Abstract
Inverted papilloma is a benign epithelial growth in the underlying stroma of the nasal cavity and paranasal sinuses. The pathogenesis of this lesion remains unclear although allergy, chronic sinusitis and viral infections have been suggested as possible causes. The tumor is well known for its invasiveness, tendency to recur and association with malignancy. Recurrence rates of inverted papilloma are unacceptably high, which actually represents residual disease in most cases. In this study, we have presented a case report and reviewed the histological features of sinonasal inverted papilloma.
Keywords: Benign nasal growth, inverted papilloma, sinonasal papilloma
INTRODUCTION
Inverted papilloma is a benign epithelial growth extending into the underlying stroma of the nasal cavity and paranasal sinus. The tumor is well known for its invasiveness, tendency to recur and association with malignancy.[1,2] In 1854, Ward first documented the occurrence of inverted papilloma in the sinonasal cavity.[3] However, in 1935, Reingertz histologically described the nature of the tumor and noted its classic inverted nature in underlying connective tissue stroma.[4] In 1971, Hymans reviewed several cases of this tumor and subdivided sinonasal papilloma into inverted, fungiform and cylindrical cell types.[1,5,6]
It is a rare benign tumor with incidence rate of 0.6 cases/100,000 people/year.[7] It comprises 0.5–4% of all primary nasal tumors.[8] It usually arises from the lateral nasal wall, in the middle meatus, often extending to the ethmoid and maxillary sinuses. In advance cases, extension into all of the ipsilateral peripheral nervous system may occur whereas intracranial growth and dural penetration are rare.[9]
The pathogenesis of this lesion remains unclear although allergy, chronic sinusitis and viral infections have been suggested as possible causes.[10]
We are presenting a case report of inverted papilloma that showed recurrence.
CASE REPORT
A 26-year-old male patient resident of Nagpur reported at a private E.N.T clinic with a chief complaint of a mass in the right nasal cavity since 3 years. The patient also gave history of right nasal blockage and nasal discharge since 3 years. It was a slow growing growth since onset. The patient gave similar history of right sided nasal mass 5 years back, for which he was operated upon in a private clinic and the mass was removed. The histopathological examination was not performed at that time. He remained alright for 2 years postoperatively. After few months, patient started experiencing intermittent nasal obstruction and nasal discharge in the absence of upper respiratory tract infection. However, as the time passed by these symptoms aggravated and he started experiencing some nasal mass inside right nasal cavity. On anterior rhinoscopy, a solitary, pinkish, pedunculated, irregular, firm mass was located in right nasal cavity. On probing, mass was attached to lateral wall of right nasal cavity and mass did not bleed on touch. Computed tomography (CT) scan was advised and it revealed that the tumor was arising from middle meatus of right nasal cavity and there was widening of osteomeatal complex of right side. On contrast, there was mild to moderate enhancement seen. This mass was quite large and measured approximately 7 cm × 6 cm. Based on the clinical features and radiographic findings, a provisional diagnosis of papilloma was given. The mass was surgically excised by lateral rhinotomy with medial maxillectomy [Figure 1] and the excised tissue was sent to the Department of Oral Pathology and Microbiology for histopathological examination. Gross examination revealed a single bit of soft tissue specimen of size approximately 3 cm × 2 cm, pinkish-white in color, irregular in shape, firm in consistency with rough surface texture. On histological examination, the hematoxylin and eosin stained section showed polypoid tissue covered with pseudostratified columnar ciliated epithelium with admixed mucocytes (goblet cells) and intraepithelial mucous cysts at places, which showed inversion into the underlying connective tissue stroma to form large clefts, ribbon and islands. The connective tissue cores were fibrocellular in nature with chronic inflammatory cells chiefly lymphocytes. Clinicopathologic correlation was suggestive of final diagnosis of inverted papilloma [Figures 2–4].
Figure 1.
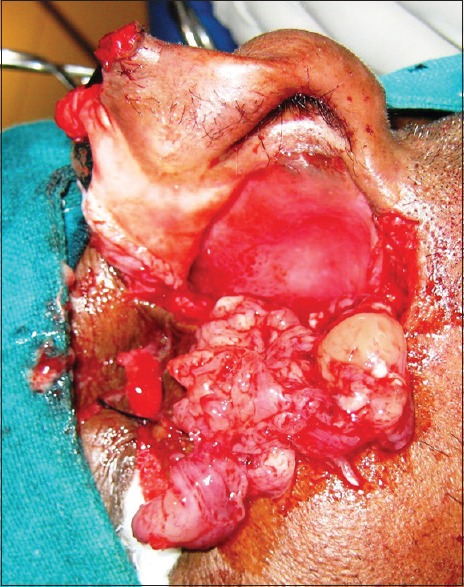
Photograph showing lateral rhinotomy incision
Figure 2.
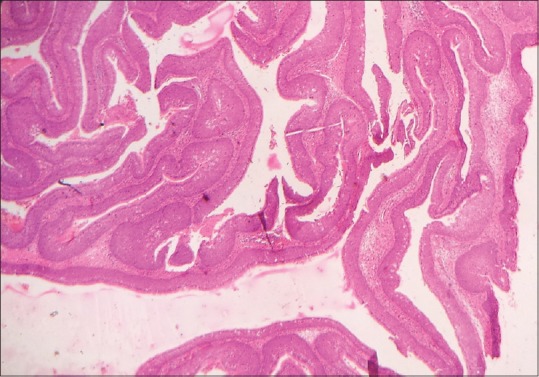
Scanner view of the section shows an endophytic or inverted growth pattern consisting of markedly thickened squamous epithelial proliferation growing downward into the underlying connective tissue stroma to form large clefts, ribbons and islands (H&E stain, x40)
Figure 4.
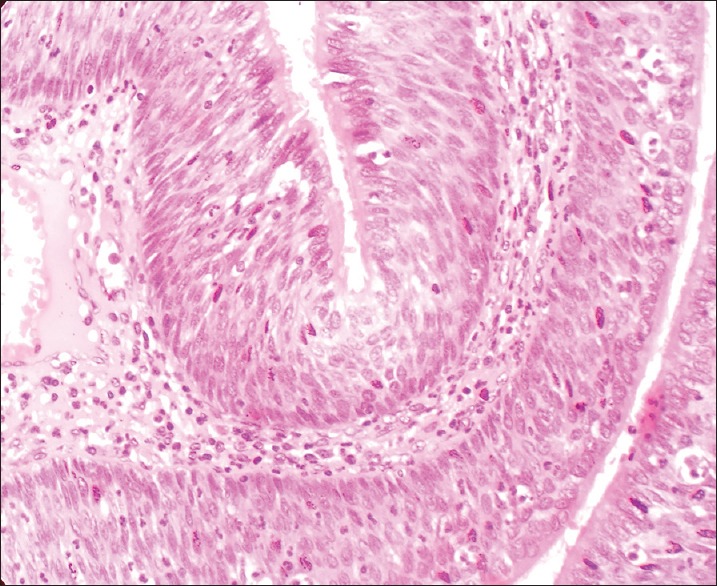
High power shows the epithelium to be composed of pseudostratified columnar cells admixed with mucocytes (goblet cells) and intraepithelial mucin microcysts (H&E stain, x400)
Figure 3.
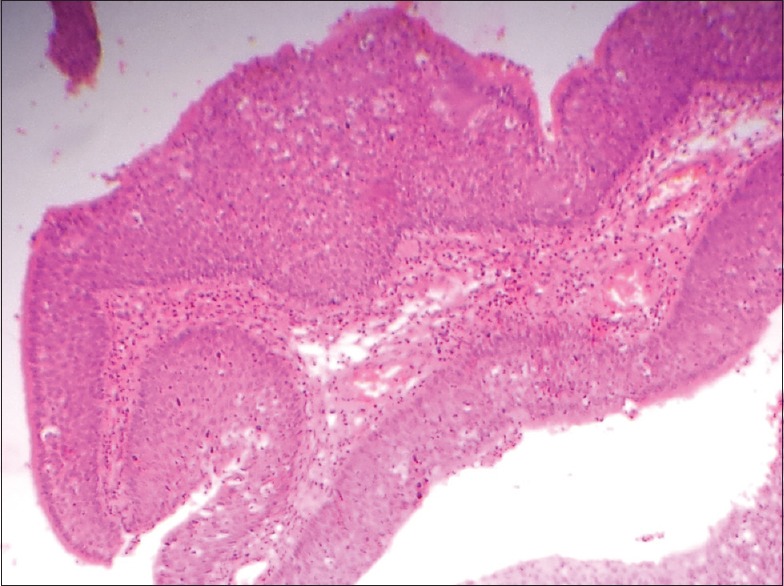
Low power view shows the inverted papillomas with an endophytic or inverted growth pattern consisting of markedly thickened pseudostratified ciliated columnar epithelium growing downward into the underlying stroma (H&E stain, x100)
DISCUSSION
Inverted papilloma (synonym: Ringertz tumor, trasitional cell papilloma, fungiform papilloma, cylindrical cell papilloma, schneiderian cell papilloma, epithelial papilloma, papillary sinusitis, soft papilloma and sinonasal-type papillomas) can be defined as a group of benign neoplasm arising from the sinonasal (Schneiderian) mucosa and is composed of squamous or columnar epithelial proliferation with associated mucous cells.[11] The ectodermally derived lining of the sinonasal tract, termed as the schneiderian membrane, may give rise to three morphologically distinct benign papillomas (Schneiderian papillomas).
Inverted
Oncocytic (cylindrical or columnar cells)
Fungiform (exophytic, septal).
Collectively, schneiderian papillomas represent <5% of all sinonasal tract tumors. The literature indicates that among sinonasal-type papillomas, the septal papilloma is the most common; however, practical experience indicates that the inverted type is the most common subtype and the oncocytic type is the least common. In general, sinonasal-type papillomas occur over a wide age range but are rare in children; however, septal papillomas tend to occur in a younger age group. Inverted papillomas occur along the lateral nasal wall (middle turbinate or ethmoid recesses), with secondary extension into the paranasal sinuses. They may originate in paranasal sinus with/without involving nasal cavity. Typically, the schneiderian papillomas are unilateral; bilateral papillomas may also occur. In the presence of bilaterality, clinical evaluation to exclude the possibility of extension from unilateral disease should be undertaken. Symptoms vary according to the site of occurrence and include airway obstruction, epistaxis and a symptomatic mass or pain. Schneiderian papillomas may occur simultaneously with nasal inflammatory polyps.
The viral etiology has been suggested in few studies and inverted papillomas are reported to be positive for human papillomavirus (HPV) on in situ hybridization and/or polymerase chain reaction. The HPV 6, HPV 11, HPV 16, HPV 18 and Epstein-–Barr virus have been isolated. Whether there is a cause and effect between the presence of HPV and the development of inverted papillomas remains to be determined.
Radiological studies are commonly used to evaluate inverted papilloma with CT scan and magnetic resonance imaging (MRI) scan being the most common. Bony changes including bowing of the bones located near the mass are common CT findings. Tumors involving the maxillary sinus may lead to widening of infundibulum on CT scan, making the uncinate process difficult to discern.[12] “Bone-remodeling” may be a better term to describe the changes that occur secondary to the constant pressure and mass effect on surrounding bony structures from inverted papilloma,[13] commonly seen at the medial wall of the maxillary sinus and lamina papyracea.[1,8] It is postulated that the bony skull base may have limited response to pressure caused by inverted papilloma, leading to more erosive changes rather than remodeling.[1,13,14] In addition, contrast CT scan may demonstrate slight enhancement and calcification.[13] MRI scan with T1-weighted images with contrast and T2-weighted images can be used to differentiate between tumor mass and postoperative secretions.[1,2,13,15,16] Sometimes, in cases without bony destruction, neither CT nor MRI is helpful in the qualitative diagnosis, but they at least provide the suspicion of a neoplasm.[17] MRI is the first imaging modality to perform in the follow-up after removal of inverted papilloma.[18] However, in this case, MRI was not performed since patient was poor. On gross appearance, the inverted papillomas appear to be exophytic, polypoidal and vascular. It is pink to gray in color, with frond-like projections extending from the bulk of the lesion,[11] vary from firm to friable in consistency.
Histologically, inverted papillomas have an endophytic or inverted growth pattern consisting of markedly thickened squamous epithelial proliferation growing downward into the underlying connective tissue stroma to form large clefts, ribbons and islands. The epithelium varies in cellularity and is composed of squamous, transitional and columnar cells (all 3 may be present in a given lesion) with admixed mucocytes (goblet cells) and intraepithelial mucin microcysts. A mixed chronic inflammatory cell infiltrate is characteristically seen within all layers of the surface epithelium. The cells are generally bland in appearance with uniform nuclei and no piling up; however, pleomorphism and cytoplasmic atypia may be present. The epithelial component may demonstrate extensive clear cell features indicative of abundant glycogen content. Mitotic figures may be seen in the basal and parabasal layers, but atypical mitotic figures are not seen. Surface keratinization may be present. The stromal components vary from myxoid to fibrous, with admixed chronic inflammatory cells and variable vascularity. Intraepithelial mucocytes show intra cytoplasmic mucin positive material, which is Mucicarmine positive and diastase – resistant, PAS – positive.
Differential diagnosis includes sinonasal inflammatory polyps, nonkeratinizing respiratory carcinoma and verrucous carcinoma. Sinonasal inflammatory polyps are clinically similar but histopathologically epithelial alterations are seen in inverted papillomas and not in the inflammatory polyps. Sometimes nonkeratinizing respiratory carcinoma mimics inverted papillomas and then they can be differentiated by the presence of dysplastic features in carcinoma. In verrucous carcinoma, characteristically, cleft-like spaces lined by a thick layer of parakeratin extending from the surface deeply into the lesion which is the hallmark of verrucous carcinoma, is seen and is absent in inverted papilloma.[19]
Inverted papilloma is a benign neoplasm with an association with squamous cell carcinoma. Krouse documents the occurrence of malignancy as 9.1% in all patients with inverted papilloma.[20] Although this association is real, the exact relationship is unclear. Squamous cell carcinoma may present in the setting of inverted papilloma in three different circumstances. First, patients may present with small foci of squamous cell carcinoma within inverted papilloma. The patient may also present with malignancy as a separate synchronous lesion and not within inverted papilloma and finally, the patient may present with metachronous carcinoma in areas of prior resection of benign inverted papilloma.[1,11,21]
This association with malignancy, along with a propensity for invasion and recurrence, drives the treatment paradigm for inverted papilloma. Treatment includes complete surgical excision, including the adjacent uninvolved mucosa, as the later is necessary as growth and extension along the mucosa results from the induction of squamous metaplasia in the adjacent sinonasal mucosa. Initially, in 18th century, inverted papillomas were excised via a transnasal closed approach with head light illumination. In fact, these early procedures mimicked polypectomies.[1,22,23] Although the intent of these procedures were curative, recurrence rates of 40–80% were unacceptably high.[2,24]
“Recurrence” actually represents residual disease in most cases, so that basic problem facing the clinician is to determine adequate treatment. Open approaches such as the lateral rhinotomy and midfacial degloving procedures allow increased tumor visualization and more complete resection including maxillectomy, which minimizes the recurrence rates.
It is mandatory to resect not only the tumor but also to remove the mucoperiosteum in areas from which the tumor originates using the drill. Intraoperatively histologic control by frozen section is strongly recommended but, unfortunately, it is not performed due to lack of knowledge or negligence on the part of surgeons.
CONCLUSION
Though the inverted papilloma comprises only 0.5–4% of all primary nasal tumor, one can suspect inverted papilloma if mass in nasal cavity seems to be arising from lateral nasal wall, with involvement of at least 1 paranasal sinus, presenting in male patient of the fifth and sixth decade of life. Common symptoms include unilateral nasal obstruction, epistaxis and sinusitis with nasal discharge. Although the intent of surgical procedures were curative, recurrence rates of 40–80% were unacceptably high. “Recurrence” actually represents residual disease in most cases, so that basic problem facing the clinician is to determine adequate treatment. Open approaches such as the lateral rhinotomy and midfacial degloving procedures allowed for increased tumor visualization and more complete resection including maxillectomy, which minimizes the recurrence rates.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Melroy CT, Senior BA. Benign sinonasal neoplasms: A focus on inverting papilloma. Otolaryngol Clin North Am. 2006;39:601–17. doi: 10.1016/j.otc.2006.01.005. x. [DOI] [PubMed] [Google Scholar]
- 2.Han JK, Smith TL, Loehrl T, Toohill RJ, Smith MM. An evolution in the management of sinonasal inverting papilloma. Laryngoscope. 2001;111:1395–400. doi: 10.1097/00005537-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Ward N. A mirror of the practice of medicine and surgery in the hospitals of London. London Hosp Lancet. 1854;2:87–99. [Google Scholar]
- 4.Ringertz N. Pathology of malignant tumors arising in the nasal and paranasal cavities and maxilla. Acta Otolaryngol. 1938;27:31–42. [Google Scholar]
- 5.Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192–206. doi: 10.1177/000348947108000205. [DOI] [PubMed] [Google Scholar]
- 6.Weissler MC, Montgomery WW, Turner PA, Montgomery SK, Joseph MP. Inverted papilloma. Ann Otol Rhinol Laryngol. 1986;95(3 Pt 1):215–21. doi: 10.1177/000348948609500301. [DOI] [PubMed] [Google Scholar]
- 7.Buchwald C, Nielsen LH, Nielsen PL, Ahlgren P, Tos M. Inverted papilloma: A follow-up study including primarily unacknowledged cases. Am J Otolaryngol. 1989;10:273–81. doi: 10.1016/0196-0709(89)90008-2. [DOI] [PubMed] [Google Scholar]
- 8.Vrabec DP. The inverted Schneiderian papilloma: A 25-year study. Laryngoscope. 1994;104(5 Pt 1):582–605. doi: 10.1002/lary.5541040513. [DOI] [PubMed] [Google Scholar]
- 9.Miller PJ, Jacobs J, Roland JT, Jr, Cooper J, Mizrachi HH. Intracranial inverting papilloma. Head Neck. 1996;18:450–3. doi: 10.1002/(SICI)1097-0347(199609/10)18:5<450::AID-HED8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Brors D, Draf W. The treatment of inverted papilloma. Curr Opin Otolaryngol Head Neck Surg. 1999;7:33–8. [Google Scholar]
- 11.Lawson W, Le Benger J, Som P, Bernard PJ, Biller HF. Inverted papilloma: An analysis of 87 cases. Laryngoscope. 1989;99:1117–24. doi: 10.1288/00005537-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Lee JT, Bhuta S, Lufkin R, Castro DJ. Isolated inverting papilloma of the sphenoid sinus. Laryngoscope. 2003;113:41–4. doi: 10.1097/00005537-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Som PM, Lawson W, Lidov MW. Simulated aggressive skull base erosion in response to benign sinonasal disease. Radiology. 1991;180:755–9. doi: 10.1148/radiology.180.3.1871290. [DOI] [PubMed] [Google Scholar]
- 14.Roobottom CA, Jewell FM, Kabala J. Primary and recurrent inverting papilloma: Appearances with magnetic resonance imaging. Clin Radiol. 1995;50:472–5. doi: 10.1016/s0009-9260(05)83163-0. [DOI] [PubMed] [Google Scholar]
- 15.Yousem DM, Fellows DW, Kennedy DW, Bolger WE, Kashima H, Zinreich SJ. Inverted papilloma: Evaluation with MR imaging. Radiology. 1992;185:501–5. doi: 10.1148/radiology.185.2.1410362. [DOI] [PubMed] [Google Scholar]
- 16.Stankiewicz JA, Girgis SJ. Endoscopic surgical treatment of nasal and paranasal sinus inverted papilloma. Otolaryngol Head Neck Surg. 1993;109:988–95. doi: 10.1177/019459989310900603. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda K, Tanno N, Suzuki H, Oshima T, Kano S, Takasaka T. Unilateral sinonasal disease without bone destruction. Differential diagnosis using diagnostic imaging and endonasal endoscopic biopsy. Arch Otolaryngol Head Neck Surg. 1997;123:198–200. doi: 10.1001/archotol.1997.01900020082012. [DOI] [PubMed] [Google Scholar]
- 18.Petit P, Vivarrat-Perrin L, Champsaur P, Juhan V, Chagnaud C, Vidal V, et al. Radiological follow-up of inverted papilloma. Eur Radiol. 2000;10:1184–9. doi: 10.1007/s003309900292. [DOI] [PubMed] [Google Scholar]
- 19.Weing B.M. Atlas of Head & Neck Pathology. Philadelphia: WB Saunders; 1993. Neoplasms of the Nasal Cavity and Paranasal Sinuses; pp. 65–72. [Google Scholar]
- 20.Krouse JH. Endoscopic treatment of inverted papilloma: Safety and efficacy. Am J Otolaryngol. 2001;22:87–99. doi: 10.1053/ajot.2001.22563. [DOI] [PubMed] [Google Scholar]
- 21.Lawson W, Ho BT, Shaari CM, Biller HF. Inverted papilloma: A report of 112 cases. Laryngoscope. 1995;105(3 Pt 1):282–8. doi: 10.1288/00005537-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Krouse JH. Development of a staging system for inverted papilloma. Laryngoscope. 2000;110:965–8. doi: 10.1097/00005537-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Wormald PJ, Ooi E, van Hasselt CA, Nair S. Endoscopic removal of sinonasal inverted papilloma including endoscopic medial maxillectomy. Laryngoscope. 2003;113:867–73. doi: 10.1097/00005537-200305000-00017. [DOI] [PubMed] [Google Scholar]
- 24.McCary WS, Gross CW, Reibel JF, Cantrell RW. Preliminary report: Endoscopic versus external surgery in the management of inverting papilloma. Laryngoscope. 1994;104:415–9. doi: 10.1288/00005537-199404000-00004. [DOI] [PubMed] [Google Scholar]


