amaryllidaceae (de Gruyter et al. 1993), while B. crinicola is mainly known from Mentha spp. or occasionally from other species also belonging to Labiatae (de Gruyter et al. 2002).
Boeremia telephii (Vestergr.) Aveskamp et al., Stud. Mycol. 65: 40. 2010.
Basionym: Ascochyta telephii Vestergr., Öfvers. Finska Vetensk.-Soc. Förh. 54: 41. 1897.
≡ Phoma telephii (Vestergr.) Kesteren, Netherlands J. Pl. Pathol. 78: 117. 1972.
Specimens examined: The Netherlands, Utrecht, from a stem of Sedum telephium, deposited in CBS Sep. 1973, G.H. Boerema, CBS 760.73 = PD 71/1616; from Sedum spectabile, deposited in CBS Jan. 2001, H. de Gruyter, CBS 109175 = PD 79/524.
Clade 5: Epicoccum
Epicoccum Link, Mag. Neuesten Entdeck. Gesammten Naturk. Ges. Naturf. Freunde Berlin 7: 32. 1815, emend. Q. Chen & L. Cai.
Conidiomata pycnidial, globose to subglobose, or to irregularly shaped, superficial on or immersed into the agar, solitary or confluent. Ostioles papillate or non-papillate, sometimes on pronounced necks. Pycnidial wall pseudoparenchymatous, 2–9-layered, outer wall brown olivaceous. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, globose to flask-shaped. Conidia variable in shape and size, hyaline or in later stages a slight brownish pigmentation may be found, smooth- and thin-walled, i.e. ovoid, ellipsoidal to oblong, (sub-)cylindrical, sometimes slightly curved, always aseptate. Synasexual morph: Sporodochia semi-immersed, scattered or aggregated, clavate. Conidia multicellular-phragmosporous, but septa being obscured by the dark verrucose wall, subglobose-pyriform, often with a basal cell, variable in dimensions, arising in gradually growing clusters as solitary, terminal elements of mycelial branches, from a more or less globose pseudoparenchymatous stroma. Chlamydospores variable and irregular, unicellular or multicellular, intercalary or terminal, solitary or in chains, smooth, verrucose or incidentally tuberculate, subhyaline to dark brown, where multicellular globose or irregular shaped, dictyosporous or botryoid (Punithalingam et al., 1972, Boerema et al., 2004, Aveskamp et al., 2010).
Type species: Epicoccum nigrum Link, Mag. Neuesten Entdeck. Gesammten Naturk. Ges. Naturf. Freunde Berlin 7: 32. 1815.
Notes: Based on our phylogenetic results, five Phoma species were recombined into the genus Epicoccum. The generic circumscription of Epicoccum is therefore emended to incorporate the morphological features of epicoccoid conidia and these newly added species, such as irregular pycnidial conidiomata and subcylindrical shaped conidia.
Epicoccum brasiliense (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814079.
Basionym: Phoma brasiliensis Aveskamp et al., Stud. Mycol. 65: 35. 2010.
Description and illustrations (Aveskamp et al. 2010).
Specimen examined: Brazil, from Amaranthus sp., Nov. 2007, E. Rosskopf (holotype CBS H-20235, culture ex-holotype CBS 120105).
Epicoccum draconis (Berk. ex Cooke) Q. Chen & L. Cai, comb. nov. MycoBank MB814080.
Basionym: Phyllosticta draconis Berk. ex Cooke, Grevillea 19: 8. 1890.
≡ Phoma draconis (Berk. ex Cooke) Boerema, Jaarb. Plziektenk. Dienst Wageningen 159: 24. 1982.
Description (de Gruyter et al. 1998).
Specimen examined: Rwanda, from a leaf of Dracaena sp., deposited in CBS Feb. 1983, G.H. Boerema, CBS H-16207, culture CBS 186.83 = PD 82/47.
Notes: In the original description of Phyllosticta draconis, the ellipsoidal conidia are cited as 7 × 3 μm (Cooke 1890). However, de Gruyter et al. (1998) described a representative culture of Phoma draconis (CBS 186.83), whose conidia measure 4–8.5 × 2–4 μm, which agrees with the holotype. CBS 186.83 clustered in the Epicoccum clade in Fig. 1, and thus we treat this taxon as a new combination in the genus Epicoccum, E. draconis.
Epicoccum henningsii (Sacc.) Q. Chen & L. Cai, comb. nov. MycoBank MB814081.
Basionym: Phoma henningsii Sacc., Syll. Fung. 10: 139. 1892.
Description (de Gruyter et al. 1993).
Specimen examined: Kenya, Maguga, from the bark of Acacia mearnsii, deposited in CBS Jan 1980, G.H. Boerema, CBS H-16354, culture CBS 104.80 = PD 74/1017.
Notes: “Phoma acacia Henn.” was the first name of this species, which was illegitimate and therefore replaced by Phoma henningsii Sacc., with conidia measuring 3.5–5 × 2 μm (Saccardo 1892). Herein a new combination in Epicoccum is proposed for this species.
Epicoccum huancayense (Turkenst.) Q. Chen & L. Cai, comb. nov. MycoBank MB814082.
Basionym: Phoma huancayensis Turkenst., Fitopatologia 13: 68. 1978.
Description (de Gruyter et al. 1998).
Specimen examined: Peru, Dep. Junin, Huancayo, near Vallis Mantaro, from a stem of Solanum sp., Feb. 1974, L.J. Turkensteen (isotype CBS H-7609, culture ex-isotype CBS 105.80 = PD 75/908).
Epicoccum nigrum Link, Mag. Neuesten Entdeck. Gesammten Naturk. Ges. Naturf. Freunde Berlin 7: 32. 1815.
= Phoma epicoccina Punith., Tulloch & Leach, Trans. Brit. Mycol. Soc. 59: 341. 1972.
Specimens examined: The Netherlands, Geleen, from human toenail, deposited in CBS Dec. 1981, CBS 125.82 = IMI 331914 = CECT 20044. USA, Oregon, from seeds of Dactylis glomerata, deposited in CBS Jan 1973, M. Tulloch (holotype of Phoma epicoccina IMI 164070, culture ex-holotype CBS 173.73 = ATCC 24428 = IMI 164070).
Notes: Sequences of the two isolates studied here were identical in LSU, ITS and tub2 (Aveskamp et al. 2010), but have 22 bp differences in rpb2, which is responsible for their distance in the phylogenetic tree. Since CBS 173.73 is the ex-type culture, further study is required to confirm if CBS 125.82 represents the same or a different species.
Epicoccum pimprinum (P.N. Mathur et al.) Aveskamp et al., Stud. Mycol. 65: 35. 2010.
Basionym: Phoma pimprina P.N. Mathur et al., Sydowia 13: 146. 1959.
Specimens examined: India, Poona, Pimpri, from soil, deposited in CBS Jun. 1960, M.J. Thirumalachar (culture ex-isotype CBS 246.60 = ATCC 22237 = ATCC 16652 = IMI 81601); from soil, 1977, PD 77/1028.
Notes: Isolate PD 77/1028 differs from the ex-type culture CBS 246.60 in one bp and 10 bp differences in LSU and tub2 respectively. Since the sequencing of the rpb2 locus of CBS 246.60 was unsuccessful, it can not be compared in the present study. If PD 77/1028 represents a different species remains to be confirmed.
Epicoccum plurivorum (P.R. Johnst.) Q. Chen & L. Cai, comb. nov. MycoBank MB814083.
Basionym: Phoma plurivora P.R. Johnst., New Zealand J. Bot. 19: 181. 1981.
Description (de Gruyter et al. 1998).
Specimen examined: New Zealand, Auckland, Mt Albert, from a leaf of Setaria sp., Feb. 1979, P.R. Johnston (holotype PDD 40397, CBS H-7624, culture ex-isotype CBS 558.81 = PDDCC 6873).
Epicoccum sorghinum (Sacc.) Aveskamp et al., Stud. Mycol. 65: 36. 2010.
Basionym: Phyllosticta sorghina Sacc., Michelia 1: 140. 1878.
≡ Phoma sorghina (Sacc.) Boerema et al., Persoonia 7: 134. 1973.
Specimens examined: France, Antibes, from a twig of Citrus sp., deposited in CBS Sep. 1968, CBS 627.68 = PD 66/926. Puerto Rico, Mayaguez, from Sorghum vulgare, deposited in CBS Apr. 1980, G.H. Boerema, CBS 179.80 = PD 76/1018.
Clade 6: Didymella
Didymella Sacc. ex Sacc., Syll. Fung. 1: 545. 1882. emend. Q. Chen & L. Cai.
= Peyronellaea Goid. ex Togliani, Ann. Sperim. Agrar. II 6: 93. 1952.
Conidiomata pycnidial, subglobose to ellipsoidal, becoming irregular, superficial on or immersed into the agar, solitary or confluent, ostiolate or poroid, sometimes with elongated necks. Micropycnidia occur in some species. Pycnidial wall pseudoparenchymatous, 2–8-layered, with a pigmented outer wall. Conidiogenous cells phialidic, hyaline, smooth, flask-shaped, ampulliform or doliiform. Conidia generally aseptate, variable in shape, smooth- and thin-walled, i.e. ellipsoidal to subglobose, cylindrical, oblong, ovoid, sometimes allantoid, hyaline, but in older cultures conidia may become pigmented, larger or septated conidia may occur in at least one species, mostly guttulate. Unicellular chlamydospores often abundantly formed in and on the agar and in the aerial mycelium, globose, intercalary, brown or (pale) olivaceous pigmented. Multicellular chlamydospores mainly alternarioid, terminal or intercalary, often in chains, brown or (pale) olivaceous . Ascomata pseudothecial, immersed or erumpent, (sub-)globose to flattened, solitary or confluent, ostiolate, 2–5(–8)-layered, composed of pseudoparenchymatous cells. Asci cylindrical to clavate or saccate, 8-spored, bitunicate, arising from a broad hymenium among pseudoparaphyses. Ascospores mostly hyaline or brownish, ellipsoidal to cymbiform, uniseptate, symmetrical or asymmetrical, constricted at the septum, or multiseptate (De Gruyter et al., 2009, Aveskamp et al., 2010, Zhang et al., 2012).
Type species: Didymella exigua (Niessl) Sacc., Michelia 2: 58. 1880.
Notes: The genus Didymella was emended to accommodate the genus Peyronellaea and several other associated phoma-like species that clustered together with type species of Didymella, i.e. D. exigua. Most species in this genus produced chlamydospores in culture.
Didymella acetosellae (A.L. Sm. & Ramsb.) Q. Chen & L. Cai, comb. nov. MycoBank MB814089.
Basionym: Phyllosticta acetosellae A.L. Sm. & Ramsb., Trans. Brit. Mycol. Soc. 4: 173. 1913.
≡ Phoma acetosellae (A.L. Sm. & Ramsb.) Aa & Boerema, Persoonia 18: 16. 2002.
Description (de Gruyter et al. 2002).
Specimen examined: The Netherlands, Baarn, from a stem of Rumex hydrolapathum, Mar. 1996, H.A. van der Aa, CBS 179.97.
Didymella aliena (Fr.) Q. Chen & L. Cai, comb. nov. MycoBank MB814090.
Basionym: Sphaeria aliena Fr., Syst. Mycol. 2: 502. 1823.
≡ Phoma aliena (Fr.) Aa & Boerema, Persoonia 16: 486. 1998.
Description (de Gruyter et al. 1998).
Specimens examined: France, Vosges, from branches of Euonymus europeus, B.D. Mougeot (neotype PAD Roum. F. gallici exs. 765). The Netherlands, from a twig of Berberis sp., deposited in CBS Jul. 1993, J. de Gruyter, CBS 379.93 = PD 82/945.
Didymella americana (Morgan-Jones & J.F. White) Q. Chen & L. Cai, comb. nov. MycoBank MB814091.
Basionym: Phoma americana Morgan-Jones & J.F. White, Mycotaxon 16: 406. 1983.
≡ Peyronellaea americana (Morgan-Jones & J.F. White) Aveskamp et al., Stud. Mycol. 65: 31. 2010.
Description (Boerema 1993).
Specimens examined: USA, Arkansas, from pod lesions of Glycine max, 1981, H.J. Walters, CBS 568.97 = ATCC 44494 = PD 94/1544; Georgia, from Zea mays, deposited in CBS Mar. 1985, G.H. Boerema, CBS H-16144, culture CBS 185.85 = PD 80/1191.
Notes: The holotype of Phoma americana is from leaves of Triticum aestivum collected by A.K. Hagan in the USA. Strains described by Boerema (1993) are morphologically similar to the original description, and our sequence data revealed that this species belongs to the genus Didymella.
Didymella anserina (Marchal) Q. Chen & L. Cai, comb. nov. MycoBank MB814092.
Basionym: Phoma anserina Marchal, Champignon Copr. 11: 1891.
≡ Peyronellaea anserina (Marchal) Aveskamp et al., Stud. Mycol. 65: 31. 2010.
= Phoma radicis-callunae R.W. Rayner, Bot. Gaz. 73: 231. 1922.
= Phoma suecica J.F.H. Beyma, Antonie van Leeuwenhoek 8: 110. 1942.
Description (de Gruyter & Noordeloos 1992).
Specimens examined: Germany, Giessen, Dec. 1979, R. Hadlok, CBS H-16562, culture CBS 253.80; former West-Germany, from plastic, deposited in CBS Dec. 1965, H. Kühlwein, CBS 397.65. The Netherlands, Ter Apel, from potato flour, 1983, CBS 360.84. UK, from Calluna sp., deposited in CBS Nov. 1929, R.W. Rayner (culture ex-holotype of “Phoma radicis-callunae” CBS 285.29).
Notes: This species was treated as new combination (Peyronellaea anserina) by Aveskamp et al. (2010), and here we recombine it into Didymella, as D. anserina. Phoma radicis-callunae was initially isolated from Calluna as endophyte (Rayner 1922), and reduced to synonymy of P. anserina (Boerema et al. 2004). Isolate CBS 397.65 was initially identified as P. suecica, which is also a synonym of P. anserina.
Didymella arachidicola (Khokhr.) Tomilin, Opredelitel' gribov roda Mycosphaerella Johans: 285. 1979.
Basionym: Mycosphaerella arachidicola Khokhr., Bolezni i vrediteli maslichnykh kul'tur 1: 29. 1934.
≡ Peyronellaea arachidicola (Khokhr.) Aveskamp et al., Stud. Mycol. 65: 31. 2010.
= Phoma arachidicola Marasas, Pauer & Boerema, Phytophylactica 6: 200. 1974.
Specimens examined: South Africa, Cape Province, Jan Kempdorp, Vaalharts Research Station, from a leaf of Arachis hypogaea, deposited in CBS May 1975, W.F.O. Marasas (isotype of Phoma arachidicola CBS H-7601, culture ex-isotype CBS 333.75 = ATCC 28333 = IMI 386092).
Notes: The sexual morph of Didymella arachidicola was originally described as Mycosphaerella arachidicola (Khokhriakov 1934), and later transferred to Didymella (Tomilin 1979) and Peyronellaea (Aveskamp et al. 2010). Here we reinstate the Didymella name based on its phylogenetic affinity.
Didymella aurea (Gruyter et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814093.
Basionym: Phoma aurea Gruyter et al., Persoonia 15: 394. 1993.
≡ Peyronellaea aurea (Gruyter et al.) Aveskamp et al., Stud. Mycol. 65: 31. 2010.
Description (de Gruyter et al. 1993).
Specimen examined: New Zealand, Auckland, from a stem of Medicago polymorpha, deposited in CBS Jan 1993, J. de Gruyter (holotype L 992.177.422, culture ex-holotype CBS 269.93 = PD 78/1087).
Didymella bellidis (Neerg.) Q. Chen & L. Cai, comb. nov. MycoBank MB814094.
Basionym: Phoma bellidis Neerg., Friesia 4: 74. 1950.
Description (de Gruyter et al. 1993).
Specimens examined: The Netherlands, from seed of Bellis perennis, deposited in CBS Nov. 1985, G.H. Boerema, CBS H-5200, culture CBS 714.85 = PD 74/265; from Bellis sp., 1994, J. de Gruyter, PD 94/886.
Notes: The type of Phoma bellidis is on Bellis perennis collected from Denmark. Conidia from the ex-type strain measure 4.5–6 × 1.5–3 μm, which is in agreement with that of CBS 714.85 as described by de Gruyter et al. (4–6.5 × 2–2.5 μm; 1993). Hence, we introduce a new combination for this species as Didymella bellidis.
Didymella boeremae (Gruyter) Q. Chen & L. Cai, comb. nov. MycoBank MB814095.
Basionym: Phoma boeremae Gruyter, Persoonia 18: 91. 2002.
Description (de Gruyter et al. 2002).
Specimen examined: Australia, Victoria, Burnley Gardens, from seed of Medicago littoralis cv. Harbinger, deposited in CBS Jan. 2002, H. de Gruyter (neotype L 996.294.536, culture ex-neotype CBS 109942 = PD 84/402).
Didymella calidophila (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814096.
Basionym: Phoma calidophila Aveskamp et al., Mycologia 101: 368. 2009.
Description (Boerema 1993).
Specimens examined: Egypt, from desert soil, deposited in CBS Jun. 1983, M.I.A. Abdel-Kader (neotype CBS H-20168, culture ex-neotype CBS 448.83). The Netherlands, Wageningen, from seeds of Cucumis sativus, RPVZ, PD 84/109.
Didymella chenopodii (P. Karst. & Har.) Q. Chen & L. Cai, comb. nov. MycoBank MB814097.
Basionym: Gloeosporium chenopodii P. Karst. & Har., J. Bot., Paris 3: 207. 1889.
≡ Phoma chenopodiicola Gruyter et al., Persoonia 15: 395. 1993.
Description (de Gruyter et al. 1993).
Specimen examined: Peru, from a stem of Chenopodium quinoa cv. Sajana, deposited in CBS Jan 1993, J, de Gruyter, CBS 128.93 = PD 79/140.
Notes: This species was initially described as Gloeosporium chenopodii, and later replaced by a nomen novum, Phoma chenopodiicola (de Gruyter et al. 1993). Here a new combination is proposed for this species as Didymella chenopodii. The type specimen was collected from Chenopodium album in France, and is preserved in PC.
Didymella coffeae-arabicae (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814098.
Basionym: Phoma coffeae-arabicae Aveskamp et al., Mycologia 101: 371. 2009.
≡ Peyronellaea coffeae-arabicae (Aveskamp et al.) Aveskamp et al., Stud. Mycol. 65: 32. 2010.
Description (Aveskamp et al. 2009a).
Specimen examined: Ethiopia, from Coffea arabica, 1984, M.M.J. Dorenbosch (holotype CBS H-20143, culture ex-holotype CBS 123380 = PD 84/1013).
Didymella curtisii (Berk.) Q. Chen & L. Cai, comb. nov. MycoBank MB814099.
Basionym: Hendersonia curtisii Berk., Nuovo Giorn. Bot. Ital. 10: 19. 1878.
≡ Stagonosporopsis curtisii (Berk.) Boerema, Verslagen Meded. Plantenziektenk. Dienst Wageningen 157: 20. 1981.
≡ Peyronellaea curtisii (Berk.) Aveskamp et al., Stud. Mycol. 65: 32. 2010.
= Phyllosticta narcissi Aderh., Centralbl. Bakteriol., 2 Abth. 6: 632. 1900.
≡ Phoma narcissi (Aderh.) Boerema et al., Persoonia 15: 215. 1993.
Description (Boerema 1993).
Specimens examined: The Netherlands, from Nerine sp., deposited in CBS May 1992, J. de Gruyter, culture CBS 251.92 = PD 86/1145; from Sprekelia sp., PD 92/1460.
Notes: This species was recombined into Peyronellaea by Aveskamp et al. (2010) as Peyronellaea curtisii, and herein we treat it as a new combination in Didymella. The two isolates have two and five bp differences in ITS and tub2 respectively, and thus may not be conspecific. Since the type material was not obtained, its taxonomy awaits future study.
Didymella dactylidis (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814100.
Basionym: Phoma dactylidis Aveskamp et al., Stud. Mycol. 65: 48. 2010.
Description and illustration (Aveskamp et al. 2010).
Specimen examined: USA, Oregon, on Dactylis glomerata, 1973 (holotype CBS H-20237, culture ex-holotype CBS 124513 = PD 73/1414).
Didymella dimorpha (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814101.
Basionym: Phoma dimorpha Aveskamp et al., Stud. Mycol. 65: 29. 2010.
Description and illustration (Aveskamp et al. 2010).
Specimen examined: Spain, Canary Isles, Gran Canaria, from phyllocladium of Opuntia sp., Oct. 1979, J.A. von Arx (holotype CBS H-20234, culture ex-holotype CBS 346.82).
Didymella eucalyptica (Sacc.) Q. Chen & L. Cai, comb. nov. MycoBank MB814103.
Basionym: Phoma eucalyptica Sacc., Syll. Fung. 3: 78. 1884.
≡ Peyronellaea eucalyptica (Sacc.) Aveskamp et al., Stud. Mycol. 65: 32. 2010.
Description (de Gruyter & Noordeloos 1992).
Specimen examined: Australia, Western Australia, from a leaf of Eucalyptus sp., deposited in CBS Jun. 1991, CBS 377.91 = PD 79/210.
Notes: Phoma eucalyptica was recombined into Peyronellaea by Aveskamp et al. (2010), as Pe. curtisii, and we here introduce the new combination Didymella eucalyptica for this species based on its phylogenetic relationship.
Didymella exigua (Niessl) Sacc., Michelia 2: 57. 1880. Fig. 13.
Fig. 13.
Didymella exigua (CBS 183.55). A. Ascomata on host. B. Surface view of ascoma. C–G. Asci with ascospores (arrow denotes pseudoparaphyse). H. Hyaline 1-septate ascospores. Scale bars: B–H = 10 μm.
Basionym: Didymosphaeria exigua Niessl, Oesterr. bot. Z. 25: 165. 1875.
≡ Cercidospora exigua (Niessl) Kuntze, Revis. gen. pl. 3: 454. 1898.
Description from ex-neotype culture (CBS 183.55): Ascomata subepidermal in the cortex of stems or in bracts of dead inflorescences, erumpent, subglobose to flattened, small, up to 170 μm diam, papillate; wall 10–15 μm thick, outer wall consisting of 2–3 layers of cells of textura angularis. Pseudoparaphyses hyaline, 1.5–2.5 μm diam, septate. Asci bitunicate, clavate to short cylindrical, 45–70 × 10–12 μm. Ascospores uni- to biseriate, ellipsoidal, straight to slightly curved, 12–16 × 4.5–6 μm, hyaline, smooth, apex obtuse, base broadly obtuse to subobtuse, medianly 1-septate, upper cell often wider than lower cell, slightly constricted at the septum.
Specimen examined: France, Menise sur Tholon, from Rumex arifolius, deposited in CBS May 1955, E. Müller (neotype CBS H-20123, culture ex-neotype CBS 183.55).
Note: Conidiomata in vivo and in vitro resemble ascomata in size, and give rise to conidia that are short cylindrical to bacilliform, 0(–1)-septate, hyaline, 9–13 × 4–6 μm (Corbaz 1957).
Didymella gardeniae (S. Chandra & Tandon) Q. Chen & L. Cai, comb. nov. MycoBank MB814104.
Basionym: Pyrenochaeta gardeniae S. Chandra & Tandon, Mycopathol. Mycol. Appl. 29: 274. 1966.
≡ Phoma gardeniae (S. Chandra & Tandon) Boerema, Verslagen Meded. Plantenziektenk. Dienst Wageningen 156: 27. 1980.
≡ Peyronellaea gardeniae (S. Chandra & Tandon) Aveskamp et al., Stud. Mycol. 65: 32. 2010.
Description (de Gruyter & Boerema 2002).
Specimen examined: India, Allahabad, from the leaf of Gardenia jasminoides, deposited in CBS Sep. 1968, S. Chandra & R.N. Tandon (isotype CBS H-7605, culture ex-isotype CBS 626.68 = IMI 108771).
Didymella glomerata (Corda) Q. Chen & L. Cai, comb. nov. MycoBank MB814105.
Basionym: Coniothyrium glomeratum Corda, Icon. Fung. (Prague) 4: 39. 1840.
≡ Phoma glomerata (Corda) Wollenw. & Hochapfel, Z. Parasitenk. 3: 592. 1936.
≡ Peyronellaea glomerata (Corda) Goid. ex Togliani, Ann. Sperim. Agrar. III 6: 93. 1952.
Description (Boerema 1993).
Specimens examined: Romania, Bucuresti, from fresco in church, Nov. 1971, I. Ionita, CBS H-16340, culture CBS 133.72. The Netherlands, from Chrysanthemum sp., deposited in CBS Sep. 1963, CBS 528.66 = PD 63/590.
Didymella heteroderae (Chen et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814106.
Basionym: Phoma heteroderae Sen Y. Chen et al., Mycologia 88: 885. 1996 (1997).
≡ Peyronellaea heteroderae (Sen Y. Chen et al.) Crous, Persoonia 32: 223. 2014.
= Phoma pomorum var. calorpreferens Boerema et al., Persoonia 15: 207. 1993.
≡ Phoma calorpreferens (Boerema et al.) Aveskamp et al., Mycologia 101: 370. 2009.
≡ Peyronellaea calorpreferens (Boerema et al.) Aveskamp et al., Stud. Mycol. 65: 31. 2010.
Description (Boerema 1993).
Specimen examined: The Netherlands, from undefined food material, 1973, G.H. Boerema (holotype L 990.290.418, culture ex-holotype CBS 109.92 = PD 73/1405).
Notes: This species was treated as Peyronellaea calorpreferens (Aveskamp et al. 2010), which was later considered as a nom. illeg., and then a new combination was introduced as Pe. heteroderae, citing the basionym as Phoma heteroderae (Crous et al. 2014).
Didymella lethalis (R. Stone) Sivan., Bitunicate Ascomycetes and their Anamorphs: 424. 1984.
Basionym: Mycosphaerella lethalis R. Stone, Ann. Mycol. 10: 587. 1912.
= Ascochyta lethalis Ellis & Barthol., Fungi Columb. 1808. 1903.
≡ Peyronellaea lethalis (Ellis & Barthol.) Aveskamp, Gruyter & Verkley, Stud. Mycol. 65: 32. 2010.
Specimen examined: Unknown origin, from unknown substrate, deposited in CBS Sep. 1925, A.W. Archer, CBS 103.25.
Notes: Sivanesan (1984) published the link between Ascochyta lethalis and Didymella lethalis. However, this connection requires molecular verification. The phylogenetic data indicated that Didymella lethalis (CBS 103.25) is closely related to D. pinodes (CBS 525.77), but they differ in seven bp in four sequenced loci. Here we tentatively retain them as two distinct species. Clarification of the relationship between the two species awaits the examination of the type specimen of Didymella lethalis.
Didymella longicolla (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814107.
Basionym: Phoma longicolla Aveskamp et al., Stud. Mycol. 65: 49. 2010.
Description and illustration (Aveskamp et al. 2010).
Specimen examined: Spain, Canary Isles, from Opuntia sp., J. de Gruyter (holotype CBS H-20238, culture ex-holotype CBS 124514 = PD 80/1189).
Didymella macrostoma (Mont.) Q. Chen & L. Cai, comb. et stat. nov. MycoBank MB814108.
Basionym: Phoma macrostoma var. macrostoma Mont., Ann. Sci. Nat. Bot. III 11: 52. 1849.
= Polyopeus purpureus var. incoloratus A.S. Horne, J. Bot. 58: 240. 1920.
≡ Phoma macrostoma var. incolorata (A.S. Horne) Boerema & Dorenb., Persoonia 6: 55. 1970. (as “macrostomum var. incolorata”)
Description (de Gruyter et al. 2002).
Specimens examined: Germany, near München, from the bark of Larix decidua, deposited in CBS Jun. 1995, L. Pehl, CBS 482.95. Switzerland, Vierwaldstättersee, near Brunnen, from a leaf of Acer pseudoplatanus, Oct. 1968, J. Gemmen, CBS H-16477, culture CBS 223.69. The Netherlands, Wageningen, from wood of Malus sylvestris, deposited in CBS Sep. 1969, G.H. Boerema, CBS H-16431, culture CBS 529.66 = PD 66/521. Unknown origin, from seed of Pinus nigra var. astriaca, deposited in CBS Aug. 1938, J.G. ten Houten, CBS 247.38.
Notes: The representative isolate of Phoma macrostoma var. incolorata (CBS 223.69) was genetically identical, and ecologically and morphologically highly similar to the representative isolates of P. macrostoma var. macrostoma (CBS 482.95, CBS 529.66). Phoma macrostoma var. incolorata only differs from the type variety in lacking hyphal pigmentation and having a negative reaction in NaOH (de Gruyter et al. 2002), which may be related to the production of cholesterol (Rajak & Rai 1983). Since these characteristics may vary under different incubation conditions and on different media for cultivation, we concluded that these two varieties should be combined to Didymella mascrostoma. Isolate CBS 247.38, which was received as Phoma libertiana, grouped with D. macrostoma in the same well-supported clade with identical sequences in all four loci, and we therefore re-identify it as D. macrostoma.
Didymella maydis (Arny & R.R. Nelson) Q. Chen & L. Cai, comb. nov. MycoBank MB814109.
Basionym: Phyllosticta maydis Arny & R.R. Nelson, Phytopathology 61: 1171. 1971.
≡ Phoma zeae-maydis Punith., Mycopathologia 112: 50. 1990. (nom. nov. for Phyllosticta maydis in Phoma)
≡ Peyronellaea maydis (Arny & R.R. Nelson) Crous, Persoonia 32: 223. 2014.
= Mycosphaerella zeae-maydis Mukunya & Boothr., Phytopathology 63: 530. 1973.
≡ Didymella zeae-maydis (Mukunya & Boothr.) Arx, Beih. Nova Hedwigia 87: 288. 1987.
≡ Peyronellaea zeae-maydis (Mukunya & Boothr.) Aveskamp et al., Stud. Mycol. 65: 33. 2010.
Description (de Gruyter 2002).
Specimens examined: USA, New York, Aurora, Cornell University, from dead Zea mays, Apr. 1972, D.M. Mukuya & C.W. Boothroyd (holotype of Mycosphaerella zeae-maydis CUP 52727); Wisconsin, Hancock, from Zea mays, Aug. 1970, D.C. Arny, culture ex-holotype of “Phyllosticta maydis” CBS 588.69.
Notes: Mukunya & Boothroyd (1973) established the sexual and asexual connection between Mycosphaerella zeae-maydis and Phyllosticta maydis. This species was recombined into Peyronellaea as Pe. zeae-maydis by Aveskamp et al. (2010), and later this treatment was corrected as a new combination Pe. maydis (Crous et al. 2014). Here we treat it based on the asexual morph and introduce a new combination, Didymella maydis.
Didymella microchlamydospora (Aveskamp & Verkley) Q. Chen & L. Cai, comb. nov. MycoBank MB814110.
Basionym: Phoma microchlamydospora Aveskamp & Verkley, Mycologia 101: 374. 2009.
Description and illustration (Aveskamp et al. 2009a).
Specimen examined: UK, from leaves of Eucalyptus sp., 1994, A.M. Ainsworth (holotype CBS H-20147, culture ex-holotype CBS 105.95).
Didymella molleriana (G. Winter) Q. Chen & L. Cai, comb. nov. MycoBank MB814102.
Basionym: Ascochyta molleriana G. Winter, Bol Soc. Brot. 1883: 26. 1884.
= Phoma digitalis Boerema, Verslagen Meded. Plantenziektenk. Dienst Wageningen 153: 19. 1979.
Description (de Gruyter et al. 2002).
Specimens examined: New Zealand, Levin, from a leaf of Digitalis purpurea, Oct. 1973, G.H. Boerema, CBS H-16201, culture CBS 229.79 = LEV 7660. The Netherlands, Ommen, from Digitalis sp., deposited in CBS Jan. 2001, H. de Gruyter, CBS 109179 = PD 90/835-1.
Note: Ascochyta molleriana Wint. was a replaced synonym of Phoma digitalis, and we recombine this species into Didymella based on its phylogeny.
Didymella musae (P. Joly) Q. Chen & L. Cai, comb. nov. MycoBank MB814111.
Basionym: Peyronellaea musae P. Joly, Rev. Mycol. 26: 97. 1961.
≡ Phoma jolyana Piroz. & Morgan-Jones, Trans. Brit. Mycol. Soc. 51: 200. 1968.
Description (Boerema 1993).
Specimen examined: India, from fruit of Mangifera indica, deposited in CBS Jun. 1969, CBS 463.69.
Didymella negriana (Thüm.) Q. Chen & L. Cai, comb. nov. MycoBank MB814112.
Basionym: Phoma negriana Thüm. “Ph. negrianum”, Die Pilze des Weinstockes, Vienna: 185. 1878.
≡ Phyllosticta negriana (Thüm.) Allesch., Rabenh. Krypt.-Fl. 1: 98. 1898.
Description (de Gruyter et al. 1998).
Specimen examined: Germany, Oberdollendorf am Rhein, from Vitis vinifera, deposited in CBS Mar. 1971, L. Kiewnik, CBS H-16511, culture CBS 358.71.
Didymella nigricans (P.R. Johnst. & Boerema) Q. Chen & L. Cai, comb. nov. MycoBank MB814113.
Basionym: Phoma nigricans P.R. Johnst & Boerema, New Zealand J. Bot. 19: 394. 1982.
≡ Peyronellaea australis Aveskamp et al., Stud. Mycol. 65: 31. 2010.
Description (de Gruyter et al. 1998).
Specimens examined: New Zealand, Auckland, Mt. Albert, from a leaf of Actinidia chinensis, Apr. 1979, P.R. Johnston (isotype CBS H-7619, culture ex-isotype CBS 444.81 = PDDCC 6546); from Actinidea chinensis, 1977, P.R. Johnston, PD 77/919.
Didymella pedeiae (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814114.
Basionym: Phoma pedeiae Aveskamp et al., Stud. Mycol. 65: 27. 2010.
Description and illustration (Aveskamp et al. 2010).
Specimen examined: The Netherlands, Aalsmeer region, on Schefflera elegantissima, 1992, isolated by J. de Gruyter (holotype CBS H-20239, culture ex-holotype CBS 124517 = PD 92/612A).
Didymella pinodella (L.K. Jones) Q. Chen & L. Cai, comb. nov. MycoBank MB814115.
Basionym: Ascochyta pinodella L.K. Jones, Bull. New York Agric. Exp. Sta., Geneva 547: 10. 1927.
≡ Phoma medicaginis var. pinodella (L.K. Jones) Boerema, Netherlands J. Pl. Pathol. 71: 88. 1965.
≡ Phoma pinodella (L.K. Jones) Morgan-Jones & K.B. Burch, Mycotaxon 29: 485. 1987.
≡ Peyronellaea pinodella (L.K. Jones) Aveskamp et al., Stud. Mycol. 65: 33. 2010.
Description (de Gruyter et al. 2002).
Specimens examined: The Netherlands, from a stem of Pisum sativum, deposited in CBS Jul. 1990, M.E. Noordeloos, CBS 318.90 = PD 81/729. USA, Minnesota, from Trifolium pratense, deposited in CBS Sep. 1966, CBS 531.66.
Didymella pinodes (Berk. & A. Bloxam) Petr., Ann. Mycol. 22: 16. 1924. Fig. 14, Fig. 15.
Fig. 14.
Didymella pinodes (K 56275). A. Type collection packet. B. Ascomata on host substrate. C. Ascomata. D. Ascospore. E. Section of ascomata. F. Asci. G. Ascus. Scale bar: B = 200 μm; C, E = 20 μm; D = 2.5 μm, F = 10 μm, G = 5 μm.
Fig. 15.
Didymella pinodes (CBS 525.77). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidia. I. Section of pycnidium. J. Section of pycnidial wall. K. Conidia. Scale bars: G = 200 μm; H = 100 μm; I = 20 μm; J–K = 10 μm.
Basionym: Sphaeria pinodes Berk. & A. Bloxam, Ann. Mag. Nat. Hist., Ser. III 7: 454. 1861.
≡ Mycosphaerella pinodes (Berk. & A. Bloxam) Vestergr., Ann. Mycol. 10: 581. 1912.
≡ Peyronellaea pinodes (Berk. & A. Bloxam) Aveskamp et al., Stud. Mycol. 65: 33. 2010.
= Ascochyta pinodes L.K. Jones, Bull. New York Agric. Exp. Sta., Geneva 547: 4. 1927.
Description from holotype (K 56275): Pseudothecia solitary, on the surface of stems, brown, uniloculate, subglobose to globose, 125–215 × 100–205 μm, ostiolate. Asci cylindrical to subclavate, 33–74 × 10–15 μm, 8-spored, biseriate. Ascospores broadly fusiform to ellipsoidal, 11–20 × 4–8 μm, smooth, straight or slightly curved, hyaline, 1-septate, slightly constricted at the septum, guttulate, upper cells usually broader and longer than the lower cells.
Description from ex-epitype culture (CBS 525.77): Conidiomata pycnidial, solitary or confluent, (sub-)globose, glabrous or with some hyphal outgrows, produced on the agar surface or immersed, (130–)170–270(–320) × 130–210(–235) μm. Ostioles 1–2, papillate. Pycnidial wall pseudoparenchymatous, 3–5-layered, 14–23 μm thick, composed of oblong to isodiametric cells, outer wall 2–3-layered, pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, 6.5–8.5 × 5–6 μm. Conidia variable in shape and size, cylindrical, allantoid to fabiform, smooth- and thin-walled, hyaline, 0–2-septate, mostly 1-septate, 7–16.5 × 4–6 μm, somewhat constricted at the septum, with 5–20 guttules per cell. Conidial matrix pale salmon.
Culture characteristics: Colonies on OA, 35–40 mm diam after 7 d, margin regular, white, floccose in concentric rings, with sparse mycelia near the centre, and an olivaceous background; reverse olivaceous, buff rings near the margin. Colonies on MEA 40–45 mm diam after 7 d, margin regular, white, with concentric rings; reverse concolourous. Colonies on PDA, 35–40 mm diam after 7 d, margin regular, densely covered by floccose, white, pale olivaceous near the centre; reverse white in outer ring, darkening towards the centre of the colony via buff, hazel to pale brown olivaceous. NaOH test negative.
Specimens examined: Belgium, Gembloux, from Pisum sativum, Sep. 1977, G. Sommereyns (epitype designated here CBS H-14681, MBT202499, culture ex-epitype CBS 525.77). UK, from stems of Pisum sativum, 1886 (holotype K 56275).
Notes: We only observed the sexual morph from the holotype specimen of Didymella pinodes. By comparing the morphological characters of the asexual morph (pycnidia, conidiogenous cells and conidia) of CBS H-14681 with the descriptions published by Punithalingam (1972) and Mel'nik (1977), we designate CBS H-14681 as epitype of this species.
Didymella pomorum (Thüm.) Q. Chen & L. Cai, comb. nov. MycoBank MB814116.
Basionym: Phoma pomorum Thüm., Fungi Pomicoli: 105. 1879.
≡ Peyronellaea pomorum var. pomorum (Thüm.) Aveskamp et al., Stud. Mycol. 65: 33. 2010.
= Peyronellaea circinata Kusnezowa, Novoste Sist. Nizsh. Rast. 8: 189. 1971.
≡ Phoma jolyana var. circinata (Kusnezowa) Boerema. & Kesteren, Kew Bull. 31: 535. 1977.
≡ Phoma pomorum var. circinata (Kusnezowa) Aveskamp et al., Mycologia 101: 377. 2009.
≡ Peyronellaea pomorum var. circinata (Kusnezowa) Aveskamp et al., Stud. Mycol. 65: 33. 2010.
= Phoma cyanea Jooste & Papendorf, Mycotaxon 12: 444. 1981.
≡ Phoma pomorum var. cyanea (Jooste & Papendorf) Aveskamp et al., Mycologia 101: 377. 2009.
≡ Peyronellaea pomorum var. cyanea (Jooste & Papendorf) Aveskamp et al., Stud. Mycol. 65: 32. 2010.
= Phoma triticina E. Müll., Phytopathol. Z. 19: 413. 1952.
Description (Boerema 1993).
Specimens examined: Russia, West Siberia, Novosibirsk, from Heracleum dissectum, deposited in CBS May 1976 (isotype of “Phoma pomorum var. circinata” CBS H-3747, culture ex-isotype CBS 285.76 = ATCC 26241 = IMI 176742 = VKM F-1843). South Africa, Heilbron, from straw of Triticum sp., 1972, W.J. Jooste (holotype of “Phoma pomorum var. cyanea” PREM 45736, culture ex-holotype CBS 388.80). Switzerland, Zürich, Oerlikon, from Triticum spelta, deposited in CBS Mar. 1952, E. Müller (culture ex-holotype of “Phoma triticina” CBS 354.52). The Netherlands, Wageningen, from Polygonum tataricum, deposited in CBS Sep. 1966, CBS H-16540, culture CBS 539.66 = ATCC 16791 = IMI 122266 = PD 64/914.
Notes: The isolates of the respective Phoma pomorum varieties, viz. vars. circinata (CBS 285.76), cyanea (CBS 388.80) and pomorum (CBS 539.66), and the species P. triticina (CBS 354.52), clustered in a well-supported clade. Sequences of these four isolates are nearly identical in all four loci, and these four taxa have only negligible differences in morphology. Thus, we regarded these four taxa to be conspecific, and treat them as a single species, Didymella pomorum.
Didymella protuberans (Lév.) Q. Chen & L. Cai, comb. nov. MycoBank MB814117. Fig. 16.
Fig. 16.
Didymella protuberans (CBS 381.96). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidium. I. Conidia. Scale bars: G = 100 μm; H = 50 μm; I = 10 μm.
Basionym: Phoma protuberans Lév., Ann. Sci. Nat. Bot. III 5: 281. 1846.
≡ Peyronellaea protuberans (Lév.) Aveskamp et al., Stud. Mycol. 65: 33. 2010.
= Didymella alectorolophi Rehm, Hedwigia 64: 294. 1923.
≡ Peyronellaea alectorolophi (Rehm.) Aveskamp et al., Stud. Mycol. 65: 31. 2010.
= Phoma alecotorolophi Boerema et al., Persoonia 16: 366. 1997.
= Phoma obtusa Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 378. 1870.
≡ Peyronellaea obtusa (Fuckel) Aveskamp et al., Stud. Mycol. 65: 33. 2010.
Description from ex-neotype culture (CBS 381.96): Conidiomata pycnidial, solitary or aggregated, irregularly globose, glabrous or covered with some hyphal outgrowths, semi-immersed or immersed, 110–280(–350) × 95–220(–295) μm. Ostioles 1–2, slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 5–7-layered, 15–25 μm thick, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 3.5–5(–6) × 3–4.5 μm. Conidia ellipsoidal, hyaline, thin-walled, smooth, aseptate, 4.5–7.5 × 3–5(–6.5) μm, egutullate or sometimes with 1(–3) small guttules. Conidial matrix whitish.
Culture characteristics: Colonies on OA, 55–60 mm diam after 7 d, margin regular, floccose, white to pale greenish olivaceous; reverse buff to white. Colonies on MEA 50–55 mm diam after 7 d, margin regular, white, with tufts of aerial mycelium; reverse olivaceous, greenish olivaceous near the centre. Colonies on PDA, 50–55 mm diam after 7 d, margin regular, white, floccose, pale leaden near the centre; reverse white to buff, olivaceous near the centre. NaOH spot test: a luteous discolouration on MEA, later changing to dull green to vinaceous-black, from the centre to outer ring.
Specimens examined: Germany, Hessen, from stalks of Daucus carota, K.W.G. Fuckel (holotype of “Phoma obtusa” G00266302 & G00266303). The Netherlands, from seed of Rhinanthus major, deposited in CBS Feb. 1996, (holotype of “Phoma alecotorolophi” L 992.167.515, culture ex-holotype CBS 132.96 = PD 93/853); from a root of Daucus carota, deposited in CBS Jul. 1993, J. de Gruyter, CBS 377.93 = PD 80/976; from Spinacia oleracea, deposited in CBS Jul. 1993, J. de Gruyter, CBS 391.93 = PD 80/87; from a leaf of Lycium halifolium, deposited in CBS Apr. 1996 (neotype of Phoma protuberans designated here HMAS 246694, MBT202500, culture ex-neotype CBS 381.96 = PD 71/706).
Notes: The type specimen of Phoma protuberans could not be traced. The original description lacks conidial dimensions. In the specimen HMAS 246694, collected from Lycium halifolium in the Netherlands, the aseptate conidia measured 4.5–7.5 × 3–5(–6.5) μm, which is, in general agreement with the description by Boerema et al. (1997), 4–10.5 × 2–5 μm in vitro. Therefore, HMAS 246694 is selected as neotype.
Strains CBS 132.96 (ex-holotype of “Phoma alecotorolophií”), CBS 377.93 and CBS 391.93, grouped in a well-supported clade together with the neotype of Didymella protuberans. Sequences used in the multi-locus analyses of these four strains are identical, and there is no detectable difference in morphology among them. Based on current data, we confirmed that these four strains represent the same species, for which the name Didymella protuberans is adopted.
Didymella rhei (Ellis & Everh.) Q. Chen & L. Cai, comb. nov. MycoBank MB814156.
Basionym: Ascochyta rhei Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 45: 160. 1893.
≡ Phoma rhei (Ellis & Everh.) Aa & Boerema, Persoonia 18: 42. 2002.
Description (de Gruyter et al. 2002).
Specimen examined: New Zealand, from a leaf of Rheum rhaponticum, deposited in CBS Jan. 2001, H. de Gruyter, CBS 109177 = LEV 15165 = PD 2000/9941.
Didymella rumicicola (Boerema & Loer.) Q. Chen & L. Cai, comb. nov. MycoBank MB814118. Fig. 17, Fig. 18.
Fig. 17.
Didymella rumicicola (PDD 50667). A. Type collection packet. B. Pycnidia on dried culture. C. Pycnidia. D. Section of pycnidial wall. E–F. Conidiogenous cells. G. Conidia. Scale bars: B = 100 μm; C = 50 μm; D, G = 10 μm; E–F = 2.5 μm.
Fig. 18.
Didymella rumicicola (CBS 683.79). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Section of pycnidial wall. I. Conidia. Scale bars: G = 200 μm; H–I = 10 μm.
Basionym: Phoma rumicicola Boerema & Loer., New Zealand J. Bot. 18: 473. 1980.
Description from holotype (PDD 50667): Conidiomata pycnidial, solitary or confluent, subglobose, glabrous, (100–)145–335(–470) × (100–)145–240(–330) μm. Ostioles 1–4, papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 3–5-layered, 18–35 μm thick, composed of isodiametric cells, outer wall 2–3-layered, pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, 3.5–5.5 × 3–4 μm. Conidia ellipsoidal to cylindrical, smooth- and thin-walled, aseptate, 6.5–11.5 × 3–4.5 μm, guttulate.
Description from ex-isotype culture (CBS 683.79): Conidiomata pycnidial, solitary or confluent, subglobose, glabrous, superficial or immersed, (75–)345–480 × (50–)250–370 μm. Ostioles 1–4, papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 2–4-layered, 20–31 μm thick, composed of isodiametric cells, outer cell layer pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, 3.5–8.5 × 3–7 μm. Conidia ellipsoidal to cylindrical, thin-walled, smooth, aseptate, 4.5–9(–12.5) × 2.5–5 μm, with many minute guttules, ca. 5–25 guttules. Conidial matrix yellowish cream.
Culture characteristics: Colonies on OA, 60–65 mm diam after 7 d, margin regular, felty, olivaceous; reverse concolourous. Colonies on MEA 55–60 mm diam after 7 d, margin regular, wooly, white, grey olivaceous near the margin; reverse buff, pale grey olivaceous near the margin. Colonies on PDA, 55–60 mm diam after 7 d, margin regular, floccose, white, abundant black pycnidia visible, giving an iron-black colour near the centre and margin; reverse dark olivaceous with some white zones. NaOH test negative.
Specimen examined: New Zealand, Levin, from Rumex obtusifolius, deposited in CBS Nov. 1979, G.F. Laundon (holotype PDD 50667, isotype CBS H-7627, culture ex-isotype CBS 683.79 = LEV 15094).
Notes: The isotype of Didymella rumicicola clustered in a well-supported clade with CBS 179.97 (D. acetosellae, originally identified as Phoma acetosellae) without any difference in the sequenced loci. These two species were both initially isolated from Rumex spp. However, D. rumicicola is distinguished from D. acetosellae in the faster growing rate (60–65 mm vs. 20–30 mm after 7 d on OA), and the smaller conidiogenous cells (3.5–8.5 × 3–7 μm in D. rumicicola vs. 5–13 × 6–12 μm in D. acetosellae; Boerema et al. 1980). Since CBS 179.97 is not the ex-type culture of D. acetosellae, the potential conspecificity of D. rumicicola and D. acetosellae remains to be confirmed.
Didymella sancta (Aveskamp et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814119.
Basionym: Phoma sancta Aveskamp et al., Mycologia 101: 377. 2009.
≡ Peyronellaea sancta (Aveskamp et al.) Aveskamp et al., Stud. Mycol. 65: 33. 2010.
Description and illustration (Aveskamp et al. 2009a).
Specimen examined: South Africa, from dead branches of Ailanthus altissima, Oct. 1982, C. Jansen (holotype CBS H-16332, culture ex-holotype CBS 281.83).
Didymella senecionicola Q. Chen & L. Cai, nom. nov. MycoBank MB814120.
≡ Phoma senecionis P. Syd., Hedwigia. 38: 136. 1899, non Didymella senecionis Hollós, 1908.
Description (de Gruyter et al. 1993).
Specimen examined: New Zealand, Raetihi, from a stem of Senecio jacobaea, deposited in CBS Jan. 1978, G.H. Boerema, CBS 160.78 = LEV 11451.
Notes: As the epithet “senecionis” was occupied in Didymella, a new name is proposed for this species. The name Didymella senecionis was based on the sexual morph, producing uniseptate ascospores arranged uniseriately into the clavate asci (Saccardo & Trotter 1913). Didymella senecionicola is presently only known from its asexual morph, producing aseptate, oblong to ellipsoidal conidia (de Gruyter et al. 1993).
Didymella sp. 1
Specimen examined: The Netherlands, Wageningen, Alphen aan de Rijn, from a leaf of Pteris sp., deposited in CBS Apr. 1996, CBS 379.96.
Notes: This isolate was incorrectly identified as “Didymella adianticola”, as it is phylogenetically distant from the authentic strains of D. adianticola (CBS 187.83 and CBS 260.92). It is probably a novel species, and will be treated after further study.
Didymella sp. 2
Specimen examined: Germany, Berlin, from a flower-stalk of Chrysanthemum roseum, deposited in CBS Sep. 1958, R. Schneider, CBS 115.58 = DSM 62044.
Notes: CBS 115.58 was originally received as “Ascochyta pyrethri”, and clustered in a distinct lineage (Fig. 1). Since the type of As. pyrethri is not available for comparison, we are unsure if CBS 115.58 represents a new species or is conspecific to As. pyrethri. This isolate awaits further study.
Didymella subglomerata (Boerema et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814121.
Basionym: Phoma subglomerata Boerema et al., Persoonia 15: 204. 1993.
≡ Peyronellaea subglomerata (Boerema et al.) Aveskamp et al., Stud. Mycol. 65: 33. 2010.
Description (Boerema 1993).
Specimen examined: USA, North Dakota, from Triticum sp., deposited in CBS Sep. 1992, J. de Gruyter, CBS 110.92 = PD 76/1010.
Didymella subherbarum (Gruyter et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814122.
Basionym: Phoma subherbarum Gruyter et al., Persoonia 15: 387. 1993.
Description (de Gruyter et al. 1993).
Specimens examined: Canada, Ontario, from overwintered seeds of Zea mays, deposited in CBS May 1992, J. de Gruyter (holotype L 992.177.439, culture ex-holotype CBS 250.92 = DAOM 171914 = PD 92/371). Peru, from Solanum sp., deposited in CBS May 1992, J. de Gruyter, CBS 249.92 = PD 78/1088.
Didymella viburnicola (Oudem.) Q. Chen & L. Cai, comb. nov. MycoBank MB814123.
Basionym: Phoma viburnicola Oudem., Ned. Kruidk. Arch. 2: 247. 1900.
Description (de Gruyter & Noordellos 1992).
Specimen examined: The Netherlands, Wageningen, Aboretum, from Viburnum cassioides, deposited in CBS May 1973, CBS H-16605, culture CBS 523.73 = PD 69/800.
Notes: Phoma viburnicola was first collected on Viburnum oxycoccus from the Netherlands, with conidia measuring 5–6 × 3.5 μm (Saccardo 1902). De Gruyter & Noordeloos (1992) confirmed the conidial size of the representative isolates as 3.5–5.5 × 1.6–2.2 μm, which agrees with the original description. We herewith treat this species as a new combination in Didymella.
Clade 7: Paraboeremia
Paraboeremia Q. Chen & L. Cai, gen. nov. MycoBank MB814061.
Etymology: Morphologically resembling the genus Boeremia, but being phylogenetically distinct.
Conidiomata pycnidial, globose to subglobose, or irregular shaped, superficial on or immersed into the agar, solitary or confluent, ostiolate, sometimes with a short neck around the ostioles. Pycnidial wall pseudoparenchymatous, 3–6-layered, outer layers pigmented. Conidiogenous cells phialidic, hyaline, smooth, globose to flask-shaped. Conidia ellipsoidal, sometimes curved, hyaline, smooth- and thin-walled, generally aseptate, guttulate, sometimes with greenish colour. Ascomata pseudothecial, subglobose to pyriform, ostiolate. Asci 8-spored, bitunicate. Ascospores subcylindrical, hyaline, 1-septate, the upper cell wider than the lower cell, constricted at the septum.
Type species: Paraboeremia selaginellae (Sacc.) Q. Chen & L. Cai.
Paraboeremia adianticola (Aa & Boerema) Q. Chen & L. Cai, comb. nov. MycoBank MB814124. Fig. 19.
Fig. 19.
Paraboeremia adianticola (CBS 260.92). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidia. I. Section of pycnidia. J. Section of pycnidial wall. K–L. Conidiogenous cells. M. Conidia. Scale bars: G = 100 μm; H–I = 50 μm; J, K, M = 10 μm; L = 5 μm.
Basionym: Didymella adianticola Aa & Boerema, Verslagen Meded. Plantenziektenk. Dienst Wageningen 159 (Jaarboek 1982): 25. 1983.
= Phyllosticta adianticola E. Young, Mycologia 7: 144. 1915.
≡ Phoma adianticola (E. Young) Boerema, Verslagen Meded. Plantenziektenk. Dienst Wageningen 159 (Jaarboek 1982): 25. 1983.
Description from culture (CBS 260.92): Conidiomata pycnidial, solitary, globose to subglobose, glabrous, semi-immersed or immersed, (150–)170–265 × (120–)140–245 μm. Ostioles 1–3, spalely papillate. Pycnidial wall pseudoparenchymatous, 4–6-layered, 13–24 μm thick, composed of isodiametric cells, outer layer brown. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to dolliform, 5.5–7 × 3–6.5 μm. Conidia ellipsoidal to cylindrical, smooth- and thin-walled, aseptate, 4–7 × 2–2.8 μm, with 2 large polar guttules. Conidial matrix white.
Culture characteristics: Colonies on OA, 55–60 mm diam after 7 d, margin regular, buff to salmon, abundant pycnidia visible; reverse pale salmon. Colonies on MEA 20–25 mm diam after 7 d, margin regular, aerial mycelium sparse, pale saffron to brown, grey near the centre; reverse pale saffron, pale brown near the centre. Colonies on PDA, 35-40 mm diam after 7 d, margin regular, floccose, white or somewhat pale pink; reverse saffron. Application of NaOH results in a greenish olivaceous discolouration of the agar.
Specimens examined: Unknown origin, from Pteris ensiformis, deposited in CBS May 1992, J. de Gruyter, CBS 260.92 = PD 86/1103. USA, Florida, from a leaf of Polystichum adiantiforme, deposited in CBS Feb. 1983, G.H. Boerema, CBS H-16142, culture CBS 187.83 = PD 82/128.
Notes: Our taxonomic treatment was based on the sexual morph. Boerema (1983) connected the sexual (Didymella adianticola) and asexual (Phoma adianticola) morphs, which however requires molecular verification.
Paraboeremia putaminum (Speg.) Q. Chen & L. Cai, comb. nov. MycoBank MB814125.
Basionym: Phoma putaminum Speg., Atti Soc. Crittog. Ital. 3: 66. 1881.
Description (de Gruyter & Noordeloos 1992).
Specimens examined: Denmark, from the rhizosphere of Malus sylvestris, deposited in CBS Feb. 1969, E. Sønderhousen, CBS 130.69 = CECT 20054 = IMI 331916. The Netherlands, from a branch of Ulmus sp., deposited in CBS Jun. 1991, G.H. Boerema, CBS 372.91 = PD 75/960.
Notes: The two representative cultures of “Phoma putaminum” (CBS 130.69 and CBS 372.91) clustered in the Paraboeremia clade, and thus a new combination Paraboeremia putaminum is proposed. This species has identical LSU sequence with the type species, Pa. selaginellae, but is distinct in two bp and three bp in ITS and tub2 sequences respectively. The clarification of their relationship awaits further study.
Paraboeremia selaginellae (Sacc.) Q. Chen & L. Cai, comb. nov. MycoBank MB814126. Fig. 20.
Fig. 20.
Paraboeremia selaginellae (CBS 122.93). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidium. I. Section of pycnidial wall. J. Conidiogenous cells. K. Conidia. Scale bars: G = 200 μm; H = 100 μm; I–J = 10 μm; K = 5 μm.
Basionym: Phyllosticta selaginellae Sacc., Malpighia 11: 304. 1897.
= Phoma selaginellicola Gruyter et al., Persoonia 15: 399. 1993.
Description from ex-neotype culture (CBS 122.93): Conidiomata pycnidial, solitary, globose to obpyriform, glabrous, semi-immersed or immersed, 130–360 × 120–320 μm. Ostioles 2–3, slightly papillate. Pycnidial wall pseudoparenchymatous, 4–7-layered, 16–23 μm thick, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 5–6.5 × 3.5–5.5 μm. Conidia ellipsoidal to cylindrical, hyaline, smooth- and thin-walled, aseptate, 2.5–5 × 1–2 μm, sometimes with 1–2 guttules. Conidial matrix whitish.
Culture characteristics: Colonies on OA, 45–50 mm diam after 7 d, margin regular, floccose, grey olivaceous, white near the margin; reverse grey olivaceous to buff near the centre. Colonies on MEA 35–40 mm diam after 7 d, margin crenate, aerial mycelium sparse, olivaceous, white near the centre; reverse concolourous. Colonies on PDA, 35–40 mm diam after 7 d, margin crenate, floccose, with concentric rings, white to pale olivaceous; reverse olivaceous to pale brown, dull green near the centre. Application of NaOH results in a brown discolouration of the agar.
Specimen examined: The Netherlands, from a leaf of Selaginella sp., deposited in CBS Jan 1993, J. de Gruyter (neotype of Phyllosticta selaginellae designated here HMAS 246693, MBT202501, culture ex-neotype CBS 122.93 = PD 77/1049).
Notes: The type specimen of Phyllosticta selaginellae could not be located, and is presumably lost. The strain CBS 122.93 from Selaginella sp. had ellipsoidal to cylindrical conidia, 2.5–5 × 1–2 μm, which is in agreement with the original description based on Selaginella helvetica, and hence this collection is designated as neotype.
Paraboeremia selaginellae has a close phylogenetic relationship to Pa. putaminum, but can be distinguished by its narrower conidia (2.5–5 × 1–2 μm). Conidia of Pa. putaminum are guttulate, 3–4 × 2–2.5 μm, and conspicuous greenish in colour (de Gruyter & Noordeloos 1992).
Clade 8: Macroventuria
Macroventuria Aa, Persoonia 6: 359. 1971.
Ascomata perithecial, globose, ostiolate, erumpent on the agar surface, setose in the upper part. Asci ellipsoidal or saccate, bitunicate, 8-spored. Ascospores mostly hyaline, ellipsoidal, 2-celled (from van der Aa 1971).
Type species: Macroventuria anomochaeta Aa, Persoonia 6: 362. 1971.
Notes: This genus was established by van der Aa (1971), accommodating two species in the family Venturiaceae which produced relatively large, nearly hyaline, two-celled ascospores, differing from Leptosphaerulina (van der Aa 1971). Later Macroventuria was placed in Pseudosphaeriaceae by Barr (1982) and then in Pleosporaceae by Eriksson & Hawksworth (1986) (Kodsueb et al. 2006). In the study of Aveskamp et al. (2010) this genus was accommodated in the Didymellaceae, which is confirmed in the present study.
Macroventuria anomochaeta Aa, Persoonia 6: 362. 1971.
Specimens examined: South Africa, Karoo Desert, from decayed canvas, deposited in CBS Aug. 1971, M.C. Papendorf (holotype CBS H-14192, culture ex-holotype CBS 525.71); Cape Province, from a trunk of Medicago sativa, Jun. 1972, W.F.O. Marasas, CBS 502.72.
Notes: Strain CBS 502.72, which was also received as “M. anomochaeta” appears to be phylogenetically distinct from the ex-holotype (CBS 525.71). Genetically, CBS 502.72 differs from CBS 525.71 in only three bp in the four loci sequenced. As we have not examined the morphology of CBS 502.72, its classification awaits further study. The type of M. wentii (CBS 526.71) differs from that of M. anomochaeta (CBS 525.71) in 19 bp in the four loci sequenced.
Macroventuria wentii Aa, Persoonia 6: 361. 1971.
Specimen examined: USA, Nevada, Death Valley, from plant litter, 1970, F.W. Went (holotype CBS H-14195, culture ex-holotype CBS 526.71).
Clade 9: Ascochyta
Ascochyta Lib., Pl. crypt. Arduenna, fasc. 1: no. 59. 1830. emend. Q. Chen & L. Cai.
Conidiomata pycnidial, subglobose or ampulliform to mammiform, sometimes irregularly shaped, superficial on or immersed into the agar, solitary or confluent, ostiolate or poroid opening formed at the end of the growing process. Pycnidial wall pseudoparenchymatous, 1–8-layered, outer wall pigmented. Conidiogenous cells annellidic or phialidic, hyaline, smooth, variable in shape, i.e. subglobose, cylindrical, flask-shaped, obpyriform, ampulliform to doliiform. Conidia variable in shape, i.e. ovoid, oblong, subcylindrical, ellipsoidal, cymbiform, allantoid, straight or slightly curved, hyaline or sometimes slightly coloured (yellow to pale brown), smooth- and thin-walled, aseptate or septate, mostly uniseptate, sometimes 2–3-septate, eguttulate or guttulate (Boerema and Bollen, 1975, Boerema et al., 2004). Chlamydospores occasionally occur in old cultures. Ascomata pseudothecial, immersed or erumpent, subglobose to flattened, or irregular, solitary or confluent, ostiolate, sometimes developing an elongated neck. Asci subcylindrical to subclavate, or saccate, sometimes slightly curved, 8-spored, bitunicate, sometimes short-stipitate. Pseudoparaphyses filamentous, hyaline, thin-walled, septate, conspicuous in immature fructifications, and disappear at maturity. Ascospores ovoid to ellipsoidal, slightly biconic, hyaline to yellowish into the ascus, may become brown when released, smooth, 1-septate, sometimes 3-septate, symmetrical or asymmetrical, constricted at the septum, uniseriate or biseriate (Jellis and Punithalingam, 1991, Trapero-Casas and Kaiser, 1992, Kaiser et al., 1997, Chilvers et al., 2009).
Type species: Ascochyta pisi Lib., Pl. crypt. Arduenna, fasc. 1: no. 59. 1830.
Notes: In most cases, the host ranges of species belonging to this genus are rather restricted, occurring mostly on the Campanulaceae, Chenopodiaceae, Leguminosae, Poaceae, Solanaceae and Umbelliferae. Some species are associated with one specific host, but may also be found on other related species of the same genus or family (Boerema & Bollen 1975). As the sexual morphs of several Ascochyta species were linked to their asexual morphs (Kaiser et al., 1997, Chilvers et al., 2009, Woudenberg et al., 2009), we incorporated these features into the generic circumscription.
Ascochyta fabae Speg. Anales Mus. Nac. Hist. Nat. Buenos Aires 6: 321. 1898–1899.
= Ascochyta pisi f. foliicola Sacc. & Marchal, Rev. Mycol. (Toulouse) 7: 148. 1885.
= Didymella fabae G.J. Jellis & Punith, Pl. Pathol. 40: 151. 1991.
Description from holotype of Didymella fabae (IMI 336944): Ascomata arranged in rows on bean straw of Vicia faba. Ascomata pseudothecial, immersed, becoming partially erumpent, dark brown to blackish brown, subglobose, solitary or confluent, 180–240 × 130–150 μm, with short necks, ostiolate. Ostiole nearly circular, 35–50 μm wide, surrounded by dark brown cells. Ascomatal wall pseudoparenchymatous, of textura angularis, 5–8 layered, outer wall 3–4-layered, dark brown. Asci arranged in a relatively flat layer, hyaline, cylindrical to subclavate, 8-spored, 55–70 × 10–14 μm, usually constricted near the base to form a distinct foot. Pseudoparaphyses hyaline, thin-walled, septate, 1–2 μm, conspicuous in immature fructifications. Ascospores irregularly biseriate, hyaline, smooth, slightly biconic, broadly ellipsoidal, 1-septate, constricted at the septum, with the upper cell broader than the lower cell, 15–18 × 5.5–6.5 μm. Naturally discharged ascospores on bean straw later turn yellowish brown to dark brown and sometimes 2-septate (from Jellis & Punithalingam 1991).
Specimens examined: Belgium, Gembloux, from Phaseolus vulgaris, Sep. 1977, G. Sommereyns, CBS H-8998, culture CBS 524.77. The Netherlands, Randwijk, from a leaf of Vicia faba, deposited in CBS Oct. 1971, G.H. Boerema, CBS 649.71; from Phaseolus vulgaris, PD 83/492. UK, Great Britain, from a dead stem of Vicia faba, Jan. 1990, G.J. Jellis (holotype of “Didymella fabae” IMI 336944).
Notes: The sexual morph of Ascochyta fabae was published by Jellis & Punithalingam (1991) as Didymella fabae, which was recorded on overwintering bean straw of Vicia faba in Cambridge. Ascochyta viciae (CBS 451.68) is phylogenetically closely related to As. fabae, but they are distinguishable based on morphology. Conidia of As. viciae are much longer and narrower than those of As. fabae (30–60 × 2.5 μm vs. 10–25 × 5–6 μm) (Saccardo, 1884, Saccardo, 1902).
Ascochyta herbicola (Wehm.) Q. Chen & L. Cai, comb. nov. MycoBank MB814127.
Basionym: Phoma herbicola Wehm., Mycologia 38: 319. 1946.
Description (de Gruyter et al. 1998).
Specimens examined: USA, Montana, Missoula, head of Seeley Lake, from water, deposited in CBS Mar. 1997, CBS H-16581, culture CBS 629.97 = PD 76/1017; Wyoming, Jackson, Glory Mountain, from stems of Syntheris dissecta, Jul. 1040, L.E. Wehmeyer (holotype 1032b).
Ascochyta lentis Vassiljevsky, Acta Inst. Bot. Acad. Sci. Pl. Crypt, ser II: 358. 1938.
= Didymella lentis W.J. Kaiser, B.C. Wang & J.D. Rogers, Pl. Dis. 81: 815. 1997.
Specimen examined: Unknown origin, from seeds of Lens culinaris, deposited in CBS Sep. 1984, G.H. Boerema, CBS H-9060, culture CBS 370.84 = PD 81/783.
Ascochyta medicaginicola var. medicaginicola Q. Chen & L. Cai, nom. nov. MycoBank MB814129.
≡ Phoma medicaginis var. medicaginis Malbr. & Roum., Rev. Mycol. 8: 91. 1886.
Description (de Gruyter et al. 2002).
Specimens examined: Czech Republic, from Medicago sativa, deposited in CBS Jul. 1990, M.E. Noordeloos, CBS 316.90 = CCM F-187. France, Rounen, from Medicago sativa, Oct. 1885, C. Roumeguère (isotype BR 5020155793119).
Notes: Ascochyta medicaginicola var. macrospora and As. medicaginicola var. medicaginicola clustered in the same branch without any difference in four sequenced loci. However, these two varieties could be distinguished based on morphology and physiology. Ascochyta medicaginicola var. medicaginicola usually produces aseptate conidia measuring (4.2–)5.7–7.2(–12.7) × (1.4–)2.1–2.3(–3.5) μm, that differ from variety A. medicaginicola var. macrospora which produces 1–3-septate, larger conidia [(2.8–)6.3–11.1(–27.8) × (1.4–)2.1–2.9(–5.8) μm] (Boerema et al. 1993), especially when incubated at low temperature. Additionally, As. medicaginicola var. macrospora showed relatively stronger specific pathogenicity to the primary host of both varieties, lucerne (Medicago sativa), than As. medicaginicola var. medicaginicola (Boerema et al. 1993). Hence, we maintain these two varieties and propose two new names.
Ascochyta medicaginicola var. macrospora (Boerema et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814128.
≡ Phoma medicaginis var. macrospora Boerema et al., Netherlands J. Pl. Pathol. 99 (Suppl. 1): 19. 1993.
Description (de Gruyter et al. 2002).
Specimens examined: Canada, Saskatchewan, Saskatoon, from seed of Medicago sativa, deposited in CBS Jun. 1965, G.H. Boerema, CBS 404.65 = IMI 116999. USA, Minnesota, from Medicago sativa, Sep. 1953, M.F. Kernkamp (holotype CBS H-16487, culture ex-holotype CBS 112.53).
Note: As the epithet “medicaginis” was occupied in Ascochyta, we introduce the new epithet “medicaginicola” for the varieties of Phoma medicaginis (see above).
Ascochyta nigripycnidia (Boerema et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814130.
Basionym: Phoma nigripycnidia Boerema et al., Persoonia 16: 356. 1997.
Description (Boerema et al. 1997).
Specimen examined: Czech Republic, from a leaf of Vicia cracca, deposited in CBS Jan 1996, M. Ondrej (holotype L 992.163.150, culture ex-holotype CBS 116.96 = CCMF 243 = PD 95/7930).
Ascochyta phacae (Corbaz) Q. Chen & L. Cai, comb. nov. MycoBank MB814131. Fig. 21.
Fig. 21.
Ascochyta phacae (ZT Myc 54988). A. Type collection packet. B. Pseudothecium. C. Section of pseudothecial wall. D. Pseudothecia on host substrate. E. Asci. F. Ascospores. Scale bars: B–C = 20 μm; D = 200 μm; E = 10 μm; F = 5 μm.
Basionym: Didymella phacae Corbaz, Sydowia 9: 229. 1955.
Description from holotype (ZT Myc 54988): Pseudothecia on stems of Phaca alpina, solitary, brown to black, uniloculate, subglobose to globose, 110–255 × 110–245 μm, ostiolate single. Ascomatal wall pseudoparenchymatous, of textura angularis, 3–5-layered, 18–23.5 μm thick. Asci cylindrical to subclavate, 40–60 × 11.5–15 μm, 8-spored, biseriate. Ascospores broadly fusiform, 11.5–14.5 × 4.5–6.5 μm, smooth, hyaline, uniseptate, slightly constricted at the septum, guttulate, upper cells usually broader than the lower cells.
Specimens examined: Switzerland, Valais, Gabi, Feehrbergen, from dead stems of Phaca alpina, deposited in CBS May 1955, E. Müller (holotype ZT Myc 54988, culture ex-holotype CBS 184.55).
Notes: Didymella phacae was linked to an ascochyta-like asexual morph (Corbaz, 1955, Corbaz, 1957, Corlett, 1981), but this morph was not formally named and described. A new combination is proposed here, as Ascochyta phacae.
Ascochyta pisi Lib., Pl. crypt. Arduenna, fasc. 1: no. 59. 1830. Fig. 22, Fig. 23.
Fig. 22.
Ascochyta pisi (BR 5020059493320). A. Type collection packet. B. Pycnidia on host substrate. C. Conidia. D. Pycnidia. E. Section of pycnidium. Scale bars: B = 100 μm; C–D = 10 μm; E = 20 μm.
Fig. 23.
Ascochyta pisi (CBS 122785). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidia. I. Section of pycnidium. J–K. Conidiogenous cells. L. Conidia. Scale bars: G = 200 μm; H–I = 20 μm; J–K = 5 μm; L = 10 μm.
≡ Septoria leguminum var. pisorum (Lib.) Desm., Ann. Sci. Nat. Bot., sér. 2, 19: 344. 1843.
= Didymella pisi Chilvers et al., Mycol. Res. 113: 396. 2009.
Description from isotype (BR 5020059493320): Leaf spots elliptical to circular, brown to black. Pycnidia on bean pod surface of Laburnum anagyroides, solitary or confluent, subglobose, 65–210 × 45–185 μm. Ostiole single. Pycnidial wall pseudoparenchymatous, 3–4-layered, 14–24 μm thick, composed of isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, doliiform. Conidia fusiform to cylindrical, smooth- and thin-walled, hyaline, uniseptate, 11–18.5 × 3–5 μm, with 2–5 guttules.
Description from holotype of Didymella pisi: Ascomata pseudothecial, globose to irregular, 200–400 μm diam, with inconspicuous ostiole, brown to blackish, soft. Asci bitunicate, cylindrical to saccate, 8-spored, 46–168 × 10–15 μm. Ascospores usually uniseriately arranged, hyaline, more or less equally bicellular, constricted at the septum, rounded at both ends or with one end more acute, smooth, 12–17.5 × 6.5–8.5 μm. Hamathecial elements sparse or absent. Pseudothecia formed on pea stems only when opposite mating types were present (from Chilvers et al. 2009).
Description from ex-epitype culture (CBS 122785): Conidiomata pycnidial, solitary, globose to subglobose, with some hyphal outgrows, produced on the agar surface and immersed, 90–195 × 75–160 μm. Ostiole single, slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 3–4 layered, 14.5–29 μm thick, composed of isodiametric cells. Conidiogenous cells annellidic, hyaline, smooth, flask-shaped to obpyriform, 5.5–8.5 × 4.5–8 μm. Conidia oblong to cylindrical, thin-walled, smooth, mainly uniseptate, incidentally aseptate or 2-septate, 7–16 × 3–5 μm, always somewhat constricted at the septum, with (4–)6–14(–16) guttules. Conidial matrix pale pink.
Culture characteristics: Colonies on OA, 45–50 mm diam after 7 d, margin regular, floccose, white, slight grey near the centre; reverse buff to pale salmon, somewhat pale olivaceous near the centre. Colonies on MEA 35–40 mm diam after 7 d, margin regular floccose, white, sparse near the margin; reverse white, pale green near the centre. Colonies on PDA, 25–30 mm diam after 7 d, margin regular, wooly, white; reverse white, buff to amber near the centre. NaOH test negative.
Specimens examined: Belgium, from pods of Pisum sativum (isotype of Ascochyta pisi BR 5020059493320). Canada, Saskatoon, from Pisum sativum, B. Gossen, CBS 122751 = ATCC 201620. The Netherlands, Venlo, from Pisum sativum, M.M.J. Dorenbosch (epitype designated here of Ascochyta pisi, HMAS 246705, MBT202502, culture ex-epitype CBS 122785 = PD 78/517); from Pisum sativum, deposited in CBS Qct. 1954, J.A. von Arx, CBS 126.54; from Juglans regia, deposited in CBS Mar. 1949, PD, CBS 108.49 = DSM 62041. USA, Idaho, from Pisum sativum, 1995, D. Webster, CBS 122750 = ATCC 201619.
Notes: Ascochyta pisi was originally described from Pisum sativum in Ardenne, on the borders of France and Belgium (Saccardo 1884). The conidia observed on the isotype (11–18.5 × 3–5 μm) and epitype (7–16 × 3–5 μm) of As. pisi are congruent with that of the original description (14–16 × 4–6 μm). Therefore, the specimen HMAS 246705 (ex CBS 122785) is designated as epitype for this species.
Didymella pisi was confirmed to be the sexual morph of As. pisi from the cross between two As. pisi isolates (CBS 122750 and CBS 122751; Chilvers et al. 2009). CBS 122750 has four bp differences in tub2 sequence from other isolates, but is identical in other loci. The isolate CBS 108.49 was initially identified as Ascochyta juglandis when deposited in CBS, but clustered with other As. pisi strains in a well-supported clade with sequences of four loci being identical to other strains in the clade. Therefore, we reclassified this strain as As. pisi.
Ascochyta rabiei (Pass.) Labr., Rev. Pathol. Vég. Entomol. Agric. France 18: 228. 1931.
Basionym: Zythia rabiei Pass., Comment. Soc. Crittog. Ital. 2: 437. 1867.
≡ Phoma rabiei (Pass.) Khune ex Gruyter, Persoonia 18: 89. 2002.
= Mycosphaerella rabiei Kovatsch. The blight of chick pea: 70. 1936.
≡ Didymella rabiei (Kovatsch.) Arx, Beitr. Kryptogamenfl. Schweiz 11: 364. 1962.
Specimens examined: Bulgaria, from Cicer arietinum, deposited in CBS Feb. 1937, I.C. Kovachevsky, ex-holotype CBS 237.37. India, from the seeds of Cicer arietinum, deposited in CBS Jun. 1965, S. Sinha, CBS 534.65. Unknown origin, from an unknown substrate, deposited in CBS Feb. 1930, F. Labrousse, CBS 206.30.
Ascochyta sp. 1
Specimens examined: Australia, from a leaf of Pisum sativum, deposited in CBS Sep. 1984, G.H. Boerema, CBS 372.84 = PD 80/1246; from a leaf of Pisum sativum, deposited in CBS Sep. 1984, G.H. Boerema, CBS H-9078, culture CBS 373.84 = PD 80/1247.
Notes: These two strains were deposited as “Ascochyta fabae”, but phylogenetically they are distinct from the authentic cultures of As. fabae (CBS 524.77, CBS 649.71 and PD 83/492). This species is probably a novel species, and will be described after further study.
Ascochyta sp. 2
Specimens examined: Sweden, Uppland, from Lathyrus vernus, May 1987, K. & L. Holm, CBS 113797 = UPSC 2222.
Notes: Isolate CBS 113797 was received as “Didymella astragalina”. However, it was distant from other Didymella species in the multi-locus phylogenetic tree, and clustered in the Ascochyta clade. The original host of D. astragalina is Astragalus cicer. Since the type of D. astragalina was unavailable for examination, it still needs to be confirmed if CBS 113797 represents a new species or is conspecific to D. astragalina.
Ascochyta syringae Bres., Hedwigia 33: 207. 1894.
Specimen examined: The Netherlands, from seed capsule of Syringa vulgaris, P.D. Wageningen, deposited in CBS Jul. 1972, G.H. Boerema, CBS 545.72.
Ascochyta versabilis (Boerema et al.) Q. Chen & L. Cai, comb. nov. MycoBank MB814132.
Basionym: Phoma versabilis Boerema et al., Persoonia 16: 154. 1996.
Description (Boerema & de Gruyter 1998).
Specimens examined: Germany, Westfalen, Oberdresselendorf, from stems of Cardamine impatiens, Oct. 1925, A. Ludwig (holotype L 995.229.369). The Netherlands, Wageningen, from a stem of Silene sp., deposited in CBS Jun. 1997, CBS 876.97 = PD 82/1008.
Notes: An authentic isolate of Phoma versabilis (CBS 876.97), which morphologically agrees well with the original description of this species (Boerema et al. 2004), grouped in the Ascochyta clade. Thus, Ascochyta versabilis was introduced as a new combination.
Ascochyta viciae Lib., Pl. crypt. Arduenna, fasc. 4: no. 356. 1837.
≡ Septoria viciae (Lib.) Westend., Herb. crypt. Belg.: no. 1151. 1857.
≡ Phyllosticta viciae (Lib.) Cooke, Handb., Brit. Fungi 1: 452. 1871.
Specimen examined: The Netherlands, Baarn, Praamgracht, from a leaf of Vicia sepium, Jun. 1968, H.A. van der Aa, CBS H-9121, culture CBS 451.68.
Ascochyta viciae-pannonicae Odřej, Biológia (Bratislava) 25: 685. 1970.
Specimen examined: Czech Republic, from a leaf of Vicia pannonica, deposited in CBS May 1992, CBS 254.92 = CCM F-241.
Clade 10: Phomatodes
Phomatodes Q. Chen & L. Cai, gen. nov. MycoBank MB814062.
Etymology: Name after its phoma-like conidia.
Conidiomata pycnidial, globose to subglobose, on agar surface or immersed, solitary or confluent, ostiolate. Pycnidial wall pseudoparenchymatous, 3–5-layered, outer wall pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform. Conidia cylindrical to allantoid, hyaline, thin-walled, smooth, aseptate, guttulate.
Type species: Phomatodes aubrietiae (Moesz) Q. Chen & L. Cai.
Phomatodes aubrietiae (Moesz) Q. Chen & L. Cai, comb. nov. MycoBank MB814133. Fig. 24.
Fig. 24.
Phomatodes aubrietiae (CBS 627.97). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidium. I–J. Conidiogenous cells. K. Conidia. Scale bars: G = 100 μm; H = 20 μm; I–K = 5 μm.
Basionym: Sclerophomella aubrietiae Moesz, Choroby Szkodn. Rosl. 3: 144. 1926.
= Phoma aubrietiae (Moesz) Boerema, Gewasbescherming 1: 66. 1970.
Description from ex-epitype culture (CBS 627.97): Conidiomata pycnidial, solitary, globose to subglobose, glabrous, semi-immersed or immersed, 110–255(–290) × 90–215(–245) μm. Ostiole single, slightly papillate. Pycnidial wall pseudoparenchymatous, 4–6 layered, 18–24.5 μm thick, composed of isodiametric cells, Conidiogenous cells phialidic, hyaline, smooth, ampulliform to dolliform, 4.5–6.5 × 3.5–5 μm. Conidia ellipsoidal to cylindrical, smooth- and thin-walled, aseptate, 6–8.5 × 2.5–3 μm, with 2(–4) large polar guttules. Conidial matrix white.
Culture characteristics: Colonies on OA, 25–30 mm diam after 7 d, margin regular, with concentric rings, woolly, grey to pale olivaceous; reverse olivaceous. Colonies on MEA 15–20 mm diam after 7 d, margin regular, fluffy, greenish olivaceous to olivaceous; reverse concolourous. Colonies on PDA, 35–40 mm diam after 7 d, margin regular, floccose, smoke-grey; reverse dark olivaceous. NaOH test negative.
Specimens examined: Albania, from dead stalks of Aubrietia gracilis (holotype BP 12773). The Netherlands, Bodegraven, from seed of Aubrietia hybrida cv. Superbissima, deposited in CBS Aug. 1967, G.H. Boerema, CBS H-16154, culture CBS 383.67 = PD 65/223; from a stem of Aubrietia sp., Mar. 1997, J. de Gruyter (epitype designated here CBS H-16155, MBT202503, culture ex-epitype CBS 627.97 = PD 70/714).
Notes: The holotype of Sclerophomella aubrietiae was collected from Aubrietia gracilis in Albania, with conidia measuring 5–10 × 2–3 μm (Boerema & Valckx 1970). The conidial dimensions of our selected epitype (CBS H-16155, ex-epitype culture CBS 627.97) agree well with that of the original description.
Phomatodes nebulosa (Pers.) Q. Chen & L. Cai, comb. nov. MycoBank MB814134. Fig. 25.
Fig. 25.
Phomatodes nebulosa (CBS 100191). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidial section. I. Section of pycnidial wall. J–K. Conidiogenous cells. L. Conidia. Scale bars: G = 200 μm; H = 20 μm; I–L = 10 μm.
Basionym: Sphaeria nebulosa Pers., Observ. Disp. Mycol. 2: 69. 1800.
≡ Phoma nebulosa (Pers.) Berk., Outl. Brit. Fung. (London): 314. 1860.
Description from culture (CBS 100191): Conidiomata pycnidial, solitary or aggregated, globose to subglobose, glabrous, produced on the agar surface or immersed, 125–185 × 105–135 μm. Ostiole single, conspicuously papillate. Pycnidial wall pseudoparenchymatous, 3–5-layered, 20–37 μm thick, brown, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 7–9 × 4.5–8(–9.5) μm. Conidia cylindrical, smooth- and thin-walled, aseptate, 5–7 × 1.5–2.5 μm, with (1–)2–6(–8) large polar guttules. Conidial exudates not recorded.
Culture characteristics: Colonies on OA, 45–50 mm diam after 7 d, margin regular, floccose, greenish olivaceous, abundant pycnidia visible near the centre of colony; reverse dark olivaceous, pale greenish olivaceous near the margin. Colonies on MEA 40–45 mm diam after 7 d, margin regular, white with a greenish olivaceous concentric ring; reverse concolourous. Colonies on PDA, 40–45 mm diam after 7 d, margin regular, floccose, white, abundant pycnidia near the centre; reverse white in outer ring, darkening towards the centre of the colony via buff, hazel to black. NaOH test negative.
Specimens examined: Poland, near Gryfice, from Thlaspi arvense, deposited in CBS Dec. 1997, collected by J. Marcinkowska, CBS 100191. The Netherlands, from a stem of Mercurialis perennis, deposited in CBS Jan 1993, J, de Gruyter, CBS 117.93 = PD 83/90; from a leaf of Armoracia rusticana, deposited in CBS Jul. 1996, collected by H.A. van der Aa, CBS 740.96.
Notes: Isolates CBS 100190 and CBS 100191 were identified as “Didymella macropodii” in Boerema et al. (2004), and two other isolates obtained in this study (CBS 740.96, PD 84/512) were also received as “D. macropodii”. In the phylogenetic analyses, CBS 100191 and CBS 740.96 clustered with the reference culture of Phomatodes nebulosa (CBS 117.93), but are distant from reference culture of D. macropodii (CBS 100190, data not shown). In addition, the morphological features of this isolate (CBS 100191) are essentially similar to that of Phomat. nebulosa (De Gruyter et al., 1993, Boerema et al., 2004), and different from D. macropodii (Boerema and de Gruyer, 1998, Boerema et al., 2004), thus we concluded that cultures CBS 100191 and CBS 740.96 were more appropriately classified as Phomat. nebulosa.
Clade 11: Calophoma
Calophoma Q. Chen & L. Cai, gen. nov. MycoBank MB814063.
Etymology: Calo = κάλλος in Greek, beauty kalos (Greek), beautiful, good; phoma = phoma-like morphology.
Conidiomata pycnidial, subglobose to irregular, on agar surface or immersed, solitary or confluent, ostiolate, or with an elongate neck in older cultures. Micropycnidia present. Pycnidial wall pseudoparenchymatous, 2–6-layered, outer wall pigmented. Conidiogenous cells phialidic, hyaline, smooth, globose to flask-shaped, ampulliform to doliiform. Conidia variable in size and shape, i.e. subglobose, subcylindrical, ellipsoidal, somewhat obclavate-fusiform, hyaline or becoming slightly brown, smooth- and thin-walled, aseptate, occasionally large 1-septate conidia occur that are eguttulate or guttulate. Chlamydospores only occur in one species, uni- or multicellular, unicellular intercalary, guttulate, thick-walled, multicellular irregular dictyo/phragmosporous, somewhat botryoid and in combination with unicellular chlamydospores.
Type species: Calophoma clematidina (Thüm.) Q. Chen & L. Cai.
Calophoma aquilegiicola (M. Petrov) Q. Chen & L. Cai, comb. nov. MycoBank MB814135.
Basionym: Phoma aquilegiicola M. Petrov, Trudy Bot. Inst. Akad. Nauk S.S.S.R, Ser. 1, Fl. Sist. Vyssh. Rast : 281. 1933.
Description (Boerema et al. 1997).
Specimens examined: New Zealand, Auckland, from fading leaves of Thalictrum dipterocarpum, Jul. 2004, C.F. Hill, CBS 116402. The Netherlands, from a stem of Aconitum pyramidale, deposited in CBS Jan 1996, CBS 107.96 = PD 73/598; from a stem of Aquilegia sp., deposited in CBS Jan 1996, CBS 108.96 = PD 79/611; from a stem of Aquilegia sp., deposited in CBS Jan 1996, CBS 109.96 = PD 83/832. Unknown origin, from Aquilegia sp., deposited in CBS Jul. 1931, R. Laubert, CBS 107.31.
Notes: The holotype of Phoma aquilegiicola was from dry stalks of Aquilegia vulgaris collected in Russia. Isolate CBS 107.31 was originally identified as Ascochyta aquilegiae, but in the phylogenetic analysis it appears indistinguishable from four representative cultures of Calophoma aquilegiicola. This species is morphologically and phylogenetically closely related to Ca. glaucii. Clarification of their relationship awaits future studies.
Calophoma clematidina (Thüm.) Q. Chen & L. Cai, comb. nov. MycoBank MB814136. Fig. 26.
Fig. 26.
Calophoma clematidina (CBS 108.79). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia sporulating on OA. H. Pycnidium. I. Swollen cells. J. Vertical section of pycnidium. K. Section of pycnidial wall. L. Conidia. M–N. Conidiogenous cells. Scale bars: G = 200 μm; H–I = 100 μm; J = 20 μm; K–M = 10 μm; N = 5 μm.
Basionym: Ascochyta clematidina Thüm., Bull. Soc. Imp. Naturalistes Moscou 55: 98. 1880.
≡ Phoma clematidina (Thüm.) Boerema, Verslagen Meded. Plantenziektenk. Dienst Wageningen (Jaarboek 1978) 153: 17. 1979.
Description from ex-epitype culture (CBS 108.79): Conidiomata pycnidial, solitary, globose to subglobose, mostly with some hyphal outgrows, produced on the agar surface or immersed, (120–)135–165 × 85–130 μm. Ostioles 1(–3), conspicuously papillate. Pycnidial wall pseudoparenchymatous, 2–4-layered, 13–21 μm thick, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 5.5–7.5 × 4–7 μm. Conidia ellipsoidal to cylindrical, smooth- and thin-walled, aseptate or occasionally 1-septate, 4.5–7 × 2–3 μm, with (0–)2–4(–8) polar guttules. Conidial matrix pale pink. Chlamydospores usually scanty, uni- or multicellular, unicellular intercalary, guttulate, thick-walled, green-brown, 8–10 μm diam, multicellular irregular dictyo/phragmosporous, somewhat botryoid and in combination with unicellular chlamydospores, tan to dark brown, 3–50 × 12–25 μm (Woudenberg et al. 2009).
Culture characteristics: Colonies on OA, 25–30 mm diam after 7 d, margin regular, felty, white, pale brown grey towards the centre; reverse buff with a hazel centric ring in the middle. Colonies on MEA 30–35 mm diam after 7 d, margin regular, wooly, white, olivaceous near the centre; reverse concolourous. Colonies on PDA, 20–25 mm diam after 7 d, margin regular, felty; white reverse buff in outer ring, darkening towards the centre of the colony via hazel to brown olivaceous. NaOH test negative.
Specimens examined: The Netherlands, Spaubeek, from the stem of Clematis sp., deposited in CBS Jan 1979, G.H. Boerema (epitype CBS H-16193, culture ex-epitype CBS 108.79 = PD 78/522). UK, England, from Clematis sp., deposited in CBS Jan. 1966, F.T. Last, CBS 102.66.
Notes: Woudenberg et al. (2009) designated an epitype (CBS H-16193 with culture CBS 108.79) for Phoma clematidina. Clematis spp. are susceptible to different Phoma s. lat. species. Calophoma clematidina (syn. Phoma clematidina) has shown host specificity to Clematis hybrids, while Didymella vitalbina was isolated exclusively from Cl. vitalba, and such isolates were initially misidentified as Phoma clematidina (Woudenberg et al. 2009).
Calophoma clematidis-rectae (Petr.) Q. Chen & L. Cai, comb. nov. MycoBank MB814137. Fig. 27.
Fig. 27.
Calophoma clematidis-rectae (CBS 507.63). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia sporulating on OA. H. Pycnidia. I. Conidiogenous cells. J. Section of pycnidial wall. K. Conidia. Scale bars: G = 200 μm; H = 40 μm; I = 5 μm; J–K = 10 μm.
Basionym: Coniothyrium clematidis-rectae Petr., Feddes Repert Spec. Nov. Regni Veg. Beih. 42: 356. 1927.
≡ Phoma clematidis-rectae (Petr.) Aveskamp et al., Stud. Mycol. 65: 25. 2010.
Description (Aveskamp et al. 2010).
Specimen examined: The Netherlands, Boskoop, from Clematis sp., deposited in CBS Nov.1963, collected by G.H. Boerema, CBS H-20275, culture CBS 507.63 = PD 07/03486747 = MUCL 9574.
Note: Aveskamp et al. (2010) recombined Coniothyrium clematidis-rectae into Phoma, and we propose a new combination for this species here, Calophoma clematidis-rectae.
Calophoma complanata (Tode) Q. Chen & L. Cai, comb. nov. MycoBank MB814138.
Basionym: Sphaeria complanata Tode, Fung. Mecklenb. Sel. (Lüneburg) 2: 21. 1791.
≡ Phoma complanata (Tode) Desm., Ann. Sci. Nat. Bot. 16: 299. 1851.
Description (Boerema & de Gruyter 1998).
Specimens examined: The Netherlands, Tilburg, from a stem of Heracleum sphondylium, Nov. 1997, H.A. van der Aa, CBS H-16194, culture CBS 100311; from a stem of Angelica sylvestris, deposited in CBS Jun. 1992 J. de Gruyter, CBS 268.92 = PD 75/3.
Calophoma glaucii (Brunaud) Q. Chen & L. Cai, comb. nov. MycoBank MB814139.
Basionym: Phoma glaucii Brunaud, “glauci”, Ann. Soc. Sci. Nat. La Rochelle 1892: 97. 1892.
Description (Boerema et al. 1997).
Specimens examined: The Netherlands, near Lisse, from Dicentra sp., deposited in CBS Jan 1996, CBS 112.96 = PD 79/765; Wageningen, from a leaf of Chelidonium majus, deposited in CBS Jan 1996, CBS 114.96 = PD 94/888.
Calophoma sp. 1
Specimen examined: Switzerland, Gabi am Simplon, from Vincetoxicum officinale, deposited in CBS May 1955, E. Müller, CBS 186.55.
Notes: This isolate resided in a single lineage, which is phylogenetically distinct from other species, and was originally identified as “Didymella vincetoxici”. Since the type of D. vincetoxici was unavailable for study, we are unsure if CBS 186.55 represents a new species or is conspecific to D. vincetoxici.
Calophoma vodakii (E. Müll.) Q. Chen & L. Cai, comb. nov. MycoBank MB814140.
Basionym: Didymella vodakii E. Müll., Sydowia 7: 332. 1953.
Specimen examined: Switzerland, Kt. Wallis, Brig, from Hepatica triloba, deposited in CBS Jun. 1953, E. Müller (holotype ZT Myc 54939, culture ex-holotype CBS 173.53).
Notes: The specimen information of CBS 173.53, such as host, locality, collection date and collector are the same as those given in the original description of Didymella vodakii when it was published as a novel species (Müller 1953). It is therefore concluded that isolate CBS 173.53 represents the ex-holotype culture of D. vodakii.
Clade 12: Phoma
Phoma Sacc., Michelia 2: 4. 1880. emend. Q. Chen & L. Cai.
= Atradidymella M.L. Davey & Currah, Amer. J. Bot. 96: 1283. 2009.
Conidiomata pycnidial, sub-globose to elongated, superficial on or immersed into the agar, solitary or confluent, ostiolate. Pycnidial wall pseudoparenchymatous, 3–7-layered, outer wall pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform. Conidia oblong to cylindrical, ellipsoidal, sometimes fusiform, hyaline, smooth- and thin-walled, aseptate, guttulate. Ascomata pseudothecial, erumpent, subglobose to pyriform, solitary, setose around ostiole, with a short neck. Hamathecium pseudoparenchymatous in young ascomata, persisting as septate filamentous remnants in mature ascomata. Asci cylindrical to clavate, 8-spored, bitunicate. Ascospores fusiform, brown, 1-septate, smooth, slightly constricted at the septum, biseriate or triseriate (Davey & Currah, 2009).
Type species: Phoma herbarum Westend., Bull. Acad. Roy. Sci. Belgique, Cl. Sci. 19: 118. 1852.
Notes: As the sexual morph (Atradidymella) of Phoma herbarum, type species of the genus Phoma, was linked here, the generic features were emended and supplemented with the characters of sexual morph.
Phoma herbarum Westend., Bull. Acad. Roy. Sci. Belgique, Cl. Sci. 19: 118. 1852. emend. Q. Chen & L. Cai. Fig. 29, Fig. 30.
Fig. 29.
Phoma herbarum (BR 5020153305384). A. Type collection packet. B. Pycnidia on host substrate. C. Section of pycnidia. D. Pycnidium with conidia. E. Conidia. F. Section of pycnidial wall. Scale bars: C = 20 μm; D, F = 10 μm; E = 5 μm.
Fig. 30.
Phoma herbarum (CBS 615.75). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidia. I. Section of pycnidial wall. J. Conidiogenous cells. K. Conidia. Scale bars: G = 100 μm; H = 50 μm; I, K = 10 μm; J = 5 μm.
= Atradidymella muscivora M.L. Davey & Currah, Amer. J. Bot. 96: 1283. 2009.
= Phoma muscivora M.L. Davey & Currah, Amer. J. Bot. 96: 1283. 2009.
= Phoma cruris-hominis Punith., Nova Hedwigia 31: 135. 1979.
Description from isotype (BR 5020153305384): Leaf spots elliptical to circular, black. Conidiomata pycnidial, solitary, subglobose, 130–220 × 55–170 μm. Ostiole single. Pycnidial wall pseudoparenchymatous, 3–5-layered, 10–30 μm thick, composed of isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, doliiform. Conidia oblong to ellipsoidal, smooth- and thin-walled, hyaline, sometimes 1-septate, 5–7.5 × 2.5–3.5 μm.
Description of sexual morph: Ascomata pseudothecial, solitary, erumpent from underlying host cell, dark brown, uniloculate, subglobose to ellipsoidal or pyriform, (75–115 × 58–95 μm) with short concolourous, occasionally septate setae around ostiole. Peridium wall pseudoparenchymatous, 3-layered, 10 μm thick. Hamathecium pseudoparenchymatous in young ascomata, persisting as septate filamentous remnants (1–3 μm) in mature ascomata. Asci cylindrical to clavate, 8-spored, bitunicate, 6–13 μm, grouped in a small fascicle of 10–20 at base of pseudothecium. Ascospores broadly fusiform, golden brown to dark brown, smooth, straight to allantoid, 1-septate, 14–20 × 4–5.5 μm, slightly constricted at septum, the upper cell sometimes shorter and broader than the lower, biseriate or triseriate (from Davey & Currah 2009).
Description from culture (CBS 615.75): Conidiomata pycnidial, solitary, globose to subglobose, glabrous, semi-immersed or immersed, 130–265 × 120–240 μm. Ostioles 1–2, slightly papillate. Pycnidial wall pseudoparenchymatous, 3–5-layered, 14–22 μm thick, composed of isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, dolliform, 5–6.5 × 4–5.5 μm. Conidia ellipsoidal to ovoid, smooth- and thin-walled, aseptate, 4.5–6 × 2–3 μm, with 1–2 guttules. Conidial matrix white.
Culture characteristics: Colonies on OA, 30–35 mm diam after 7 d, margin regular, abundant pycnidia in concentric rings, giving a salmon colour to the colonies, pale brown near the centre; reverse pale greenish olivaceous in outer ring, towards the centre of the colony via buff to olivaceous. Colonies on MEA 35–40 mm diam after 7 d, margin irregular, flattened, white to greenish olivaceous; reverse greenish olivaceous, white near the margin. Colonies on PDA, 35–40 mm diam after 7 d, margin regular, felty, white near the margin, darkening towards the centre, via hazel to grey-brown; reverse hazel to brown. NaOH test negative.
Specimens examined: Belgium, Vlaams-Brabant, Tervuren, from a stem of Solanum lycopersicum (isotype of Phoma herbarum BR 5020153305384). Switzerland, Kt. Graubünden, from Achillea millefolium, deposited in CBS Mar. 1951, E. Müller, CBS 304.51. The Netherlands, Emmeloord, from the stem of Rosa multiflora cv. Cathayensis, deposited in CBS Dec. 1975, G.H. Boerema, CBS 615.75 = PD 73/665 = IMI 199779; Naaldwijk, from a stem base of Nerium sp., deposited in CBS Sep. 1991, J. de Gruyter, CBS 502.91 = PD 82/276. UK, from a leg of woman, Apr. 1977, Y.M. Clayton, holotype of “Phoma cruris-hominis” IMI 213845, culture ex-holotype of “Phoma cruris-hominis” CBS 377.92 = IMI 213845; near Dumfries, from die-back of Picea excelsa, deposited in CBS Oct. 1937, T.R. Peace, CBS 274.37. USA, Michigan, Wolf Lake, from dried gametophytes of Funaria hygrometrica, 2008, M.L. Davey, culture ex-holotype of “Atradidymella muscivora” UAMH 10909 = CBS 127589; from gametophytes of Polytrichum juniperinum growing on the base of an uprooted Picea mariana tree, 2008, M.L. Davey, culture ex-holotype of “Atradidymella muscivora” UAMH 10909 = CBS 127589 = Pj8-D.
Notes: Atradidymella muscivora was introduced as the sexual morph of a new species “Phoma muscivora”, which is morphologically similar to P. herbarum (Davey & Currah 2009). However, based on the description of P. muscivora, there are no significant morphological differences from P. herbarum, thus we conclude that they are conspecific. Phoma muscivora and At. muscivora are treated as synonyms of Phoma herbarum here, which by default makes it the first report of a sexual morph of the type species of the genus Phoma. The culture ex-holotype of Phoma cruris-hominis (CBS 377.92), isolated from a lesion on the leg of a woman in London (Punithalingam 1979b), was shown to be genetically identical to the ex-type species of P. herbarum. Two isolates deposited as Phoma acuum (CBS 274.37) and Leptosphaeria millefolii (CBS 304.51) clustered with P. herbarum in the phylogenetic tree, with only two bp differences in tub2 from the authentic strains of P. herbarum (CBS 502.91, CBS 615.75). Due to the similarity based on their DNA sequences, we re-identified these isolates as P. herbarum.
Phoma neerlandica Q. Chen & L. Cai, sp. nov. MycoBank MB814141. Fig. 28.
Fig. 28.
Phoma neerlandica (CBS 134.96). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H–I. Pycnidia. J. Section of pycnidial wall. K. Conidia. Scale bars: G = 200 μm; H–I = 50 μm; J–K = 10 μm.
Etymology: Epithet derived from the country of origin, the Netherlands.
Description from ex-holotype culture (CBS 134.96): Conidiomata pycnidial, solitary or aggregated, globose to subglobose, glabrous, produced on the agar surface or immersed, 95–350(–430) × 80–300 μm. Ostioles 1–2(–3), papillate. Pycnidial wall pseudoparenchymatous, 5–7-layered, 20–35 μm thick, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, 4–7 × 3–6.5 μm. Conidia ellipsoidal to cylindrical, sometimes fusiform, smooth- and thin-walled, aseptate, occasionally 1-septate, 4.5–8.5(–12) × 2–3.5 μm, with 1–6 minute guttules. Conidial matrix rosy-buff to pale salmon.
Culture characteristics: Colonies on OA, 40–45 mm diam after 7 d, margin regular, flattened, olivaceous to grey, abundant pycnidia produced in concentric rings; reverse alternate olivaceous and salmon concentric rings. Colonies on MEA 40–45 mm diam after 7 d, margin regular, wooly, dull green; reverse concolourous. Colonies on PDA, 35–40 mm diam after 7 d, margin regular, wooly, pale olivaceous to hazel, white near the margin, black pycnidia produced in some sectors; reverse pale brown olivaceous with salmon patches, white near the margin. NaOH test negative.
Specimen examined: The Netherlands, Emmeloord, from a leaf of Delphinium sp., deposited in CBS Feb. 1996 (holotype HMAS 246691, culture ex-holotype CBS 134.96 = PD 84/676).
Notes: Isolate CBS 134.96 was initially identified as “Phoma delphinii”. However, we have been unable to trace the type material. In the original description, conidial dimensions of Phoma delphinii were given as 3–4 × 2 μm, while Boerema et al. (1997) indicated that conidia of CBS 134.96 were notably variable in shape and size, mostly 4–15 × 1.5–5 μm, and including some 1-septate large conidia (15.5–22 × 4–5 μm). In our observations, the conidial size of CBS 134.96 agrees with that reported by Boerema et al. (1997). Since the conidial dimensions of CBS 134.96 and Phoma delphinii differ markedly, we prefer to describe this isolate as a new species, Phoma neerlandica.
Phoma neerlandica is phylogenetically most closely related to the type species of Phoma, P. herbarum. They can be morphologically distinguished from each other by the longer, uniseptate conidia in P. neerlandica [4.5–8.5(–12) × 2–3.5 μm] compared to the aseptate conidia in P. herbarum (4.5–6 × 2–3 μm).
Clade 13: Leptosphaerulina
Leptosphaerulina McAlpine, Fungus Diseases of stone-fruit trees in Australia: 103. 1902.
Ascomata pseudothecial, immersed or erumpent, obpyriform to subglobose, ostiolate. Asci clavate to ovoid, or obovoid, saccate, oblong, bitunicate, 8-spored. Ascospores muriform, oblong, ellipsoidal to obovoid, subfusoid, hyaline to brown, 1(–6)-septate, slightly constricted at the septum, biseriate or triseriate (Saccardo, 1905, Inderbitzin et al., 2000, Abler, 2003, Crous et al., 2011).
Type species: Leptosphaerulina australis McAlpine, Fungus Diseases of stone-fruit trees in Australia: 103. 1902.
Notes: The genus Leptosphaerulina was introduced to accommodate the type species L. australis (McAlpine 1902), which was isolated from Prunus armeniaca (Saccardo 1905). The genus currently comprises about 25 species (McAlpine, 1902, Graham and Luttrell, 1961, Irwin and Davis, 1985, Roux, 1986, Inderbitzin et al., 2000). Leptosphaerulina was first accommodated in the Pleosporaceae (Inderbitzin et al., 2000, Kodsueb et al., 2006), but later found to be related to Didymella (Kodsueb et al. 2006). Our analysis showed that Leptosphaerulina grouped in a distinct clade in the Didymellaceae, but that it is distant from Didymella.
Leptosphaerulina americana (Ellis & Everh.) J.H. Graham & Luttr., Phytopathology 51: 686. 1961.
Basionym: Pleospora americana Ellis & Everh., N. Amer. Pyren. (Newfield): 336. 1892.
Specimen examined: USA, Georgia, from Trifolium pratense, deposited in CBS May 1955, E.S. Luttrell, CBS 213.55.
Leptosphaerulina arachidicola W.Y. Yen et al., J. Agric. Forest. 10: 167. 1956.
Specimen examined: China, Taiwan, from a leaf of Arachis hypogaea, deposited in CBS May 1959, K.T. Huang, CBS 275.59 = ATCC 13446.
Leptosphaerulina australis McAlpine, Fungus Diseases of stone-fruit trees in Australia: 103. 1902.
On synthetic nutrient-poor agar: Ascomata pseudothecial, solitary to aggregated in clusters, brown, superficial on agar medium, obpyriform to subglobose, 100–150 × 150–200 μm; ostiole central, up to 30 μm diam; outer wall covered with short, brown hyphal setae, 5–15 × 3–5 μm, with obtuse ends. Asci 100–120 × 35–45 μm, 8-spored, hyaline, obovoid, bitunicate with strongly developed apical chamber, 5–7 × 2–3 μm. Ascospores multiseriate in asci, hyaline, smooth, with mucoid sheath, 4 transverse septa, and 2–3 vertical, and 1–2 oblique septa, constricted at second vertical septum from apex, ellipsoidal to obovoid, tapering from middle of upper part of ascospore (widest point) to an acutely rounded apex, base obtusely rounded; hamathecial tissue dissolving among asci, and pseudoparaphyses not observed, (32–)33–27(–40) × (12–)13–14(–15) μm.
Culture characteristics: Colonies on OA, 20–25 mm diam after 7 d, lobate margins, dirty white near the centre, olivaceous grey to iron-grey near the margin. Colonies on MEA, 20–25 mm diam after 7 d, lobate margins, dirty white near the centre, sienna near the margin; reverse sienna. Colonies on PDA, 20–25 mm diam after 7 d, lobate margins, dirty white near the centre, olivaceous grey near the margin; reverse iron-grey (from Crous et al. 2011).
Specimen examined: Kenya, on leaves of Protea sp., 1999, culture CBS 116307 = CPC 3712. Indonesia, Lampung, from Eugenia aromatica, Dec. 1982, H. Vermeulen, CBS 317.83.
Notes: Leptosphaerulina australis was originally isolated from Prunus armeniaca in Australia (Saccardo 1905). The culture collected from Kenya is the first record from Proteaceae (Crous et al. 2011).
Leptosphaerulina trifolii (Rostr.) Petr., Sydowia 13: 76. 1959.
Basionym: Sphaerulina trifolii Rostr., Bot. Tidsskr. 22: 265. 1899.
Specimen examined: The Netherlands, from Trifolium sp., deposited in CBS Jul. 1958, CBS 235.58.
Clade 14: Neoascochyta
Neoascochyta Q. Chen & L. Cai, gen. nov. MycoBank MB814064.
Etymology: Morphologically resembling the genus Ascochyta, but phylogenetically distinct.
Conidiomata pycnidial, globose to subglobose, or irregularly shaped, superficial on or immersed into the agar, solitary or confluent, ostiolate, sometimes with a short neck. Pycnidial wall pseudoparenchymatous, 2–7-layered, outer wall pigmented, thick. Conidiogenous cells phialidic, hyaline, smooth, globose to flask-shaped, short obpyriform, or ampulliform to doliiform. Conidia variable in shape, hyaline, smooth- and thin-walled, i.e. fusoid to cylindrical, obclavate-ovoid to ellipsoidal, incidentally slight curved, uniseptate or aseptate, eguttulate or guttulate. Ascomata pseudothecial immersed or erumpent, solitary or confluent, globose to subglobose, ostiolate. Asci cylindrical to subclavate, slightly curved, short pedicellate or sessile, 8-spored, bitunicate. Ascospores cylindrical to ovoid, ellipsoidal, hyaline, 1-septate, symmetrical or asymmetrical, constricted at the septum, biseriate or irregular uniseriate.
Type species: Neoascochyta exitialis (Morini) Q. Chen & L. Cai.
Neoascochyta desmazieri (Cavara) Q. Chen & L. Cai, comb. nov. MycoBank MB814142. Fig. 31.
Fig. 31.
Neoascochyta desmazieri (CBS 297.69). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidia. I. Section of pycnidium. J. Section of pycnidial wall. K–L. Conidiogenous cells. M. Conidia. Scale bars: G = 200 μm; H = 100 μm; I = 20 μm; J, M = 10 μm; K–L = 5 μm.
Basionym: Ascochyta desmazieri Cav., Z. Pflanzenkrankh 3: 21. 1893. (as “desmazieresii”).
Description from ex-neotype culture (CBS 297.69): Conidiomata pycnidial, solitary or sometimes aggregated, globose to subglobose, mostly with some hyphal outgrows, immersed, 115–280 × 95–165(–235) μm. Ostiole single, papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 2–4(–5)-layered, 15–28 μm thick, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 6–8.5 × 7.5–11 μm. Conidia cylindrical, hyaline, smooth- and thin-walled, mostly 1-septate, 8.5–18 × 2.5–4 μm, with 4–10(–13) guttules per cell. Conidial exudates not recorded.
Culture characteristics: Colonies on OA, 20–25 mm diam after 7 d, margin regular, felty, with concentric rings, white, pale greenish olivaceous near the centre; reverse white in outer ring, darkening towards the centre of the colony via pale salmon, buff to hazel. Colonies on MEA 35–40 mm diam after 7 d, margin regular, felty, whitish, grey greenish olivaceous near the centre; reverse white in outer ring, darkening towards the centre of the colony via buff, hazel to olivaceous. Colonies on PDA, 35–40 mm diam after 7 d, margin regular, similar as on MEA. NaOH test negative.
Specimens examined: Austria, Landwirtschaftl, from Poaceae, Mar. 1979, E. Lengauer, CBS H-8993, culture CBS 247.79. Germany, Hohenlieth, from Lolium perenne, deposited in CBS Apr. 1969, U.G. Schlösser (neotype designated here HMAS 246690, MBT202505, culture ex-neotype CBS 297.69). Norway, Oclo, from hay, Feb. 1997, M. Torp, CBS H-8935, culture CBS 758.97.
Notes: Attempts to locate the type specimen of Ascochyta desmazieri were unsuccessful. This species was first published as Septoria graminium var. lolii based on the examination of Pl. crypt. No. 1919 of Desmazières, and later was placed in Ascochyta by Cavara (1893) as As. desmazieri, with conidia measuring 20–30 × 2 μm. Sprague (1944) emended the conidial range of As. desmazieri as 15–20 × 2.8–3.5 μm after examining Desmazières's exsiccatum No. 2169 (Punithalingam 1979a). Punithalingam (1979a) clarified the confusion surrounding As. desmazieri, Septoria sp. and Phoma lolii, and suggested to retain As. desmazieri as a single species. The morphology of the neotype (HMAS 246690; 8.5–18 × 2.5–4 μm), which we designated here, agrees with the description of As. desmazieri by Sprague (1944).
The sole isolate deposited in CBS as “Ascochyta agrostidis” (CBS 758.97) was genetically identical to the culture ex-neotype of Neoascochyta desmazieri (CBS 297.69). Therefore, we reclassify isolate CBS 758.97 as Neoa. desmazieri.
Neoascochyta exitialis (Morini) Q. Chen & L. Cai, comb. nov. MycoBank MB814143. Fig. 32.
Fig. 32.
Neoascochyta exitialis (CBS 389.86). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidia. I. Pycnidial section. J. Section of pycnidial wall. K–L. Conidiogenous cells. M. Conidia. Scale bars: G = 200 μm; H–I = 20 μm; J–M = 10 μm.
Basionym: Sphaerella exitialis Morini, Nuovo Glorn. Bot. Ital. 18: 37. 1886.
≡ Didymella exitialis (Morini) E. Müll., Phytopathol. Z. 19: 407. 1952.
Description from culture (CBS 389.86): Conidiomata pycnidial, solitary, globose to subglobose, mostly with some hyphal outgrows, superficial on or immersed into the agar, 95–150 × 75–120 μm. Ostiole single, papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 3–5-layered, 22–40 μm thick, composed of isodiametric or sometimes irregular cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, 6–8 × 6–9.5 μm. Conidia broadly fusoid to cylindrical, incidentally slightly curved, smooth- and thin-walled, hyaline, uniseptate, 15.5–25 × 4–7 μm, with many minute guttules, ca. 15–30 guttules per cell. Conidial exudates not recorded.
Culture characteristics: Colonies on OA, 20–25 mm diam after 7 d, margin regular, floccose, white, grey olivaceous near the margin; reverse white in outer ring, olivaceous near the centre. Colonies on MEA 35–40 mm diam after 7 d, margin regular, wooly, pale greenish olivaceous, olivaceous near the centre; reverse concolourous. Colonies on PDA, 30–35 mm diam after 7 d, margin regular, wooly, whitish, hazel near the centre; reverse dull green. NaOH test negative.
Specimens examined: Germany, Monheim, from a leaf of Secale cereale, May 1984, M. Hossfeld, CBS 811.84; from a leaf of Hordeum vulgare, deposited in CBS Dec. 1984, CBS H-8939, culture CBS 812.84. Sweden, Uppland, from Allium sp., Sep. 1986, O. Constantinescu, CBS 113693 = UPSC 1929. Switzerland, Utzenstorf, from Triticum aestivum, deposited in CBS Sep. 1986, CBS 389.86 = INIFAT C86 = MW I 1343. The Netherlands, Gelderland, Laren, from Triticum sp. variety Tower, deposited in CBS Mar. 2002, I. de Vires, CBS 110124. Unknown origin, unknown substrate, deposited in CBS Aug. 1940, K. Röder, CBS 118.40.
Notes: Isolate CBS 118.40 was initially identified as “D. arcuata”, CBS 811.84 and CBS 812.84 as “As. avenae”, CBS 110124 as “As. skagwayensis”, and CBS 113693 as “As. allii”. The multi-locus analysis revealed no phylogenetic differences among these isolates. Genetically there was nearly no difference among these strains, except a single bp difference of CBS 113693 in tub2. Here we reclassified all these isolates as Neoascochyta exitialis.
Neoascochyta graminicola (Punith.) Q. Chen & L. Cai, comb. nov. MycoBank MB814144. Fig. 33.
Fig. 33.
Neoascochyta graminicola (CBS 102789). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidium. I–J. Conidiogenous cells. K. Conidia. Scale bars: G = 100 μm; H = 50 μm; I–J = 5 μm; K = 10 μm.
Basionym: Didymella graminicola Punith., Mycol. Pap. 119: 2. 1970.
Description from culture (CBS 102789): Conidiomata pycnidial, solitary, subglobose, glabrous, superficial on or immersed into the agar, 195–325 × 145–270(–300) μm. Ostioles 1–2, slightly papillate. Pycnidial wall pseudoparenchymatous, 3–5-layered, 17–24 μm thick, composed of isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to dolliform, 8.5–10.5 × 6.5–9.5 μm. Conidia cylindrical, smooth- and thin-walled, 1-septate, 12.5–17.5 × 4.5–6.5 μm, with 4–8 guttules. Conidia matrix white.
Culture characteristics: Colonies on OA, 15–20 mm diam after 7 d, margin regular, floccose, white to hazel, pale olivaceous near the centre; reverse pale olivaceous, white near the margin. Colonies on MEA 15–20 mm diam after 7 d, margin crenate, flattened, pale greenish olivaceous; reverse concolourous. Colonies on PDA, 35–40 mm diam after 7 d, margin dendritic, floccose, white to pale greenish olivaceous; reverse olivaceous. NaOH test negative.
Specimens examined: Belgium, Gembloux, from Hordeum vulgare, deposited in CBS Sep. 1979, J. Fraselle, CBS H-9007, culture CBS 586.79. Germany, Kiel-Kitzeberg, Schlosskoppelweg, from seeds of Lolium perenne or L. multiflorum, 1968, U.G. Schlösser (holotype IMI 136404); from seed of Lolium multiflorum, deposited in CBS Apr. 1969, U.G. Schlösser, CBS 301.69; from Triticum aestivum, Apr. 1982, G.M. Hoffmann, CBS H-1614, culture CBS 447.82; Eschweiler, from a leaf of Hordeum vulgare, May 1984, M. Hossfeld, CBS H-9017, culture CBS 815.84; Monheim, from a leaf of Hordeum vulgare, May 1984, M. Hossfeld, CBS H-9016, culture CBS 816.84. New Zealand, Canterbury Province, from a leaf of Lolium perenne, Dec. 1999, S. Ganev, culture CBS 102789.
Notes: According to the original literature (Punithalingam 1969), the holotype of Didymella graminicola was collected from Lolium perenne or L. multiflorum in Germany. The culture CBS 301.69 was previously deposited as “Ascochyta sorghi”, CBS 447.82 as “D. exitialis”, CBS 586.79 as “As. graminea”, CBS 815.84 and CBS 816.84 as “As. hordei var. americana”. In the phylogenetic analysis, these cultures clustered together in a well-supported clade and their sequences of four loci are genetically identical to the authentic culture of Neoascochyta graminicola (CBS 102789). As As. sorghi was reported to be restricted to sorghum (Punithalingam 1979a), isolate CBS 301.69 from Lolium multiflorum was misidentified. Isolate CBS 447.82 clustered distantly from the ex-type of D. exitialis (CBS 389.86). Ascochyta graminea was originally reported from Cynodon dactylon in Italy (Punithalingam 1979a), whereas the isolate CBS 586.79 was from a different host, Hordeum vulgare, which belongs to the same host family as Neoa. graminicola (syn. D. graminicola). According to the original description of As. hordei var. americana, its conidia (15–20 × 4–5(–5.5) μm; Punithalingam 1979a) are hyaline to yellowish brown, wider than those of Neoa. graminicola (hyaline, 14–18(–20) × 3–4 μm; Punithalingam 1969), which suggests that they are two distinct species. Although isolates CBS 815.84 and CBS 816.84 were both isolated from Hordeum vulgare, the same host of As. hordei var. americana, they were phylogenetically identical to Neoa. graminicola, and re-identified as such.
Neoascochyta europaea (Punith.) Q. Chen & L. Cai, comb. et stat. nov. MycoBank MB814145. Fig. 34, Fig. 35.
Fig. 34.
Neoascochyta europaea (IMI 164252). A. Type collection packet. B. Pycnidia. C. Pycnidia on host substrate. D. Conidia. Scale bars: B = 50 μm; C = 200 μm; D = 10 μm.
Fig. 35.
Neoascochyta europaea (CBS 820.84). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidium. I. Pycnidial section. J. Section of pycnidial wall. K. Conidiogenous cells. L. Conidia. Scale bars: G = 200 μm; H = 100 μm; I = 20 μm; J–L = 10 μm.
Basionym: Ascochyta hordei var. europaea Punith., Mycol. Pap. 142: 95. 1979.
Description from holotype (IMI 164252): Leaf spots elliptical to circular, rosy buff with brown border. Pycnidia immersed in leaf surface of Hordeum vulgaris, solitary or confluent, subglobose, 50–290 × 40–250 μm. Ostioles 1(–2) on a short neck. Pycnidial wall pseudoparenchymatous, 2-layered, composed of isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, doliiform. Conidia fusoid to cylindrical, sometimes ellipsoidal, smooth- and thin-walled, hyaline to pale brown, 1-septate, 12.5–19.5 × 3–5 μm, with 2–10 guttules per cell.
Description from ex-epitype culture (CBS 820.84): Pycnidia mostly solitary or sometimes confluent, globose to subglobose, with some hyphal outgrows, produced on the agar surface or immersed, (190–)215–450(–565) × (150–)200–350(–420) μm. Ostioles 1–4 on a short neck. Pycnidial wall pseudoparenchymatous, 3–5-layered, 27–50 μm thick, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 7.5–11.5 × 6–9 μm. Conidia fusoid to cylindrical, incidentally slight curved, smooth- and thin-walled, hyaline to pale buff, 1-septate, 14.5–20.5 × 4–5 μm, with many minute guttules, ca. 10–20 guttules per cell. Conidial exudates not recorded.
Culture characteristics: Colonies on OA, 40–45 mm diam after 7 d, margin regular, floccose, dark grey, pycnidia semi-immersed in concentric rings near the margin, grey olivaceous; reverse concolourous. Colonies on MEA 35–40 mm diam after 7 d, margin regular, wooly, pale greenish, olivaceous near the centre, white near the margin; reverse concolourous. Colonies on PDA, 40–45 mm diam after 7 d, margin regular, floccose, smoke-grey with a pale ring near the margin, black pycnidia produced near the centre and a concentric ring; reverse dull green. NaOH test negative.
Specimens examined: Germany, Eschweiler, from a leaf of Hordeum vulgare, May 1984, M. Hossfeld, CBS H-9024, culture CBS 819.84; from a leaf of Hordeum vulgare, May 1984, M. Hossfeld (epitype designated here CBS H-9025, MBT202506, culture ex-epitype CBS 820.84). UK, from leaves of Hordeum vulare., Feb. 1972, T. Fozzard (holotype IMI 164252).
Notes: Conidia from the holotype are mostly 1-septate, 12.5–19.5 × 3–5 μm, hyaline to pale brown, which agrees well with the original description with conidia 14–16 × 3–4.5(–5) μm. The morphology of specimens selected in this study agrees with the type as well, and thus CBS H-9025 is chosen as epitype, with the living culture ex-epitype CBS 820.84. Neoascochyta europaea mainly occurs in Europe and especially in Great Britain on barley, rye and wheat (Punithalingam 1979a).
Neoascochyta paspali (P.R. Johnst.) Q. Chen & L. Cai, comb. nov. MycoBank MB814147.
Basionym: Phoma paspali P.R. Johnst., New Zealand J. Bot. 19: 181. 1981.
Description (de Gruyter et al. 1998).
Specimen examined: New Zealand, Auckland, Kaikohe, from a dead leaf of Paspalum dilatatum, Jan. 1979, P.K. Buchanan (isotype CBS H-7623, culture ex-isotype CBS 560.81 = PD 92/1569).
Neoascochyta sp. 1
Specimen examined: Argentina, Tandil, from a leaf of Triticum aestivum, Oct. 2002, CBS 112524.
Notes: CBS 112524 was initially identified as “Ascochyta hordei” and grouped in the same clade with CBS 516.81, another misidentified culture, in the phylogenetic tree. Since the type material of As. hordei could not be obtained, the identity of CBS 112524 remains uncertain, and requires further study.
Neoascochyta sp. 2
Specimen examined: Italy, Cenreo Richerche sul riso, Mortara, from Oryza sativa, Aug. 1981, CBS H-11964, culture CBS 516.81.
Notes: This isolate was incorrectly identified as “Didymella graminicola”, and is phylogenetically distant from the authentic culture of this species (CBS 102789). This is a potential new species, and will be described elsewhere.
Neoascochyta sp. 3
Specimen examined: Norway, Oslo, from hay, deposited in CBS Apr. 1997, M. Torp, CBS H-9005, culture CBS 689.97.
Notes: Isolate CBS 689.97 was deposited as “Ascochyta festucae” and represents a single branch, which was distant from other species in the tree. Since the type of As. festucae is unavailable, we could not confirm if CBS 689.97 represents a new species, or is conspecific to As. festucae.
Neoascochyta sp. 4
Specimen examined: South Africa, Heilbron, from Triticum aestivum, deposited in CBS Sep. 1974, W.J. Jooste, CBS H-9008, culture CBS 544.74.
Notes: Isolate CBS 544.74, originally identified as “Ascochyta hordei”, clustered sister to Neoascochyta sp. 5. This culture was collected from Triticum aestivum, while the type of As. hordei was from Hordeum sativum (Punithalingam 1979a). Since the type material of As. hordei was unavailable, the identity of this isolate remains uncertain.
Neoascochyta sp. 5
Specimen examined: South Africa, Potchefstroom, from straw, deposited in CBS Oct. 1972, M.C. Papendorf, CBS H-8974, culture CBS 876.72.
Notes: Isolate CBS 876.72, originally identified as “Ascochyta brachypodii”, clustered sister to Neoascochyta sp. 4, which is distinct from other species in the phylogenetic tree. Since the type material of As. brachypodii was unavailable, the identity of this isolate remains uncertain.
Clade 15: Xenodidymella
Xenodidymella Q. Chen & L. Cai, gen. nov. MycoBank MB814065.
Etymology: Xeno = ξένος in Greek, alien, distinct; didymella = didymella-like conidia.
Conidiomata pycnidial, globose to subglobose, on agar surface or immersed, solitary or confluent, ostiolate. Pycnidial wall pseudoparenchymatous, 3–9-layered, outer wall pigmented. Conidiogenous cells phialidic, hyaline, smooth, globose to flask-shaped, ampulliform. Conidia variable in shape, hyaline, smooth- and thin-walled, i.e. ellipsoidal to allantoid, subcylindrical, oblong, pyriform, usually aseptate or occasionally 1-septate in vivo, mostly guttulate. Chlamydospores occasionally present, brown, intercalary, in spiral chains, unicellular, globose to subglobose. Ascomata pseudothecial, immersed or erumpent, globose to subglobose, solitary or confluent, ostiolate or poroid. Asci cylindrical to subclavate, 8-spored, bitunicate. Ascospores obovoid to oblong, clavate, ellipsoidal, sometimes slightly curved, hyaline, 1-septate, symmetrical or asymmetrical, constricted at the septum, biseriate.
Type species: Xenodidymella applanata (Niessl) Q. Chen & L. Cai.
Xenodidymella applanata (Niessl) Q. Chen & L. Cai, comb. nov. MycoBank MB814148. Fig. 36, Fig. 37.
Fig. 36.
Xenodidymella applanata (M 0275818). A. Type collection packet. B. Pseudothecia on host substrate. C. Pseudothecium. D. Section of pseudothecial wall. E. Asci. F. Ascospores. Scale bars: B = 100 μm; C, D = 50 μm; E–F = 5 μm.
Fig. 37.
Xenodidymella applanata (CBS 195.36). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidium. I–J. Conidiogenous cells. K–L. Conidia. Scale bars: G = 100 μm; H = 50 μm; I–L = 5 μm.
Basionym: Didymosphaeria applanata Niessl, Oesterr. Bot. Z. 25: 129. 1875.
≡ Didymella applanata (Niessl) Sacc., Syll. Fung. 1: 546. 1882.
= Phyllosticta argillacea Bres., Hedwigia 33: 206. 1894.
≡ Phoma argillacea (Bres.) Aa & Boerema, Persoonia 18: 17. 2002.
Description from holotype (M 0275818): Leaf spots circular, brown to black. Pseudothecia on leaf surface, solitary, globose to subglobose, 225–265 × 210–260 μm. Ostioles single. Asci cylindrical, 50–60 × 10.5–14.5 μm, 8-spored, biseriate. Pseudothecia wall pseudoparenchymatous, composed of isodiametric cells, 5–7-layered, 30–41 μm thick. Ascospores broadly fusiform, 11.5–15.5 × (4–)5.5–7.5 μm, smooth, straight or slightly curved, hyaline, 1-septate, slightly constricted at the septum, upper cells usually broader than the lower cells.
Description from ex-epitype culture (CBS 195.36): Conidiomata pycnidial, solitary, globose to subglobose, glabrous, produced on the agar surface or semi-immersed, 85–175 × 60–145 μm. Ostiole single, slightly papillate. Pycnidial wall pseudoparenchymatous, 5–7-layered, 20–25 μm thick, composed of isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to dolliform, 5.5–8 × 4.5–6 μm. Conidia ellipsoidal to ovoid, smooth- and thin-walled, aseptate, 5–7 × 2–3 μm, with several guttules. Conidia matrix white.
Culture characteristics: Colonies on OA, 20–25 mm diam after 7 d, margin regular, crenate, floccose, white, pale olivaceous near the centre; reverse buff to pale brown. Colonies on MEA, 15–20 mm diam after 7 d, margin regular, floccose, white, pale greenish olivaceous near the margin; reverse buff. Colonies on PDA, 15–20 mm diam after 7 d, margin regular, floccose, white; reverse pale brown olivaceous. Application of NaOH results in a pale reddish discolouration of the agar.
Specimens examined: Germany, near Köningstein, from leaves of Rubus idaeus, Aug. 1893, W. Krieger (holotype of “Phyllosticta argillacea” Fungi saxon. 1187, S). Sweden, Umeå, Västerhiske, from a shoot of Rubus idaeus, Jan. 2000, S. Hellqvist, CBS 115577; from Rubus arcticus subsp. × stellarcticus, Jan. 2000, S. Hellqvist, CBS 115578. The Netherlands, Baarn, from Rubus idaeus cv. ‘Rode Radbout’, deposited in CBS Apr. 1963, J.A. von Arx, CBS H-11941, culture CBS 205.63; Breda, from stem of Rubus idaeus, 1936, Rietsema (epitype of Didymosphaeria applanata designated here HMAS 246688, MBT202507, culture ex-epitype CBS 195.36). UK, Shrewsbury, from Rubus idaeus, 1875, Plowright (holotype of Didymosphaeria applanata M 0275818).
Notes: A phoma-like asexual morph of Didymella applanata has been described by Corbaz (1957) and Corlett (1981), and later identified by de Gruyter et al. (2002) as Phoma argillacea. The original description of the asexual morph reported a conidial size of 6–9 × 2–3 μm, which agrees with the epitype (5–7 × 2–2.8 μm) designated in the present study. Xenodidymella applanata is a pathogen of raspberry (Rubus idaeus) that was in the past commonly recorded as a sexual morph on this host. Furthermore, it also occasionally occurred on other species of Rubus (de Gruyter et al. 2002). Strain CBS 115578 showed certain distance from the other three representative strains of Xenodidymella applanata, with two bp differences in four sequenced loci.
Xenodidymella asphodeli (E. Müll.) Q. Chen & L. Cai, comb. nov. MycoBank MB814149. Fig. 38, Fig. 39.
Fig. 38.
Xenodidymella asphodeli (ZT Myc 56445). A. Type collection packet. B. Pycnidia on host substrate. C. Pycnidium. D. Section of pycnidial wall. E. Conidia. Scale bars: C = 20 μm; D–E = 10 μm.
Fig. 39.
Xenodidymella asphodeli (CBS 375.62). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H–I. Pycnidia. J. Pycnidial section. K. Section of pycnidial wall. L. Chlamydospores in chains. M–N. Conidiogenous cells. O. Conidia. Scale bars: G = 200 μm; H–J, L = 50 μm; K = 20 μm; M–N = 5 μm; O = 10 μm.
Basionym: Didymella asphodeli E. Müll., Sydowia 12: 245. 1958 (1959).
= Ascospora solieri Mont., Ann. Sci. Nat. Bot., sér. 3, 11: 48. 1849.
≡ Phoma solieri (Mont.) Sacc., Michelia 1: 525. 1879.
Description from holotype (ZT Myc 56445): Leaf spots elliptical, pale brown to black. Pycnidia abundant, on leaf surface of Asphodelus albus, solitary, globose, (85–)180–280(–360) × (70–)150–320 μm. Ostiole single, distinctly papillate. Pycnidial wall pseudoparenchymatous, 3–4-layered, 15–30 μm thick, composed of oblong to isodiametric cells, outer wall 2–3-layered, brown. Conidiogenous cells phialidic, hyaline, smooth, ampulliform. Conidia broad cylindrical, smooth- and thin-walled, aseptate, 16.5–26 × 5.5–8 μm, guttulate.
Description from ex-epitype culture (CBS 375.62): Conidiomata pycnidial, solitary, globose, with some hyphal outgrows, superficila on or immersed into the agar, 160–385(–445) × 135–350(–400) μm. Ostiole single, distinct papillate. Pycnidial wall pseudoparenchymatous, 3–7-layered, 30–65 μm thick, composed of oblong to isodiametric cells, outer wall two-layered, brown. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, 8.5–12 × 6.5–11 μm. Conidia variable in shape and size, broadly obovoid, pyriform to cylindrical, smooth- and thin-walled, aseptate, 14–27(–34) × 4.5–11(–15) μm, with 20–40 large guttules. Conidial matrix pale pink. Chlamydospores unicellular, produced in and on the agar, brown, intercalary, in spiral chains, globose to subglobose, 14.5–41.5 × 10–37 μm, thick-walled.
Culture characteristics: Colonies on OA, 40–45 mm diam after 7 d, margin regular, flattened, olivaceous, black pycnidia produced in concentric rings; reverse iron-grey to olivaceous in concentric rings. Colonies on MEA 35–40 mm diam after 7 d, margin regular, floccose, greenish olivaceous to dark leaden-black, white tufts near the centre; reverse greenish olivaceous to dark leaden-black, hazel near the centre. Colonies on PDA, 40–45 mm diam after 7 d, margin regular, floccose, white to iron-black; reverse hazel to iron-black in concentric rings. NaOH test negative.
Specimens examined: France, Aples Maritimes, Tende, from Asphodelus albus, deposited in CBS Jan. 1962, E. Müller (epitype of Didymella asphodeli designated here HMAS 246689, MBT202508, culture ex-epitype CBS 375.62). Italy, Sardinie, from a wilting leaf of Asphodelus ramosus, May 1974, W. Gams & J. Stalpers, CBS 499.72. Switzerland, Monte Generoso, Bella Vista, from dead stems of Asphodelus albus, May 1956, Kt. Tessin (holotype of Didymella asphodeli ZT Myc 56445).
Notes: When Didymella asphodeli was introduced, a description of a sexual morph was provided (Müller 1958). However, in the examination of the holotype, we only observed the asexual morph with large conidia, 16.5–26 × 5.5–8 μm, which agrees with the conidial morphology of the epitype designated here, 14–27(–34) × 4.5–11(–15) μm. Müller (1958) also reported a connection between D. asphodeli and a pycnidial fungus which was identified as Phyllostictina solieri (currently Phoma solieri). However, this sexual-asexual link requires molecular verification. The two isolates (CBS 375.62 and CBS 499.72) showed certain distance in phylogeny, and further study is needed to confirm if the two strains represent different species.
Xenodidymella catariae (Cooke & Ellis) Q. Chen & L. Cai, comb. nov. MycoBank MB814150.
Basionym: Sphaeria catariae Cooke & Ellis, Grevillea 5: 95. 1877.
≡ Didymella catariae (Cooke & Ellis) Sacc., Syll. Fung. (Abellini) 1: 557. 1882.
= Ascochyta nepeticola Melnik, Novosti Sist. Nizsh. Rast. 5: 178. 1968.
≡ Phoma nepeticola (Melnik) Dorenb. & Gruyter, Persoonia 18: 18. 2002.
Description (de Gruyter et al. 2002).
Specimen examined: The Netherlands, from the stem of Nepeta catenaria, deposited in CBS Mar. 2000, CBS 102635 = PD 77/1131.
Notes: This species was first reported from Nepeta catenaria in New Jersey, with ascospores described as biseriate, ellipsoidal, uniseptate, 20 × 8 μm (Cooke & Ellis 1877). The asexual and the sexual morphs were reported from the same host, with conidia (4–)5–7(–11.5) × 2.5–5 μm in vitro, and 8–15(–17) × (2.5–)3–(4.5–)5 μm in vivo (de Gruyter et al. 2002).
Xenodidymella humicola (J.C. Gilman & E.V. Abbott) Q. Chen & L. Cai, comb. nov. MycoBank MB814151.
Basionym: Phoma humicola J.C. Gilman & E.V. Abbott, Iowa State Coll. J. Sci. 1: 266. 1927.
Description (de Gruyter et al. 1998).
Specimen examined: USA, Nevada, Death Valley, from a dead leaf of Franseria sp., deposited in CBS Apr. 1985, G.H. Boerema, CBS H-16390, culture CBS 220.85 = PD 71/1030.
Clade 16: Neodidymelliopsis
Neodidymelliopsis Q. Chen & L. Cai, gen. nov. MycoBank MB814066.
Etymology: Neo = νέο in Greek, new; in reference to the morphologically similarity with the genus Didymella.
Conidiomata pycnidial, globose to subglobose, ellipsoidal, later irregular, superficial on or immersed into the agar, solitary or confluent, ostiolate, or with an elongated neck. Pycnidial wall pseudoparenchymatous, 2–7-layered, outer wall pigmented. Conidiogenous cells phialidic, hyaline, smooth, flask-shaped, ampulliform to short cylindrical. Conidia variable in shape, smooth- and thin-walled, i.e. ovoid to ellipsoidal, cylindrical, allantoid, hyaline to pale brown, or pale yellowish, usually aseptate or occasionally 1-septate in vivo, mostly guttulate. Chlamydospores observed in some species, intercalary or terminal, globose to oval, single or in chains, brown, smooth, sometimes dictyochlamydospores. Ascomata pseudothecial, immersed or erumpent, subglobose to pyriform, solitary or confluent, ostiolate. Asci cylindrical to clavate, sessile or stipitate, 8-spored, bitunicate. Pseudoparaphyses filamentous, 0(–3)-septate. Ascospores subovoid to oblong, ellipsoidal, hyaline, smooth, 1(–3)-septate, symmetrical or asymmetrical, constricted at the septum, bi- to triseriate.
Type species: Neodidymelliopsis cannabis (G. Winter) Q. Chen & L. Cai.
Neodidymelliopsis cannabis (G. Winter) Q. Chen & L. Cai, comb. nov. MycoBank MB814152.
Basionym: Sphaerella cannabis G. Winter, Hedwigia 11: 145. 1872.
≡ Didymella cannabis (G. Winter) Arx, Beitr. Kryptogamenfl. Schweiz 11: 365. 1962.
= Depazea cannabis L.A. Kirchn., Lotos 6: 183. 1856.
≡ Phoma cannabis (L.A. Kirchn.) McPartl., Mycologia 86: 871. 1994.
= Didymella urticicola Aa & Boerema, Trans. Brit. Mycol. Soc. 67: 303. 1976.
= Phoma urticicola Aa & Boerema, Trans. Brit. Mycol. Soc. 67: 303. 1976.
Description and illustrations (McPartland 1994).
Specimens examined: The Netherlands, Baarn, from a leaf of Urtica dioica, Dec. 1967, H.A. van der Aa, CBS H-11956, culture CBS 591.67; Wageningen, from a dead stem tip of Urtica dioica, Mar. 1973, G.H. Boerema (holotype of “Didymella urticicola” CBS H-11971, culture ex-holotype CBS 121.75 = ATCC 32164 = IHEM 3403 = IMI 194767 = PD 73/584); Zeist, from packing material, Nov. 1976, G.A. Harrewijn, CBS H-11959, culture CBS 629.76. Unknown origin, from Cannabis sativa, deposited in CBS Oct. 1937, K. Röder, CBS 234.37.
Notes: Cannabis is the only known host of Neod. cannabis, and records of this species are mainly from countries in Eurasia and North America (McPartland 1994). Initially isolates CBS 121.75 and CBS 591.67 were respectively identified as “Didymella urticicola” and “D. eupyrena”. Sequences of all four loci were identical to that of the authentic cultures of Neod. cannabis (CBS 629.76 and CBS 234.37). Furthermore, morphologically there were no significant differences between D. urticicola [conidia (3–)4–6.5(–8.5) × (1.5–)2–3(–3.5) μm; Boerema 1976] and D. cannabis (conidia 3–8 × 2–3 μm; McPartland 1994). We re-identified CBS 121.75 and CBS 591.67 as Neod. cannabis, and treated Didymella urticicola and its asexual morph Phoma urticicola as synonyms of Neod. cannabis. This new combination was proposed based on the sexual morph of the taxon, and the sexual-asexual connection should be further confirmed. A neotype of the asexual morph of Neod. cannabis was designated by McPartland (1994), which was from Germany and deposited in BPI. The asexual stage of Neod. cannabis often produces septate conidia in vivo, which was considered as a “pseudo-ascochyta” form. Although the oldest epithet for this species is that of Depazea cannabis L.A. Kirchn. 1856, we have been unable to confirm this synonymy.
Neodidymelliopsis polemonii (Cooke) Q. Chen & L. Cai, comb. nov. MycoBank MB814153. Fig. 40, Fig. 41.
Fig. 40.
Neodidymelliopsis polemonii (K 197453). A, D. Type collection packet. B. Pycnidia on host substrate. C. Pycnidium. E. Conidiogenous cells. F. Section of pycnidial wall. G. Section of pycnidium. H. Conidia. Scale bars: B = 200 μm; C = 50 μm; E = 2.5 μm; F–G = 10 μm; H = 5 μm.
Fig. 41.
Neodidymelliopsis polemonii (CBS 109181). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidia. I. Section of pycnidium. J. Conidiogenous cells. K. Conidia. Scale bars: G = 200 μm; H = 100 μm; I = 20 μm; J–K = 10 μm.
Basionym: Phoma polemonii Cooke, Grevillea 13: 94. 1885.
Description from isotype (K 197453): Caulicolous, associated with stem lesions. Conidiomata pycnidial, ellipsoidal to subglobose, on the surface of stems, 148–388 × 120–287 μm. Ostiole single, papillate. Pycnidial wall pseudoparenchymatous, 3–5-layered, 14.5–30.5 μm thick, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, 3.5–5.5 × 3.5–5 μm. Conidia ellipsoidal to cylindrical, thin-walled, smooth, hyaline, 4.5–7 × 2–3 μm, eguttulate.
Description from ex-epitype culture (CBS 109181): Conidiomata pycnidial, solitary or confluent, globose to subglobose, or irregular, covered with hyphal outgrowths, semi-immersed or immersed, 100–340 × 75–235 μm. Ostioles 1–3, with wide openings or developing to elongated necks, slightly papillate or non-papillate. Pycnidial wall pseudoparenchymatous, 2–7-layered, 14–19 μm thick, composed of oblong to isodiametric cells, outer layers pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 3.5–7 × 2.5–6 μm. Conidia ellipsoidal to cylindrical, sometimes allantoid, hyaline, smooth- and thin-walled, aseptate, 5.5–7(–7.5) × 1.5–3 μm, with 2(–4) small polar guttules. Conidial matrix whitish.
Culture characteristics: Colonies on OA, 30–35 mm diam after 7 d, margin regular, floccose, white, hazel near the colony margin; reverse buff, pale brown near the margin. Colonies on MEA 25–30 mm diam after 7 d, margin regular, floccose, white to pale olivaceous; reverse olivaceous. Colonies on PDA, 20–25 mm diam after 7 d, margin regular, floccose, white to pale greenish olivaceous; reverse dull green. NaOH test negative.
Specimens examined: The Netherlands, from Polemonium caeruleum, deposited in CBS Jan. 2001, H. de Gruyter, (epitype designated here HMAS 246687, MBT202509, culture ex-epitype CBS 109181 = PD 83/757); Valkenswaard, from Polemonium caeruleum, Oct. 1967, H.A. van der Aa, CBS H-9081, culture CBS 375.67. UK, Surrey, from stems of Polemonium coeruleum, Mar. 1885, M.C. Cooke (isotype K 197453).
Notes: According to the original literature, Phoma polemonii was described from the stems of Polemonium caeruleum in the UK, with ellipsoidal conidia, 10 × 3 μm. The conidial dimensions observed in the type specimen in K are 4.5–7 × 2–3 μm, which is quite different from the original description. We have repeated the measurement several times using 90 conidia in total, and confirmed the conidial dimensions of the isotype as 4.5–7 × 2–3 μm. Morphological characters of our selected epitype (HMAS 246687, ex-epitype CBS 109181) from Polemonium caeruleum are consistent with the isotype specimen, although 1-septate, larger conidia occasionally occur. Isolate CBS 375.67 was initially identified as “Ascochyta polemonii”, but phylogenetically it clustered with Neodidymelliopsis polemonii, and was morphologically similar and from the same host, Polemonium caeruleum. Therefore, we re-identified this isolate as Neod. polemonii.
Neodidymelliopsis sp. 1
Specimen examined: Canada, British Columbia, from a leaf of Achlys triphylla, Jun. 1976, J. Gremmen, CBS 256.77.
Notes: Isolate CBS 256.77, originally identified as “Ascochyta achlydis”, was phylogenetically distinct from other species in the genus Neodidymelliopsis. This isolate occurred on Achlys triphylla, which is the same original host of Ascochyta achlydis. Since the type of Ascochyta achlydis was unavailable, it was unclear if CBS 256.77 represented a new species, or was conspecific to As. achlydis.
Neodidymelliopsis sp. 2
Specimen examined: Israel, En Avdat, Negev desert, from soil in desert, Feb. 1996, A. van Iperen, CBS 382.96.
Notes: Isolate CBS 382.96, deposited as “Ascochyta scotinospora”, represented a distinct lineage in the phylogenetic tree. Since the type of As. scotinospora was unavailable, it was unclear if CBS 382.96 represented a new species, or was conspecific to As. scotinospora.
Neodidymelliopsis xanthina (Sacc.) Q. Chen & L. Cai, comb. nov. MycoBank MB814154. Fig. 42.
Fig. 42.
Neodidymelliopsis xanthina (CBS 383.68). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H. Pycnidia. I. Conidiogenous cells. J. Conidia. Scale bars: G = 200 μm; H = 100 μm; I–J = 10 μm.
Basionym: Phoma xanthina Sacc., Michelia 1: 359. 1878.
≡ Macrophoma xanthina (Sacc.) Berl. & Voglino, Atti Soc. Veneto-Trentino. Sci. Nat. Padova 10: 181. 1887.
≡ Ascochyta xanthina (Sacc.) Petr. & P. Syd., Ann. Mycol. 22: 347. 1924.
Description from ex-neotype culture (CBS 383.68): Conidiomata pycnidial, solitary or confluent, globose to subglobose, glabrous, superficial on or immersed into the agar, (310–)345–535(–600) × 285–530(–565) μm. Ostioles single, papillate. Pycnidial wall pseudoparenchymatous, 2–3-layered, 13–31 μm thick, composed of oblong to isodiametric cells. Conidiogenous cells phialidic, hyaline, smooth, ampulliform, 7–12.5 × 5.5–12.5 μm. Conidia ellipsoidal to allantoid, incidentally slight curved, smooth- and thin-walled, hyaline to pale yellowish, mainly aseptate, (5–)6.5–11.5 × 2–4.5 μm, with (0–)2–12(–15) minute polar guttules, occasionally with larger 1-septate conidia. Conidial matrix pale brown.
Culture characteristics: Colonies on OA, 45–50 mm diam after 7 d, margin regular, floccose, white, pale grey to olivaceous near the centre; reverse grey-brown to hazel, white near the margin. Colonies on MEA 40–45 mm diam after 7 d, margin regular, floccose, white, pale greenish olivaceous near the centre; reverse concolourous. Colonies on PDA, 35–40 mm diam after 7 d, margin regular, floccose, whitish, black pycnidia visible near the centre and concentric rings; reverse buff in outer ring, darkening towards the centre of the colony via amber, hazel to brown zones. Application of NaOH resulted in a slight greenish to reddish discolouration.
Specimens examined: The Netherlands, Baarn, from leaves of Delphinium sp., May 1968, H.A. van der Aa (neotype designated here CBS H-8938, MBT202512, culture ex-neotype CBS 383.68); from a leaf of Delphinium sp., Jun. 1969, H.A. van der Aa, CBS H-8939, culture CBS 168.70.
Notes: The type of Phoma xanthina was from Delphinium sp. in France. Loan requests for the type specimen were unsuccessful, and we assume that it has been lost. De Gruyter (2002) provided a description of a representative culture of P. xanthina (CBS 383.68 from Delphinium sp. in the Netherlands), which was also examined in the present study. CBS 383.68 is chosen as neotype due to its morphological congruence with the original description of this species.
Isolate CBS 168.70 was previously identified as “Ascochyta aquilegiae”, and found to cluster with Neod. xanthina in the present phylogenetic study. Hence, it is considered as conspecific to Neod. xanthina.
Clade 17: Nothophoma
Nothophoma Q. Chen & L. Cai, gen. nov. MycoBank MB814060.
Etymology: Notho = nothus in Greek, fake, close but different; phoma = phoma-like morphology.
Conidiomata pycnidial, globose to elongated, or irregular, superficial on or immersed into the agar, solitary or confluent, ostiolate, sometimes with a short neck. Pycnidial wall pseudoparenchymatous, 2–9-layered, outer wall pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, sometimes flask-shaped. Conidia variable in shape, hyaline but incidentally brown, smooth- and thin-walled, aseptate, i.e. ovoid, oblong to ellipsoidal, eguttulate or guttulate.
Type species: Nothophoma infossa (Ellis & Everh.) Q. Chen & L. Cai.
Nothophoma anigozanthi (Tassi) Q. Chen & L. Cai, comb. nov. MycoBank MB814084. Fig. 11, Fig. 12.
Fig. 11.
Nothophoma anigozanthi (N 3622). A. Type collection packet. B. Ascomata on host substrate. C. Asci. D. Section of ascomata. E. Ascospores. Scale bar: C–E = 10 μm.
Fig. 12.
Nothophoma anigozanthi (CBS 381.91). A–B. Colony on OA (front and reverse). C–D. Colony on MEA (front and reverse). E–F. Colony on PDA (front and reverse). G. Pycnidia forming on OA. H–I. Pycnidia. J. Section of pycnidial wall. K–L. Conidiogenous cells. M. Conidia. Scale bars: G = 200 μm; H = 40 μm; I = 20 μm; J, M = 10 μm; K–L = 5 μm.
Basionym: Phoma anigozanthi Tassi, Bull. Labor. Ort. Bot. Siena 2: 148. 1899.
≡ Phyllosticta anigozanthi (Tassi) Allesch, Rabenh. Krypt.-Fl. [ed. 2], Pilze 7: 754. 1903.
Description from holotype (N 3622): Leaf spots elliptical to circular, black. Pseudothecia solitary, on the surface of leaves, brown, uniloculate, subglobose to globose, 85–125 × 70–100 μm, ostiolate. Asci obpyriform to fusiform, 55–73 × 17–26 μm, 8-spored, irregular uniseriate. Ascospores broadly fusiform to ellipsoidal, 14–20 × 3.5–5.5 μm, smooth, straight or slightly curved, hyaline, uniseptate, slightly constricted at the septum, guttulate, upper cells usually broader and longer than the lower cells.
Description from ex-epitype culture (CBS 381.91): Conidiomata pycnidial, solitary or aggregated, globose to subglobose, glabrous, olivaceous buff, superficial on or semi-immersed in the agar, (65–)70–130 μm diam; conidiomata with age becoming black, broadly globose to irregular, with some white hyphal outgrows and with a clear elongated neck around the ostioles, (145–)155–280(–300) × (120–)140–230(–250) μm. Ostioles 1–4(–6), on a distinctly elongated neck (up to 170 μm). Pycnidial wall pseudoparenchymatous, 3–6-layered, 16–41 μm thick, composed of isodiametric cells, outer wall 2–3-layered, pigmented. Conidiogenous cells phialidic, hyaline, smooth, ampulliform to doliiform, 5–9 × 4.5–7.5 μm. Conidia ellipsoidal, smooth- and thin-walled, aseptate, 3.5–5 × 1.5–2.5 μm, sometimes with several very small guttules. Conidial matrix creamy white.
Culture characteristics: Colonies on OA, 40–45 mm diam after 7 d, margin regular, powdery due to the abundant pycnidia produced in concentric rings, olivaceous to grey olivaceous; reverse concolourous. Colonies on MEA 40–45 mm diam after 7 d, margin regular, flattened, greenish olivaceous, pale salmon near the margin; reverse concolourous. Colonies on PDA, similar as on OA, but somewhat slower growing, 30–35 mm diam after 7 d, hazel to olivaceous. NaOH spot test: a luteous discolouration on MEA, later changing to dull green to vinaceous-black, from the centre to outer ring.
Specimen examined: Italy, on leaves of Anigozanthos flavidus, Feb. 1862 (holotype N 3622 in SIENA). The Netherlands, from a leaf of Anigozanthus maugleisii, deposited in CBS Jun. 1991, H. Cevat (epitype designated here CBS H-5199, MBT202498, culture ex-epitype CBS 381.91 = PD 79/1110).
Notes: The original description of Phoma anigozanthi indicated that this fungus produces aseptate conidia, 4–4.5 × 2 μm, which is in agreement with our observation of the specimen CBS H-5199 (3.5–5 × 1.5–2.5 μm). CBS H-5199 is therefore designated as epitype. Sphaerella millepunctata was recorded as the spermogonial state of Nothophoma anigozanthi (syn. Phoma anigozanthi; Saccardo 1902), and we did observe the asci and ascospores from the holotype of “Phoma anigozanthi” from Anigozanthos flavidus preserved in herbarium SIENA in Italy. An emended description of the sexual morph of P. anigozanthi is therefore provided.
Nothophoma arachidis-hypogaeae (V.G. Rao) Q. Chen & L. Cai, comb. nov. MycoBank MB814085.
Basionym: Phyllosticta arachidis-hypogaeae V.G. Rao, Sydowia 16: 275. 1962 (1963).
≡ Phoma arachidis-hypogaeae (V.G. Rao) Aa & Boerema, Persoonia 15: 388. 1993.
Description (de Gruyter et al. 1993).
Specimens examined: India, Poona, from leaves of Arachis hypogaea, Sep. 1962, V. Rao (holotype M.A.C.S. No. 134); Madras, from a leaf of Arachis hypogaea, deposited in CBS Jan 1993, J, de Gruyter, CBS 125.93 = PD 77/1029.
Notes: Nothophoma arachidis-hypogaeae clustered with No. infossa (CBS 123395), but they can be distinguished based on morphology and phylogeny. Conidia of No. arachidis-hypogaeae are narrower than that of No. infossa (3.2–5.2 × 1.8–2.4 μm vs. 4.5–6 × 2.5–3.5 μm) (De Gruyter et al., 1993, Aveskamp et al., 2009a). In the four sequenced loci, CBS 125.93 differs from CBS 123395 in 20 bp.
Nothophoma gossypiicola (Gruyter) Q. Chen & L. Cai, comb. nov. MycoBank MB814087.
Basionym: Phoma gossypiicola Gruyter, Persoonia 18: 96. 2002.
Description (de Gruyter 2002).
Specimen examined: USA, Texas, from a leaf of Gossypium sp., deposited in CBS Aug. 1967, G.H. Boerema, CBS H-9006, culture CBS 377.67.
Notes: This species was first described as Ascochyta gossypii Woron. in 1914, the holotype of which was collected by N. Woronichin on leaves of Gossypium sp. near Abazinka, the former Soviet Union (de Gruyter 2002). However, this name was illegitimate and replaced by a nomen novum, Phoma gossypiicola (de Gruyter 2002). Here this species is transferred to the new genus Nothophoma.
Nothophoma infossa (Ellis & Everh.) Q. Chen & L. Cai, comb. nov. MycoBank MB814088.
Basionym: Phoma infossa Ellis & Everh., J. Mycol. 4: 102. 1888.
Description and illustrations (Aveskamp et al. 2009a).
Specimen examined: Argentina, Buenos Aires Province, La Plata, from leaves of Fraxinus pennsylvanica, 2008, A.E. Perello (neotype CBS H-20145, culture ex-neotype CBS 123395).
Nothophoma quercina (Syd.) Q. Chen & L. Cai, comb. nov. MycoBank MB814086.
Basionym: Cicinobolus quercinus Syd., Ann. Mycol. 13: 42. 1915.
≡ Ampelomyces quercinus (Syd.) Rudakov, Mikol. Fitopatol. 13: 109. 1979.
≡ Phoma fungicola Aveskamp et al., Stud. Mycol. 65: 26. 2010.
Description (Aveskamp et al. 2010).
Specimen examined: Ukraine, Crimea, in the vicinity of Feodosiya, on Microsphaera alphitoides from Quercus sp., deposited in CBS Dec. 1992, CBS H-20276, culture CBS 633.92 = ATCC 36786 = VKM MF-325.
Notes: This species, originally published as Cicinobolus quercinus, was transferred to Ampelomyces, and later treated as a nomen novum in the genus Phoma by Aveskamp et al. (2010). According to the phylogenetic analysis in the present study, it clustered in the Nothophoma clade, and thus Nothophoma quercina was proposed as a new combination.
Microsphaeropsidaceae Q. Chen, L. Cai & Crous, fam. nov. MycoBank MB814155.
Conidiomata pycnidial, immersed or erumpent, subglobose, solitary or confluent, ostiolate. Pycnidial wall of textura angularis. Conidiogenous cells phialidic, hyaline, ampulliform to doliiform or subcylindrical, or somewhat irregular. Conidia thin-walled, smooth or (sometimes) with ornamentations, pale brown to yellowish or greenish brown, variable in shape, ovoid, globose, cylindrical to bacilliform, ellipsoidal to oblong, 0–1-septate.
Type genus: Microsphaeropsis Höhn., Hedwigia 59: 267. 1917.
Microsphaeropsis Höhn., Hedwigia 59: 267. 1917.
Conidiomata pycnidial, immersed or erumpent, subglobose, solitary or confluent, ostiolate. Pycnidial wall of textura angularis. Conidiogenous cells phialidic, hyaline, ampulliform to doliiform or subcylindrical, with a prominent apical periclinal thickening. Conidia thin-walled, smooth or finely roughened, hyaline when young, becoming pale brown to yellowish or greenish brown, variable in shape, ovoid, globose, cylindrical to bacilliform, ellipsoidal to oblong, straight to slightly curved, 0–1-septate.
Type species: Microsphaeropsis olivacea (Bonord.) Höhn., Hedwigia 59: 267. 1917.
Notes: Microsphaeropsis was established by von Höhnel, and was originally placed in the Montagnulaceae (von Höhnel 1917). Our phylogenetic analysis clearly indicated that Microsphaeropsis is basal to Didymellaceae, from which it appears to have a significant evolutionary distance. Conidia of Microsphaeropsis usually differ from those of Didymellaceae in surface ornamentation and darker colour. For this reason the Microsphaeropsidaceae is herewith introduced to accommodate Microsphaeropsis.
Microsphaeropsis olivacea (Bonord.) Höhn., Hedwigia 59: 267. 1917. Fig. 43.
Fig. 43.
Microsphaeropsis olivacea (BPI 797151). A. Conidiomata on host tissue. B. Section through conidiomatal wall, showing chains of chlamydospores. C–E. Conidiogenous cells. F. Brown, 0(–1)-septate conidia. G. Aseptate conidia. Scale bars: A = 200 μm; B–G = 10 μm.
Basionym: Coniothyrium olivaceum Bonord., Jahrb. Nassauischen Vereins Naturk. 23–24: 377. 1869.
Description from holotype (BPI 797151): Conidiomata pycnidial, up to 200 μm diam, solitary, dark brown, immersed, becoming erumpent and somewhat papillate at central ostiole, up to 80 μm diam. Pycnidial wall pseudoparenchymatous, 4–8-layered, of textura angularis, brown, giving rise to chains of brown chlamydospores extending into the host tissue, brown, smooth, thick-walled, ellipsoidal to globose, 6–10 μm diam. Conidiophores reduced to conidiogenous cells lining the inner cavity of conidioma. Conidiogenous cells hyaline, smooth, subcylindrical to doliiform, 5–7 × 4–7 μm; apex with prominent periclinal thickening. Conidia solitary, initially hyaline, smooth, becoming pale brown and finely roughened, 1–2-guttulate, ellipsoidal to subcylindrical with obtuse ends, straight to slightly curved, 0(–1)septate, (5–)6–7(–8.5) × (3–)3.5–4 μm.
Specimens examined: Austria, on stem of Hedera helix (holotype BPI 797151, ex herb. Fuckel, ex herb. Boiss). France, Nancy, from needles of Pirus laricio, deposited in CBS Apr. 1977, M. Morelet, CBS H-10854, culture CBS 233.77. The Netherlands, Valkenswaard, from dead twigs and pods of Sarothamnus sp., Feb 1971, H.A. van der Aa, CBS H-10870, culture CBS 432.71.
Notes: The two cultures studied here closely resemble M. olivacea, in having smooth to finely roughened, pale brown, ellipsoidal to subcylindrical, straight to slightly curved conidia, (5–)6–7 × 3–4 μm (in vitro). Because they occur on different hosts, however, we refrain from designating any one of these isolates as ex-epitype.
Microsphaeropsis proteae (Crous & Denman) Crous & Denman, Persoonia 27: 32. 2011.
Basionym: Coniothyrium proteae Crous & Denman, S. African J. Bot. 64: 139. 1998.
Description and illustrations (Crous et al. 2011).
Specimen examined: South Africa, Western Cape Province, from Protea nitida, Aug. 1996, S. Denman (culture ex-type CBS 111319 = CPC 1425).
Discussion
This study was prompted by the question of how to delineate natural genera in the Ascochyta-Didymella-Phoma complex, which represents a dilemma to plant pathologists and mycologists alike (Chilvers et al., 2009, Aveskamp et al., 2010, Hyde et al., 2013). Based on the previous studies by Aveskamp et al., 2009a, Aveskamp et al., 2009b, Aveskamp et al., 2010 and De Gruyter et al., 2009, De Gruyter et al., 2012, we combined the multi-locus data of rpb2 with LSU, ITS and tub2 for phylogenetic analysis, and added more isolates of previously unstudied species. The topology of the single rpb2 phylogeny is highly similar to the combined four loci tree. In this regard, the rpb2 gene showed better resolution at the species and generic level than ITS, LSU or tub2. Unfortunately, the success rate of the amplification of rpb2 was not satisfactory.
The family Didymellaceae was established to accommodate the majority of species in Phoma s. lat. and related genera by de Gruyter et al. (2009), based on its type genus Didymella. Aveskamp et al. (2010) revised the taxonomy of some monophyletic clades in Didymellaceae. An interesting result generated in the present study was that a well-supported clade comprising Microsphaeropsis species clustered outside the Didymellaceae. That was inconsistent with previous studies, which indicated that the type species of Microsphaeropsis, Mi. olivacea, grouped in Didymellaceae (De Gruyter et al., 2009, De Gruyter et al., 2012, Aveskamp et al., 2010). This is not so surprising, as previous studies were mostly based on LSU / SSU (e.g. de Gruyter et al. 2009) which lacked necessary resolution at genus level and resulted in unresolved polytomies (e.g. Aveskamp et al. 2010). Microsphaeropsis is characterised by small, predominantly aseptate conidia, formed on pycnidial phialides, which are morphologically similar to some species of Phoma and Coniothyrium (Jones, 1976, Carisse and Bernier, 2002). However, Microsphaeropsis produces pale greenish brown, finely roughened conidia, that differ significantly from the mainly hyaline, smooth conidia observed in Phoma species, and the usually 0–1-septate, verrucose conidia produced from annellides in Coniothyrium s. str. (Morgan-Jones, 1974, Carisse and Bernier, 2002, Aveskamp et al., 2010, De Gruyter et al., 2012). Additionally, in the study of de Gruyter et al. (2012), Coniothyrium s. str. clustered with the type genus Leptosphaeria in Leptosphaeriaceae, which was in agreement with the results obtained in the present study. Since many species of Microsphaeropsis are still unknown from culture or DNA sequence, further work is needed to resolve species boundaries in this genus.
The genera Boeremia, Leptosphaerulina, Macroventuria and Stagonosporopsis cluster in Didymellaceae, which agrees with the results of Aveskamp et al. (2010). Five Phoma species lacking of chlamydospores were also included in Epicoccum. Species in the former genus Peyronellaea and some that resided in several other lineages (named Groups G, H, I, J sensu Aveskamp et al. 2010) were recombined into Didymella. Furthermore, we demarcated the genera Ascochyta, Didymella and Phoma on the basis of their phylogeny of their respective generic type species, whilst we also introduced nine new genera, which were well-supported in the molecular phylogenetic analyses, i.e. Allophoma, Calophoma, Heterophoma, Neoascochyta, Neodidymelliopsis, Nothophoma, Paraboeremia, Phomatodes and Xenodidymella. Among the currently studied 17 genera in Didymellaceae, with the exception of Didymella, the sexual morph is only known from nine genera, i.e. Ascochyta, Leptosphaerulina, Macroventuria, Neoascochyta, Neodidymelliopsis, Paraboeremia, Phoma, Stagonosporopsis and Xenodidymella. Presently, all former Didymella species are known from their sexual morphs, although this will change as asexual taxa can now also be accommodated in this genus. The delimitation of Ascochyta, Didymella and Phoma is clarified by the present findings, in which the type species are separated into distinct monophyletic lineages, and all three genera were linked to sexual morphs.
The genera Ampelomyces, Ascochyta (De Gruyter et al., 2009, Aveskamp et al., 2010), Boeremia (Aveskamp et al. 2010), Chaetasbolisia (De Gruyter et al., 2009, Aveskamp et al., 2010, Wijayawardene et al., 2012, Zhang et al., 2012), Dactuliochaeta (Wijayawardene et al., 2012, Zhang et al., 2012), Didymella, Epicoccum, Leptosphaerulina, Macroventuria, Microsphaeropsis, Peyronellaea, Phoma (Aveskamp et al. 2010), Piggotia, Pithoascus (Wijayawardene et al., 2012, Zhang et al., 2012) and Stagonosporopsis (Aveskamp et al. 2010) were formerly placed in the family Didymellaceae. However, Ampelomyces, with the type species Ampelomyces quisqualis, was accommodated in Phaeosphaeriaceae (de Gruyter et al. 2009); Chaetasbolisia needs to be restudied including more taxa (Aveskamp et al. 2010); Microsphaeropsis grouped sister to the Didymellaceae in the Microsphaeropsidaceae in the present study; Pithoascus was recently placed in Microascaceae (Sandoval-Denis et al. 2016); while Dactuliochaeta and Piggotia require more molecular data to validate their taxonomic placements (Hyde et al. 2013). Hence, it was not possible to presently accept these three doubtful genera (Chaetasbolisia, Dactuliochaeta and Piggotia) in Didymellaceae. Moreover, Platychora was previously assigned to Venturiaceae (Barr 1968), but in a later study by Winton et al. (2007) and Zhang et al. (2012), the generic type Platychora ulmi was shown to cluster in Didymellaceae. This genus and the type species should also be re-evaluated based on new collections and epitypification (Zhang et al. 2012). We concluded that 17 genera viz. Allophoma, Ascochyta, Boeremia, Calophoma, Didymella, Epicoccum, Heterophoma, Leptosphaerulina, Macroventuria, Neoascochyta, Neodidymelliopsis, Nothophoma, Paraboeremia, Phoma, Phomatodes, Stagonosporopsis and Xenodidymella can presently be supported as members of Didymellaceae.
Morphological characteristics have proven to be relatively conserved in Phoma s. lat., including features such as shape and dimensions of pycnidia, conidiogenous cells and conidia. The relatively simple asexual morphological features of these species could not provide sufficient distinctions for species delimitation. Although these species clustered in different phylogenetic lineages, they share some overlapping morphological features (Table 2), which is similar to the situation in the genus Septoria for which it was concluded that reliable identification in future should be based on DNA sequence data linked to morphology and ecology (Quaedvlieg et al., 2013, Verkley et al., 2013).
In previous years, conidiogenesis and conidial septation used to be regarded as the most important criteria to discriminate species of Phoma and allied genera, especially between Ascochyta and Phoma (Morgan-Jones, 1974, Boerema and Bollen, 1975, Jones, 1976, Punithalingam, 1979a, De Gruyter et al., 2009, De Gruyter et al., 2012, Aveskamp et al., 2010). However, conidiogenesis of species in the same genus was later found to differ, such as the annellidic conidiogenous cells in As. pisi (Boerema & Bollen 1975), versus the phialidic conidiogenous cells in As. fabae (Punithalingam 1975). Punithalingam (1979a) elucidated that the annellidic state was the initial stage during pycnidial development in Ascochyta, and that the phialidic state was the final stage that could be observed once pycnidia matured. Under the conditions employed in the present study, we observed all species accommodated in the Didymellaceae to exhibit phialidic conidiogenesis.
Several species belonging to phoma-related genera are known to exhibit some level of host-specificity. For instance, Ascochyta fabae showed pathogenic specialisation for faba bean (Vicia faba), while As. lentis is specific to lentil (Lens culinaris) (Kaiser et al. 1997), Nothophoma infossa (syn. Phoma infossa) is often associated with ash trees (Fraxinus sp.) and No. gossypiicola (syn. Phoma gossypiicola) is reported only on cotton plants (Gossypium spp.) (Aveskamp et al. 2010). However, not all fungal-host associations in Didymellaceae are clearly defined. Although the strains used in the present study were collected globally, cultures for each species are still limited in number and mainly arise from collections made in Europe and the USA. Generally, Asia, Africa and Latin America have been rather poorly represented in previous studies. For many old names, ex-type cultures are lacking, and holotype specimens could not be traced. To truly elucidate the taxonomy of phoma-like genera, therefore, a conserted global effort is called for not only to recollect previously described species, but also to add isolates from continents that have been largely neglected or undersampled by mycologists and plant pathologists in the past.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (NSFC 31322001), China. Qian Chen acknowledges the external Cooperation Program of the Chinese Academy of Sciences (GJHZ1310) and NSFC (31110103906) for supporting her visit to CBS. The CBS-KNAW is acknowledged for providing cultures to facilitate this study. Drs Joyce HC Woudenberg, JZ (Ewald) Groenewald and Lorenzo Lombard, and Ms Mieke Starink-Willemse are thanked for support to Q.C. during her visit to CBS. The various fungaria cited in the Materials and Methods section are acknowledged for providing specimens for morphological studies.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
References
- Abler S.W. Virginia Polytechnic Institute and State University; Blacksburg, Virginia, USA: 2003. Ecology and taxonomy of Leptosphaerulina spp. associated with turfgrasses in the United States. [Google Scholar]
- Aveskamp M.M., de Gruyter J., Crous P.W. Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Diversity. 2008;31:1–18. [Google Scholar]
- Aveskamp M.M., de Gruyter J., Woudenberg J.H.C. Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology. 2010;65:1–60. doi: 10.3114/sim.2010.65.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveskamp M.M., Verkley G.J.M., de Gruyter J. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia. 2009;101:363–382. doi: 10.3852/08-199. [DOI] [PubMed] [Google Scholar]
- Aveskamp M.M., Woudenberg J.H.C., de Gruyter J. Development of taxon-specific sequence characterized amplified region (SCAR) markers based on actin sequences and DNA amplification fingerprinting (DAF): a case study in the Phoma exigua species complex. Molecular Plant Pathology. 2009;10:403–414. doi: 10.1111/j.1364-3703.2009.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr M.E. The Venturiaceae in North America. Canadian Journal of Botany. 1968;46:799–864. [Google Scholar]
- Barr M.E. On the Pleomassariaceae (Pleosporales) in North America. Mycotaxon. 1982;15:345–348. [Google Scholar]
- Berner D., Cavin C., Woudenberg J.H.C. Assessment of Boeremia exigua var. rhapontica, as a biological control agent of Russian knapweed (Rhaponticum repens) Biological Control. 2015;81:65–75. [Google Scholar]
- Boerema G.H. The Phoma species studied in culture by Dr R.W.G. Dennis. Transactions of the British Mycological Society. 1976;67:289–319. [Google Scholar]
- Boerema G.H. Mycologisch-taxonomisch onderzoek. Phoma-soorten van de sectie Peyronellaea. Verslagen en Mededelingen Plantenziektenkundige Dienst Wageningen. 1983;159:21–25. [Google Scholar]
- Boerema G.H. Contributions towards a monograph of Phoma (Coelomycetes) – II. Section Peyronellaea. Persoonia. 1993;15:197–221. [Google Scholar]
- Boerema G.H. Contributions towards a monograph of Phoma (Coelomycetes) – V. Subdivision of the genus in sections. Mycotaxon. 1997;64:321–333. [Google Scholar]
- Boerema G.H., Bollen G.J. Conidiogenesis and conidial septation as differentiating criteria between Phoma and Ascochyta. Persoonia. 1975;8:111–444. [Google Scholar]
- Boerema G.H., de Gruyter J. Contributions towards a monograph of Phoma (Coelomycetes) – VII. Section Sclerophomella: taxa with thick-walled pseudoparenchymatous pycnidia. Persoonia. 1998;17:81–95. [Google Scholar]
- Boerema G.H., de Gruyter J., Noordeloos M.E. Contributions towards a monograph of Phoma (Coelomycetes) – IV. Section Heterospora: taxa with large sized conidial dimorphs, in vivo sometimes as Stagonosporopsis synanamorphs. Persoonia. 1997;16:335–371. [Google Scholar]
- Boerema G.H., de Gruyter J., Noordeloos M.E. CABI Publishing; Wallingford, UK: 2004. Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. [Google Scholar]
- Boerema G.H., Loerakker W.M., Laundon G.F. Phoma rumicicola sp. nov., a cause of leaf spots on Rumex obtusifolius. New Zealand Journal of Botany. 1980;18:473–476. [Google Scholar]
- Boerema G.H., Pieters R., Hamers M.E.C. Check-list for scientific names of common parasitic fungi. Series 2c,d (additions and corrections): fungi on field crops: pulse (legumes), forage crops (herbage legumes), vegetables and cruciferous crops. Netherlands Journal of Plant Pathology. 1993;99(Supplement 1):1–32. [Google Scholar]
- Boerema G.H., Valckx A.G.M. Enkele bijzondere schimmelaantastingen. III. Gewasbescherming. 1970;1:65–68. [Google Scholar]
- Boerema G.H., Verhoeven A.A. Check-list for scientific names of common parasitic fungi. Series 2c: fungi on field crops: pulse (legumes), and forage crops (herbage legumes) Netherlands Journal of Plant Pathology. 1979;85:151–185. [Google Scholar]
- Carisse O., Bernier J. Microsphaeropsis ochracea sp. nov. associated with dead apple leaves. Mycologia. 2002;94:297–301. [PubMed] [Google Scholar]
- Cavara F. Über einige parasitische Pilze auf dem Getreide. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. 1893;3:16–26. [Google Scholar]
- Chen Q., Zhang K., Zhang G.Z. A polyphasic approach to characterise two novel species of Phoma (Didymellaceae) from China. Phytotaxa. 2015;197:267–281. [Google Scholar]
- Chilvers M.I., Rogers J.D., Dugan F.M. Didymella pisi sp. nov., the teleomorph of Ascochyta pisi. Mycological Research. 2009;113:391–400. doi: 10.1016/j.mycres.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Cooke M.C. New British fungi. Grevillea. 1890;19:8. [Google Scholar]
- Cooke M.C., Ellis J.B. New Jersey fungi. Grevillea. 1877;5:89–95. [Google Scholar]
- Corbaz R. Sur Didymella phacae Corbaz. Sydowia. 1955;9:229–230. [Google Scholar]
- Corbaz R. Recherches sur le genre Didymella Sacc. Phytopathologische Zeitschrift. 1957;28:275–414. [Google Scholar]
- Corlett M. A taxonomic survey of some species of Didymella and Didymella-like species. Canadian Journal of Botany. 1981;59:2016–2042. [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Shivas R.G., Quaedvlieg W. Fungal Planet description sheets: 214–280. Persoonia. 2014;32:184–306. doi: 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Summerell B.A., Swart L. Fungal pathogens of Proteaceae. Persoonia. 2011;27:20–45. doi: 10.3767/003158511X606239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z. Centraalbureau voor Schimmelcultures; Utrecht, Netherlands: 2009. Fungal Biodiversity. [CBS Laboratory manual series 1.] [Google Scholar]
- Cubero O.F., Crespo A., Fatehi J. DNA extraction and PCR amplification method suitable for fresh, herbariumstored, lichenized, and other fungi. Plant Systematics and Evolution. 1999;216:243–249. [Google Scholar]
- Davey M.L., Currah R.S. Atradidymella muscivora gen. et sp. nov. (Pleosporales) and its anamorph Phoma muscivora sp. nov.: a new pleomorphic pathogen of boreal bryophytes. American Journal of Botany. 2009;96:1281–1288. doi: 10.3732/ajb.0900010. [DOI] [PubMed] [Google Scholar]
- De Gruyter J. Contributions towards a monograph of Phoma (Coelomycetes) – IX Section Macrospora. Persoonia. 2002;18:85–102. [Google Scholar]
- De Gruyter J., Aveskamp M.M., Woudenberg J.H.C. Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycological Research. 2009;113:508–519. doi: 10.1016/j.mycres.2009.01.002. [DOI] [PubMed] [Google Scholar]
- De Gruyter J., Boerema G.H. Constributions towards a monograph of Phoma (Coelomycetes) – VIII. Section Paraphoma: taxa with setose pycnidia. Persoonia. 2002;17:541–561. [Google Scholar]
- De Gruyter J., Boerema G.H., van der Aa H.A. Contributions towards a monograph of Phoma (Coelomycetes) VI – 2. Section Phyllostictoides: outline of its taxa. Persoonia. 2002;18:1–53. [Google Scholar]
- De Gruyter J., Noordeloos M.E. Constributions towards a monograph of Phoma (Coelomycetes) – I. 1. Section Phoma: taxa with very small conidia in vitro. Persoonia. 1992;15:71–92. [Google Scholar]
- De Gruyter J., Noordeloos M.E., Boerema G.H. Contributions towards a monograph of Phoma (Coelomycetes) – I. 2. Section Phoma: additional taxa with very small conidia and taxa with conidia up to 7 μm long. Persoonia. 1993;15:369–400. [Google Scholar]
- De Gruyter J., Noordeloos M.E., Boerema G.H. Contributions towards a monograph of Phoma (Coelomycetes) – I. 3. Section Phoma: taxa with conidia longer than 7 μm. Persoonia. 1998;16:471–490. [Google Scholar]
- De Gruyter J., Woudenberg J.H.C., Aveskamp M.M. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia. 2010;102:1066–1081. doi: 10.3852/09-240. [DOI] [PubMed] [Google Scholar]
- De Gruyter J., Woudenberg J.H.C., Aveskamp M.M. Redisposition of Phoma-like anamorphs in Pleosporales. Studies in Mycology. 2012;75:1–36. doi: 10.3114/sim0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog G.S., Gerrits van den Ende A.H.G. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Diedicke H. Die Abteilung Hyalodidymae der Sphaerioideen. Annales Mycologici. 1912;10:135–152. [Google Scholar]
- Echandi E. La Quema de los cafetos causada por Phoma costarricensis n. sp. Revista de biologia tropicale, Valparaiso. 1957;5:81–102. [Google Scholar]
- Eriksson O.E., Hawksworth D.L. Outline of the Ascomycetes – 1986. Systema Ascomycetum. 1986;5:185–324. [Google Scholar]
- Farr D.F., Rossman A.Y. ARS, USDA; 2015. Fungal databases, systematic mycology and microbiology laboratory.http://nt.ars-grin.gov/fungaldatabases/ [Google Scholar]
- Graham J.H., Luttrell E.S. Species of Leptosphaerulina on forage plants. Phytopathology. 1961;51:680–693. [Google Scholar]
- Holm L. Nomenclatural notes on Pyrenomycetes. Taxon. 1975;24:475–488. [Google Scholar]
- Hyde K.D., Jones E.B.G., Liu J.K. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Inderbitzin P., Jones E.B.G., Vrijmoed L.L.P. A new species of Leptosphaerulina from decaying mangrove wood from Hong Kong. Mycoscience. 2000;41:233–237. [Google Scholar]
- Irwin J.A.G., Davis R.D. Taxonomy of some Leptosphaerulina spp. on legumes in Eastern Australia. Australian Journal of Botany. 1985;33:233–237. [Google Scholar]
- Jellis G.J., Punithalingam E. Discovery of Didymella fabae sp. nov., the teleomorph of Ascochyta fabae, on faba bean straw. Plant Pathology. 1991;40:150–157. [Google Scholar]
- Jones J.P. Ultrastructure of conidium ontogeny in Phoma pomorum, Microsphaeropsis olivaceum, and Coniothyrium fuckelii. Canadian Journal of Botany. 1976;54:831–851. [Google Scholar]
- Kaiser W.J., Wang B.C., Rogers J.D. Ascochyta fabae and A. lentis: host specificity, teleomorphs (Didymella), hybrid analysis, and taxonomic status. Plant Disease. 1997;81:809–816. doi: 10.1094/PDIS.1997.81.7.809. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhriakov M. Vol. 1. [Diseases and pests of oil-yielding plants]. 1934. Bolezni i vrediteli maslichnykh kul'tur; p. 29. [Google Scholar]
- Kodsueb R., Dhanasekaran V., Aptroot A. The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequences analyses of partial 28S rDNA. Mycologia. 2006;98:571–583. doi: 10.3852/mycologia.98.4.571. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Mason-Gamer R.J., Kellogg E.A. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae) Systematic Biology. 1996;45:524–545. [Google Scholar]
- McAlpine D. Agriculture Department of Victoria; Australia, Melbourne: 1902. Fungus diseases of stone-fruit trees in Australia, and their treatment. [Google Scholar]
- McPartland J.M. Cannabis pathogens X: Phoma, Ascochyta and Didymella species. Mycologia. 1994;86:870–878. [Google Scholar]
- Mel'nik V.A. 1977. Opredelitel' gribov roda Ascochyta Lib. Leningrad, USSR. [Google Scholar]
- Moesz G. Mykologiai közlemények. Botanikai közlemények. 1925;22:39–52. [Google Scholar]
- Morgan-Jones G. Concerning some species of Microsphaeropsis. Canadian Journal of Botany. 1974;52:2575–2579. [Google Scholar]
- Mukunya D.M., Boothroyd C.W. Mycosphaerella zeae-maydis sp. n., the sexual stage of Phyllosticta maydis. Phytopathology. 1973;65:529–532. [Google Scholar]
- Müller E. Kulturversuche mit Ascomyceten I. Sydowia. 1953;7:325–334. [Google Scholar]
- Müller E. Über die Hauptfruchtform von Phyllostictina solieri (Mont.) Petr. et Syd. Sydowia. 1958;12:244–246. [Google Scholar]
- Müller E., von Arx J.A. Die Gattungen der didymosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz. 1962;11:1–922. [Google Scholar]
- Nylander J.A.A. Evolutionary Biology, Centre Uppsala University; 2004. MrModeltest v2. Program distributed by the author. [Google Scholar]
- Peever T.L., Barve M.P., Stone L.J. Evolutionary relationships among Ascochyta species infecting wild and cultivated hosts in the legume tribes Cicereae and Vicieae. Mycologia. 2007;99:59–77. doi: 10.3852/mycologia.99.1.59. [DOI] [PubMed] [Google Scholar]
- Punithalingam E. Studies on Sphaeropsidales in culture. Mycological Papers. 1969;119:1–24. [Google Scholar]
- Punithalingam E. 1972. Mycosphaerella pinodes. CMI descriptions of pathogenic fungi and bacteria. No. 340. [Google Scholar]
- Punithalingam E. 1975. Ascochyta fabae. CMI descriptions of pathogenic fungi and bacteria. No. 461. [Google Scholar]
- Punithalingam E. Graminicolous Ascochyta species. Mycological Papers. 1979;142:1–214. [Google Scholar]
- Punithalingam E. Sphaeropsidales in culture from humans. Nova Hedwigia. 1979;31:119–158. [Google Scholar]
- Punithalingam E., Tulloch M., Leach C.M. Phoma epicoccina sp. nov. on Dactylis glomerata. Transactions of the British Mycological Society. 1972;59:341–345. [Google Scholar]
- Quaedvlieg W., Verkley G.J.M., Shin H.-D. Sizing up Septoria. Studies in Mycology. 2013;75:307–390. doi: 10.3114/sim0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajak R.C., Rai M.K. Cholesterol content in twenty species of Phoma. National Academy of Sciences and Letters. 1983;6:113–114. [Google Scholar]
- Rayner M.C. Nitrogen fixation in Ericaceae. Botanical Gazette. 1922;73:226–235. [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute and British Mycological Society; Kew, Surrey, UK: 1970. A mycological colour chart. [Google Scholar]
- Rehner S.A., Samuels G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research. 1994;98:625–634. [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux C. Leptosphaerulina chartarum sp. nov., the teleomorph of Pithomyces chartarum. Transactions of the British Mycological Society. 1986;86:319–323. [Google Scholar]
- Saccardo P.A. Conspectus genera fungorum Italiae inferiorum nempe ad Sphaeropsideas, Melanconieas et Hyphomyceteas pertinentium systemate sporologico dispositorum. Michelia. 1880;2:1–38. [Google Scholar]
- Saccardo P.A. Sylloge Sphaeropsidearum et Melanconiearum omnium hucusque cognitorum. Sylloge Fungorum. 1884;3:1–860. Padova, Italy. [Google Scholar]
- Saccardo P.A. Vol. 10. Italy; Padova: 1892. Sylloge Fungorum omnium hucusque cognitorum: Supplementum Universale, Pars II; pp. 1–964. [Google Scholar]
- Saccardo P.A. Vol. 16. Italy; Padova: 1902. Sylloge Fungorum omnium hucusque cognitorum: Supplementum Universale, Pars V; pp. 1–1291. [Google Scholar]
- Saccardo P.A. Vol. 17. Italy; Padova: 1905. Sylloge Fungorum omnium hucusque cognitorum: Supplementum Universale, Pars VI; pp. 1–991. [Google Scholar]
- Saccardo P.A., Trotter A. Vol. 22. Italy; Padova: 1913. Sylloge Fungorum omnium hucusque cognitorum: Supplementum Universale, Pars IX; pp. 1–1612. [Google Scholar]
- Sandoval-Denis M., Gené J., Sutton D.A. Redefining Microascus, Scopulariopsis and allied genera. Persoonia. 2016;36:1–36. doi: 10.3767/003158516X688027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan A. J. Cramer; Vaduz, Germany: 1984. The Bitunicate Ascomycetes and their anamorphs. [Google Scholar]
- Smith H., Wingfield M.J., Crous P.W. Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany. 1996;62:86–88. [Google Scholar]
- Sprague R. Oregon State College Press; Corvallis, Oregon: 1944. Septoria disease of Gramineae in Western United States. [Google Scholar]
- Stamatakis A., Alachiotis N. Time and memory efficient likelihood-based tree searched on phylogenomic alignments with missing data. Bioinformatics. 2010;26:i132–i139. doi: 10.1093/bioinformatics/btq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.Y., Qi Y.L., Cai L. Induction of sporulation in plant pathogenic fungi. Mycology. 2012;3:195–200. [Google Scholar]
- Sung G.-H., Sung J.-M., Hywel-Jones N.L. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution. 2007;44:1204–1223. doi: 10.1016/j.ympev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Sutton B.C. 1st edn. Commonwealth Mycological Institute; UK: 1980. The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata. [Google Scholar]
- Tamura K., Stecher G., Peterson D. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution. 2013;31:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomilin B.A. ‘Nauka’ Publishing House; Leningrad, USSR: 1979. Opredelitel' gribov roda Mycosphaerella Johansen. [Google Scholar]
- Trapero-Casas A., Kaiser W.J. Development of Didymella rabiei, the teleomorph of Ascochyta rabiei, on Strawchickpea straw. Phytopathology. 1992;82:1261–1266. [Google Scholar]
- Vaghefi N., Pethybridge S.J., Ford R. Stagonosporopsis spp. associated with ray blight disease of Asteraceae. Australasian Plant Pathology. 2012;41:675–686. [Google Scholar]
- Van der Aa H.A. Macroventuria, a new genus in the Venturiaceae. Persoonia. 1971;6:359–363. [Google Scholar]
- Van der Aa H.A., Boerema G.H., de Gruyter J. Contributions towards a monograph of Phoma (Coelomycetes) – VI-1. Section Phyllostictoides: characteristics and nomenclature of its type species Phoma exigua. Persoonia. 2000;17:435–456. [Google Scholar]
- Verkley G.J.M., Quaedvlieg W., Shin H.-D. A new approach to species delimination in Septoria. Studies in Mycology. 2013;75:213–305. doi: 10.3114/sim0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Arx J.A., Müller E. A re-evaluation of the bitunicate Ascomycetes with keys to families and genera. Studies in Mycology. 1975;9:1–159. [Google Scholar]
- Von Höhnel F. Fungi imperfecti. Beiträge zur Kenntnis derselben. Hedwigia. 1917;59:236–284. [Google Scholar]
- Von Höhnel F. Mycologische Fragmente CCVI. Über die Gattung Didymella Saccardo. Annales Mycologici. 1918;16:64–65. [Google Scholar]
- White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego, California, USA: 1990. pp. 315–322. [Google Scholar]
- Wijayawardene D.N.N., McKenzie E.H.C., Hyde K.D. Towards incorporating anamorphic fungi in a natural classification – checklist and notes for 2011. Mycosphere. 2012;3:157–228. [Google Scholar]
- Winton L.M., Stone J.K., Hansen E.M. The systematic position of Phaeocryptopus gaeumannii. Mycologia. 2007;99:240–252. doi: 10.3852/mycologia.99.2.240. [DOI] [PubMed] [Google Scholar]
- Woudenberg J.H.C., Aveskamp M.M., de Gruyter J. Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia. 2009;22:56–62. doi: 10.3767/003158509X427808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudenberg J.H.C., Groenewald J.Z., Binder M. Alternaria redefined. Studies in Mycology. 2013;75:171–212. doi: 10.3114/sim0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Crous P.W., Schoch C.L. Pleosporales. Fungal Diversity. 2012;53:1–221. doi: 10.1007/s13225-011-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]