Abstract
Negative pressure wound therapy (NPWT) has been demonstrated to be effective at preventing biofilm-associated infections; however, its role in biofilm prevention is unknown. The present study evaluated the effect of NPWT on biofilm prevention when rapidly initiated following wound contamination. Full-thickness dermal wounds (8 mm) were created in rabbit ears and inoculated with green fluorescent protein-labeled Staphylococcus aureus (S. aureus). At 6 h following inoculation, continuous NPWT at −125 mmHg was initiated, with the wounds on the contralateral ear left untreated in order to serve as self-controls. S. aureus rapidly formed mature biofilms in the wound beds post-inoculation, with a persistent bacterial burden of ~105−107 colony-forming units (CFUs)/wound and impaired wound healing. Compared with the untreated group, NPWT resulted in a significant reduction in biofilm matrix, which was verified by scanning electron microscopy and epifluorescence. A reduction in bacterial counts followed (P<0.05) with ~103 CFUs/wound on postoperative day 13 and improvement in all healing parameters (P<0.05) relative to control wounds. The results of the present investigation suggest that NPWT is an effective strategy to impeding the formation of S. aureus wound biofilms when initiated rapidly following bacterial contamination. The early application of NPWT, aimed at biofilm prevention, may improve wound care.
Keywords: acute wound, bacterial contamination, negative pressure wound therapy, early application, biofilm prevention
Introduction
Bacterial biofilms have long been considered to be one of the most difficult problems in the field of wound care (1–8). Defined as complex microbial communities irreversibly attached to the wound surface and embedded in self-secreted extracellular polymeric substances (EPS), biofilms provide bacteria with an effective barrier against host immune cells and antimicrobial agents (6,9). As the most common pathogen of wound infection, Staphylococcus aureus (S. aureus) has been widely studied by researchers (10–12). The EPS of staphylococcal biofilms are primarily composed of polysaccharide intercellular adhesin (PIA), extracellular DNA (eDNA), protein and cellular debris (13,14). In particular, PIA and eDNA are the main components of the extracellular matrix of biofilms, and their roles in intercellular adhesion and biofilm formation have been investigated by numerous studies (14–20). Furthermore, the compromised open wounds create an ideal microenvironment for bacterial colonization and biofilm development (6). A series of in vitro and in vivo studies have revealed that wound-associated biofilms grow rapidly and mature within 24 h post-infection (12,21–23). Once bacteria form a mature biofilm they are difficult to eradicate (8). Despite traditional therapies such as serial debridement and lavage that can remove the majority of mature biofilms and necrotic tissue, residual bacteria may rapidly reestablish a robust biofilm architecture, causing pain to the patients during the process (24,25). The ultimate consequence is a delay in wound healing and reepithelialization (8,26–28).
The durability of a mature biofilm highlights the importance of preventing biofilm formation at the early stages of wound infection. Although specialized dressings for wound care do have an inhibitory effect on biofilms, the efficacy varies greatly with the type, concentration and release of active compound (29). Antibiofilm agents, such as dispersin B and DNase I, have been demonstrated to be effective in the degradation of biofilm matrix both in vitro and in vivo, although these are lacking in clinical application and standard treatment regimens (9,30). In addition, numerous small molecules targeting bacterial signaling pathways, such as autoinducing peptide, have demonstrated antibiofilm biological activities, although the clinical efficacy and safety of these compounds has not been sufficiently evaluated (9,26,31). Despite considerable research, a significant improvement in biofilm prevention in the clinical setting remains lacking.
Physical therapies have emerged in biofilm management due to their satisfactory efficacy and low risk for microbial resistance (32). In particular, negative pressure wound therapy (NPWT) has been shown to improve the healing process of infected wounds and to avoid biofilm-associated infections when applied as early as possible (33–38). Previous studies have attributed these benefits to the secondary effects of NPWT, including fluid removal, modulation of inflammation, and the stimulation of wound healing signaling pathways (38–41). However, investigations into the role of NPWT in biofilm formation remain limited. Despite individual studies suggesting its compression effect on established mature biofilms in vitro (42–44), the efficacy of NPWT on biofilm prevention remains unclear, particularly in vivo.
The aim of the present study was to evaluate and validate the potential effect of NPWT on biofilm prevention when initiated rapidly following wound contamination. Subsequent changes in bacterial burden and wound healing secondary to NPWT were also investigated. The results may provide a better understanding of the therapeutic regimen required for wound care.
Materials and methods
Ethical statement
All animal experiments in the present study were approved by the Medical Ethics Committee of the Chinese PLA General Hospital (Beijing, China) in compliance with the Guidelines for Care and Use of Animals in Research (45).
Animals
A total of 18 adult female Japanese large-ear white rabbits (age, 3–6 months; weight, ~3 kg; purchased from the Laboratory Animal Center of the Academy of Military Medical Sciences, Beijing, China) were used for this study. The rabbits were acclimated to standard housing and fed ad libitum under constant temperature (22°C) and humidity (45%) with a 12-h light/dark cycle.
Bacterial strains and culture
S. aureus strain RN6390 constitutively expressing green fluorescent protein (obtained the from Chinese PLA Institute for Disease Control and Prevention, Beijing, China) was utilized for wound inoculation. S. aureus was grown overnight and subcultured in Luria-Bertani broth (AOBOX Biotechnology Co. Ltd., Beijing, China) at 37°C until log phase. Cells were harvested by centrifugation at 4°C (5,000 × g), and washed three times with phosphate-buffered saline (PBS). The final bacterial resuspension was diluted with PBS to an optical density of 1.0 at 600 nm equivalent to 105 colony-forming units (CFUs)/µl empirically (11).
Wound protocol and bacterial inoculation
The wounding and bacterial inoculation protocol was based on the previously published wound model with minor modifications (12). Briefly, rabbits were anesthetized by intramuscular injection of a ketamine (45 mg/kg; Gutian Pharma Co., Ltd., Fujian, China) and xylazine (5 mg/kg; Huamu Animal Health Product Co., Ltd., Jilin, China) mixture. Ears were shaved and sterilized twice with 70% ethanol. Following local anesthesia with 1% lidocaine (Yimin Pharmaceutical Co., Ltd, Beijing, China), six standardized 8 mm diameter full-thickness dermal wounds were created by an experienced surgeon on each ventral ear down to the perichondrium using a scalpel. Following hemostasis, the wounds were dressed with semiocclusive IV3000 Transparent Adhesive Film Dressing (Smith & Nephew Healthcare Ltd., Hull, UK). On postoperative day (POD) 3, each wound was inoculated with 1×106 CFUs of S. aureus at a volume of 10 µl. Planktonic bacteria were allowed to proliferate in vivo under the semiocclusive transparent film for a minimum of 6 h to ensure bacterial adhesion and colonization (12,41,46,47).
Study design and treatment protocol
All rabbit ears were used to create acute S. aureus-contaminated wounds (6 wounds/ear; 36 ears). For each animal, the two ears were respectively and randomly assigned to the ‘untreated control group’ and the ‘NPWT group’ (18 ears/group), with the 6 wounds on each ear following the same protocol (32).
The wounds were treated under the previously published protocol with minor modifications (48). Briefly, 6 h postinoculation on POD 3, the wounds were dressed with a standard NPWT dressing (consisting of polyvinyl alcohol foam, semiocclusive transparent dressing and suction tube; Wuhan VSD Medical Science & Technology Co., Ltd., Wuhan, China) trimmed in advance to the appropriate size. The suction tube was then connected to a vacuum pump device (provided by Professor Lei Hu, Beihang University, Beijing, China). Wounds treated with NPWT were subjected to continuous negative pressure at −125 mmHg throughout the study (41,47). Dressings were checked daily and changed on PODs 4, 6, 8 and 10, as recommended for infected wounds by the manufacturer. Animals were sacrificed via an overdose of intravenous pentobarbital sodium (100–240 mg/kg; Sigma-Aldrich, St. Louis, MI, USA) on PODs 4 (n=8), 6 (n=2), 8 (n=2), 10 (n=2) and 13 (n=4). Wounds were harvested using an 8-mm dermal biopsy punch (Miltex, Inc., York, PA, USA) for PIA, eDNA, viable bacterial count and scanning electron microscopy (SEM) analyses, or a scalpel for epifluorescence and histological analyses.
Detection of PIA and eDNA in wound biofilms
The dorsal side of the wound was removed to eliminate the interference of non-specific bacteria outside of the wound surface (32). The tissue samples were placed in centrifuge tubes with 1 ml PBS, and sonicated for 2 min to remove bacterial biofilms from the tissue (32,49). The insoluble material was discarded by centrifugation at 4°C (13,400 × g). In total, 0.5 ml supernatant was used for the measurement of PIA, and the remainder was used for eDNA extraction. An improved Elson-Morgan assay was performed subsequently to measure the levels of PIA (13,19,20,50). Briefly, 0.5 ml supernatant was supplemented with 0.1 ml potassium tetraborate (0.8 mol/l; Vetec; Sigma-Aldrich) in a test tube. Tubes were heated in a boiling water bath for 3 min and were subsequently cooled using tap water. A total of 3 ml Ehrlich's reagent (Vetec; Sigma-Aldrich) was added and, immediately after mixing, the tubes were placed in a water bath at 37°C for 20 min. The reaction was terminated by cooling the tubes with tap water. The results were expressed as the absorbance of the final reaction solution at 585 nm as determined using a spectrophotometer (GeneQuant 1300; GE Healthcare Life Sciences, Logan, UT, USA) (50).
The remaining supernatant was used for the extraction of eDNA using the TIANamp Micro DNA kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer's protocol. The eDNA levels/wound were expressed as the DNA concentration, quantified using a Qubit® 2.0 fluorometer with a Qubit® dsDNA BR Assay kit (both Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol (30).
SEM
Following 2.5% glutaraldehyde and 1% osmium tetroxide fixation, the tissue samples were dehydrated through a series of graded ethanol (30, 50, 70, 80, 90 and 100%) and dried with a critical point dryer (HCP-2; Hitachi, Ltd., Tokyo, Japan) by flooding with liquid carbon dioxide at 5°C for 20 min and raising the temperature to the critical point (~35°C). Subsequently, samples were mounted using double-sided tape and coated with gold in an auto sputter coater (E-1010; Hitachi, Ltd., Tokyo, Japan). Imaging of the tissue samples was performed by SEM (S-3400N; Hitachi, Ltd., Tokyo, Japan) operated at the scanning voltage of 15 kV.
Fluorescent staining and fluorescence light microscopy
The wounds were bisected at the largest diameter and embedded in optimal cutting temperature (O.C.T.) Compound (Sakura Finetek USA, Inc., Torrance, CA, USA), snap-frozen, and stored in liquid nitrogen until cryosectioning. Tissue sections (6 µm) were obtained with a Leica CM1950 freezing microtome (Leica Microsystems GmbH, Wetzlar, Germany). Visualization of biofilm matrix was accomplished by staining with Concanavalin A Texas Red® Conjugate (Invitrogen; Thermo Fisher Scientific, Inc.), specific for S. aureus exopolysaccharides, for 15 min in the dark at room temperature (12). The tissue sections were then rinsed three times in PBS and incubated with 4′,6-diamidino-2-phenylindole dilactate (Invitrogen; Thermo Fisher Scientific, Inc.) for 5 min to visualize the host cells. Fluorescence microscopy was performed with an Olympus BX51 microscope (Olympus Corporation, Tokyo, Japan).
Viable bacterial count measurements
Wounds were pretreated as described in the protocol of PIA measurement. Tissue specimens were homogenized into 1 ml suspension with sterile PBS, followed by sonication for 2 min to disrupt bacterial aggregates in the biofilm. Subsequently, the homogenates were serially diluted with sterile PBS (ranging from 10−1, 10−2, 10−3 to 10−6 times the concentration of the homogenates), plated on Staphylococcus Isolation Agar (Hardy Diagnostics, Santa Maria, CA, USA) and incubated at 37°C for 24 h. A standard colony-counting method was conducted and the results were expressed as the logarithm of CFUs/wound (12,41).
Measurement of wound closure
From POD 0 (the day of wounding), images of the wounds were captured with a digital camera (IXUSi; Canon, Inc., Tokyo, Japan) on each day of dressing change. Wound size was then determined by quantifying the wound area using Image-Pro Plus version 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). The rate of wound closure was expressed as a percentage of the initial wound area (51).
Histological analysis
Wounds were bisected at the maximum diameter and fixed in 10% neutral formalin (Yili Fine Chemicals Co., Ltd., Beijing, China). The tissue samples were then embedded in paraffin, sectioned into 5-µm sections and stained with hematoxylin and eosin. The observation of the tissue sections was performed with an Olympus MVX10 macro-microscope (Olympus Corporation). Images of the stained wounds were captured and analyzed using Image-Pro Plus software, version 6.0. The morphometric parameters included the epithelial and granulation gaps (the distance between the leading edges of newly formed epithelial or granulation tissue) and the epithelial or granulation area (the sum of the area of newly formed epithelial or granulation tissue on the two sides of the wound bed) (28). The measurements were carried out by two independent observers who were blinded to the treatment, and an average result was calculated.
Statistical analysis
Data are reported in graphical form as mean ± standard deviation. Student's t-test (two-tailed and paired) was used to compare the PIA/eDNA content, viable bacterial counts and histological parameters between NPWT-treated wounds and their untreated control. Wound closure was analyzed using repeated-measures analysis of variance, followed by the least significant difference post-hoc test to evaluate the statistical difference between groups at each time point. All analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA) at a significance level of α=0.05.
Results
NPWT reduces PIA and eDNA levels and inhibits the production of extracellular matrix
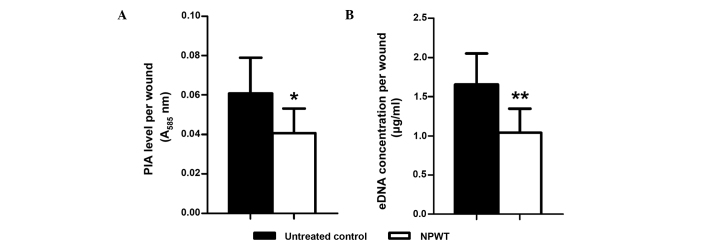
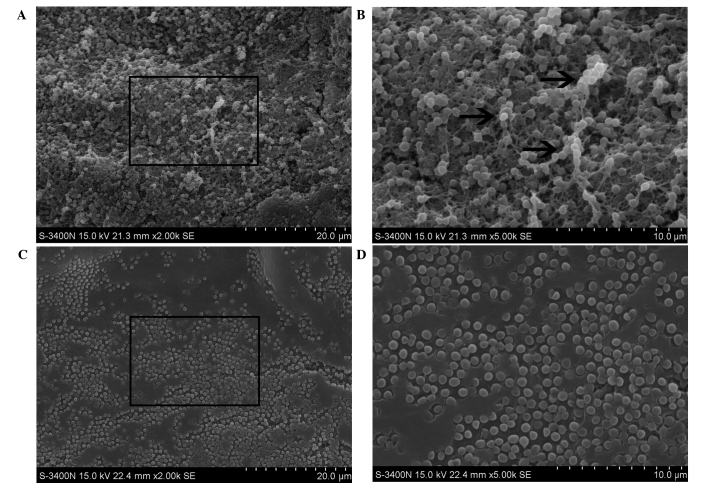
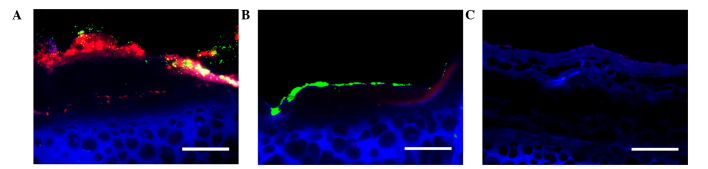
With the previously established rabbit ear wound model, S. aureus-contaminated wounds were treated with or without NPWT from 6 h postinoculation. For the main components of S. aureus biofilm, namely PIA and eDNA, NPWT resulted in a significant reduction in their levels in the wound beds (PIA, P<0.05; eDNA, P<0.01) (Fig. 1). This reduction could be visualized through SEM (Fig. 2). The untreated wounds manifested a relatively intact biofilm structure (Fig. 2A) with large amounts of cocci-shaped S. aureus embedded within the lattice-like extracellular matrix (arrows, Fig. 2B). Conversely, wounds treaded with NPWT showed comparable amounts of bacteria but a lack of extracellular matrix (Fig. 2C), which was more visible at a higher magnification (Fig. 2D). To verify the results, epifluorescence microscopy was performed, showing a mature biofilm with large amounts of exopolysaccharide matrix surrounding the bacteria in untreated wounds (Fig. 3A). By comparison, bacteria in the NPWT group spread over the wound beds with sparse exopolysaccharide matrix (Fig. 3B). An uncolonized area of the wound tissue showed background staining of Concanavalin A, and served as a negative control (Fig. 3C).
Figure 1.
Detection of components of the biofilm matrix from Staphylococcus aureus-infected wounds with and without NPWT treatment. NPWT resulted in significant reductions in (A) PIA and (B) eDNA content relative to untreated wounds. *P<0.05 and **P<0.01 vs. untreated control. Data are presented as mean ± standard deviation (n=10–12 wounds/group). NPWT, negative pressure wound therapy; PIA, polysaccharide intercellular adhesin; eDNA, extracellular DNA; A585, absorbance at 585 nm.
Figure 2.
Scanning electron microscopy of Staphylococcus aureus-infected wounds with and without NPWT treatment. (A) The untreated wounds exhibited a mature biofilm structure. (B) Higher magnification of the same visual field showed large amounts of cocci-shaped Staphylococcus aureus embedded within the lattice-like extracellular matrix (arrows). (C) Conversely, wounds treated with NPWT presented approximately equal amounts of bacteria but lack of extracellular matrix, (D) which was more explicit at higher magnification. NPWT, negative pressure wound therapy.
Figure 3.
Fluorescence light microscopy of NPWT untreated and treated Staphylococcus aureus-infected wounds counterstained with ConA and DAPI. (A) GFP-labeled Staphylococcus aureus (green) formed a mature biofilm on the untreated wound surface (blue), showing a complex structure with large amounts of exopolysaccharide (red) around the bacteria. (B) Staphylococcus aureus in the NPWT group spread over the wound bed with sparse exopolysaccharide matrix. (C) As a negative control, an area of wound tissue with no bacterial aggregates was shown to exhibit background staining of ConA. Scale bar = 100 µm. NPWT, negative pressure wound therapy; ConA, Concanavalin A; DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein.
Prolonged NPWT reduces bacterial load
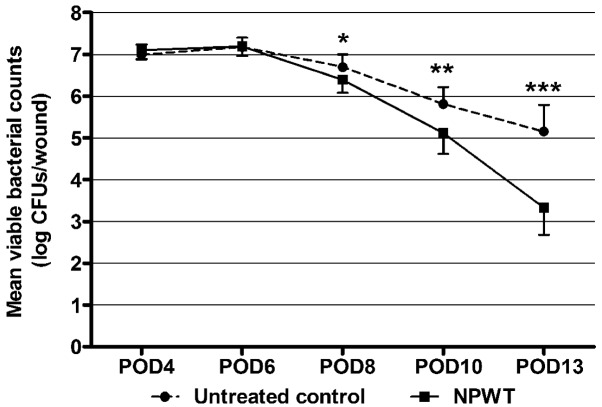
Viable bacterial counts were measured over time to investigate the effect of early application of NPWT on bacterial burden (Fig. 4). On POD 4 and 6, the bacterial load in the wounds treated with NPWT was not significantly different from that in the untreated controls with ~107 CFUs/wound. However, from POD 8 there was a significant decline of the bacterial load in the NPWT group (POD 8, P<0.05; POD 10, P<0.01). At the end of the study (POD 13), NPWT resulted in a significant reduction in the bacterial count by two-log fold compared with the untreated wounds (P<0.001).
Figure 4.
Viable bacterial counts from Staphylococcus aureus-infected wounds with and without NPWT treatment. Serial bacterial counts from untreated wounds revealed a persistent bacterial burden averaging between 105−107 CFUs/wound. However, wounds treated with NPWT showed a gradual but significant reduction in viable bacteria compared with untreated controls, with ~103 CFUs/wound on POD 13. *P<0.05, **P<0.01 and ***P<0.001 vs. the untreated control. Data are presented as mean ± standard deviation (n=10–12 wounds/group/time-point). NPWT, negative pressure wound therapy; CFUs, colony-forming units; POD, postoperative day.
NPWT enhances wound healing
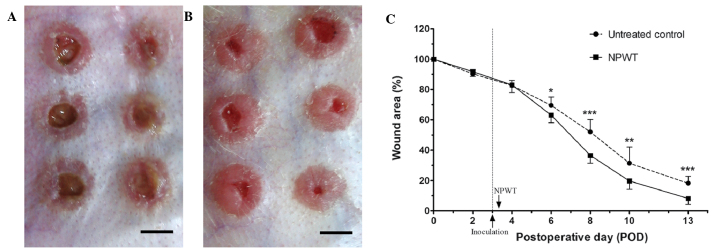
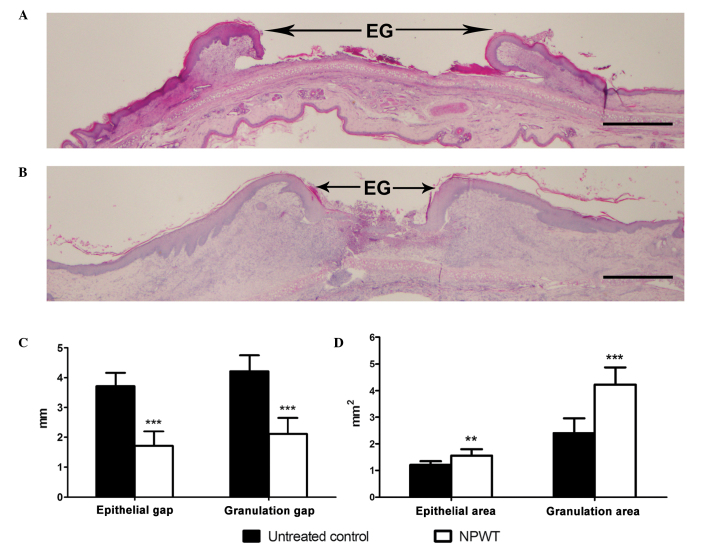
The reduction in bacterial burden secondary to NPWT correlated with a synchronous enhancement in wound healing. Gross appearance on POD 13 manifested a marked difference between the two groups. Compared with the film-like exudates overlying the untreated wounds (Fig. 5A), NPWT accelerated wound closure and epithelialization, with clean granulation tissue beds (Fig. 5B). The rate of wound closure in the NPWT group was significantly higher compared with that of the untreated group (Fig. 5C). Images of histological tissue sections showed increased amounts of new epithelial and granulation tissue in NPWT-treated wounds (Fig. 6B) compared with untreated controls (Fig. 6A). Trends in wound healing were quantified by measuring the epithelial and granulation gaps and areas. NPWT led to a significant reduction in epithelial and granulation gaps (P<0.001) and increase in new epithelial (P<0.01) and granulation (P<0.001) areas compared with the untreated wounds (Fig. 6C and D).
Figure 5.
Appearance of wounds and wound closure rates. (A) The untreated wounds on POD 13 manifested a delay in healing and film-like exudates overlying the wound surfaces. (B) Conversely, NPWT accelerated wound closure and epithelialization, with clean granulation tissue beds. (C) The wound closure rate, shown as the percentage of initial wound area, was significantly increased in the NPWT group compared with the untreated control group from POD 6. (A and B) Scale bar = 5 mm. *P<0.05, **P<0.01 and ***P<0.001 vs. the untreated control. Data are presented as mean ± standard deviation (n=12 wounds/group). NPWT, negative pressure wound therapy; POD, postoperative day.
Figure 6.
Comparison of histological sections and healing parameters between NPWT untreated and treated Staphylococcus aureus-infected wounds. Histological tissue sections, stained with hematoxylin and eosin on POD 13, showed that compared with (A) the untreated control, the amounts of new epithelial and granulation tissue in (B) the NPWT-treated wounds were increased. As determined by quantitative analysis, NPWT was shown to result in improvements in all healing parameters, (C) with a significant reduction in epithelial and granulation gaps and (D) increase in new epithelial and granulation areas. Scale bar =1 mm. **P<0.01 and ***P<0.001 vs. the untreated control. Data are presented as mean ± standard deviation (n=10–12 wounds/group). EG, epithelial gap; NPWT, negative pressure wound therapy; POD, postoperative day.
Discussion
Bacterial biofilm remains a challenging issue in the field of wound care (1–8). Although there is a greater understanding of the necessity of biofilm prevention, the therapeutic strategies to prevent biofilm formation are limited in clinical practice (8,9,24–31). In an effort to seek safe and effective wound care modalities, early application of NPWT has been widely acknowledged for its considerable efficacy and low incidence of biofilm-associated infections (32–38). However, despite an increasing number of studies investigating this type of therapy, the potential role of NPWT in biofilm prevention has yet to be elucidated (37–44). The present study examined and evaluated the effect of NPWT on biofilm prevention when initiated rapidly following wound contamination.
The results indicated that the early application of NPWT may be an effective approach for the prevention of biofilm formation in S. aureus-contaminated wounds. Although the exact mechanism underlying the effects of NPWT has yet to be established, a possible explanation for the benefits observed following treatment with NPWT may be the lack of biofilm matrix. As the structural components of staphylococcal biofilms, PIA and extracellular DNA have an important role in bacterial aggregation and biofilm formation (15–18). Scavenging these matrix components or inhibiting their biosynthesis can effectively prevent biofilm formation and development (9). Previous studies have demonstrated the effectiveness of NPWT in fluid removal and wound cleaning due to the continuous suction (39). The continuous suction may also clear PIA and eDNA from the contaminated wounds before these components are able to form extensive intermolecular crosslinks around the bacteria. In addition, due to the physical effect of negative pressure, NPWT leads to an essentially different microenvironment for wound healing, characterized by hypoxia and microstress (38). These environmental factors, which may stimulate host gene expression and cell proliferation through a variety of signaling pathways (38,40), may also have an effect on the bacteria. Therefore, another possible mechanism is that the environmental factors secondary to NPWT inhibit the biosynthesis of bacterial biofilm components, such as PIA and eDNA. Therefore, the early application of NPWT may prevent biofilm formation in S. aureus-contaminated wound beds.
Without the protection of the biofilm matrix, the bacteria may be eliminated by host immune cells more effectively (9), as shown by viable bacterial counts. Notably, the reduction of the bacterial burden should not be attributed to physical suction, as the bacterial counts did not significantly decrease at the early stages of NPWT. The immune response is more likely to have a leading role in bacterial clearance, as previously demonstrated (41,46). Subsequently, a reduction in biofilm matrix and bacterial burden secondary to NPWT may be beneficial to wound healing, since both of these factors are thought to inhibit the healing process (26–28). In addition, NPWT may also improve wound healing through its ability to stimulate host gene expression and cell proliferation (38–40).
Previous studies have investigated the effect of topical negative pressure used with or without bactericide on established mature biofilms in vitro, and demonstrated the presence of several changes in biofilm morphometric parameters and a reduction in bacterial counts (42–44). However, a compression (perhaps fragmentation) effect on biofilm architecture alone was inferred from the results (42,44). There was no evidence that NPWT was able to remove an established mature biofilm. Unlike these previous studies, the present investigation predominantly focused on the initial stages of wound infection, and examined the efficacy of NPWT on biofilm formation and development. For clinicians and researchers, processes that impede biofilm formation at the initial stage have long been regarded as a promising approach to prevent biofilm-associated infections and chronic infections (25,37). Bacteria without the protection of a biofilm matrix are more susceptible to immune cells and antimicrobial treatment (9,25). The results of the present study suggested that the early use of NPWT rapidly following wound contamination may be more effective than delayed application in impeding biofilm formation. These results are concordant with those of previous studies that have demonstrated a satisfactory efficacy of NPWT on managing acute wounds with contamination when initiated early (33–36). Although further investigations are required in order to clarify the role of biofilm matrix reduction in wound care, the present study provides a possible mechanism of action for this therapy.
Despite rigorous methods and significant results, there were limitations to the study. In particular, the effect of NPWT on S. aureus alone was investigated. As the majority of clinical cases are mixed infections, the investigation of other bacterial species is required during further studies to validate the results discussed herein. In addition, compared with previous studies, NPWT was not combined with traditional therapies, such as bactericide or irrigation. Although biofilm formation and development were inhibited by NPWT alone, viable bacterial counts did not decrease until immune cells infiltrated the wounds. Combined treatment is may have the potential to enhance the elimination of bacteria.
In view of the fact that mature biofilms are difficult to eradicate, the demand for effective and practical measures to avoid biofilm-associated infections is rising in clinical settings. Early application of NPWT to wounds contaminated with S. aureus has shown a significant efficacy on biofilm prevention in the present study. With an increasing number of clinical trials to validate its role in wound infection management, NPWT appears to reduce the accumulation of biofilm matrix, providing more opportunities for host cells or antimicrobial treatments to eliminate the unprotected bacteria. A better understanding of the potential role of NPWT in wound management and biofilm prevention should contribute to innovations in the field of wound care.
Acknowledgements
The present study was supported by a grant from the National Natural Science Foundation of China (grant no. 81472112).
References
- 1.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 3.Parsek MR, Singh PK. Bacterial biofilms: An emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 4.Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17:91–96. doi: 10.1097/00001432-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsay D, von Holy A. Bacterial biofilms within the clinical setting: What healthcare professionals should know. J Hosp Infect. 2006;64:313–325. doi: 10.1016/j.jhin.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 7.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, Costerton JW, Stewart PS. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 8.Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N, Givskov M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008;16:2–10. doi: 10.1111/j.1524-475X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 9.Boles BR, Horswill AR. Staphylococcal biofilm disassembly. Trends Microbiol. 2011;19:449–455. doi: 10.1016/j.tim.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: Report from the SENTRY Antimicrobial Surveillance Program (1998-2004) Diagn Microbiol Infect Dis. 2007;57:7–13. doi: 10.1016/j.diagmicrobio.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Seth AK, Geringer MR, Nguyen KT, Agnew SP, Dumanian Z, Galiano RD, Leung KP, Mustoe TA, Hong SJ. Bacteriophage therapy for Staphylococcus aureus biofilm-infected wounds: A new approach to chronic wound care. Plast Reconstr Surg. 2013;131:225–234. doi: 10.1097/PRS.0b013e31827e47cd. [DOI] [PubMed] [Google Scholar]
- 12.Gurjala AN, Geringer MR, Seth AK, Hong SJ, Smeltzer MS, Galiano RD, Leung KP, Mustoe TA. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen. 2011;19:400–410. doi: 10.1111/j.1524-475X.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- 13.Sadovskaya I, Vinogradov E, Flahaut S, Kogan G, Jabbouri S. Extracellular carbohydrate-containing polymers of a model biofilm-producing strain, Staphylococcus epidermidis RP62A. Infect Immun. 2005;73:3007–3017. doi: 10.1128/IAI.73.5.3007-3017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33:5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Bayles KW. The biological role of death and lysis in biofilm development. Nat Rev Microbiol. 2007;5:721–726. doi: 10.1038/nrmicro1743. [DOI] [PubMed] [Google Scholar]
- 17.Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Gara JP. ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270:179–188. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Wang Y, Tao L. Sulfhydryl compounds reduce Staphylococcus aureus biofilm formation by inhibiting PIA biosynthesis. FEMS Microbiol Lett. 2011;316:44–50. doi: 10.1111/j.1574-6968.2010.02190.x. [DOI] [PubMed] [Google Scholar]
- 20.Sadovskaya I, Chaignon P, Kogan G, Chokr A, Vinogradov E, Jabbouri S. Carbohydrate-containing components of biofilms produced in vitro by some staphylococcal strains related to orthopaedic prosthesis infections. FEMS Immunol Med Microbiol. 2006;47:75–82. doi: 10.1111/j.1574-695X.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 21.Schaber JA, Triffo WJ, Suh SJ, Oliver JW, Hastert MC, Griswold JA, Auer M, Hamood AN, Rumbaugh KP. Pseudomonas aeruginosa forms biofilms in acute infection independent of cell-to-cell signaling. Infect Immun. 2007;75:3715–3721. doi: 10.1128/IAI.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison-Balestra C, Cazzaniga AL, Davis SC, Mertz PM. A wound-isolated Pseudomonas aeruginosa grows a biofilm in vitro within 10 hours and is visualized by light microscopy. Dermatol Surg. 2003;29:631–635. doi: 10.1046/j.1524-4725.2003.29146.x. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama H, Huh WK, Yamasaki O, Oono T, Iwatsuki K. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in mouse skin: Does S. aureus generally produce a biofilm on damaged skin? Br J Dermatol. 2002;147:879–885. doi: 10.1046/j.1365-2133.2002.04962.x. [DOI] [PubMed] [Google Scholar]
- 24.Seth AK, Geringer MR, Gurjala AN, Hong SJ, Galiano RD, Leung KP, Mustoe TA. Treatment of Pseudomonas aeruginosa biofilm-infected wounds with clinical wound care strategies: A quantitative study using an in vivo rabbit ear model. Plast Reconstr Surg. 2012;129:262e–274e. doi: 10.1097/PRS.0b013e31823aeb3b. [DOI] [PubMed] [Google Scholar]
- 25.Wolcott RD, Rumbaugh KP, James G, Schultz G, Phillips P, Yang Q, Watters C, Stewart PS, Dowd SE. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care. 2010;19:320–328. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]
- 26.Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17:354–359. doi: 10.1111/j.1524-475X.2009.00489.x. [DOI] [PubMed] [Google Scholar]
- 27.Roche ED, Renick PJ, Tetens SP, Ramsay SJ, Daniels EQ, Carson DL. Increasing the presence of biofilm and healing delay in a porcine model of MRSA-infected wounds. Wound Repair Regen. 2012;20:537–543. doi: 10.1111/j.1524-475X.2012.00808.x. [DOI] [PubMed] [Google Scholar]
- 28.Seth AK, Geringer MR, Galiano RD, Leung KP, Mustoe TA, Hong SJ. Quantitative comparison and analysis of species-specific wound biofilm virulence using an in vivo, rabbit-ear model. J Am Coll Surg. 2012;215:388–399. doi: 10.1016/j.jamcollsurg.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Brackman G, De Meyer L, Nelis HJ, Coenye T. Biofilm inhibitory and eradicating activity of wound care products against Staphylococcus aureus and Staphylococcus epidermidis biofilms in an in vitro chronic wound model. J Appl Microbiol. 2013;114:1833–1842. doi: 10.1111/jam.12191. [DOI] [PubMed] [Google Scholar]
- 30.Watters C, Everett JA, Haley C, Clinton A, Rumbaugh KP. Insulin treatment modulates the host immune system to enhance Pseudomonas aeruginosa wound biofilms. Infect Immun. 2014;82:92–100. doi: 10.1128/IAI.00651-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan JB. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J Dent Res. 2010;89:205–218. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seth AK, Nguyen KT, Geringer MR, Hong SJ, Leung KP, Mustoe TA, Galiano RD. Noncontact, low-frequency ultrasound as an effective therapy against Pseudomonas aeruginosa-infected biofilm wounds. Wound Repair Regen. 2013;21:266–274. doi: 10.1111/wrr.12000. [DOI] [PubMed] [Google Scholar]
- 33.Costello JP, Amling JK, Emerson DA, Peer SM, Afflu DK, Zurakowski D, Jonas RA, Nath DS. Negative pressure wound therapy for sternal wound infections following congenital heart surgery. J Wound Care. 2014;23:31–36. doi: 10.12968/jowc.2014.23.1.31. [DOI] [PubMed] [Google Scholar]
- 34.Cheng HT, Hsu YC, Wu CI. Risk of infection with delayed wound coverage by using negative-pressure wound therapy in Gustilo Grade IIIB/IIIC open tibial fracture: An evidence-based review. J Plast Reconstr Aesthet Surg. 2013;66:876–878. doi: 10.1016/j.bjps.2012.11.030. [DOI] [PubMed] [Google Scholar]
- 35.Steingrimsson S, Gottfredsson M, Gudmundsdottir I, Sjögren J, Gudbjartsson T. Negative-pressure wound therapy for deep sternal wound infections reduces the rate of surgical interventions for early re-infections. Interact Cardiovasc Thorac Surg. 2012;15:406–410. doi: 10.1093/icvts/ivs254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anagnostakos K, Mosser P. Negative pressure wound therapy in the management of postoperative infections after musculoskeletal tumour surgery. J Wound Care. 2014;23:191–194. doi: 10.12968/jowc.2014.23.4.191. 196-197. [DOI] [PubMed] [Google Scholar]
- 37.Bradley BH, Cunningham M. Biofilms in chronic wounds and the potential role of negative pressure wound therapy: An integrative review. J Wound Ostomy Continence Nurs. 2013;40:143–149. doi: 10.1097/WON.0b013e31827e8481. [DOI] [PubMed] [Google Scholar]
- 38.Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg. 2014;51:301–331. doi: 10.1067/j.cpsurg.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Orgill DP, Manders EK, Sumpio BE, Lee RC, Attinger CE, Gurtner GC, Ehrlich HP. The mechanisms of action of vacuum assisted closure: More to learn. Surgery. 2009;146:40–51. doi: 10.1016/j.surg.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Morykwas MJ, Simpson J, Punger K, Argenta A, Kremers L, Argenta J. Vacuum-assisted closure: State of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117(7 Suppl):121S–126S. doi: 10.1097/01.prs.0000225450.12593.12. [DOI] [PubMed] [Google Scholar]
- 41.Liu D, Zhang L, Li T, Wang G, Du H, Hou H, Han L, Tang P. Negative-pressure wound therapy enhances local inflammatory responses in acute infected soft-tissue wound. Cell Biochem Biophys. 2014;70:539–547. doi: 10.1007/s12013-014-9953-0. [DOI] [PubMed] [Google Scholar]
- 42.Ngo QD, Vickery K, Deva AK. The effect of topical negative pressure on wound biofilms using an in vitro wound model. Wound Repair Regen. 2012;20:83–90. doi: 10.1111/j.1524-475X.2011.00747.x. [DOI] [PubMed] [Google Scholar]
- 43.Phillips PL, Yang Q, Schultz GS. The effect of negative pressure wound therapy with periodic instillation using antimicrobial solutions on Pseudomonas aeruginosa biofilm on porcine skin explants. Int Wound J. 2013;10(Suppl 1):48–55. doi: 10.1111/iwj.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valente PM, Deva A, Ngo Q, Vickery K. The increased killing of biofilms in vitro by combining topical silver dressings with topical negative pressure in chronic wounds. Int Wound J. doi: 10.1111/iwj.12248. doi: 10.1111/iwj.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Division of Research Resources: Guide for the care and use of laboratory animals. 8th. Washington, DC: National Academies Press; 2011. Institute of Laboratory Animal Resources (US). Committee on Care, Use of Laboratory Animals, and National Institutes of Health (US) [Google Scholar]
- 46.Lalliss SJ, Stinner DJ, Waterman SM, Branstetter JG, Masini BD, Wenke JC. Negative pressure wound therapy reduces Pseudomonas wound contamination more than Staphylococcus aureus. J Orthop Trauma. 2010;24:598–602. doi: 10.1097/BOT.0b013e3181ec45ba. [DOI] [PubMed] [Google Scholar]
- 47.Assadian O, Assadian A, Stadler M, Diab-Elschahawi M, Kramer A. Bacterial growth kinetic without the influence of the immune system using vacuum-assisted closure dressing with and without negative pressure in an in vitro wound model. Int Wound J. 2010;7:283–289. doi: 10.1111/j.1742-481X.2010.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen SZ, Li J, Li XY, Xu LS. Effects of vacuum-assisted closure on wound microcirculation: An experimental study. Asian J Surg. 2005;28:211–217. doi: 10.1016/S1015-9584(09)60346-8. [DOI] [PubMed] [Google Scholar]
- 49.Burian M, Rautenberg M, Kohler T, Fritz M, Krismer B, Unger C, Hoffmann WH, Peschel A, Wolz C, Goerke C. Temporal expression of adhesion factors and activity of global regulators during establishment of Staphylococcus aureus nasal colonization. J Infect Dis. 2010;201:1414–1421. doi: 10.1086/651619. [DOI] [PubMed] [Google Scholar]
- 50.Reissig JL, Storminger JL, Leloir LF. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955;217:959–966. [PubMed] [Google Scholar]
- 51.Lim Y, Levy MA, Bray TM. Dietary supplementation of N-acetylcysteine enhances early inflammatory responses during cutaneous wound healing in protein malnourished mice. J Nutr Biochem. 2006;17:328–336. doi: 10.1016/j.jnutbio.2005.08.004. [DOI] [PubMed] [Google Scholar]