Abstract
Background:
Fixation is the most imperative step in the practice of diagnostic histopathology, which is intimately linked to 10% formalin. As a result of increasing concerns about the potential carcinogenicity of the formaldehyde, attempt to find safer alternatives is necessary. Honey has been shown to possess antimicrobial, antiviral and antimutagenic properties. Many studies have reported that honey possesses dehydrating and preserving effects also.
Aims and Objectives:
To study the fixative properties of processed and unprocessed honey in oral tissues followed by comparision with formalin.
Materials and Methods:
The study group comprised 12 different tissues. Each tissue was cut into 3 segments and were immediately fixed in bottles containing 10% unprocessed honey, 10% processed honey and 10% formalin, respectively, for 24 h at room temperature. After fixation, tissues were processed using the routine standard processing protocol followed by hematoxylin and eosin staining. Data were statistically analyzed using descriptive statistics such as mean, standard deviation and percentage. Kruskal–Wallis test followed by post hoc Dunn's multiple comparisons test and Chi-square test or Fisher's exact test for small sample size. A P < 0.05 was considered as significant. Data analysis was done by using software Minitab v14.0.
Results:
When all the stained sections were assessed for the parameters, there was no statistically significant difference between tissues fixed in processed and unprocessed honey compared to formalin (P = 0.004). The tissue morphology and staining adequacy for diagnosis in honey fixed tissue was at par with formalin fixed tissue. Hence, our results suggest that both processed honey and unprocessed honey can be used as a safe alternative for formalin.
Keywords: Formalin substitutes, non-formalin fixatives, processed honey, tissue fixatives, unprocessed honey
INTRODUCTION
Fixation is the most imperative step in the practice of diagnostic pathology for processing of biopsy tissue specimen to examine and for the archival preservation. Fixation should preserve cellular architecture along with the protein, carbohydrate and other bioactive moieties in their spatial relationship to the cell.[1]
An ideal fixative is expected to impart mechanical rigidity to withstand tissue processing, prevent decomposition, putrefaction and autolysis. Fixation is a physiochemical process that is gradual and complex, involving diffusion of fixative that perfectly preserves cellular morphology yet does not modify the specimen composition. Due of this concern, the selection of a particular fixative generally warrants multiple and careful consideration.[2]
Formaldehyde was first discovered in 1859 by the Russian chemist Alexander M. Butlerov. Later, it was Ferdinand Blum in the 19th century who while working on formaldehyde for disinfection accidentally found that it can “fix” the tissue. Formalin became the fixative of choice in just a few years.[3] Neutral buffered formalin (NBF) is a 10% solution of formalin buffered at pH 7.2–7.4 with phosphate salts to prevent its acidification. The recent concern in this regard is the various deleterious effect of formalin, such as irritation of eyes, nose, throat and allergic skin reaction.[4] Formaldehyde is regulated as a carcinogen by California/Occupational Safety Health Assocation and California/Environmental Protection Agency. Owing to these demerits of formalin the quest to find a safer alternative has been of concern for most of the pathologist.[5]
According to Codex Alimentarius, “Honey is the natural sweet substance, produced by honeybees from the nectar of plants or from secretions of living parts of plants, or excretions of plant-sucking insects on the living parts of plants, which the bees collect, transform by combining with specific substances of their own, deposit, dehydrate, store and leave in honeycombs to ripen and mature.” Honey is a mixture of sugars and other compounds. It contains tiny amounts of several compounds including chrysin, pinobanksin, vitamin C, catalase and pinocembrin.[6] The various properties of honey include antioxidant, antimicrobial and antiautolytic effects. It has the quality of penetrating the deepest tissue and can prevent autolysis and putrefaction.[2]
Honey has been found to prevent autolysis as tissues put in it for up to 30 days did not show any sign of putrefaction and autolysis. The tissue hardening property makes it similar in action to fixatives which acts by hardening the tissues.[2] It has been said that honey that is not well filtered (unprocessed) may contain various artifacts in it, including viable spores such as clostridia which may cause false positive reactions, unlike the filtered processed honey.[2] Thus, the present study which is first of its kind was conducted using both unprocessed and processed honey and with the following aims and objective.
Aims and objective
To test the hypothesis that the tissues fixed in Unprocessed Honey is better than/at par with the conventional formalin fixative
To test the hypothesis that the tissues fixed in Processed Honey is better than/at par with the conventional formalin fixative
To compare the efficacy of unprocessed honey, with processed honey for the fixation of histological tissue.
MATERIALS AND METHODS
Tissue preparation
The study group comprising of 36 human tissues including oral epithelium, lymphoid, salivary gland, fat, muscle and skin that were taken from the Department of Oral Pathology. Twelve different tissues were cut into three segments and was immediately fixed in a bottle containing 10% unprocessed honey (Group A),10% processed honey (Group B) and bottle containing 10% NBF (Group C) for 24 h at room temperature. The present experimental study was carried out to know if unprocessed honey and processed honey have any fixative properties in comparison with NBF.
Preparation of fixatives
Group A - 10% unprocessed honey (pH: 3.6) - 10 ml of pure, unprocessed honey (pH - 3.6) was mixed with 100 ml of distilled water.
Group B - 10% processed honey (pH: 5.05) - 10 ml of pure, processed honey (Dabur; pH-4.6) was mixed with 100 ml of distilled water.
Group C - 10% NBF (pH 7.2-7.4).
After fixation, tissues were processed using the routine standard processing protocol; followed by hematoxylin and eosin staining (H and E). Two oral pathologists examined the coded stained slides and allotted the scores.
The H and E stained sections were graded based on the following parameters [Table 1].[7,8]
Table 1.
Histomorphological criteria

Evaluation
A total score of 3–5 was considered adequate for diagnosis and score of ≤2 was considered inadequate for diagnosis.
Statistical analysis
The results were statistically analyzed, and data were expressed in terms of median ± IQ (Inter-Quartile deviation) and percentage. Comparison between three groups was done by Kruskal–Wallis test followed by post hoc Dunn's multiple comparisons test and Chi-square test or Fisher's exact test for small sample size. A P < 0.05 was considered as significant. Data analysis was done by using software Minitab v14.0.
RESULTS
When all the stained sections were assessed for the nuclear staining, it showed 100% fixing and staining efficiency for processed honey, unprocessed honey and NBF. On evaluation of cytoplasmic stain, there was 92% of adequate staining pattern in processed and unprocessed honey as compared to NBF. There was 75% adequacy for tissue morphology in processed and unprocessed honey as compared to NBF, which showed 92%. There is no statistically significant difference in clarity and uniformity of staining pattern among all the three fixatives [Table 2]. There was no statistically significant difference among the three groups for adequacy of diagnosis [Table 3]. On assessment of artifacts, our study showed a statistically significant difference between unprocessed honey and processed honey when compared with formalin (P = 0.004) [Table 4]. However, processed honey has better fixative properties compared to unprocessed honey [Table 5].
Table 2.
Staining pattern in Group A, B and C

Table 3.
Scores for adequacy of diagnosis for Group A, B and C

Table 4.
Score for artifacts in Group A, B and C

Table 5.
Sensitivity, specificity, positive and negative predictive value for accuracy of diagnosis in Group A and Group B

However, the limitation in this study was the subjective nature of the criteria for assessing the quality by scores.
DISCUSSION
NBF is a well-known fixative and widely used fixative in histopathological laboratories for routine histopathology and immunohistochemistry. Various studies have been conducted in surge of an ideal fixative but till date, there is not even a single fixative that fulfils all the criteria. Though formalin is well established, it has been shown to produce deleterious effects on humans. The two well-known disadvantages of formalin are. First, formalin is highly toxic.[1] In the biological system, formaldehyde reacts with hydrochloric acid in the presence of water to form bis-chloromethyl ether, a known carcinogen for humans.[4] Lu et al.[9] found strong evidence that can support a genotoxic and cytotoxic mode of action for the carcinogenesis of inhaled formaldehyde in the respiratory nasal epithelium. The International Agency for Research on Cancer branch of the World Health Organization classifies formaldehyde as carcinogenic in humans and it has been demonstrated to contribute to respiratory pathologies, allergies and respiratory tract cancers.[10,11] Second, the chemical action of formalin severely binds DNA, RNA and proteins, which makes them difficult or impossible to extract in a useful form.[1]
Fixatives without formaldehyde are available and have generated interest recently because of their lesser likelihood of affecting health.[1,12,13] Honey is the natural sweet substance produced by honeybees from the nectar of flowers. Modern medicine has ignored the useful properties of honey until the appearance of multi-resistant bacteria which led to a rediscovery of its wound healing, tissue preservation and antibiotic properties.[1,14,15] Honey has been shown to inhibit the growth of a wide range of bacteria, fungi, protozoa and viruses. The antibacterial effect of honey depends on its osmotic effect (high sugar and low water content), acidity, hydrogen peroxide formed by enzymatic reaction and phytochemical factors.[16] Most unprocessed honey, when diluted slowly, generates hydrogen peroxide owing to activation of the enzyme, glucose oxidase, which oxidizes glucose to gluconic acid and hydrogen peroxide. Hydrogen peroxide has antibacterial properties.[17] The present study was undertaken to assess and document the efficacy of processed honey and unprocessed honey as an alternative fixative for histological sections.
Of the sections studied, nuclear staining showed 100% fixing and staining efficiency in processed honey, unprocessed honey and NBF fixatives. There was no significant difference in all the three fixative methods. Thus nuclear morphology and details were well preserved in all three fixatives [Table 2 and Figure 1].
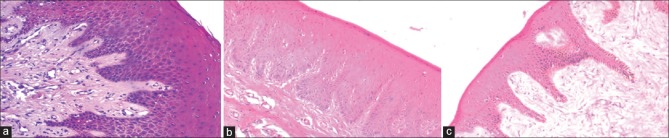
Figure 1.
Photomicrograph showing adequacy of nuclear and cytoplasmic staining in epithelial tissue (a) conventional neutral buffered formalin, (b) processed honey and (c) unprocessed honey (H&E stain, ×100)
When cytoplasmic staining was evaluated, 92% of unprocessed and processed honey showed adequate staining pattern as compared with the NBF. No statistically significant difference was seen, suggesting that they were equivalent to NBF fixative [Table 2 and Figure 1].
When tissue morphology was evaluated, 75% of unprocessed and processed honey showed adequate staining pattern when compared with NBF that showed 92% adequacy. No statistically significant difference was seen, suggesting that they were equivalent to NBF fixative [Table 2].
The architecture of all the tissues such as epithelium, salivary gland, lymphoid and fat was accurately preserved. Although few of the sections fixed with unprocessed honey, showed overall compromised morphology and architecture for the cellular details when in relation to NBF [Figures 2–4].
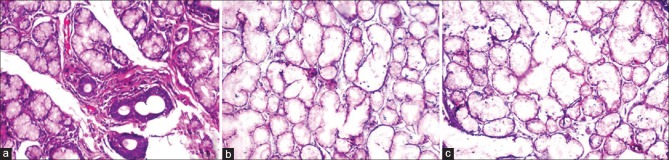
Figure 2.
Photomicrograph showing adequacy of tissue morphology and clarity in salivary gland tissue (a) conventional neutral buffered formalin (H&E stain, ×100), (b) processed honey (H&E stain, ×200) and (c) unprocessed honey (H&E stain, ×200)
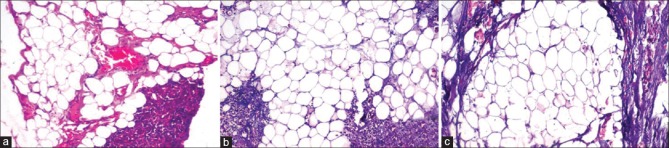
Figure 4.
Photomicrograph showing adequacy of tissue morphology and clarity in adipose tissue (a) conventional neutral buffered formalin, (b) processed honey and (c) unprocessed honey (H&E stain, ×100)
Figure 3.
Photomicrograph showing adequacy of tissue morphology and clarity in lymphoid tissue (a) conventional neutral buffered formalin, (b) processed honey and (c) unprocessed honey (H&E stain, ×100)
On analysis of clarity and uniformity of staining pattern, no statistically significant difference was seen, suggesting that they were equivalent to NBF fixative.
In few areas of the studied section, “out-of-focus” areas were in epithelial tissue. Collagen fibres were more hyalinized in tissues fixed with processed honey and unprocessed honey. Ozkan et al.[12] and Avwirio et al.[13] also confirmed a similar finding in their study where honey was substituted for formalin. These changes could be due to the low pH of both unprocessed (pH-3.6) and processed honey (pH-5.05) fixative solution that did not favor the well preservation of cytoplasm and collagen tissues.
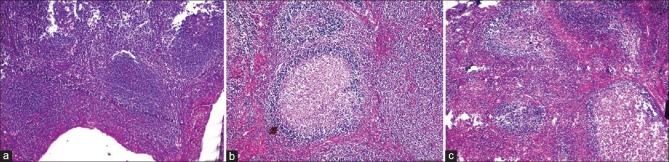
On analysis for artifacts, there was significant difference between unprocessed honey and NBF as compared to processed honey. More number of fixation artifacts such as slit-like spaces at the basement membrane of epithelium and homogeneous eosinophillic stroma in few areas of connective tissue could be due to the shrinkage of tissue during fixation [Figures 5 and 6]. The staining artifact such as over staining and out of focus area were noticed in few areas in all three fixative which could be due to improper removal of wax or fold in the section [Table 4].
Figure 5.
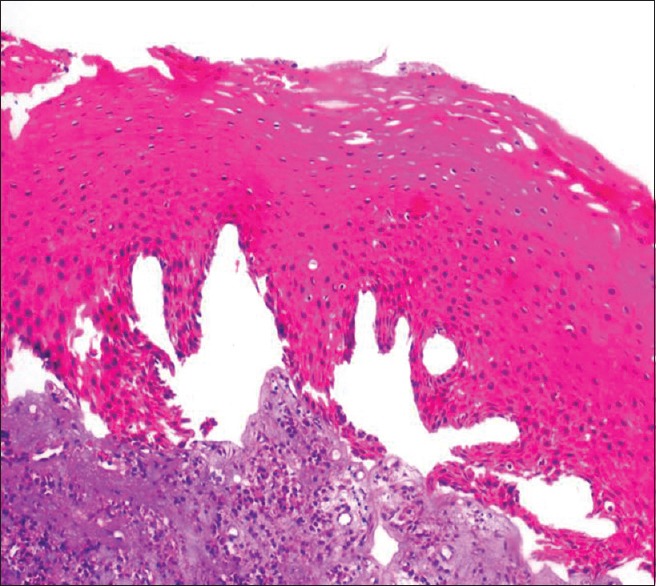
Photomicrograph showing artifact shrinkage in epithelial tissue in unprocessed honey (H&E stain, ×100)
Figure 6.
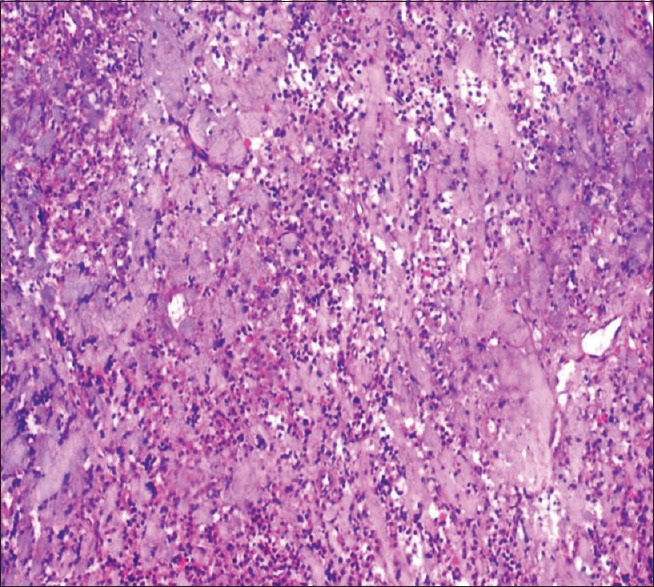
Photomicrograph showing artifactual hyalinized connective tissue in unprocessed honey (H&E stain, ×100)
When the scores for the adequacy of diagnosis were analyzed, there was no statistically significant difference among all the three fixatives, indicating that the tissue architecture and morphology were very well preserved in all the three groups without hampering the appreciation of tissue proper [Table 3]. However, the positive predictive value was better in processed honey compared to unprocessed honey [Table 5]. Based on all these observations from the present study, it can be deduced that both processed honey and unprocessed honey have all the fixative properties that an ideal fixative should have and can be used as an alternative fixative to formalin.
CONCLUSION
To go organic is a theme of the present day; everyone is trying to explore it in their own field to combat the global warming. An attempt was made to explore the natural substance honey as a substitute for fixation of tissues. Apart from homogenization of connective tissue, the quality of fixation with unprocessed and processed honey was comparable to formalin. Processed honey was superior to unprocessed honey. With an added benefit of honey being eco-friendly, easily availabe, cost effective, nontoxic and noninflammable, it can also be used as an effective alternative.
Scope of the study
A follow-up analysis has to be done after a period of 12 months and to check the effectiveness of honey with special stains, IHC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 6th ed. Edinburgh: Churchill Livingstone; 2007. pp. 56–9. [Google Scholar]
- 2.Culling CF, Allison RT, Barr WT. Cellular Pathology Technique. 4th ed. London: Butter Worth and Co., Publishers; 1985. pp. 29–61. [Google Scholar]
- 3.Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem. 1985;33:845–53. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 4.Jayalakshmi K, Ravikumar H, Naidu J, Raghavendra R. A silent killer in the laboratory – Formaldehyde: Review of effects and management. Int J Oral Maxillofac Pathol. 2011;2:13–9. [Google Scholar]
- 5.Clark RP. Formaldehyde in pathology departments. J Clin Pathol. 1983;36:839–46. doi: 10.1136/jcp.36.8.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson S, Jadon N. CSE Study: Antibiotic Residues in Honey. 2010:1–10. [Google Scholar]
- 7.Ankle MR, Joshi PS. A study to evaluate the efficacy of xylene-free hematoxylin and eosin staining procedure as compared to the conventional hematoxylin and eosin staining: An experimental study. J Oral Maxillofac Pathol. 2011;15:161–7. doi: 10.4103/0973-029X.84482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramulu S, Koneru A, Ravikumar S, Sharma P, Ramesh D, Patil R. Liquid dish washing soap: An excellent substitute for xylene and alcohol in Hematoxylin and Eosin staining procedure. J Orofac Sci. 2012;4:37–42. [Google Scholar]
- 9.Lu K, Collins LB, Ru H, Bermudez E, Swenberg JA. Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol Sci. 2010;116:441–51. doi: 10.1093/toxsci/kfq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerns WD, Pavkov KL, Donofrio DJ, Gralla EJ, Swenberg JA. Carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure. Cancer Res. 1983;43:4382–92. [PubMed] [Google Scholar]
- 11.Swenberg JA, Kerns WD, Mitchell RI, Gralla EJ, Pavkov KL. Induction of squamous cell carcinomas of the rat nasal cavity by inhalation exposure to formaldehyde vapor. Cancer Res. 1980;40:3398–402. [PubMed] [Google Scholar]
- 12.Ozkan N, Salva E, Cakalagaoglu F, Tüzüner B. Honey as a substitute for formalin? Biotech Histochem. 2012;87:148–53. doi: 10.3109/10520295.2011.590155. [DOI] [PubMed] [Google Scholar]
- 13.Avwioro G, Bankole J, Iyiola S, Avwioro T, Akinola G. One of the properties of honey in wound healing is prevention of autolysis. Der Pharm Lett. 2010;2:321–5. [Google Scholar]
- 14.Molan PC. Why honey is effective as a medicine. 1. Its use in modern medicine. Bee World. 1999;80:80–92. [Google Scholar]
- 15.Subramanian M. Storage of skin grafts in honey. Lancet. 1993;341:63–4. doi: 10.1016/0140-6736(93)92547-7. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy J. The antibacterial effects of honey. Am Bee J. 1995;1:171–2. [Google Scholar]
- 17.Molan P. Antibacterial properties of honey. Hivelights. 1996;15:19. [Google Scholar]